Abstract
Although, green tea has numerous health benefits, adverse effects with excessive consumption have been reported. Using Drosophila melanogaster, a decrease in male fertility with green tea was evidenced. Here, the extent of green tea toxicity on development and reproduction was investigated. Drosophila melanogaster embryos and larvae were exposed to various doses of green tea polyphenols (GTP). Larvae exposed to 10 mg/mL GTP were slower to develop, emerged smaller, and exhibited a dramatic decline in the number of emerged offspring. GTP protected flies against desiccation but sensitized them to starvation and heat stress. Female offspring exhibited a decline in reproductive output and decreased survival while males were unaffected. GTP had a negative impact on reproductive organs in both males and females (e.g., atrophic testes in males, absence of mature eggs in females). Collectively, the data show that high doses of GTP adversely affect development and reproduction of Drosophila melanogaster.
Keywords: development, Drosophila, green tea, reproduction, toxicity
1. Introduction
Green tea, derived from the plant Camellia sinensis, is not only a popular drink worldwide but it is also considered a nutraceutical (Mak, 2012) as a neuroprotective (Weinreb, Mandel, Amit, & Youdim, 2004), cardioprotective (Basu & Lucas, 2007; Zhong, Huan, Cao, & Yang, 2015) and anti-carcinogenic agent (Fujiki, Sueoka, Watanabe, & Suganuma, 2015). In spite of reported health benefits with green tea, there are some reports on possible adverse effects with its excessive consumption. In 2008, the US Pharmacoepia identified 34 cases of cytolytic and cholestatic liver damage associated with long term use or high usage of green tea products (Sarma, Ko, & Dog, 2008; Schonthal, 2011). Furthermore, green tea components, particularly its primary active flavonoid, epigallocatechin gallate (EGCG), has been shown to interact with and influence metabolism of a number of drugs (Andersen et al., 2013; Cuzzolin, Zaffani, & Benoni, 2006) and cytochrome P450 substrates (Schonthal, 2011; Yang & Pan, 2012), which could result in therapeutic failure or toxic drug levels. During investigations of green tea’s actions on different organ systems, unwarranted effects have been cited at high doses suggesting limitations to its use and questioning its safety. Such effects include liver (Mazzanti, Di Sotto, & Vitalone, 2015), gastrointestinal (Schonthal, 2011), hematological and renal toxicities (Wu, Yao, & Boring, 2011), as well as decreased hormonal levels (Chandra, Choudhury, De, & Sarkar, 2011; Kao, Hiipakka, & Liao, 2000), sperm counts (Chandra et al., 2011; De Amicis, Santoro, Guido, Russo, & Aquila, 2012) and atrophy of reproductive organs (Kapetanovic et al., 2009; Wu et al., 2011). While such toxicities have been cited in a number of reports, they are often over-looked, and their mechanisms are not fully understood. In line with some of these observations, we have previously reported that while green tea polyphenols (GTP) increased the lifespan of Drosophila melanogaster, it resulted in a reduction in male fertility (Lopez et al., 2014).
The fruit fly, Drosophila melanogaster, with its evolutionary conserved biological pathways, is a commonly used model organism in biomedical research (Jafari, Long, Mueller, & Rose, 2006) and an emerging model to screen for adverse drug reactions (Avanesian, Semnani, & Jafari, 2009). Evaluation of drug induced developmental and reproductive effects is commonly used to assess adverse drug reactions (Siddique et al., 2009; Weisbrot, Lin, Ye, Blank, & Goodman, 2003).
The Drosophila life cycle consists of distinct developmental stages that include embryogenesis, 1st, 2nd and 3rd larval instars, pupae and adults (Kozlova & Thummel, 2000). Each stage is highly regulated by transcriptional control in response to nutritional, environmental and hormonal cues (Kozlova & Thummel, 2000). Considering the highly conserved pathways between fruit fly and mammalian reproductive systems, the fruit fly is considered an excellent model system for the evaluation of drug toxicities (Avanesian et al., 2009). Reproductive phenotypes, including egg production, mating behavior and fertility, are notable measurable characteristics. Female fecundity, defined as egg production, is a complex yet established phenotype to evaluate toxicity and has been used to evaluate the reproductive adverse effects of chemotherapeutic agents such as methotrexate (Affleck, Neumann, Wong, & Walker, 2006; Kislukhin, King, Walters, Macdonald, & Long, 2013). In addition, Drosophila male fertility, defined as production of viable offspring, is also considered a phenotype to evaluate drug induced toxicity since it has been shown to be influenced by a number of factors such as mating behavior, hormones, testes development, reproductive morphology, and spermatogenesis (Tiwari, Pragya, Ram, & Chowdhuri, 2011).
In this study, we observed that GTP at a high dose of 10 mg/mL resulted in delayed emergence, smaller offspring, morphological abnormalities in reproductive organs and reduced reproductive output. Collectively, our findings indicate that green tea at high doses can negatively impact development and reproductive physiology.
2. Materials and Methods
2.1 Green tea
Green tea polyphenols (GTP) were purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). HPLC grade standards, individual catechins, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and included epigallocatechin gallate (98% EGCG), epicatechin gallate (98% ECG), epigallocatechin (95% EGC), epicatechin (98% EC) and internal standard ethylgallate (≥96% EG). Standards were dissolved in water/methanol (1:1, v/v) solution and quantified by ESI-LC/MS/MS using a Micromass Waters Quattro Premier XE (Waters Corp., Milford, MA, USA) coupled with an Acquity UPLC BEH C18 Column (Waters Corp.). The injection volume was 20 μL with an eluent flow rate of 0.3 mL/min. Gradient elution solvent A consisted of a mixture of water with 2% acetonitrile (ACN) and 0.2% ammonium acetate (5mM AA). Solvent B was ACN with 0.2% AA. The eluent gradient was ramped from 10% to 90% B in one minute. All acquisitions were performed in negative ion mode. Data acquisition and processing was performed using Waters MassLynx 4.1 (Waters Corp.).
