Abstract
Objective
Despite the benefits of vaccination and guidelines for their use, influenza and pneumococcal vaccination rates remain below the 90% goal set by Healthy People 2010 for persons aged 65 years and older. Standing order programs (SOPs) authorize vaccination administration without physician orders. Here we examine the cost-effectiveness of SOPs to improve both pneumococcal and influenza vaccination rates in outpatient settings for individuals aged 65 years and older.
Study Design
Decision analysis-based cost-effectiveness analysis.
Methods
A Markov model was constructed to estimate the incremental cost-effectiveness of outpatient SOPs for PPSV and influenza vaccination in hypothetical 65-year-old and older US population cohorts. Vaccination rate improvement data were obtained from the medical literature. CDC Active Bacterial Core surveillance data and U.S. national databases were used to estimate costs and outcomes.
Results
SOPs cost $14,171 per quality adjusted life-year (QALY) gained compared to no program from a third-party payer perspective. In one-way sensitivity analyses, the SOP strategy cost less than $50,000/QALY if SOPs increased absolute vaccination rates by 4% or more (base case: 18%), annual SOP costs were less than $21 per person (base case: $4.60), or annual influenza incidence was 4% or more (base case: 10%). Model results were insensitive to other individual parameter variation, and were supported by a probabilistic sensitivity analysis.
Conclusions
SOP used to improve PPSV and influenza vaccination rates in outpatient settings is a promising and economically favorable investment, with cost-effectiveness analysis results remaining robust to parameter variation over clinically plausible ranges.
Pneumonia and influenza continue to be among the leading causes of death in the United States (U.S.).1 Influenza is estimated to cause an average of 200,000 hospitalizations and 36,000 deaths annually.2 Because individuals 65 years and older are at increased risk for influenza complications, seasonal influenza vaccination is important. Invasive pneumococcal disease (IPD), which includes bacteremia and/or infection of the meninges, joints, bones, or body cavities, is a relatively common outcome following influenza, particularly among individuals with chronic illnesses.3,4 Each year, pneumococcus causes about 500,000 cases of pneumonia, 50,000 cases of bacteremia, 3,000 cases of meningitis, and up to 7,000 - 12,500 deaths.5
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) has recommended influenza vaccination for all persons aged six months and older; pneumococcal polysaccharide vaccine (PPSV) is recommended for all persons aged 65 years and older, and for persons with chronic medical conditions aged 18 years and older.6 Among persons aged 65 years and older in 2010, the annual seasonal influenza vaccination rate was 63.6%,7 while 59.4% reported ever receiving PPSV.8 Despite the benefits of adult vaccination and the availability of usage guidelines, vaccination rates for seasonal influenza and PPSV remain below the Healthy People 2020 goal of 90% for each vaccine among persons aged 65 years and older.9
Standing orders authorize nurses and pharmacists to administer vaccinations according to a protocol approved by an institution or physician without an individual order or examination by the physician.10 The Task Force on Community Preventive Services reviewed the evidence for standing orders and strongly recommended them.11,12 Several studies have reported the successful use of SOPs.13-15 Because both PPSV and influenza vaccination are recommended for persons aged 65 years and older, co-administration is another strategy to raise vaccination rates. Although Smith et al. found that dual PPSV and influenza vaccination of all 50-year olds was economically reasonable,4 the cost-effectiveness of SOPs for vaccination of PPSV and influenza vaccine administered in outpatient settings for persons aged 65 years and older is unknown.
Methods
A Markov model was constructed to estimate, from the third-party payer and societal perspectives, the incremental cost-effectiveness of an SOP intervention for PPSV and influenza immunization. The intervention is implementation of SOPs in outpatient practice, and the comparison is between a base case of current practice (including some SOP-using practices) and broader SOP implementation in primary care practices for hypothetical cohorts of 65 year old and older patients in the U.S.
