Abstract
Concern has been raised over the association of diacetyl with lung disease clinically resembling bronchiolitis obliterans in food manufacturing workers. This has resulted in the need for identification of alternative chemicals to be used in the manufacturing process. Structurally similar chemicals, 2,3-pentanedione, 2,3-hexanedione, 3,4-hexanedione and 2,3-heptanedione, used as constituents of synthetic flavoring agents have been suggested as potential alternatives for diacetyl, however, immunotoxicity data on these chemicals are limited. The present study evaluated the dermal irritation and sensitization potential of diacetyl alternatives using a murine model. None of the chemicals were identified as dermal irritants when tested at concentrations up to 50%. Similar to diacetyl (EC3 = 17.9%), concentration-dependent increases in lymphocyte proliferation were observed following exposure to all four chemicals, with calculated EC3 values of 15.4% (2,3-pentanedione), 18.2% (2,3-hexanedione), 15.5% (3,4-hexanedione) and 14.1% (2,3-heptanedione). No biologically significant elevations in local or total serum IgE were identified after exposure to 25–50% concentrations of these chemicals. These results demonstrate the potential for development of hypersensitivity responses to these proposed alternative butter flavorings and raise concern about the use of structurally similar replacement chemicals. Additionally, a contaminant with strong sensitization potential was found in varying concentrations in diacetyl obtained from different producers.
Keywords: Diacetyl, Butter flavourings, Hypersensitivity, LLNA
1. Introduction
Reports of severe lung disease in employees at a microwave popcorn plant were investigated by scientists from the National Institute for Occupational Safety and Health (NIOSH) (Kanwal et al., 2006; Kreiss et al., 2002). An association was identified between the production of butter-flavored microwave popcorn and bronchiolitis obliterans (BO), a rare lung disease characterized sometimes by airway obstruction, inflammation and scarring occurring in the airways of the lung, resulting in severe shortness of breath, dry cough and sometimes death (Kanwal, 2008; Kreiss, 2007; Kullman et al., 2005; van Rooy et al., 2007). Artificial butter flavorings are proprietary mixtures which can contain more than 100 different volatile chemicals. Extensive environmental sampling initially identified the diketone diacetyl as the predominant component of the butter flavoring in plants with identified cases of BO (Boylstein et al., 2006; Martyny et al., 2008; White et al., 2010).
Published toxicity data for artificial butter flavorings and diacetyl are limited. However, several studies have used animal models to investigate the association between diacetyl exposure and BO. Hubbs et al. found that damage to intrapulmonary airways in rats inhaling butter flavoring vapors occurred after 6-h exposures to concentrations of vapors containing 285 ppm or greater of the diacetyl component (Hubbs et al., 2002). Subsequent studies indicated that pulmonary toxicity, including cellular degeneration and epithelial damage was associated with acute exposure to diacetyl (295 ppm and greater) as a single agent (Hubbs et al., 2008). Intratracheal instillation with a single dose of diacetyl (125 mg/kg) in rats also resulted in airway specific injury, increased airway resistance, lung fluid neutrophilia and extensive intraluminal airway fibrosis characteristic of BO (Palmer et al., 2011). These findings were further supported by inhalation studies in mice which demonstrated significant epithelial injury and peribronchial lymphocytic inflammation (Morgan et al., 2008). Recently, bronchiolitis obliterans-like changes were described in rats inhaling diacetyl vapors at concentrations >150 ppm for 2 weeks (Morgan et al., 2012a). Differences exist in the anatomical location of sites of greatest diacetyl damage in rodents and man which could be explained by species differences in sites of absorption within the respiratory tract (Morris and Hubbs, 2009; Gloede et al., 2011).
Diacetyl (also known as 2,3-butanedione, dimethyl diketone, and 2,3-diketobutane) is commonly used in the flavoring industry to add a buttery odor and flavor to food products. It is currently listed as a “high priority” chemical by the Flavor and Extract Manufacturers Association of the United States (FEMA) indicating that the chemical may pose a respiratory hazard if handled in an unsafe manner. Published toxicity studies also suggest that diacetyl and/or butter flavorings may present a health hazard. Diacetyl does not currently have an Occupational Safety and Health Association (OSHA) permissible exposure limit (PEL). However, the American Conference of Industrial Hygienists (ACGIH) has a threshold limit value (TLV; 0.01 ppm) and a short term exposure limit (STEL; 0.02 ppm) and the National Institute for Occupational Safety and Health (NIOSH) has proposed a recommended exposure limit (REL; 5 ppb) and a STEL (25 ppb) (NIOSH, 2011). Prevention measures such as substitution, engineering controls and respiratory protection aimed at lowering personal exposure levels need to be executed.
As a result of the data indicating diacetyl toxicity, many food manufacturers have turned to flavor alternatives (Barrera, 2011). However, substitutes for diacetyl may also be toxic and both OSHA and NIOSH have expressed concerns regarding their potential toxicity (OSHA, 2010; NIOSH, 2013). The toxicity of one such substitute, 2,3-pentanedione, has been investigated and the results suggest that pulmonary exposure in rats causes airway epithelia damage similar to that produced by diacetyl. Wistar-Han rats exposed to 2,3-pentanedione up to 200 ppm for 6 h/day, 5 day/week for up to 2 weeks had intraluminal and intramural fibrotic airway lesions similar to that of human BO (Morgan et al., 2012b). Studies conducted by Hubbs et al. investigating 2,3-pentanedione also found that following inhalation exposure Sprague–Dawley rats exhibited respiratory epithelial injury and respiratory toxicity comparable to that for diacetyl-induced injury (Hubbs et al., 2012). A recent analysis of butter flavorings used at a microwave popcorn plant identified the presence of 2,3-hexanedione, 2,3-heptanedione and 2,3-pentanedione in one or more of the samples using quantitative and semi-quantitative analysis (Boylstein, 2012). These alternative flavorings are being used in spite of the lack of thorough toxicological investigations (Day et al., 2011).
Our lab has previously shown that diacetyl is a chemical sensitizer when tested in the murine local lymph node assay (LLNA) (Anderson et al., 2007). In addition, reports suggest that flavoring chemicals may be associated with work-related asthma and skin diseases (Sahakian et al., 2008; Akpinar-Elci et al., 2004). This correlation suggests that in addition to BO, diacetyl and other butter flavoring chemicals may play a role in asthma and allergic disease.
The work described in this manuscript evaluates the skin sensitization potential of structurally similar alternative flavoring chemicals to begin to assess their potential role in the development of allergic disease and their safety as alternatives for diacetyl.