2.2 Fruit fly strains and experimental conditions
All assays were performed using w1118 flies (FBID #3605) obtained from the Bloomington Drosophila Stock Center (BDSC) at Indiana University, Bloomington, IN, USA through FlyBase. Flies were maintained at 23°C ± 1°C and 60–70% humidity under 12-h light –12-h dark cycles. Standard banana–molasses food, composed of 9% carbohydrate content and 3.6% yeast content, was used in all feeding assays. For developmental feedings, which included the exposure of embryos and larvae to GTP, food was prepared by mixing the treatment within banana-molasses food and refrigerated for 24h. Unless stated, the GTP dose that was used for all the experiments was 10 mg/ml in fly food. In our work, this dose resulted in lifespan extension.
2.3 Toxicity and developmental assay
Toxicity assays were performed by preparing larval food as described above at 0, 2.5, 5, and 10 mg/mL concentrations of GTP. For each concentration, 6 flies per sex were placed in each vial (n=120 per treatment) for egg laying. After 24 h, flies were removed and the number of eggs laid was recorded. Larval development was checked every 24 h, and the number of pupae and emerged offspring, including dates of occurrence, were recorded.
2.4 Size and weight of emerged offspring
For size measurements, 6 flies per treatment (control or 10 mg/mL GTP) per sex were randomly selected from the population of emerged offspring. Flies were imaged using a ruler for scale, and pictures were analyzed using the open access Image Processing and Analysis in Java program (Image J, NIH, Bethesda, MD, USA). Fly length was estimated with one line from the top of the head to the end of the abdomen. Scaling in image J was set to pixels/centimeter. To determine weight, flies were sedated on a CO2 plate and weighed using a Sartorius SE2 Ultra Micro Balance (Bradford, MA, USA) in groups of 10 (n=60 per treatment, per sex).
2.5 Measurement of DNA content
A total of 5 flies per sex, per treatment, were weighed and flash frozen. Flies were homogenized in DNA isolation buffer (50 mM Tris-HCL, pH 8.0, 5 mM ethylenediaminetetraacetic acid (EDTA), 100 mM NaCl, 0.5% SDS) and supplemented with proteinase K (0.5 mg/mL final concentration) and digested overnight at 55°C with mild shaking. The samples were then extracted by standard phenol-chloroform procedures and precipitated by ethanol. DNA content was quantified and normalized to fly weight.
2.6 Fertility assay
Male fertility was performed as outlined in our previous work, Lopez et al. (2014), with the following modifications: 6 male and 6 female mating pairs were used per vial instead of 1 mating pair. Embryos and larvae were exposed to GTP or control throughout their development. Offspring were collected for fertility assays.
2.7 Dissections of testes and ovaries
Six flies per sex from each treatment (control or GTP) were randomly selected for dissection. Using fine forceps, the internal reproductive organs were teased out of the abdomen into a drop of phosphate buffer saline (PBS). Samples were stained with DAPI nuclei stain (Sigma-Aldrich, St. Louis, MO, USA) and were visualized under fluorescence using a Zeiss Axio Scope.A1. (Carl Zeiss Industrial Metrology LLC., Maple Grove, MN, USA)
2.8 Measurement of water, lipid and protein contents
Water, lipid and soluble protein levels were measured as previously described in Schriner et al., (Schriner et al., 2013). Flies were collected and weighed as described above. Water content was determined after drying flies for 48 h at 70°C, re-weighing them and taking the difference in weights. Lipid content was determined after lipid extraction with diethyl ether. Samples were dried and re-weighed. Fat content was determined by taking the difference in the weights before and after diethyl ether extraction divided by the initial weight. Soluble protein was determined from the supernatant of homogenized flies and measured by reaction with Coomassie Brilliant Blue normalized to fly weight.
2.9 Stress assays
A total of 120 flies per sex per treatment were used for each stress assay. For desiccation, flies were housed in empty vials and deaths were recorded every 2 hours. For starvation, flies were housed in vials containing 2% agarose to provide moisture without any food and deaths were recorded every 4 hours. To evaluate heat tolerance, flies were housed at 37° C and deaths recorded every hour. To evaluate the protection against superoxide, flies were exposed to 12.5 mM of paraquat (98% methyl viologen dichloride hydrate, Sigma-Aldrich, St. Louis, MO, USA) mixed in standard banana molasses food and deaths were recorded every 4 h. Survival analysis for all stress assays was determined by log-rank Mantel-Cox test.
2.10 Gene expression assay
Heat shock protein expressions were performed as described in Schriner et al. (Schriner et al., 2013). In brief, flies were frozen in groups of 10 and RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Samples were treated with DNase (New England Biolabs, Ipswich, MA, USA) and converted to cDNA by the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Quantitative PCR was performed on a MiniOpticon realtime PCR system with SYBR green dye (Bio-Rad). Relative amplification was calculated by the threshold cycle of each respective gene divided by the threshold cycle of the reference gene, RNA polymerase II. Primer sequences and product sizes were as follows. RNA pol II forward: AGGGCGGCGAGGACATGGAT, and reverse: CGACGGCTGGTAGTGACCGC. HSP70 forward: ACCAAGGGGTGTGCCCCAGA, and reverse: CTTGGCCTTGCCCGTGCTCA. HSP22 forward: TTGGCGGATGGCCGAGGAGA, and reverse: AGCGCCACACTCCAAACGGG. Primers were designed by NCBI/Primer-BLAST.
2.11 Lifespan assay
A total of 120 emerged offspring from control and GTP were collected and utilized for lifespan assays. For this particular assay, the flies were no longer exposed to GTP-treated food throughout their life; the only exposure to GTP was during development as described above. Flies were transferred every 2 days to fresh food and deaths were recorded until all flies died.
2.12 Statistical Analysis
All statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA). Statistical evaluation of differences between treatment groups was analyzed by Student’s t-test. Data analysis of green tea and control groups separated by sex was analyzed by two-way ANOVA. The Mantel–Cox log-rank test was used to evaluate fly survival in stress and lifespan assays. P values < 0.05 were considered statistically significant.