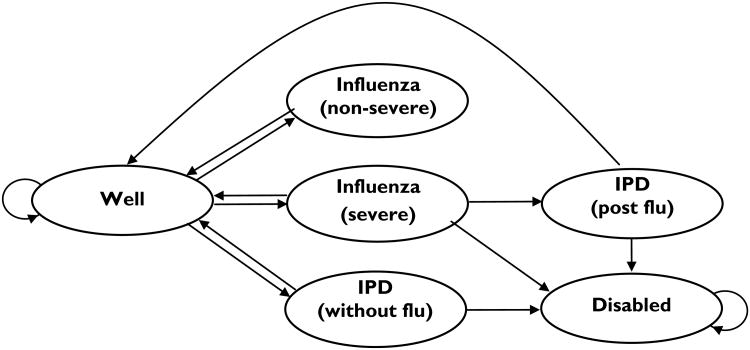
Figure 1 presents a state transition diagram illustrating the Markov model, which was adapted from a prior study.4 During each monthly cycle, a person may stay well, develop non-severe influenza, severe influenza, or IPD without influenza. Severe influenza was defined as requiring inpatient treatment while non-severe influenza was defined as those cases not requiring inpatient therapy. Inpatients with severe influenza may develop IPD, become disabled, or die due to influenza or other causes. We assumed that patients with non-severe influenza would recover and not go on to worse outcomes, depicted by arrows from illness states to the well state. In the model, influenza occurs in 5-month seasons each year, with annual influenza vaccination at the start of each season, equal monthly incidence within that time frame, and constant yearly incidence over time. PPSV was given, based on the likelihood of vaccination, when patients entered the model; we assumed that no PPSV was given later in the model and no repeat PPSV vaccination. Patients who develop IPD may recover without disability, become disabled, or die. The cohort was followed monthly over their lifetimes until death. Age-specific mortality not associated with illness was based on U.S. mortality tables.16
Figure 1. Web Archive.
The State Diagram of Markov Model for Influenza and Pneumococcal Polysaccharide Vaccines in Persons ≥65 Years of Age. Not shown in the diagram: patients in all states can transition to the Dead stage, based on age- and/or disease-specific mortality.
Generally, U.S. national databases and published sources were used to estimate costs and outcomes, which were discounted at an annual rate of 3%.17,18 PPSV effectiveness was estimated based on an expert panel, consisting of present and former CDC ACIP members or liaisons and other pneumococcal disease experts (Table 1), as described previously.19 Because PPSV is generally thought to have little or no effectiveness against non-bacteremic pneumonia,20,21 we conservatively assumed no PPSV effectiveness against pneumonia. IPD data were obtained from the CDC's Active Bacterial Core (ABC) surveillance data (Table 2).22 Costs were derived from the medical literature, Medicare physician fees,23 and 2006 Nationwide Inpatient Sample (NIS) data. Hospitalization charges from NIS data were adjusted using cost-to-charge ratios from the Medicare cost report.24 Parameter values used in the model are summarized in Table 3. In addition, the following assumptions were included in the model: (1) equal likelihood and severity of side effects for each vaccine; (2) patients with immuno-compromising conditions gain no benefit from PPSV4,19,25; (3) 60.1% vaccine uptake for both vaccines, based on NHIS data26, (4) yearly SOP costs remain constant through the lifetime of the modeled patient cohort, and (5) vaccination rates remain constant at the improved rate resulting from SOP use.
Table 1. Expert panel estimates of PPSV effectiveness against susceptible pneumococcal serotypes.
| Years post vaccination | Base Case (%) | Range | |
|---|---|---|---|
|
| |||
| Low (%) | High (%) | ||
| 1 | 80 | 60 | 90 |
| 3 | 73 | 50 | 83 |
| 5 | 58 | 31 | 80 |
| 7 | 33 | 13 | 48 |
| 10 | 0 | 0 | 10 |
| 15 | 0 | 0 | 10 |
Table 2. Characteristics of invasive pneumococcal infections based on active bacterial core surveillance system (ABC).
| Age 65-69 | Age 70-79 | Age 80+ | Source | |
|---|---|---|---|---|
| IPD rates (per 100,000 per year) | 38.7 | 38.7 | 38.7 | 2009 ABCs |
| IPD outcome rates (per 100,000 per year) | ||||
| Mortality | 6.56 | 6.56 | 6.56 | 2009 ABCs |
| Meningitis | 1.61 | 1.27 | 1.26 | 2007-8 ABCs |
| PPSV vaccine serotype coverage (%) | 74.1 | 65.8 | 62.9 | 2007-8 ABCs |
Source: Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs).22
IPD: invasive pneumococcal disease; PPSV: pneumococcal polysaccharide vaccine
Table 3. Parameter values and ranges examined in sensitivity analyses.