2. Materials and methods
2.1. Animals
Female BALB/c mice were used for the murine models. This mouse strain has a Th2 bias and is commonly used to evaluate IgE-mediated sensitization (Klink and Meade, 2003; Woolhiser et al., 2000). The mice were purchased from Taconic (Germantown, NY) at 6–8 weeks of age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to treatment group, weighed, and individually identified via tail marking using a permanent marker or tattoo. A preliminary analysis of variance on body weights was performed to ensure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of 5 per cage in ventilated plastic shoebox cages with hardwood chip bedding, NIH-31 modified 6% irradiated rodent diet (Harlan Teklad), and tap water was provided from water bottles, ad libitum. The temperature in the animal facility was maintained between 68 and 72 °F and the relative humidity between 36% and 57%. The light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
2.2. Chemicals
The following butter flavoring chemicals were purchased from Sigma–Aldrich Chemical Company, Inc. (Milwaukee, WI) and used to generate data for Figs. 1, 2 and Table 1: 2,3-hexanedione (90%, CAS# 3848-24-6), 3,4-hexanedione (95%, CAS# 4437-51-8), 2,3-heptanedione (>97%, CAS# 96-04-8), 2,3-butanedione/diacetyl (>97%, CAS# 431-03-8), 2,3-pentanedione, (97%, CAS# 600-14-6). Diacetyl was also purchased from the producers TCI America (98%; Portland, OR), Fluka (99%; Milwaukee, WI) and Acros Organics (99%; Morris Plains, NJ) for comparison purposes to evaluate a potential contaminant (Fig. 3). Additional chemicals used for these studies were purchased from Sigma–Aldrich and include: α-hexylcinnamaldehyde (HCA, CAS 101-86-0), 2,4-dinitrofluorobenzene (DNFB, CAS 70-34-8), O-(2,3,4,5,6-pentafluoro-benzyl)hydroxylamine hydrochloride (PFBHA) (98+%), furfuryl propionate (>98%), 2-ethoxyethyl acetate (98%), 2-butoxyethyl acetate (99%), toluene 2,4-diisocyanate (TDI, CAS 584-84-9), 2,2,2-trifluoroethylamine hydrochloride (TFEA, 98%), N-(3-dimethylaminopropyl)–N′-ethylcarbodiimide hydrochloride (EDC ≥ 98%), pyridine (99.8%), tert-butyl methyl ether (MTBE, ≥ 99.8%). HPLC grade methanol and hexane Optima grade (95%) were purchased from Fisher Scientific (Pittsburgh, PA). Water (DI H2O) was distilled, deionized to a resistivity of 18 MΩ cm and filtered using a Milli-Q® filter system (Billerica, MA). Helium (UHP grade), the carrier gas, and Nitrogen (UHP grade), were supplied by Butler Gas (McKees Rocks, PA) and used as received. Experiments were carried out at (297 ± 3) K and 1 atmosphere pressure. Helium (UHP grade), the GC carrier gas and nitrogen (UHP grade) were supplied by Amerigas (Sabraton, WV) and used as received.
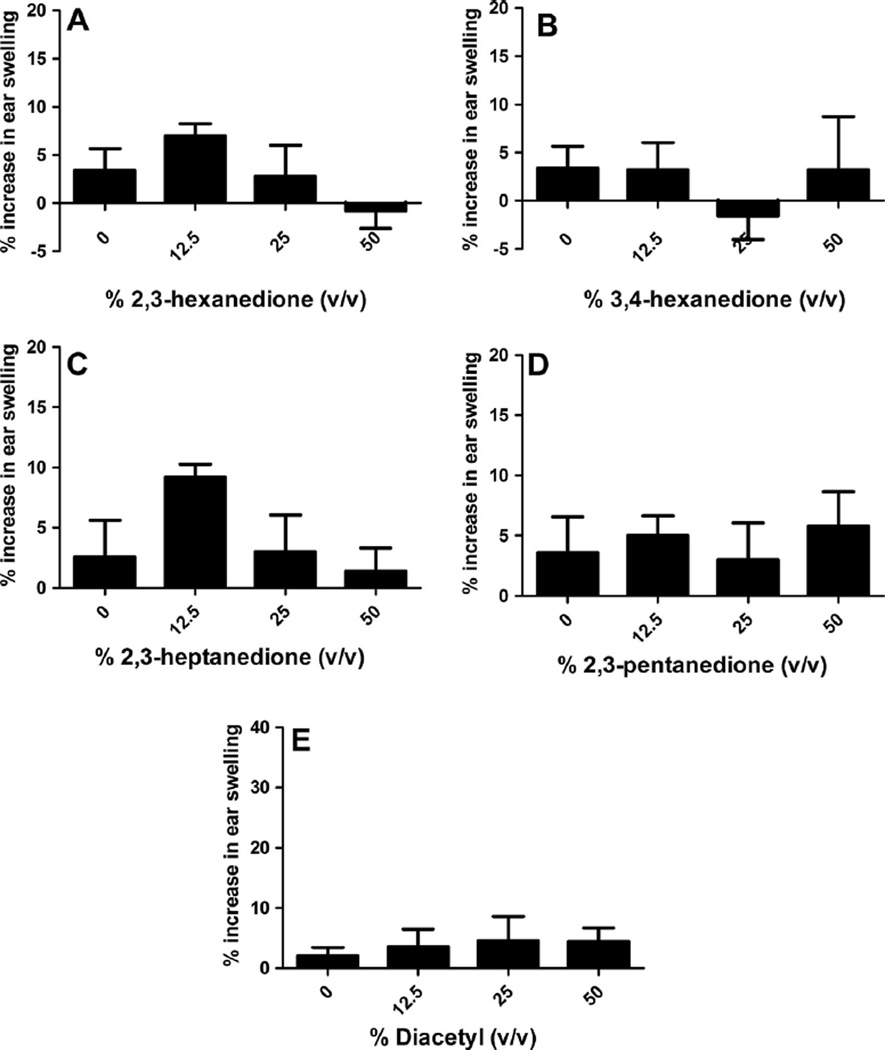
Fig. 1.
Ear swelling as a result of dermal exposure to butter flavorings. Analysis of irritation after exposure to 2,3-hexanedione (A), 3,4-hexanedione (B), 2,3-heptanedione (C), 2,3-pentanedione (D), and diacetyl (E). Bars represent means ± SE of 5 mice per group.