3. RESULTS
3.1 Composition of green tea polyphenols
Analysis of the GTP by ESI-MS revealed 54% EGCG, 11% ECG, 8% EC, 6% EGC and <0.1% caffeine as summarized in Table 1. The composition of the green tea that we used in this study was consistent with the composition of standardized green tea extracts used in scientific studies (Svoboda, Vlčková, & Nováková, 2015).
Table 1.
Composition of green tea polyphenols
| Analyte | % in GTP | RT (mins) | Transitions [M-H]- | Mean ± SD (μg/mL) | % RSD |
|---|---|---|---|---|---|
| Epigallocatechin gallate (EGCG) | 54 | 0.83 | 458 > 169 | 5.38 ± 0.16 | 3.0 |
| Epicatechin gallate (ECG) | 11 | 0.90 | 441 > 289 | 2.18 ± 0.093 | 4.2 |
| Epigallocatechin (EGC) | 6 | 0.68 | 305 > 125 | 2.46 ± 0.071 | 2.9 |
| Epicatechin (EC) | 8 | 1.45 | 289 > 245 | 1.90 ± 0.18 | 9.8 |
GTP, green tea polyphenols; RT, retention time; SD, standard deviation; RSD, relative standard deviation
3.2 Green tea polyphenols impair development and reduce offspring size and weight at a high dose
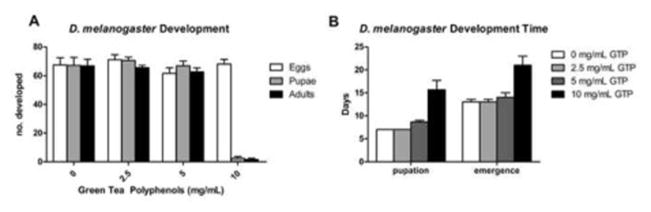
The effect of green tea on Drosophila development was evaluated by exposing embryonic and larval stages to a mixture of GTP at varying doses. The number of eggs laid by adults exposed to GTP was normal at all doses (Fig. 1A). No effects on development involving pupation and emergence were observed at the 0, 2.5 or 5 mg/mL doses but GTP at 10 mg/mL significantly inhibited the formation of pupae and subsequent offspring emergence (Fig. 1A). A surprising finding was the extended delay in pupation and emergence of offspring as doses of GTP increased (Fig. 1B).
Fig. 1. The effect of green tea polyphenols on development.
Green tea polyphenols fed to larvae during development had no effect on adult emergence at 0, 2.5, and 5 mg/mL doses (P>0.05). However, a significant reduction in pupae and adults was observed at the 10 mg/mL dose (P<0.0001) (A). Time to development was significantly increased as doses of GTP were increased (P<0.05) (B). Data are presented as means ± SEM, n=10 vials with 6 mating pairs (A) and n=3 experimental trials (B) and analyzed by two-way ANOVA.
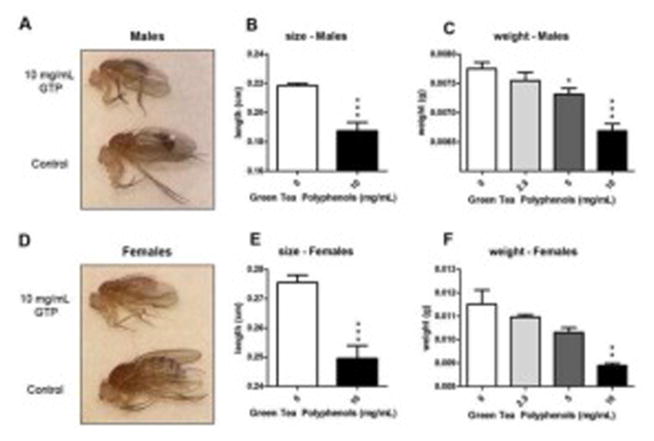
Male and females from the GTP treatment group emerged as smaller adult flies compared to controls (Fig. 2A and 2D). Recorded lengths from a random sample from each treatment showed a significant reduction in fly length by GTP for both males (Fig. 2B) and females (Fig. 2E). This observation was further confirmed by measuring weights from each group. A dose-dependent reduction in weight was observed in both sexes with the greatest reduction occurring at 10 mg/mL of GTP (Fig. 2C and 2F).
Fig. 2. Phenotypic effects of emerged offspring from food treated with green tea polyphenols.
Emerged offspring from the 10 mg/mL dose of green tea polyphenols were noticeably smaller in size than controls (A, D). Lengths of both males (B) and females (E) were significantly smaller after being exposed to 10 mg/mL GTP during development (n=6 per treatment per sex, ***P=0.0005, student’s t-test). Weight of emerged offspring also decreased in a dose dependent manner for both males (C) and females (F) with the greatest reduction occurring at the 10 mg/mL dose (n=60 per treatment per sex, *P =0.01, **P=0.001 and ***P<0.001).
3.3 Green tea polyphenols reduce cell numbers
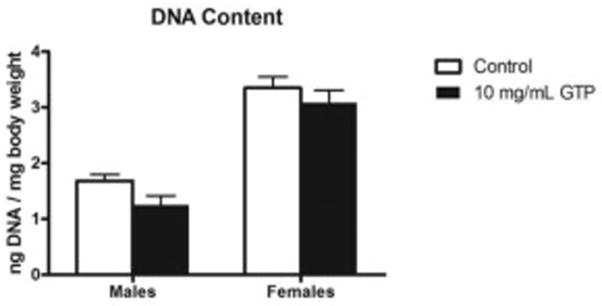
To evaluate whether the emergence of smaller offspring by GTP is the result of reduced cell size or reduction of cell numbers, we measured the total DNA content relative to fruit fly weights. We found no significant increase in the total DNA content/body weight ratio in either sex (Fig. 3) which is consistent with fewer cells in GTP treated flies.
Fig. 3. DNA content in flies treated with green tea polyphenols.