| Description | Base Case | Range | Distribution | Source | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Yearly influenza risk (%) | 10 | 2.7 | 21.4 | Beta | 45,47-50 |
| Hospitalization risk with influenza (%) | 3.1 | 1.7 | 5.7 | Beta | 51 |
| IPD risk in hospitalized influenza patients (%) | 10 | 7.5 | 12.5 | Beta | 47 |
| Influenza vaccine effectiveness (%) | 59 | 51 | 67 | Beta | 50 |
| PPSV effectiveness | Table 1 | Table 1 low range | Table 1 high range | Triangular | Table 1 |
| Relative risk of pneumococcal infection with PPSV serotypesb | 1 | 0.9 | 1.1 | Uniform | Table 2 |
| Impact of SOP on vaccination rate (%) | 18 | 8 | 27 | Beta | 52 |
| Vaccine adverse events | |||||
| Duration of symptoms (days) | 3 | 1 | 8 | Exponential | 40 |
| Probability (%) | 3.2 | 2.2 | 4.6 | Beta | 40 |
| Disability | |||||
| Excess mortality per year | 0.1 | 0 | 1 | Uniform | Estimated |
| Relative riskb | 1 | 0.5 | 1.5 | Uniform | Table 2a |
| IPD case-fatality odds ratio for patients with immunocompromising or other comorbid conditions | 1.5 | 1.3 | 1.8 | Log normal | 53 |
| Utility weights | |||||
| Age ≥ 65 years | 0.84 | 0.68 | 1 | Uniform | 54 |
| Invasive pneumococcal disease (applied for 34 days) | 0.2 | 0.1 | 0.5 | Uniform | 27 |
| Influenza – hospitalized (applied for 14 days) | 0.2 | 0.1 | 0.5 | Uniform | Estimate 27 |
| Influenza – outpatient (applied for 5 days) | 0.8 | 0.7 | 0.9 | Uniform | Estimate 54 |
| Disabled (applied for remaining lifetime) | 0.4 | 0.2 | 0.6 | Uniform | Estimate 54 |
| Vaccine adverse event (applied for 3 days) | 0.9 | 0.8 | 0.99 | Uniform | Estimate 54 |
| Costs (in 2006 US Dollars) | |||||
| Influenza vaccine and administration | $32 | $26 | $40 | Gamma | 21,28 |
| PPSV and administration | $43 | $33 | $60 | Gamma | 27 |
| Standing order program cost per patient (per year) | $4.60 | $0 | $35 | Gamma | Estimate 15 |
| Influenza | |||||
| Hospitalized | $4,723 | $4,397 | $5,075 | Gamma | NIS |
| Outpatient | $167 | $129 | $209 | Gamma | NIS |
| Invasive pneumococcal disease | |||||
| Discharged alive | |||||
| Age 65-74 years | $20,416 | $18,374 | $22,458 | Gamma | NIS |
| Age ≥ 75 years | $17,166 | $15,449 | $18,883 | Gamma | NIS |
| Died | |||||
| Age 65-74 years | $29,263 | $26,337 | $32,189 | Gamma | NIS |
| Age ≥ 75 years | $20,750 | $18,675 | $22,825 | Gamma | NIS |
| Disability (per year) | $10,000 | $8,000 | $10,000 | Gamma | Estimate 4 |
IPD: invasive pneumococcal disease; PPSV: pneumococcal polysaccharide vaccine; NIS, National Inpatient Survey.
Using meningitis rates as a proxy for disability incidence;
Relative risk compared to Table 2 values.