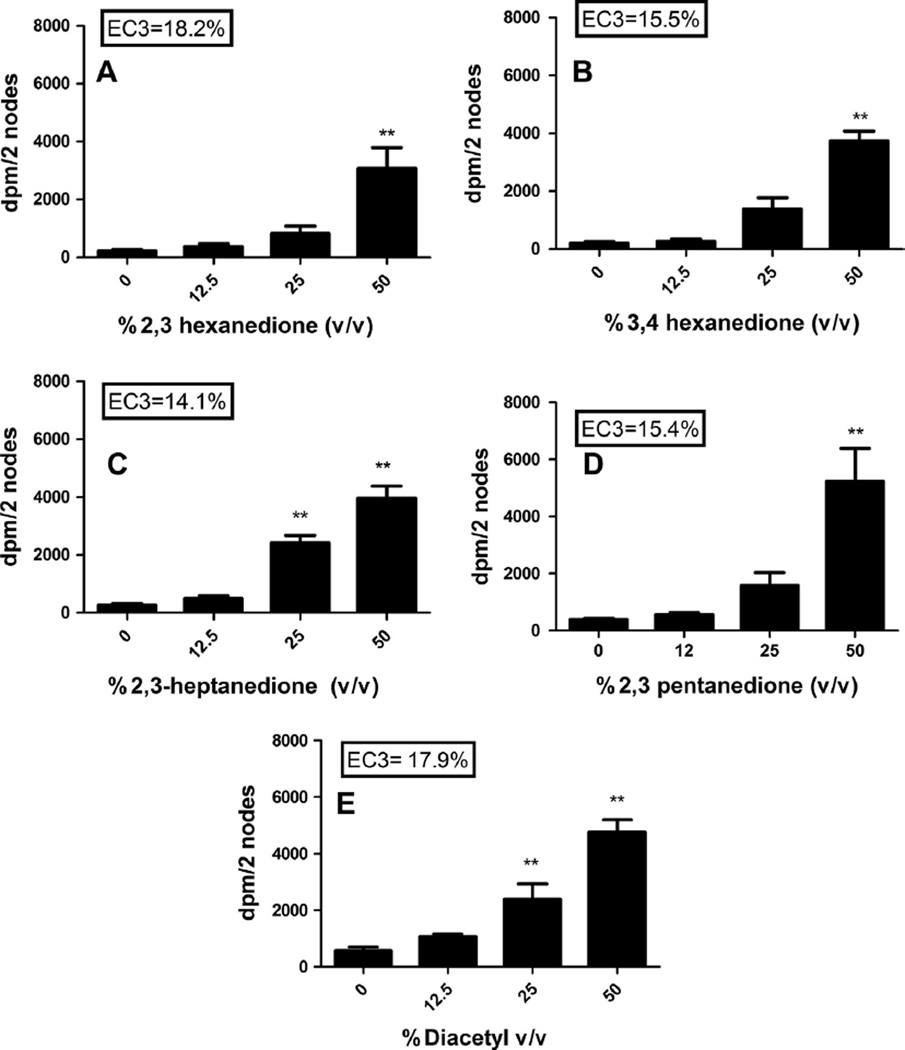
Fig. 2.
Sensitization potential after dermal exposure to butter flavorings. Analysis of the sensitization potential of 2,3-hexanedione (A), 3,4-hexanedione (B), 2,3- heptanedione (C), 2,3-pentanedione (D), and diacetyl (E) using the LLNA. 3H-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentration of butter flavoring shown above. Bars represent means ± SE of 5 mice per group. Levels of statistical significance are denoted as **(p ≤ 0.01) as compared to acetone vehicle.
Table 1.
LLNA/phenotypic analysis and total IgE dose response studies.
| Dose group | LLNA (DPM) | % IgE + B220 + (% lymphocyte population) | %B220 + (% lymphocyte population) | Total IgE (ng/ml) |
|---|---|---|---|---|
| Acetone | 208 ± 63 | 1.08 ± 0.49 | 13.91 ± 0.82 | 446 ± 106 |
| 2,3-Hexanedione | ||||
| 12.5% | 385 ± 93 | 0.60 ± 0.31 | 16.22 ± 0.98 | 347 ± 78 |
| 25% | 829 ± 257 | 1.39 ± 0.57 | 17.26 ± 0.94 | 409 ± 25 |
| 50% | 3075 ± 720** | 10.04 ± 2.69* | 26.73 ± 1.03* | 371 ± 133 |
| Acetone | 208 ± 63 | 1.08 ± 0.49 | 13.91 ± 0.82 | 445 ± 106 |
| 3,4-Hexanedione | ||||
| 12.5% | 273 ± 77 | 0.95 ± 0.61 | 17.14 ± 1.46 | 353 ± 62 |
| 25% | 1385 ± 390 | 4.18 ± 1.01 | 24.02 ± 2.38 | 325 ± 61 |
| 50% | 3745 ± 334** | 21.66 ± 3.11** | 29.21 ± 1.80** | 401 ± 45 |
| Acetone | 274 ± 49 | 1.23 ± 0.28 | 14.88 ± 1.59 | 354 ± 103 |
| 2,3-Heptanedione | ||||
| 12.5% | 497 ± 97 | 1.71 ± 0.27 | 12.69 ± 1.22 | 491 ± 146 |
| 25% | 2421 ± 252** | 4.62 ± 0.92 | 17.05 ± 1.31 | 338 ± 54 |
| 50% | 3970 ± 415** | 22.26 ± 0.81** | 26.26 ± 1.28* | 587 ± 189 |
| Acetone | 389 ± 45 | 1.27 ± 0.11 | 10.29 ± 10.37 | 292 ± 110 |
| 2,3-Pentanedione | ||||
| 12.5% | 560 ± 77 | 1.73 ± 0.47 | 12.20 ± 1.21 | 374 ± 118 |
| 25% | 1584 ± 453 | 2.04 ± 1.18 | 15.00 ± 1.56 | 337 ± 120 |
| 50% | 5230 ± 1152** | 15.56 ± 1.14** | 20.55 ± 2.21* | 483 ± 143 |
| Acetone | 578 ± 116 | 1.50 ± 0.75 | 15.40 ± 1.05 | 332 ± 117 |
| Diacetyl (Aldrich) | ||||
| 12.5% | 1060 ± 110 | 2.59 ± 0.97 | 17.09 ± 1.29 | 387 ± 65 |
| 25% | 2392 ± 536** | 7.06 ± 1.54* | 20.15 ± 1.91 | 450 ± 144 |
| 50% | 4770 ± 430** | 14.86 ± 4.32** | 28.89 ± 1.56* | 521 ± 133 |
Bars represent means ± SE of 5 mice per group.
Levels of statistical significance are denoted as *(p ≤ 0.05) and **(p ≤ 0.01) as compared to acetone vehicle.
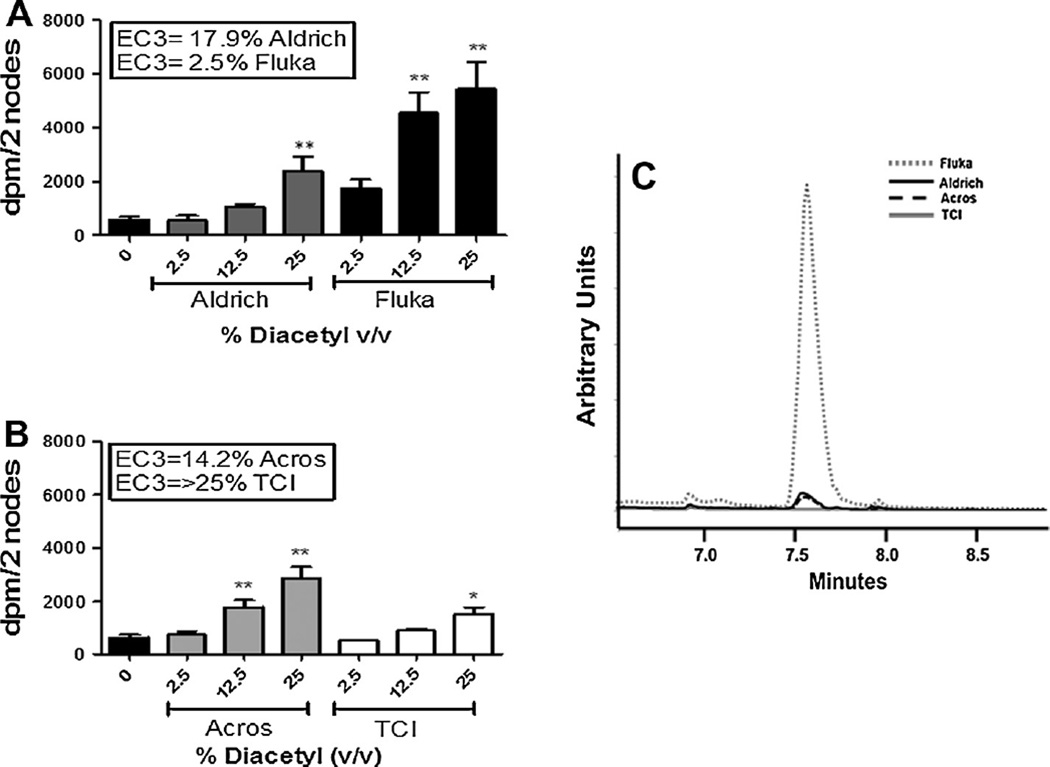
Fig. 3.