DNA content isolated from treated flies normalized to body weight was used to determine whether cell numbers were affected by GTP treatment. Flies treated with GTP did not exhibit an increase in DNA content normalized to body weight. Data are presented as means ± SEM, n=30 per treatment, per sex and analyzed by two-way ANOVA.
3.4 Green tea polyphenols fed during larval stages impact subsequent reproductive output of adult females
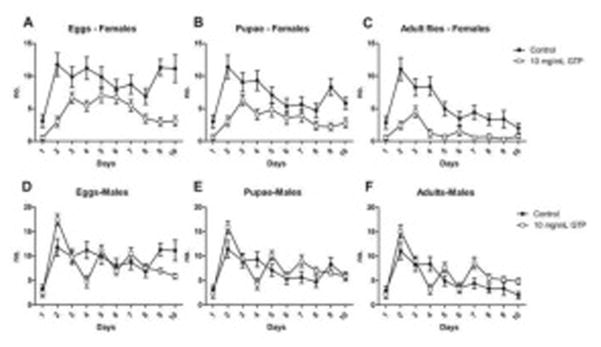
It is well established that reproductive output in flies, egg production from females or viable offspring from males, is a marker of toxicity in Drosophila (Avanesian et al., 2009; Tiwari et al., 2011). We evaluated the reproductive output of emerged offspring after being treated with GTP during development. Female offspring exposed to GTP exhibited a significant reduction in reproduction as adults compared to control (Fig. 4A, 4B, 4C). The fertility of male flies, or number of eggs laid by treated males upon mating with females, was not affected by the treatment (Fig. 4D, 4E). Interestingly, an increase in offspring numbers was observed (Fig. 4F).
Fig. 4. Reproductive output of adult Drosophila melanogaster fed green tea polyphenols during larval stages.
The fertility of emerged offspring from GTP-treated food was determined by measuring the number of eggs, pupae and adults formed from each sex over a 10-day mating period. Female flies exhibited a significant reduction in overall reproductive output including eggs laid (A), pupae formed (B) and emerged offspring (C) (P<0.0001). Male flies showed no difference in fertility by the number of eggs laid (D) or pupae (E) formed (P>0.05) however, an increase in the number of emerged flies was observed (P=0.01) (F). Data are presented as means ± SEM (n=60 per treatment per sex) and analyzed by two-way ANOVA. P >0.05 are not significant.
3.5 Green tea polyphenols cause morphological defects of reproductive organs
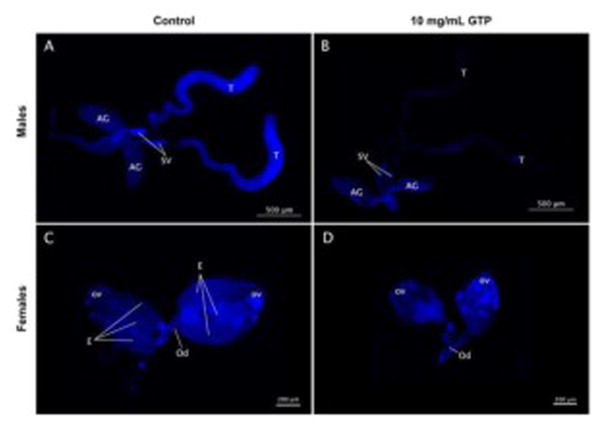
Reduced fly fertility can be associated with a number of factors including morphological defects in reproductive organs (Anderson, 1945; Siddique et al., 2009). Male offspring emerged from control food had normal reproductive structures such as full testes, seminal vesicles and accessory glands (Fig. 5A). However, male offspring from the GTP group exhibited atrophy of reproductive organs and a dramatic reduction of the number of nuclei in testes (Fig. 5B). Similarly, control females had normal ovaries and recognizable structures including ovarioles and mature eggs (Fig. 5C), whereas emerged females from GTP group revealed smaller ovarian structures and absence of mature eggs (Fig. 5D).
Fig. 5. The effect of green tea polyphenols on reproductive organs.
Reproductive organs of emerged offspring from GTP-treated food evaluated by DAPI nuclei stain revealed morphological abnormalities. Male reproductive organs from control males (A) showed normal and full structures of accessory glands (AG), seminal vesicles (SV) and testes (T). Males emerged from GTP food exhibited an overall reduction in organ size, atrophy of testes and reduced number of nuclei (B). Ovaries from control females (C) revealed normal organ structures including the presence of ovarioles (ov), mature eggs (E) and ovarian duct (Od). Females exposed to GTP exhibited a dramatic reduction in ovary size and absence of mature eggs (D).
3.6 Green tea polyphenols increase water content and decrease lipid levels without effecting protein levels
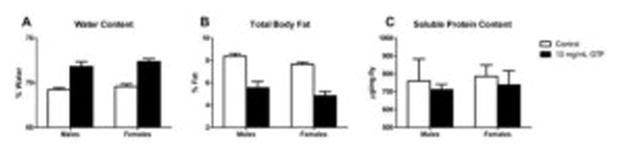
Increased water, lipid and protein levels are often associated with enhanced stress resistance and increased survival (Chippindalet, 1998). Offspring emerged from GTP food had increased water (Fig. 6A) but reduced lipid (Fig. 6B) contents in both sexes. No changes in soluble protein levels were observed (Fig. 6C).
Fig. 6. The effect of green tea polyphenols on water, lipid and protein content.
Larval fed GTP offspring exhibited higher levels of water (A) and lower total body fat content (B) than controls (P<0.0001). Offspring did not exhibit significant changes in soluble protein content (P>0.05) (C). Data are presented as means ± SEM (n=60 per treatment per sex) and analyzed by two-way ANOVA with Bonferroni post-test. P>0.05 are not significant.
3.7 Green tea polyphenols confer a modest protection against desiccation but sensitize flies to starvation, heat and oxidative stress
Flies emerged from GTP food were evaluated for their ability to confer protection against stress, a marker of health and survival in Drosophila melanogaster (Rose, Vu, Park, & Graves, 1992). Flies emerged from GTP were resistant to desiccation (Fig. 7A and 7B) but sensitized to starvation (Fig. 7C and 7D) and heat stress (Fig. 7E and 7F).