The numerator of the cost-effectiveness ratio represents per patient change in resources associated with the SOP including vaccine and administration costs, disease costs, and SOP costs. Details about the estimation of those costs are published elsewhere.4,15,21,24,27,28 SOP costs are derived from time and motion studies of an inpatient program,15 and thus may over- or under-estimate the costs of outpatient programs; for this reason, these costs were varied widely in sensitivity analyses. SOP costs in the model are per person in contact with the implemented program, not per person vaccinated. The denominator of cost-effectiveness ratio represents differences in adjusted quality of life years (QALYs) resulting from increased vaccination rates due to SOP use. QALYs account for changes in both duration and quality of life, and are the product of time spent in a health state and the quality of life utility value for that health state summed overall health states and over time.
In addition to the base case cost-effectiveness analysis, one-way sensitivity analyses and Monte Carlo probabilistic sensitivity analyses were performed to examine the robustness of cost-effectiveness estimates. One-way sensitivity analyses were conducted for all model parameters in Table 1 varying them over their listed ranges to evaluate influence on model results. In these analyses, the parameter of interest was varied while all other variables remained unchanged from their base case values. The probabilistic sensitivity analysis varied all input parameters simultaneously across their ranges; 10,000 model iterations were performed over specific distributions selected based on the level of parameter value certainty. TreeAge Pro 2009 (TreeAge Software, Williamstown, MA) was used to perform the analysis.
Results
From a third-party payer perspective, SOPs cost $14,171 per QALY gained compared to no SOP when the SOP-related absolute increase in vaccination rate was at its base case level, 18% (Table 4, top). When the societal perspective taken, which adds costs that patients incur while seeking or receiving care,18 SOPs cost $12,718 per QALY gained. We report all subsequent results from the third-party payer perspective.
Table 4. Incremental Cost-Effectiveness Ratios for SOP by Base Case (18%) and in Sensitivity Analyses on Vaccination Rate Increases for Influenza and Pneumococcal Polysaccharide Vaccines in Persons ≥65 Years of Age.
| Strategy | Increase in Vaccination Rate (%) | Cost (C) | Incremental Cost (ΔC) | Effectiveness (E, QALY) | Incremental Effectiveness (ΔE) | ICER (ΔC/ΔE) |
|---|---|---|---|---|---|---|
| No SOP | 18 | $740.47 | 11.4605 | |||
| SOP | $831.45 | $90.99 | 11.4669 | 0.0064 | $14,171 | |
| No SOP | 0 | $740.47 | 11.4605 | |||
| SOP | $803.25 | 11.4605 | (Dominated) | |||
| No SOP | 1 | $740.47 | 11.4605 | |||
| SOP | $804.80 | $64.33 | 11.4608 | 0.0004 | $180,429 | |
| No SOP | 2 | $740.47 | 11.4605 | |||
| SOP | $806.36 | $65.89 | 11.4612 | 0.0007 | $92,395 | |
| No SOP | 3 | $740.47 | 11.4605 | |||
| SOP | $807.92 | $67.45 | 11.4615 | 0.0011 | $63,052 | |
| No SOP | 5 | $740.47 | 11.4605 | |||
| SOP | $811.04 | $70.57 | 11.4623 | 0.0018 | $39,579 | |
| No SOP | 10 | $740.47 | 11.4605 | |||
| SOP | $818.86 | $78.40 | 11.464 | 0.0036 | $21,982 | |
| No SOP | 20 | $740.47 | 11.4605 | |||
| SOP | $834.62 | $94.15 | 11.4676 | 0.0071 | $13,196 |
QALY: quality adjusted life year; ICER: Incremental cost-effectiveness ratio ($/QALY); SOP: standing order program.