Identification of a potential diacetyl contaminant. Analysis of the sensitization potential of diacetyl produced by Aldrich & Fluka (A) and Acros & TCI (B) using the LLNA and of diacetyl products. 3H-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentration of butter flavoring shown above. Bars represent means ± SE of 5 mice per group. Levels of statistical significance are denoted as *(p ≤ 0.05) and **(p ≤ 0.01) as compared to acetone vehicle. (C) Overlaid gas chromatograms of prepared 0.082 M diacetyl solutions (see text) showing the variation in contaminant concentration. All chromatograms are on the same scale.
2.3. Concentration range finding studies
Concentration range finding studies were performed to select the concentrations of the butter flavorings to be used for dermal exposures. Mice (3 per group) were exposed topically to acetone vehicle or increasing concentrations of test article (up to 50%) in acetone on the dorsal surface of each ear (25 µl per ear) for 3 consecutive days. Acetone was selected as the appropriate vehicle based on solubility, historical control data, and accepted use in skin sensitization studies (NIEHS, 1999). Animals were allowed to rest for 2 days following the last exposure and then weighed and examined for signs of overt toxicity including loss of body weight, fatigue/lack of activity, and ungroomed fur.
2.4. Combined irritancy and local lymph node assay
To determine the irritancy and sensitization potential of the butter flavorings, a combined LLNA was conducted as previously described (Anderson et al., 2007) and according to the method described in the ICCVAM Peer Review Panel report (NIEHS, 1999) with minor modifications. Briefly, mice (5 per group) were exposed topically to acetone vehicle, increasing concentrations of test agent, or positive control (HCA) on the dorsal surface of each ear (25 µl per ear) for three consecutive days. HCA (30% HCA) is an accepted and well characterized positive control for the LLNA (NIEHS, 1999). DNCB (0.3%) was used as a positive control for the irritancy portion of the experiment. For irritancy evaluation, the thickness of the right and left ear pinnae of each mouse was measured using a modified engineer’s micrometer (Mitutoyo Co., Japan) before the first chemical administration and 24 h following the final exposure. The mean percentage of ear swelling was calculated based on the following equation: [(mean post-challenge ear thickness – mean pre-challenge ear thickness)/mean pre-challenge thickness] × 100. Animals were allowed to rest for 2 days following the last exposure. On day 6, mice were injected intravenously via the lateral tail vein with 20 µCi 3H-thymidine (Dupont NEN; specific activity 2 Ci/mmol). Five hours after 3H-thymidine injection, animals were euthanized via CO2 inhalation, and the left and right auricular draining lymph nodes (DLN; drain site of chemical application) located at the bifurcation of the jugular vein were excised and pooled for each animal. Single cell suspensions were made and incubated overnight in 5% trichloroacetic acid and samples were counted using a Packard Tri-Carb 2500TR liquid scintillation analyzer. Stimulation indices (SI) were calculated by dividing the mean disintegrations per minute (DPM) per test group by the mean DPM for the vehicle control group. EC3 values (concentration of chemical required to induce a 3-fold increase over the vehicle control) were calculated based on the equation from Basketter et al. (1999). Dosing concentrations (12.5–50%) were selected based on the results from the concentration range finding studies. The concentration of chemical required to induce a 3-fold increase over the vehicle control (EC3) was calculated based on the equations from Basketter et al. (1999).
2.5. Phenotypic analysis of draining lymph node cells
To further evaluate the mechanisms of the hypersensitivity response, the number of IgE + B220+ cells in the DLN was quantitated using flow cytometry. Soluble IgE bound to the B-cell surface via the low affinity IgE receptor (CD23) is dependent on the level of soluble IgE present in the local DLN environment (representative of local IgE levels) and changes in this population following allergen exposure have been detected earlier than serum IgE levels. Manetz and Meade (1999) have shown that select chemicals capable of inducing Th2-mediated allergic responses, have similar peak increases in the percent IgE + B220 + and B220 + populations and these populations tend to become significantly elevated at equivalent concentrations. For the phenotypic analysis, mice (5 per group) were exposed to 25 µl/ear of the acetone vehicle, increasing concentrations of test article (12.5%, 25%, and 50%), or positive control (2.5% TDI) once daily for 4 consecutive days. Animals were weighed and examined for gross pathology at the end of the experiment (Day 10). DLN were collected (2 nodes/animal/3 ml PBS) and dissociated using the frosted ends of 2 microscope slides, and phenotypes were analyzed using flow cytometry as described by Manetz and Meade (1999). The following organs were also removed, cleaned of connective tissue and weighed: liver, spleen, kidneys, and thymus. Serum was collected for total IgE analysis (see below).
2.6. Ige antibody levels
Following euthanasia of animals included in the phenotyping study, blood samples were collected via cardiac puncture. Sera were separated by centrifugation and frozen at −20 °C for subsequent analysis of total IgE by ELISA. A standard colorimetric sandwich ELISA was performed as previously described (Anderson et al., 2007).
2.7. Statistical analysis
For analysis, data was first tested for homogeneity using the Bartlett’s Chi Square test. If homogeneous, a one-way analysis of variance (ANOVA) was conducted. If the ANOVA showed significance at p < 0.05 or less, the Dunnett’s Multiple Range t test was used to compare treatment groups with the control group. Linear trend analysis was performed to determine if the test articles had exposure concentration-related effects for the specified endpoints. Statistical analysis was performed using Graph Pad Prism version 5.0 (San Diego, CA). Statistical significance is designated by *(p ≤ 0.05) and **(p ≤ 0.01).
2.8. Diacetyl contaminant identification investigation
Due to variations in the sensitization response between the results of these studies and those previously reported (Anderson et al., 2007); diacetyl from different producers was evaluated for the presence of a contaminant. Separate solutions (0.082 M) were prepared with diacetyl from each producer (Fluka, Aldrich, TCI, and Acros) by adding 14 µL of the chemical to 2.0 mL of methanol. A 1 µL sample of each prepared solution was used for gas chromatograph/mass spectrometric analysis as described below. Results are shown in Fig. 3C.