Fig. 7. The effect of green tea polyphenols on the tolerance towards desiccation, starvation, heat and paraquat.
Offspring emerged from GTP-treated food exhibited a modest protection against desiccation in male (A) and female (B) flies. However, GTP sensitized offspring to starvation (C, D), and heat stress (E, F). Male offspring from GTP-treated food did not exhibit any significant reduction in survival with exposure to paraquat (G). Female offspring from GTP-treated food exhibited a significant reduction in survival when exposed to paraquat (H). The sample sizes were n=120 per sex and per treatment. P values were calculated by Mantel-Cox log rank test.
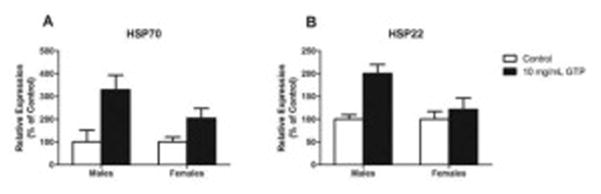
To evaluate the protective ability of GTP against oxidative stress, we subjected flies to paraquat, a superoxide generator. While we observed no significant differences in survival for males from paraquat exposure (Fig. 7G), female survival was significantly reduced (Fig. 7H). The expression levels of heat shock proteins (HSPs), specifically HSP70 and HSP22, were measured as a marker of cellular damage under a stress-induced environment, in this case caused by GTP. We observed that GTP caused a significant up-regulation in the expression levels of HSP70 (Fig. 8A) and HSP22 (Fig. 8B) in male flies. While an increase in HSP70 and HSP22 may be present in females as well, the effect was not statistically significant.
Fig. 8. The effect of green tea polyphenols on heat shock proteins.
Male offspring emerged from GTP-treated food exhibited a significant upregulation in the expression levels of HSP70 (P=0.002) (A) and HSP22 (P=0.004) (B). Although an increase in the expression levels of HSP70 and HSP22 was observed in females as well, the effect was not significant (P>0.05). Data are presented as means ± SEM (n=60 per treatment per sex) and analyzed by two-way ANOVA with Bonferroni post-test.
3.8 Green tea polyphenols reduce adult fly survival in females but has no effect on male lifespan
Flies were fed GTP only during early development and the lifespan of emerged offspring was examined. Female flies exhibited a 17% reduction in overall lifespan (Fig. 9A), whereas males were unaffected (Fig. 9B).
Fig. 9. Drosophila male and female lifespan after treatment with green tea polyphenols during development.
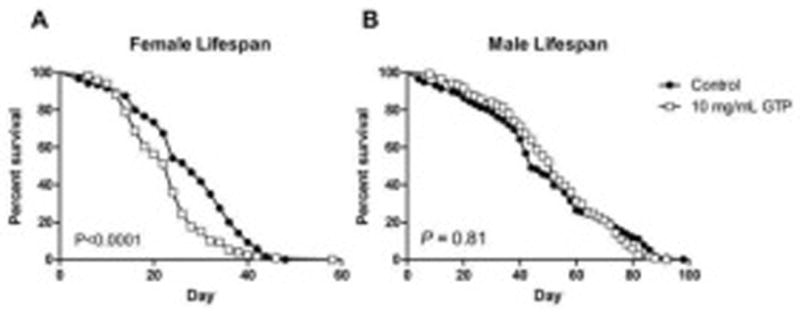
Female Drosophila exposed to a 10 mg/mL treatment of GTP during larval stages subsequently exhibited a 17% decrease in adult lifespan (A). Emerged male offspring from treated food were not affected from the treatment (B). The sample sizes were n=120 per sex and per treatment. P values were calculated by Mantel-Cox log rank test.
4 DISCUSSION
Previously, we reported that green tea polyphenols (GTP) fed to adult Drosophila melanogaster increased male mean lifespan by up to 19% but resulted in a decrease in male fertility at doses ≥10 mg/mL (Lopez et al., 2014). This adverse effect on male fertility was surprising and warranted further investigation on the extent of toxicity caused by green tea. In this work, we explored the impact of GTP on health by exposing Drosophila melanogaster to GTP during early stages of development. We surmised that since developing embryos and larvae are highly susceptible to environmental factors, exposing flies to GTP during early development would amplify subsequent negative phenotypic effects in emerged adults. Our results showed a number of phenotypic effects by GTP, most notably a delay in emergence, smaller offspring, reduced reproductive output by females and abnormalities of reproductive organs in both sexes. Moreover, we evaluated whether GTP provided any protection against stress and found that emerged GTP-treated offspring were resistant to desiccation but were sensitized to starvation and heat stress, phenotypes consistent with water and lipid changes in flies. Lastly, female flies emerged from GTP-treated food exhibited a significant decrease in survival whereas male fly lifespan was unaffected.
Numerous studies, particularly those investigating the effects of environmental pollutants on Drosophila development, have revealed that flies exposed to adverse food conditions emerge as smaller adults and require longer time periods for development (Böhni et al., 1999; Gupta et al., 2007; Siddique et al., 2009). It has been suggested that these observations are the result of somatic effects caused by the relevant treatment (Siddique et al., 2009). However, studies evaluating adverse effects of green tea on organismal development are scarce. Epicatechin gallate (ECG) and epigallocatechin gallate (EGCG), which make up more than half of green tea’s polyphenolic composition, have been shown to induce embryonic toxicity and impair development in mouse blastocysts. These compounds also induced apoptosis with a corresponding decrease in cell numbers, decreased implantation rates, and resulted in embryos with lower fetal weight (Fan & Chan, 2014; Tu, Chen, & Chan, 2010). These observations are consistent with our results showing that Drosophila embryos subjected to green tea-treated food had a remarkable reduction in offspring numbers, and the larvae that successfully emerged into adults were dramatically smaller in body size than respective controls. We questioned whether the small body size was the result of a decrease in individual cell sizes or a decrease in cell numbers. If there was a decrease in cell size, the GTP treated flies would have the same number of cells, and hence total DNA content, as controls. Since GTP-treated flies are smaller compared to controls, this would result in an increased DNA content/body weight ratio, which we did not see. Therefore, our results are consistent with either elevated apoptosis or decreased cell division or both. The striking developmental defects by green tea can be attributed to green tea’s pro-oxidant and pro-apoptotic activity. In fact, green tea’s ability to induce apoptosis is the basis of its reported classification as an anti-carcinogenic agent by initiating cell-cycle arrest and growth inhibition in carcinoma cells (Ghasemi-Pirbaluti et al., 2015; Gupta, Ahmad, Nieminen, & Mukhtar, 2000; Lambert & Elias, 2010). This mechanism is likely the cause of green tea’s impairment of fly development since Drosophila development is highly dependent on cell cycle progression and cell survival, events which are influenced in part by environmental factors (Böhni et al., 1999).