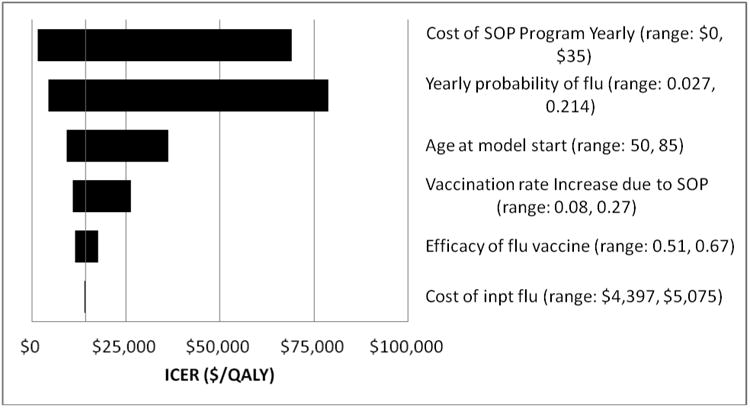
In one-way sensitivity analyses, individual variation of the vaccination rate increase due to SOPs (Table 4) showed that the incremental cost-effectiveness ratio remained less than $50,000 per QALY gained if absolute vaccination rates increased ≥4%. The effects of varying other selected parameter values in one-way sensitivity analyses are shown in Figure 2. Of these, program costs and annual influenza probability had the greatest effects on results, however SOPs cost less than $50,000/QALY if program costs were not greater than $21 per person per year (base case = $4.6015) or annual influenza incidence was ≥4% (base case 10%). Varying PPSV effectiveness had little impact on model results, given the relatively low incidence of invasive pneumococcal disease when compared to influenza incidence. With PPSV effectiveness at its low range estimate (Table 1), SOPs cost $14,694/QALY gained, $523 more than the base case value; if PPSV was completely ineffective, SOPs cost $15,577/QALY. Individual variation of the other listed parameters had little effect on model results.
Figure 2. Tornado Diagram at SOP vs. No SOP for Influenza and Pneumococcal Polysaccharide Vaccines in Persons ≥65 Years of Age.
In the probabilistic sensitivity analysis, which varied all parameter values simultaneously over distributions, SOPs were favored in greater than 82% of model iterations if the willingness-to-pay acceptability threshold was $50,000/QALY or more. In this analysis, SOPs were cost saving in 9.6% of iterations and were more costly and less effective than no program in 0.7% of the model iterations.
Discussion
We found that SOPs for influenza and pneumococcal vaccination were cost-effective under a wide range of assumptions. When using the frequently cited $50,000 per QALY gained acceptability threshold, which probably underestimates willingness to pay for health care gains in the US,19,29,30 $21 per person spent on program costs or 4% absolute increases in vaccination rates would still meet this criterion. The analysis was relatively insensitive to variation of other parameters, and simultaneous variation of all parameters in a probabilistic sensitivity analysis showed a high likelihood that SOPs would be favored.
Missed opportunities for vaccination during outpatient visits contribute to low vaccination rates and unnecessary disease burden. Failure to assess and offer vaccines at visits, as well as low rates of preventive care visits contribute to missed opportunities to vaccinate.31,32 SOPs are a powerful way to reduce missed opportunities and to raise immunization rates. The CDC has recommended SOPs for adult vaccination since 2000.10 However, the Center for Medicare & Medicaid Services (CMS) prohibited SOPs for all medications until 2002 when CMS allowed SOPs for influenza and pneumococcal polysaccharide vaccines.33 The ACIP,10 the Task Force for Community Preventive Services,12 and the Southern California Evidence-Based Practice Center-RAND34 have endorsed SOPs for improving immunization rates.
However, only 42% of primary care physicians who immunized adults in their practices reported consistent use of SOPs.35 Factors associated with consistent use of SOPs include awareness about CDC/CMS stance on standing orders policies, physician perception about the power of SOPs, staffing levels (i.e., number of assistants to help each clinician), and use of electronic medical records (EMRs).35,36 Record keeping and tracing of vaccination status is facilitated by the EMR. In some settings, the EMR can send alerts, make ordering and billing of vaccinations easy, or pull the most recent vaccination status into the nursing, thereby facilitating the use of SOP protocols by nursing personnel.36 CMS has incentives for EMR usage which may further facilitate SOPs.
Given the SOPs are effective in raising vaccination rates and economically reasonable, why are they not used more? Physicians and practice managers may be unaware of the economics of SOPs, which we estimate will cost less than $5 per person per year to implement; in contrast, the administration fee by Medicare for influenza and pneumococcal vaccines is about $21, depending on the locale.37 Several benefits can occur through SOP use, including reduced office visits for respiratory infections, decreasing both patient illness burden and strains on office manpower and flow during the influenza season. Another benefit is that adult immunization is a quality measure that can lead to bonus payments38 in some settings. The balance between SOP cost and the reimbursement that can occur through their use appears sufficient to justify SOPs.