To identify possible oxygenated compounds (i.e., aldehydes, ketones, and dicarbonyls) present in the purchased diacetyl, 100 µL of O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine hydrochloride (PFBHA) (250 mM in deionized water) was added to 10 µL of diacetyl(Fluka or TCI)/methanol solution described above in 2 mL of deionized water. This solution was heated for approximately 1 h in a 70 °C water bath to accelerate the derivatization reaction. The solution was removed from the water bath and allowed to cool, then derivatization for carboxylic acid groups was initiated by addition of 1 mL of 0.8 M EDC in H2O, 1 mL of 0.8 M TFEA in H2O, and 50 µL of pyridine to the aqueous sample and then letting the samples react for 15 min (Ford et al., 2007). This solution was then extracted with 3 mL of methyl tert-butyl ether (MTBE). The MTBE layer, containing the derivatized compounds (oximes and/or 2,2,2-trifluoroethylamides) was removed (ca. 2.5 mL) and blown to dryness with clean air and reconstituted in 100uL methanol. It should be noted that diacetyl is also derivatized by PFBHA resulting in diacetyl oxime peaks in the chromatogram. Samples were analyzed using a Varian (Palo Alto, CA) 3800/Saturn 2000 gas chromatograph/mass spectrometer (GC/MS) system operated in the electron impact (EI) mode. Compound separation was achieved by a Restek (Bellefonte, PA) Rtx-5MS (0.25 mm I.D., 30-m long, 1 µm film thickness) column and the following GC oven parameters: 40 °C for 2 min, then 10 °C min−1 to 140 °C, then 20 °C min−1 to 280 °C and held for 8 min. The GC injector was initially held at 60 °C for 2 min, then ramped at 200 °C min−1 300 °C. Each sample (1 µL) was injected in the splitless mode and the injector was returned to split mode 1 min after sample injection. The Saturn 2000 ion trap mass spectrometer was tuned using perfluorotributylamine (FC-43). Full-scan EI spectra were collected from m/z 40–650. Each sample was analyzed in duplicate. Acetonitrile was the chemical ionization (CI) reagent used for all CI spectra.
3. Results
3.1. Toxicity
There were no chemical-related deaths, changes in body weights, or clinical signs of toxicity in mice following exposure concentrations as high as 50% of 2,3-pentanedione, diacetyl, 2,3-hexanedione, 3,4-hexanedione, or 2,3-heptanedione (data not shown). There were no biologically significant changes in liver, kidney, thymus or spleen weights following exposure to any of the butter flavorings (data not shown).
3.2. Irritancy as indicated by ear swelling
Dermal exposure to 2,3-hexanedione, 3,4-hexanedione, 2,3- heptanedione, 2,3-pentanedione, or diacetyl did not induce significant ear swelling 24 h post final chemical exposure (Fig. 1). DNFB (0.3%) was used as a positive control for irritancy studies and resulted in an average significant increase of 129% ear swelling post exposure for all studies (data not shown).
3.3. Sensitization potential determined by the llna
The butter flavorings, 2,3-hexanedione, 3,4-hexanedione, 2,3-heptanedione, 2,3-pentanedione, and diacetyl all tested positive in the LLNA with EC3 values of 18.2%, 15.5%, 14.1%, 17.9%, and 15.4%, respectively (Fig. 2). The concentrations tested were determined based on the results of the concentration range finding studies. Dose responsive increases (Linear Trend Test p < 0.05) in lymphocyte proliferation were observed following exposure to all butter flavorings reaching statistical significance at 25% for 2,3-heptanedione and diacetyl and 50% for 2,3-hexanedione, 2,3-pentanedione and 3,4-hexanedione (Table 1). HCA (30%) was used as a positive control for these experiments and resulted in an average stimulation index (SI) value of 9.8 for the two studies (data not shown).
3.4. Lymph node phenotyping and analysis of total serum ige
Phenotypic analysis of the draining lymph nodes of mice exposed to all butter flavorings showed dose responsive increases (Linear Trend Test p < 0.05) in both the B220+ and IgE+B220+ cell populations (Table 1). Consistent with the LLNA results, statistical significance in the% B220+ cells was reached following exposure to 2,3-pentanedione (20.55 ± 1.03 2.21; 50%), 2,3-hexanedione (26.73 ± 1.03; 50%), 2,3-heptanedione (26.26 ± 1.28; 50%) 3,4-hexanedione (24.02 ± 2.38; 25%) and diacetyl (28.89 ± 1.56; 50%). Statistical significance in the percent IgE + B220 + cells was also reached following exposure to each butter flavoring (15.56 ± 1.42 for 2,3-pentanedione (50%); 10.04 ± 2.69 for 2,3-hexanedione (50%); 22.26 ± 0.81 for 2,3-heptanedione (50%); 21.66 ± 3.11 for 3,4-hexanedione (50%); and 7.06 ± 1.54 for diacetyl (25%)), although based on the paradigm described by Manetz and Meade, only 3,4-hexanedione and 2,3-heptanedione were considered to be biologically significant (1999). TDI (1.5%) was used as a positive control for these experiments and resulted in significant elevations of IgE+B220+ (28.31 ± 1.08) and B220+ (31.27 ± 1.09) cell populations. Elevations in serum IgE is commonly used as an indicator of a type I hypersensitivity response to dermal sensitizers. Exposure to 2,3-pentanedione, 2,3-hexanedione, 3,4-hexanedione, 2,3-heptanedione, and diacetyl did not produce elevations in total serum IgE levels (Table 1). TDI (1.5%) was used as a positive control for these experiments and resulted in a significant elevation of total IgE (~2100 ng/ml) when compared to vehicle (~446 ng/ml).
3.5. Identification of diacetyl contaminant
Earlier studies conducted in this laboratory revealed a SI of 1.9% for diacetyl. Given the inconsistency with the results from these studies, the sensitization potential of diacetyl purchased from different vendors was evaluated (Fig. 3C). The sensitization potential of diacetyl obtained from all vendors except Fluka was found to be similar to those reported above (EC3 values of 14.2–25%). However, the EC3 value for the diacetyl obtained from Fluka was much lower and consistent with previously published data, suggesting the presence of a possible contaminant. Chemical characterization of the diacetyl from different vendors identified a chromatographic peak that was not attributable to diacetyl at retention time 7.6 min for each of the diacetyl samples (Fig. 3C). Interestingly the EC3 values of diacetyl purchased from different vendors appeared to be proportional to the level of the contaminant. Based on Fig. 3C it was determined that the peak area of the contaminant, which is proportional to concentration, varied over seven orders of magnitude across the diacetyl samples. The highest contaminant concentration (largest peak area) was detected in the Fluka sample which also had the lowest EC3 value (indicating the greatest potency).
When characterizing the contaminant, the chromatographic peak was found to have ions of m/z (relative abundance): 43 (11%), 111 (14%), 129 (10%), 154.8 (100%), 179 (14%) (Supplemental Fig. 1). Using acetonitrile for chemical ionization an M + 1 ion of m/z of 154.7 (100%) with an additional 179 (25%) was observed. A molecular weight of 154 was assumed for this compound as the 179 ion is likely the result of a trap reaction in the mass spectrometer which can be common in ion trap mass spectrometers. The PFBHA derivatization technique (described above) used to detect carbonyl structures resulted in the detection of an oxime derivative present in the Fluka diacetyl (Supplemental Fig. 2). The three observed peaks are due to geometric isomers of the suspect contaminant. Identification of multiple peaks of the same oxime compound is relatively simple since the mass spectra for each chromatographic peak are almost identical or very similar.