Hormetic effects of GTP may provide an explanation about these observed adverse effects. Hormesis is a phenomenon in which low dose exposure to a potential toxic compound is beneficial but higher doses of the same compound become harmful (Murakami, 2014; Son, Camandola, & Mattson, 2008). In flies, beneficial effects after exposure to hormetic compounds can lead to increased resistance against further stressors such as environmental and oxidative stress (Birringer, 2011; Mattson, 2008). With the exception of desiccation, emerged offspring from GTP were more susceptible to heat, starvation and oxidative stress. The protective effect exhibited by GTP against desiccation and sensitization to starvation could be explained by the increased levels in water and decreased levels in lipid content, respectfully, since such phenotypes are known to be positively correlated with one another (Chippindalet, 1998). Green tea’s inability to protect against superoxide free radicals could be due to a higher dose of GTP resulting in green tea’s pro-oxidant toxic effect (Forester & Lambert, 2011). Some of our results may appear to disagree with our previous work where we did not observe protective effects against desiccation, and GTP did not increase water content (Lopez et al., 2014). The reason for this discrepancy is likely due to feeding GTP to adult flies in our previous work versus exposing embryos to GTP in this work. Presumably, green tea could act through different mechanisms at different stages of fly development. Early embryonic development is a phase dependent on cell cycle progression and larval transitions (Kozlova & Thummel, 2000; Lehner & O’Farrell, 1989) but in adult stages, flies are primarily post-mitotic (Lehner & O’Farrell, 1989). Studies have shown that green tea constituents can bind to DNA and RNA, inhibit replication, increase topoisomerase II-mediated DNA cleavage, induce apoptosis and attenuate cell cycle progression at the G1/G0 phase (Bandele & Osheroff, 2008; Gupta et al., 2000; Kuzuhara, Sei, Yamaguchi, Suganuma, & Fujiki, 2006; Saiko et al., 2015). It is plausible that GTP is acting in this manner to inhibit growth and development of Drosophila melanogaster.
We observed remarkable abnormalities in the reproductive organs of both male and female flies. While male testes showed fewer nuclei and were smaller in size, female ovaries appeared to be almost entirely absent of mature eggs indicating that females emerged from GTP-treated food were more susceptible to the effects of GTP than males. The toxic effects of GTP on the female reproductive system were further evident by the reduced number of eggs laid and viable offspring produced. Male fertility did not appear to be largely affected. These sex-specific effects on male and female reproduction could be due to the difference in the numbers and sizes of the male and female gametes. Since sperm are so small and numerous, a significant reduction in gamete production could result in a nearly undetectable effect on the fertility and organ morphology. Whereas in females, eggs make up a large proportion of the female reproductive system, and similar degree of impairment in gamete production could result in marked morphological and reproductive effects.
Hormetic compounds, such as those found in green tea, are known to induce heat shock proteins (HSPs), stress proteins that function during harmful conditions to aid cell survival by binding and refolding damaged proteins (Mattson, 2008). Studies evaluating the toxic effects of chemical pollutants on Drosophila utilize the expression levels of HSPs, particularly the induction of HSP70, as a marker of stress response and cellular damage (Siddique et al., 2009). The small mitochondrial heat shock protein, HSP22, is essential in the maintenance of protein homeostasis and up-regulated in response to heat and oxidative stress (Bhole, Allikian, & Tower, 2004; Morrow & Tanguay, 2015). Additionally, both HSP70 and HSP22 are up-regulated during Drosophila aging (Tower, 2011). We observed a sex-specific effect of GTP on HSP70 and HSP22. Emerged male offspring from GTP-treated food exhibited an up-regulation in HSP70 and HSP22. The effect was not observed in females. Previous studies have shown that green tea and its primary active flavonoid, EGCG, induce HSPs as a protective mechanism to combat stress-induced environments and increase survival (Abbas & Wink, 2009; Zhang, Jie, Zhang, & Zhao, 2009). In our study, HSPs were up-regulated by GTP treatment alone which is indicative of stress caused by the treatment itself.
Additionally, we explored the effect of GTP on HSP22 as it has been suggested as a predictive biomarker for Drosophila survival (Yang & Tower, 2009). Yang and Tower (2009) identified that flies robustly expressing HSP22 at a younger age tended to die sooner. Additionally, over-expression of HSP22 made flies more sensitive to heat and oxidative stress and reduced fly lifespan (Bhole et al., 2004). Similar to our results, flies exposed to GTP exhibited higher levels of HSP22 which sensitized flies to stress and compromised survival. Female flies, in particular, were more susceptible to oxidative stress by paraquat than male flies. In addition to sex-specific differences that affect fly lifespan (Adler, Cassidy, Fricke, & Bonduriansky, 2013; Maklakov et al., 2008) and those described above, we speculate the sensitivity to oxidative stress induced by GTP contributed to an overall shorter lifespan for females. Lastly, the observed difference in HSP expression between control and GTP-treated groups suggested that at high doses, GTP is detrimental and leads to cell loss and subsequent morphological changes.