Another possible reason for limited SOP use is unfamiliarity with resources. Peer-reviewed SOP toolkits, suggested related resources and protocols are available at www.immunizationed.org 39 and protocols for SOP for various vaccines are available at www.immunize.org/standingorders.
Strengths and Limitations
Although inpatient SOP costs have been published for PPSV,15 to our knowledge, this is the first paper examining the cost-effectiveness of outpatient SOPs for both PPSV and influenza vaccination. The results of our study should facilitate planning by health care providers and administrators, office managers, insurers and government officials.
Limitations include a number of estimated variables as well as SOP cost and cost-effectiveness estimates that may not remain stable during these times of substantial change in health care. In addition, certain parameters, such as vaccine effectiveness estimates, are controversial.40-45 For these reasons, we varied all parameters in sensitivity analyses, finding, in particular, that PPSV effectiveness values had little influence on model results. Models based on national data provide estimates but do not necessarily reflect the costs in a particular locale. We assume that yearly SOP costs, and the improved vaccination rates that occur through their use, remain constant, thus our analysis will not be correct if SOP costs or effects change significantly over time. Finally, although a new vaccine, the pneumococcal conjugate vaccine is now licensed in the United States,46 the ACIP has thus far declined to make recommendations for its routine use in adults; for this reason we have not considered it in our analysis.
With these limitations in mind, we conclude that SOP implementation for both PPSV and influenza vaccination in outpatient settings, targeting patients aged 65 and older, is a promising and economically favorable investment, with cost-effectiveness analysis results remaining robust to parameter variation over clinically plausible ranges.
Take-Away Points
We examined the cost-effectiveness of using standing order programs, which allow influenza and pneumococcal vaccination without a physician order, to improve the suboptimal vaccination rates in older US populations
Administering pneumococcal and influenza vaccines in outpatient settings under standing order programs was economically favorable and can impact public health through higher vaccination rates.
Results were robust to parameter variation over clinically plausible ranges.
In a time of health care reform and physician shortages, our results support wider use of standing order programs.
Contributor Information
Chyongchiou Jeng Lin, Department of Family Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States.
Richard K. Zimmerman, Department of Family Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States.
Kenneth J. Smith, Department of Internal Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States.
References
- 1.U.S. Department of Health and Human Services, CDC/NCHS. Deaths and Mortality. [Accessed September 15, 2011];2011 URL: http://www.cdc.gov/nchs/fastats/deaths.htm.
- 2.Aldrich N, Keyser CM, Benson WF. CDC Says Immunizations Reduce Deaths From Influenza and Pneumococcal Disease Among Older Adults. 2008 URL: http://www.cdc.gov/vaccines/vpd-vac/pneumo/downloads/influ-pneu-disease-2008-508.pdf.
- 3.McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith KJ, Lee BY, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine. 2010;28(48):7620–7625. doi: 10.1016/j.vaccine.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Foundation for Infectious. Diseases. Facts about Pneumococcal Disease for Adults. [Accessed July 13, 2012]; URL: http://www.adultvaccination.com/vpd/pneumococcal/facts.html.
- 6.U.S. Department of Health and Human Services, CDC/NCHS. CDC's Advisory Committee on Immunization Practices (ACIP) Recommends Universal Annual Influenza Vaccination. [Accessed September 1, 2011];2010 URL: http://www.cdc.gov/media/pressrel/2010/r100224.htm.
- 7.U.S. Department of Health and Human Services, CDC/NCHS. Annual Percentage of Adults Aged 65 Years and Over who Had Received Influenza Vaccination During Past 12 Months. [Accessed September 10, 2011];2011 URL: http://www.cdc.gov/nchs/data/nhis/earlyrelease/201106_04.pdf.
- 8.U.S. Department of Health and Human Services, CDC/NCHS. Annual Percentage of Adults Aged 65 Years and Over who Had Ever Received a Pneumococcal Vaccination. [Accessed September 10, 2011];2011 URL: http://www.cdc.gov/nchs/data/nhis/earlyrelease/201106_05.pdf.