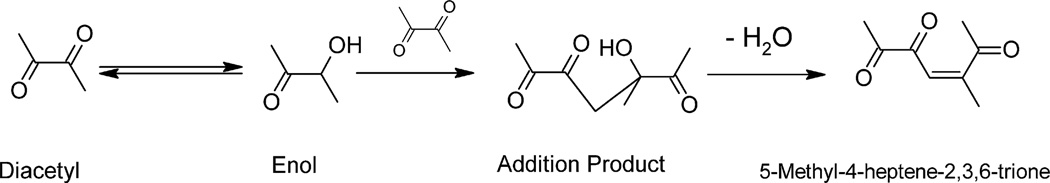
The chromatographic retention times of this oxime were 21.6, 21.9 and 22.2 min with ions of m/z (relative abundance): 43 (48%), 181 (100%), 307 (60%), 321 (76%), 501 (97%), 545 (15%) (Supplemental Fig. 3). Chemical ionization of these peaks yielded an M + 1 ion of 545 (Supplemental Fig. 3). Using the molecular weight of the contaminant (154) plus the molecular contribution of the PFBHA derivative (195) results in a molecular weight of 544 (154 + 2(195)) suggesting there are two derivatizable carbonyl entities on the molecule. Derivatization of carboxylic acid using EDC/TFEA did not result in any observable 2,2,2-trifuoroethylamide derivative, suggesting that the contaminant does not have a carboxylic acid moiety. Based on the molecular weight and structural information gleaned from the chemical derivatization, the compound 5-methyl-4-heptene-2,3,6-trione is proposed as the diacetyl contaminant. This compound is predicted to be the result of a condensation reaction (aldol condensation) between two diacetyl molecules (Personal communication: A. Lopes, CiVentiChem, Cary, NC; J.M. Matthews, RTI Int., Research Triangle Park, NC.) (Fig. 4). While it is reasonable to expect all three carbonyl moieties would be derivatized by PFBHA, it is possible that the carbon double bond could influence the electron density of the carbonyl at the C-6 position to prevent derivatization. By comparison, both of the carbonyls in diacetyl are derivatized by PFBHA even though they are adjacent to one another.
Fig. 4.
Proposed diacetyl contaminant. Possible mechanism leading to the aldol condensation product, 5-methyl-4-heptene-2,3,6-trione, between two diacetyl molecules.
4. Discussion
The studies described here used a standardized murine model to begin to evaluate the sensitization potential of 2,3-pentanedione, 2,3-hexanedione 3,4-hexanedione, and 2,3-heptanedione. Neither dermal irritation nor systemic toxicity were induced following in vivo skin exposure to any of the flavorings tested. Similar to diacetyl, 2,3-pentanedione, 2,3-hexanedione, 3,4-hexanedione and 2,3-heptanedione were identified as weak sensitizing chemicals. In addition, biologically significant increases in B220+ and IgE + B220+ cells in the DLNs, in the absence of significant increases in total serum IgE were observed only for 3,4-hexanedione and 2,3-heptanedione. This provides evidence of increases in local IgE that did not reach systemic levels following 3 days of dermal exposures.
Previous published studies conducted in our laboratory investigating the allergic sensitization potential of diacetyl (purchased from Fluka) resulted in a calculated EC3 value of 1.9% (Anderson et al., 2007). These findings were incongruent with other published studies and the results published here. Roberts et al., reported the EC3 value for diacetyl (producer not reported) to be 11.3% (Roberts et al., 1999) and analytical studies conducted by Dworak et al. calculated a higher EC3 value and weak sensitization potential for diacetyl (Dworak et al., 2013). In an attempt to resolve this issue, chemical characterization and skin sensitization potential of diacetyl purchased from numerous producers was evaluated. Consistent with the earlier publication (Anderson et al., 2007) an EC3 value of 2.5% (Fig. 1A) was obtained for the chemical purchased from Fluka which was much lower than that obtained for the diacetyl purchased from the other sources. A contaminant, with abundance inversely proportional to the calculated EC3 values for diacetyl purchased from different vendors, was discovered. The findings described here suggest that this contaminant may be the result of a producer specific synthesis or storage process, and emphasize the importance of chemical quality control in toxicity testing. It also raises the question of the potential significance of the contaminant in the skin disease observed in a few workers exposed to diacetyl (Akpinar-Elci et al., 2004). Although the exact concentration of the contaminant is not known, based on the producers’ reported diacetyl purity (97–99%) and our chemical analysis it is thought to be small. However, even a small amount of this contaminant was shown to influence the potency of diacetyl as a skin sensitizer to a magnitude that could change the potency classification from other than strong to strong based on the criteria set forth by ICCVAM (2011).
Chemical potency in allergic sensitization has been shown to correlate with hapten reactivity (Chipinda et al., 2011). While all chemicals with reactive electrophilic centers will form covalent adduct with proteins, mechanistic pathways differ and this may influence chemical potency. Diacetyl and the alternative flavorings belong to the Schiff base former mechanistic applicability domain and will only react with amines to form Schiff bases (Roberts et al., 2006). Based on the structure of the proposed diacetyl contaminant (5-methyl-4-heptene-2,3,6-trione), it would be expected to bind proteins through a Michael acceptor mechanism, binding to thiol and amine moieties (Roberts and Natsch, 2009). Patlewicz et al. (2002) observed that most Schiff base aldehydes were not strong sensitizers, while those that were Michael acceptors were generally more potent. These structure activity relationships provide further support for 5-methyl-4-heptene-2,3,6-trione as the contaminant in the test articles used in these studies. The increased reactivity (via Michael addition) of 5-methyl-4-heptene-2,3,6-trione compared to diacetyl is consistent with the lower EC3 values obtain from the LLNA when samples with higher amounts of the contaminant were evaluated.
The sensitization potency (EC3) was similar for all butter flavorings tested (Table 2). All of the chemicals investigated share the same dicarbonyl structure in which one carbonyl moiety is next to the other (─C(═O)C(═O)─) with the structural differences between the chemicals consisting of differing carbon chain lengths. These structural similarities may result in similar types of human health effects. Recent animal studies have demonstrated that exposure to 2,3-pentanedione produces pulmonary effects similar to diacetyl suggesting that it might not be a suitable replacement (Morgan et al., 2012). Also, in vitro studies conducted in non-neuronal cell lines identified similar toxicities for the alpha-diketones, 2,3-hexanedione and 3,4-hexanedione (Coleman et al., 2008). Studies conducted by Zimmermann et al., have also shown that 2,3-butanedione and 2,3-hexanedione both induce chromosome loss in Saccharomyces cerevisiae (Zimmermann and Mohr, 1992).
Table 2.