In summary, our results demonstrate that high concentrations of green tea impair development and reproduction of Drosophila melanogaster, as demonstrated by delayed emergence, small offspring sizes, reduced offspring numbers and atrophy of reproductive organs. The conserved biological pathways, including similarities between reproductive and developmental genes and hormones in flies and humans, make flies a valid model organism to evaluate drug induced adverse effects (Avanesian et al., 2009; Kislukhin et al., 2013; Kozlova & Thummel, 2000). Our results indicate that when consumed at high doses, green tea could potentially be detrimental to human physiological processes such as development and reproduction. However, it is difficult to draw direct conclusions on human implications since the mechanisms of bioavailability of polyphenols in both mammals and Drosophila remain poorly understood.
HIGHLIGHTS.
Fruit fly larvae were exposed to green tea polyphenols (GTP) only during development
A high dose delayed development, reduced offspring sizes and impaired reproduction
Morphological abnormalities of reproductive organs were observed
High doses of GTP may cause developmental adverse effects in humans
Acknowledgments
This study was supported in part by the National Institutes of Health, grant R21AT004987.
Abbreviations
- GTP
green tea polyphenols
- EGCG
epigallocatechin gallate
- ECG
epicatechin gallate
- EGC
epigallocatechin
- EC
epicatechin
- EG
ethylgallate
- HSP
heat shock protein
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas S, Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009;75(3):216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- Adler MI, Cassidy EJ, Fricke C, Bonduriansky R. The lifespan-reproduction trade-off under dietary restriction is sex-specific and context-dependent. Experimental gerontology. 2013;48(6):539–548. doi: 10.1016/j.exger.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Affleck JG, Neumann K, Wong L, Walker VK. The effects of methotrexate on Drosophila development, female fecundity, and gene expression. Toxicological Sciences. 2006;89(2):495–503. doi: 10.1093/toxsci/kfj036. [DOI] [PubMed] [Google Scholar]
- Andersen MR, Sweet E, Lowe KA, Standish LJ, Drescher CW, Goff BA. Dangerous combinations: Ingestible CAM supplement use during chemotherapy in patients with ovarian cancer. J Altern Complement Med. 2013;19(8):714–720. doi: 10.1089/acm.2012.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RC. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics. 1945;30(3):280. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanesian A, Semnani S, Jafari M. Can Drosophila melanogaster represent a model system for the detection of reproductive adverse drug reactions? Drug Discov Today. 2009;14(15–16):761–766. doi: 10.1016/j.drudis.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chemical research in toxicology. 2008;21(4):936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Lucas EA. Mechanisms and Effects of Green Tea on Cardiovascular Health. Nutrition Reviews. 2007;65(8):361–375. doi: 10.1301/nr.2007.aug.361-375. [DOI] [PubMed] [Google Scholar]
- Bhole D, Allikian MJ, Tower J. Doxycycline-regulated over-expression of hsp22 has negative effects on stress resistance and life span in adult Drosophilamelanogaster. Mechanisms of ageing and development. 2004;125(9):651–663. doi: 10.1016/j.mad.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Birringer M. Hormetics: dietary triggers of an adaptive stress response. Pharm Res. 2011;28(11):2680–2694. doi: 10.1007/s11095-011-0551-1. [DOI] [PubMed] [Google Scholar]
- Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1–4. Cell. 1999;97(7):865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Chandra AK, Choudhury SR, De N, Sarkar M. Effect of green tea (Camellia sinensis L.) extract on morphological and functional changes in adult male gonads of albino rats. Indian Journal of Experimental Biology. 2011;49:689–697. [PubMed] [Google Scholar]
- Chippindalet AK. Metabolic reserves and evolved stress resistance in Drosophila melanogaster. Physiological Zoology. 1998;71(5):584–594. doi: 10.1086/515963. [DOI] [PubMed] [Google Scholar]
- Cuzzolin L, Zaffani S, Benoni G. Safety implications regarding use of phytomedicines. Eur J Clin Pharmacol. 2006;62(1):37–42. doi: 10.1007/s00228-005-0050-6. [DOI] [PubMed] [Google Scholar]
- Sarma Dandapantula N, MLB, Chavez Mary L, Gardiner Paula, Ko Richard, GBM, Marles Robin J, Pellicore Linda S, Dog GIGaTL. Safety of green tea extracts: A systematic review by the US Pharmacopeia. Drug Safety. 2008;31(6):469–484. doi: 10.2165/00002018-200831060-00003. [DOI] [PubMed] [Google Scholar]
- De Amicis F, Santoro M, Guido C, Russo A, Aquila S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol Nutr Food Res. 2012;56(11):1655–1664. doi: 10.1002/mnfr.201200190. [DOI] [PubMed] [Google Scholar]
- Fan YC, Chan WH. Epigallocatechin gallate induces embryonic toxicity in mouse blastocysts through apoptosis. Drug Chem Toxicol. 2014;37(3):247–254. doi: 10.3109/01480545.2013.838778. [DOI] [PubMed] [Google Scholar]
- Forester SC, Lambert JD. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol Nutr Food Res. 2011;55(6):844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki H, Sueoka E, Watanabe T, Suganuma M. Primary cancer prevention by green tea, and tertiary cancer prevention by the combination of green tea catechins and anticancer compounds. J Cancer Prev. 2015;20(1):1–4. doi: 10.15430/JCP.2015.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi-Pirbaluti M, Pourgheysari B, Shirzad H, Motaghi E, Dehkordi NA, Surani Z, Pirayesh A. Effect of Epigallocatechin-3-gallate (EGCG) on cell proliferation inhibition and apoptosis induction in lymphoblastic leukemia cell line. Journal of HerbMed Pharmacology. 2015;4(2) [Google Scholar]
- Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicology and applied pharmacology. 2000;164(1):82–90. doi: 10.1006/taap.1999.8885. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Siddique HR, Mathur N, Mishra RK, Saxena DK, Chowdhuri DK. Adverse effect of organophosphate compounds, dichlorvos and chlorpyrifos in the reproductive tissues of transgenic Drosophila melanogaster: 70kDa heat shock protein as a marker of cellular damage. Toxicology. 2007;238(1):1–14. doi: 10.1016/j.tox.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Jafari M, Long AD, Mueller LD, Rose MR. The Pharmacology of Aging in Drosophila. Current Drug Targets. 2006;7(10):1479–1483. doi: 10.2174/1389450110607011479. [DOI] [PubMed] [Google Scholar]
- Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology. 2000;141(3):980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Crowell JA, Krishnaraj R, Zakharov A, Lindeblad M, Lyubimov A. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260(1–3):28–36. doi: 10.1016/j.tox.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislukhin G, King EG, Walters KN, Macdonald SJ, Long AD. The genetic architecture of methotrexate toxicity is similar in Drosophila melanogaster and humans. G3: Genes| Genomes| Genetics. 2013;3(8):1301–1310. doi: 10.1534/g3.113.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova T, Thummel CS. Steroid regulation of postembryonic development and reproduction in Drosophila. Trends in Endocrinology & Metabolism. 2000;11(7):276–280. doi: 10.1016/s1043-2760(00)00282-4. [DOI] [PubMed] [Google Scholar]
- Kuzuhara T, Sei Y, Yamaguchi K, Suganuma M, Fujiki H. DNA and RNA as new binding targets of green tea catechins. Journal of Biological Chemistry. 2006;281(25):17446–17456. doi: 10.1074/jbc.M601196200. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501(1):65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, O’Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56(6):957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez T, Schriner SE, Okoro M, Lu D, Chiang BT, Huey J, Jafari M. Green tea polyphenols extend the lifespan of male drosophila melanogaster while impairing reproductive fitness. J Med Food. 2014;17(12):1314–1321. doi: 10.1089/jmf.2013.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JC. Potential role of green tea catechins in various disease therapies: progress and promise. Clin Exp Pharmacol Physiol. 2012;39(3):265–273. doi: 10.1111/j.1440-1681.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology. 2008;18(14):1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti G, Di Sotto A, Vitalone A. Hepatotoxicity of green tea: an update. Arch Toxicol. 2015 doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- Morrow G, Tanguay RM. The Big Book on Small Heat Shock Proteins. Springer; 2015. Drosophila small heat shock proteins: an update on their features and functions; pp. 579–606. [Google Scholar]
- Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys. 2014;557:3–10. doi: 10.1016/j.abb.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Rose MR, Vu LN, Park SU, Graves JL. Selection on stress resistance increases longevity in Drosophila melanogaster. Experimental gerontology. 1992;27(2):241–250. doi: 10.1016/0531-5565(92)90048-5. [DOI] [PubMed] [Google Scholar]
- Saiko P, Steinmann M-T, Schuster H, Graser G, Bressler S, Giessrigl B, Bago-Horvath Z. Epigallocatechin gallate, ellagic acid, and rosmarinic acid perturb dNTP pools and inhibit de novo DNA synthesis and proliferation of human HL-60 promyelocytic leukemia cells: Synergism with arabinofuranosylcytosine. Phytomedicine. 2015;22(1):213–222. doi: 10.1016/j.phymed.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Schonthal AH. Adverse effects of concentrated green tea extracts. Mol Nutr Food Res. 2011;55(6):874–885. doi: 10.1002/mnfr.201000644. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Lee K, Truong S, Salvadora KT, Maler S, Nam A, Jafari M. Extension of Drosophila lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLoS One. 2013;8(5):e63886. doi: 10.1371/journal.pone.0063886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique HR, Mitra K, Bajpai VK, Ravi Ram K, Saxena DK, Chowdhuri DK. Hazardous effect of tannery solid waste leachates on development and reproduction in Drosophila melanogaster: 70kDa heat shock protein as a marker of cellular damage. Ecotoxicol Environ Saf. 2009;72(6):1652–1662. doi: 10.1016/j.ecoenv.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromolecular Med. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Vlčková H, Nováková L. Development and validation of UHPLC–MS/MS method for determination of eight naturally occurring catechin derivatives in various tea samples and the role of matrix effects. Journal of Pharmaceutical and Biomedical Analysis. 2015;114:62–70. doi: 10.1016/j.jpba.2015.04.026. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Pragya P, Ram KR, Chowdhuri DK. Environmental chemical mediated male reproductive toxicity: Drosophila melanogaster as an alternate animal model. Theriogenology. 2011;76(2):197–216. doi: 10.1016/j.theriogenology.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46(5):355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HC, Chen CP, Chan WH. Epicatechin gallate decreases the viability and subsequent embryonic development of mouse blastocysts. Taiwanese Journal of Obstetrics and Gynecology. 2010;49(2):174–180. doi: 10.1016/S1028-4559(10)60037-X. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J Nutr Biochem. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Weisbrot D, Lin H, Ye L, Blank M, Goodman R. Effects of mobile phone radiation on reproduction and development in Drosophila melanogaster. J Cell Biochem. 2003;89(1):48–55. doi: 10.1002/jcb.10480. [DOI] [PubMed] [Google Scholar]
- Wu KM, Yao J, Boring D. Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. Int J Toxicol. 2011;30(1):19–20. doi: 10.1177/1091581810387445. [DOI] [PubMed] [Google Scholar]
- Yang CS, Pan E. The effects of green tea polyphenols on drug metabolism. Expert Opinion on Drug Metabolism and Toxicology. 2012;8(6):677–689. doi: 10.1517/17425255.2012.681375. [DOI] [PubMed] [Google Scholar]
- Yang J, Tower J. Expression of hsp22 and hsp70 transgenes is partially predictive of drosophila survival under normal and stress conditions. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64(8):828–838. doi: 10.1093/gerona/glp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jie G, Zhang J, Zhao B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic Biol Med. 2009;46(3):414–421. doi: 10.1016/j.freeradbiomed.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Zhong W, Huan XD, Cao Q, Yang J. Cardioprotective effect of epigallocatechin-3-gallate against myocardial infarction in hypercholesterolemic rats. Exp Ther Med. 2015;9(2):405–410. doi: 10.3892/etm.2014.2135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]