- 9.U.S. Department of Health and Human Services. 2020 Topics & Objectives. [Accessed February 1, 2012]; http://www.healthypeople.gov/2020/topicsobjectives2020/pdfs/HP2020objectives.pdf.
- 10.U.S. Department of Health and Human Services. Use of standing orders programs to increase adult vaccination rates. Morbidity and Mortality Weekly Report. 2000;49(1):15–26. [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults. A report on recommendations of the Task Force on Community Preventive Services. Morbidity and Mortality Weekly Report. 1999;48:1–15. [PubMed] [Google Scholar]
- 12.Task Force on Community Preventive Services. Task Force on Community Preventive Services Vaccine-preventable diseases: improving vaccination coverage in children, adolescents, and adults. 2000 [Google Scholar]
- 13.Rhew DC, Glassman PA, Goetz MB. Improving pneumococcal vaccine rates. Nurse protocols versus clinical reminders. J Gener Intern Med. 1999;14:351–356. doi: 10.1046/j.1525-1497.1999.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dexter PR. Inpatient Computer-Based Standing Orders vs. Physician Reminders to Increase Influenza and Pneumococcal Vaccination Rates. J Am Med Assoc. 2004;292(19):2366–2371. doi: 10.1001/jama.292.19.2366. [DOI] [PubMed] [Google Scholar]
- 15.Middleton DB, Lin CJ, Smith KJ, et al. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect Control Hosp Epidemiol. 2008;29(5):385–394. doi: 10.1086/587155. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. National Vital Statistics Reports. 2010;58(21):7–8. [Google Scholar]
- 17.Gold M, Siegel J, Russell LB, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 18.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. J Am Med Assoc. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 19.Smith KJ, Zimmerman RK, Lin CJ, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26(11):1420–1431. doi: 10.1016/j.vaccine.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. Canadian Med Ass J. 2009;180(1):48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Db Syst Rev. 2008;(1) doi: 10.1002/14651858.CD000422.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) [Accessed February 10, 2012]; http://www.cdc.gov/abcs/index.html.
- 23.Medicare Physician Fee Schedule look-up (CPT 90471) Data year: 2006. [Accessed March 23, 2012]; http://www.cms.gov/apps/physician-fee-schedule/
- 24.Agency for Healthcare Research and Quality. Cost-to-Charge Ratio Files. Healthcare Cost and Utilization Project (HCUP) [Accessed July 1, 2007];2007 http://www.hcup-us.ahrq.gov/db/state/costtocharge.jsp.
- 25.Jackson LA, Neuzil KM. Pneumococcal polysaccharide vaccines. In: Plotkin SA, O W, Offit PA, editors. Vaccines. Fifth. Saunders Elsevier Inc; 2008. pp. 569–604. [Google Scholar]
- 26.U.S. Department of Health and Human Services, CDC/NCHS. Self-reported pneumococcal vaccination coverage trends 1989 - 2008 among adults by age group, risk group, race/ethnicity, health-care worker status, and pregnancy status, United States, National Health Interview Survey (NHIS) [Accessed September 1, 2011]; http://www.cdc.gov/flu/professionals/vaccination/pdf/NHIS89_08ppvvaxtrendtab.pdf.
- 27.Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med. 2003;138(12):960–968. doi: 10.7326/0003-4819-138-12-200306170-00007. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. National Immunization Program. CDC Price list. [Accessed March 1, 2007];2006 http://www.cdc.gov/vaccines/programs/vfc/downloads/archived-pricelists/2006.pdf.