Summary of results.
| Chemical | Structure | Irritancy | LLNA | EC3 | Phenotyping | IgE | |
|---|---|---|---|---|---|---|---|
| %IgE + B220+≊%B220+* | B220+ | ||||||
| 2,3-Pentanedione | − | + | 15.4% | No | + | − | |
| 2,3-Hexanedione | − | + | 18.2% | No | + | − | |
| 3,4-Hexanedione | − | + | 15.5% | No | + | − | |
| 2,3-Heptanedione | − | + | 14.1% | No | + | − | |
| Diacetyl/2,3-butadiene (Aldrich) | − | + | 17.9% | No | + | − | |
At peak concentration, paradigm described by Manetz and Meade (1999).
Studies investigating other types of chemicals also suggest that structurally similar chemicals may not be the best replacement chemicals. For example, glutaraldehyde has been the primary chemical used for disinfecting heat-sensitive medical devices for the last 40 years; however, it has been reported to induce occupational asthma and other health effects (Quirce et al., 1999). Recently, structurally similar ortho-Phthalaldehyde has been proposed as a less toxic alternative. However, studies evaluating the allergenicity of these chemicals demonstrated the sensitizing potential for ortho-Phthalaldehyde is comparable to that of glutaraldehyde suggesting that it may not be a safe alternative (Anderson et al., 2010b). Similar to diacetyl, other structurally similar dicarbonyls including glyoxal, methylglyoxal, and 4-oxopentanal have been evaluated in the LLNA and were all identified as sensitizing chemicals (Anderson et al., 2007). Investigations of the adverse pulmonary health effects of dicarbonyls (diacetyl, 4-oxopentanal, glyoxal, methyl glyoxal and glutaraldehyde) were conducted using an in vitro indoor air exposure system. Similar changes in inflammatory cytokine expression (IL-8, Il-6, TNF-α, and GM-CSF) were identified in a pulmonary epithelial cell line (A549) following exposure (Anderson et al., 2010a).
The majority of chemical substances in the workplace have no established occupational exposure limits (OEL). In the absence of established OELs, employers and workers often lack the necessary guidance on the extent to which occupational exposures should be controlled. Alternative strategies such as health hazard banding, systematic categorization of chemicals in bands using predefined criteria based on the physicochemical and toxicological properties have been proposed. Criteria typically cover a variety of toxicological endpoints ranging from acute toxicity to systematic toxicity from repeated exposures to special endpoints of concern (i.e., irritation, sensitization, development toxicity and carcinogenicity). Health hazard banding is a potentially valuable tool for risk management of source chemical agents and other occupational hazards and may be beneficial to situations like the one described in this manuscript.
5. Conclusion
In summary, based on calculated EC3 values from the Local Lymph Node Assay, these studies demonstrated a similar dermal sensitization potential (other than strong), based on the criteria set forth by ICCVAM (ICCVAM, 2011) for the butter flavoring substitutes, 2,3-pentanedione, 2,3-hexanedione, 2,3-heptanedione, 3,4-hexanedione. This laboratory had previously published data identifying diacetyl as a strong sensitizer. Analysis of diacetyl from several producers demonstrated variable potencies, with the potency correlating to the amount of a previously unrecognized contaminant. Diacetyl produced by Aldrich, TCI and Acros was found to contain low to undetectable amounts of the contaminant and yielded an EC3 value which was congruent with previously published data from other labs and resulted in the same potency classification (other than strong) as the butter flavoring substitutes. Since the responses were comparable to those for diacetyl, this suggests that structurally similar chemicals may not be safe alternatives in regards to hypersensitivity responses. While sensitization has no known role in the pulmonary disease which has been associated with butter flavorings exposure, dermatitis has been reported. Our finding that skin sensitization potential is a shared characteristic of diacetyl and structurally-related potential substitutes supports the NIOSH recommendation that potential substitutes for hazardous flavorings should be evaluated for toxicity before they are used (NIOSH, 2013).
Supplementary Material
Acknowledgements
The authors thank Paul Siegel and Ann F. Hubbs for their contributions to this manuscript. This work was supported by an interagency agreement with the National Institute of Environmental Health Sciences (Y1-ES0001-06). The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.fct.2013.08.053.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Contributor Information
Stacey E. Anderson, Email: sanderson4@cdc.gov.
Jennifer Franko, Email: Jfranko@bethanywv.edu.
J.R. Wells, Email: RWells@cdc.gov.
Ewa Lukomska, Email: Elukomska@cdc.gov.
B. Jean Meade, Email: JMeade@cdc.gov.
References
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur. Respir. J. 2004;24:298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007;97:355–363. doi: 10.1093/toxsci/kfm043. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Jackson LG, Franko J, Wells JR. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicol. Sci. 2010a;115:453–461. doi: 10.1093/toxsci/kfq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Umbright C, Sellamuthu R, Fluharty K, Kashon M, Franko J, Jackson LG, Johnson VJ, Joseph P. Irritancy and allergic responses induced by topical application of ortho-phthalaldehyde. Toxicol. Sci. 2010b;115:435–443. doi: 10.1093/toxsci/kfq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera M. No substitute for safety. Occup. Health Saf. 2011;80(32):34–36. [PubMed] [Google Scholar]
- Basketter DA, Lea LJ, Dickens A, Briggs D, Pate I, Dearman RJ, Kimber I. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J. Appl. Toxicol. 1999;19:261–266. doi: 10.1002/(sici)1099-1263(199907/08)19:4<261::aid-jat572>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Boylstein R. Identification of diacetyl substitutes at a microwave popcorn production plant. J. Occup. Environ. Hyg. 2012;9:D33–D34. doi: 10.1080/15459624.2011.639234. [DOI] [PubMed] [Google Scholar]
- Boylstein R, Piacitelli C, Grote A, Kanwal R, Kullman G, Kreiss K. Diacetyl emissions and airborne dust from butter flavorings used in microwave popcorn production. J. Occup. Environ. Hyg. 2006;3:530–535. doi: 10.1080/15459620600909708. [DOI] [PubMed] [Google Scholar]
- Chipinda I, Hettick JM, Siegel PD. Haptenation: chemical reactivity and protein binding. J. Allergy. 2011;2011:839682. doi: 10.1155/2011/839682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MD, Zilz TR, Griffiths HR, Woehrling EK. A comparison of the apoptotic and cytotoxic effects of hexanedione derivatives on human nonneuronal lines and the neuroblastoma line SH-SY5Y. Basic Clin. Pharmacol. Toxicol. 2008;102:25–29. doi: 10.1111/j.1742-7843.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- Day G, LeBouf R, Grote A, Pendergrass S, Cummings K, Kreiss K, Kullman G. Identification and measurement of diacetyl substitutes in dry bakery mix production. J. Occup. Environ. Hyg. 2011;8:93–103. doi: 10.1080/15459624.2011.547148. [DOI] [PubMed] [Google Scholar]