- 29.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Archives Intern Med. 2003;163(14):1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 30.Braithwaite RS, Meltzer DO, King JT, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per Quality-Adjusted Life-Year decision rule? Med Care. 2008 Apr;46(4):349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 31.Nowalk MP, Zimmerman RK, Feghali J. Missed opportunities for adult immunization in diverse primary care office settings. Vaccine. 2004 Sep 3;22(25-26):3457–3463. doi: 10.1016/j.vaccine.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Nowalk MP, Zimmerman RK, Cleary SM, Bruehlman RD. Missed opportunities to vaccinate older adults in primary care. J Am Board Fam Pract. 2005;18(1):20–27. doi: 10.3122/jabfm.18.1.20. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare and Medicaid Services. Medicare and Medicaid programs; conditions of participation: immunization standards for hospitals, long-term care facilities, and home health agencies: final rule with comment period. Fed Reg. 2002;67:61808–61814. [PubMed] [Google Scholar]
- 34.Administration HCF. Evidence Report and Evidence-Based Recommendations: Interventions that Increase the Utilization of Medicare-Funded Preventive Service for Persons Age 65 and Older. Baltimore, MA: Health Care Financing Administration; 1999. [Google Scholar]
- 35.Zimmerman RK, Albert SM, Nowalk MP, Yonas MA, Ahmed F. Use of standing orders for adult influenza vaccination: A national survey of primary care physicians. Am J Prev Med. 2011;40(2):144–148. doi: 10.1016/j.amepre.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerman RK, Nowalk MP, Raymund M, et al. Tailored interventions to increase influenza vaccination in neighborhood health centers serving the disadvantaged. Am J Public Health Oct. 2003;93(10):1699–1705. doi: 10.2105/ajph.93.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Services HM. Reimbursement Fees – Flu, Pneumococcal and Hepatitis B Vaccines. 2011. [Accessed November 23, 2011];2011 https://www.highmarkmedicareservices.com/partb/reimbursement/flu-pnu-hep-11.html.
- 38.Agency for Healthcare Research and Quality. Using Quality Measures. [Accessed March 1, 2012]; http://www.qualitymeasures.ahrq.gov/selecting-and-using/using.aspx.
- 39.Centers for Disease Control and Prevention. STFM Group on Immunization Education. [Accessed February 1, 2012]; http://www.immunizationed.org/Default.aspx.
- 40.Jackson LA, Benson P, Sneller VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. J Am Med Assoc. 1999;281(3):243–248. doi: 10.1001/jama.281.3.243. [DOI] [PubMed] [Google Scholar]
- 41.Dear K, Holden J, Andrews R, Tatham D. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2003;(4):CD000422. doi: 10.1002/14651858.CD000422. [DOI] [PubMed] [Google Scholar]
- 42.Fedson DS, Musher DM. Pneumococcal Polysaccharide Vaccine. In: Plotkin SA, O W, Offit PA, editors. Vaccines. 4th. Philadephia: Elsevier; 2004. pp. 529–588. [Google Scholar]
- 43.Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine. 2004 Feb 25;22(8):927–946. doi: 10.1016/j.vaccine.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Vila-Corcoles A, Ochoa-Gondar O, Hospital I, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: The EVAN-65 Study. Clin Infect Dis. 2006;43(7):860–868. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 45.Jefferson TO, Rivetti D, Pietrantonj DC, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults (Review) Cochrane Db Syst Rev. 2007;(2) doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 46.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. J Am Med Assoc. 2012 Feb 22;307(8):804–812. doi: 10.1001/jama.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khazeni N, Hutton DW, Garber AM, Owens DK. Effectiveness and Cost-Effectiveness of Expanded Antiviral Prophylaxis and Adjuvanted Vaccination Strategies for an Influenza A (H5N1) Pandemic. Ann Intern Med. 2009;151(12):840–U843. doi: 10.1059/0003-4819-151-12-200912150-00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee BY, Brown ST, Cooley PC, et al. A Computer Simulation of Employee Vaccination to Mitigate an Influenza Epidemic. Am J Prev Med. 2010;38(3):247–257. doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee BY, Tai JHY, Bailey RR, Smith KJ. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009;27(50):7110–7115. doi: 10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Community Preventive Services Task Force. Universally Recommended Vaccinations: Standing Orders. [Accessed June 5, 2012];2012 http://www.thecommunityguide.org/vaccines/universally/RRstandingorders.html.
- 53.IOM Report. Patient safety - achieving a new standard for care. Acad Emerg Med. 2005;12(10):1011–1012. doi: 10.1197/j.aem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses - Using national measures to create condition-specific values. Med Care. 1998;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]