- Dworak JJ, Roberts DW, Calter MA, Fields CA, Borak J. Is diacetyl a respiratory sensitizer? A reconsideration using QSAR, QMM, and competition experiments. Chem. Res. Toxicol. 2013 doi: 10.1021/tx400097v. [DOI] [PubMed] [Google Scholar]
- Ford QL, Burns JM, Ferry JL. Aqueous in situ derivatization of carboxylic acids by an ionic carbodiimide and 2,2,2-trifluoroethylamine for electron-capture detection. J. Chromatogr. A. 2007;1145:241–245. doi: 10.1016/j.chroma.2007.01.096. [DOI] [PubMed] [Google Scholar]
- Gloede E, Cichocki JA, Baldino JB, Morris JB. A validated hybrid computational fluid dynamics-physiologically based pharmacokinetic model for respiratory tract vapor absorption in the human and rat and its application to inhalation dosimetry of diacetyl. Toxicol. Sci. 2011;123:231–246. doi: 10.1093/toxsci/kfr165. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, et al. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol. Appl. Pharmacol. 2002;185:128–135. doi: 10.1006/taap.2002.9525. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Goldsmith WT, Kashon ML, Frazer D, Mercer RR, Battelli LA, Kullman GJ, Schwegler-Berry D, Friend S, Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague–Dawley rats. Toxicol. Pathol. 2008;36:330–344. doi: 10.1177/0192623307312694. [DOI] [PubMed] [Google Scholar]
- Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, Frazer DG, Fedan JS, Goravanahally MP, Castranova V, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague–Dawley rats. Am. J. Pathol. 2012;181:829–844. doi: 10.1016/j.ajpath.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICCVAM. Use of the Murine Local Lymph Node Assay for Potency Categorization. ICCVAM Test Method Evaluation Report. Usefulness and Limitations of the Murine Local Lymph Node Assay for Potency Categorization of Chemicals Causing Allergic Contact Dermatitis in Humans. 2011 NIH, Publication No. 11–7709. [Google Scholar]
- Kanwal R. Bronchiolitis obliterans in workers exposed to flavoring chemicals. Curr. Opin. Pulm. Med. 2008;14:141–146. doi: 10.1097/MCP.0b013e3282f52478. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, Fedan K, Kreiss K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J. Occup. Environ. Med. 2006;48:149–157. doi: 10.1097/01.jom.0000194152.48728.fb. [DOI] [PubMed] [Google Scholar]
- Klink KJ, Meade BJ. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicol. Sci. 2003;75:89–98. doi: 10.1093/toxsci/kfg171. [DOI] [PubMed] [Google Scholar]
- Kreiss K. Flavoring-related bronchiolitis obliterans. Curr. Opin. Allergy Clin. Immunol. 2007;7:162–167. doi: 10.1097/ACI.0b013e3280298235. [DOI] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. New Engl. J. Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Kullman G, Boylstein R, Jones W, Piacitelli C, Pendergrass S, Kreiss K. Characterization of respiratory exposures at a microwave popcorn plant with cases of bronchiolitis obliterans. J. Occup. Environ. Hyg. 2005;2:169–178. doi: 10.1080/15459620590923091. [DOI] [PubMed] [Google Scholar]
- Manetz TS, Meade BJ. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol. Sci. 1999;48:206–217. doi: 10.1093/toxsci/48.2.206. [DOI] [PubMed] [Google Scholar]
- Martyny JW, Van Dyke MV, Arbuckle S, Towle M, Rose CS. Diacetyl exposures in the flavor manufacturing industry. J. Occup. Environ. Hyg. 2008;5:679–688. doi: 10.1080/15459620802368463. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol. Sci. 2008;103:169–180. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Johnson CL, Gwinn WM, Price HC, Flake GP. Bronchial fibrosis in rats exposed to 2,3-butanedione and 2,3-pentanedione vapors. The Toxicologist. 2012a doi: 10.1177/0192623311431946. Abstract 866. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: implications for bronchiolitis obliterans in humans. Toxicol. Pathol. 2012b doi: 10.1177/0192623311431946. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hubbs AF. Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol. Sci. 2009;108:173–183. doi: 10.1093/toxsci/kfn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Draft Criteria for a Recommended Standard: Occupational Exposure to Diacetyl and 2,3-pentanedione. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. 2011 DHHS (NIOSH) Publication No. 2011-XXX [< http://www.cdc.gov/niosh/docket/archive/pdfs/NIOSH-245/0245-081211-draftdocument.pdf>].
- NIOSH. Flavorings-Related Lung Disease. 2013 < http://www.cdc.gov/niosh/topics/flavorings/>.
- OSHA, Occupational Safety and Health Administration. OSHA Bulletin and Alert Recommends Safety Measure to Protect Workers From Exposure to Diacetyl and Diacetyl Substitutes. United States Deapratment of Labor, Office of Communications. 2010 [Google Scholar]
- Palmer SM, Flake GP, Kelly FL, Zhang HL, Nugent JL, Kirby PJ, Foley JF, Gwinn WM, Morgan DL. Severe airway epithelial injury, aberrant repair and bronchiolitis obliterans develops after diacetyl instillation in rats. PLoS One. 2011;6:e17644. doi: 10.1371/journal.pone.0017644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlewicz GY, Wright ZM, Basketter DA, Pease CK, Lepoittevin JP, Arnau EG. Structure-activity relationships for selected fragrance allergens. Contact Dermatitis. 2002;47:219–226. doi: 10.1034/j.1600-0536.2002.470406.x. [DOI] [PubMed] [Google Scholar]
- Quirce S, Gomez M, Bombin C, Sastre J. Glutaraldehyde-induced asthma. Allergy. 1999;54:1121–1122. doi: 10.1034/j.1398-9995.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Natsch A. High throughput kinetic profiling approach for covalent binding to peptides: application to skin sensitization potency of Michael acceptor electrophiles. Chem. Res. Toxicol. 2009;22:592–603. doi: 10.1021/tx800431x. [DOI] [PubMed] [Google Scholar]
- Roberts DW, York M, Basketter DA. Structure-activity relationships in the murine local lymph node assay for skin sensitization: alpha, beta-diketones. Contact Dermatitis. 1999;41:14–17. doi: 10.1111/j.1600-0536.1999.tb06201.x. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Aptula AO, Patlewicz G. Mechanistic applicability domains for non-animal based prediction of toxicological endpoints. QSAR analysis of the schiff base applicability domain for skin sensitization. Chem. Res. Toxicol. 2006;19:1228–1233. doi: 10.1021/tx060102o. [DOI] [PubMed] [Google Scholar]
- Sahakian N, Kullman G, Lynch D, Kreiss K. Asthma arising in flavouring-exposed food production workers. Int. J. Occup. Med. Environ. Health. 2008;21:173–177. doi: 10.2478/v10001-008-0019-7. [DOI] [PubMed] [Google Scholar]
- van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am. J. Resp. Crit. Care Med. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- White KL, Heikkila K, Williams R, Levin L, Lockey JE, Rice C. Diacetyl exposures at four microwave popcorn plants. J. Occup. Environ. Hyg. 2010;7:185–193. doi: 10.1080/15459620903554870. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Munson AE, Meade BJ. Comparison of mouse strains using the local lymph node assay. Toxicology. 2000;146:221–227. doi: 10.1016/s0300-483x(00)00152-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann FK, Mohr A. Formaldehyde, glyoxal, urethane, methyl carbamate, 2,3-butanedione, 2,3-hexanedione, ethyl acrylate, dibromoacetonitrile and 2-hydroxypropionitrile induce chromosome loss in Saccharomyces cerevisiae. Mutat. Res. 1992;270:151–166. doi: 10.1016/0027-5107(92)90126-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.