Abstract
Several classes of diversely substituted styryl type dyes have been synthesized with the goal of extending their expected fluorescent properties as much towards red as possible given the constraint that they maintain drug-like properties and retain high affinity binding to their biological target. We report on the synthesis, optical properties of a series of styryl dyes (d1-d14), and the anomalous photophysical behavior of several of these Donor-Acceptor pairs separated by long conjugated π-systems (d7-d10). We further describe an unusual dual emission behavior with two distinct ground state conformers which could be individually excited to locally excited (LE) and twisted intramolecular charge transfer (TICT) excited state in push-pull dye systems (d7, d9 and d10). Additionally, unexpected emission behavior in dye systems d7 and d8 wherein the amino- derivative d7 displayed a dual emission in polar medium while the N,N-dimethyl derivative d8 and other methylated derivatives d12-d14 showed only LE emission but did not show any TICT emission. Based on photophysical and nerve binding studies, we down selected compounds that exhibited the most robust fluorescent staining of nerve tissue sections. These dyes (d7, d9, and d10) were subsequently selected for in-vivo fluorescence imaging studies in rodents using the small animal multispectral imaging instrument and the dual-mode laparoscopic instrument developed in-house.
Keywords: Styryl dyes, TICT emission, nerve labeling, dyes, laparoscopy, in vivo and ex vixo imaging, fluorescence imaging of nerves
Introduction
Nerves are often difficult to visualize during surgery, due to their intricacy and anatomical variations in their location.1 As a result, unintended nerve injury has been recognized as an important cause of morbidity associated with several lifesaving surgical procedures such as prostatectomy,2,3 thyroidectomy,4,5 rhytidectomy,6 and breast cancer surgery.7 The need for guidance during surgery has been recognized for about a decade, beginning with the evaluation of techniques employing pre-operative CT and MR imaging.8-11 However, real time decision making has directed intraoperative identification efforts towards fluorescence imaging,12 including approaches relying on visualizing the nerves based on contrast agents designed to elicit selective nerve fluorescence during surgery, reviewed recently.13
To facilitate clinical use, the ideal in vivo nerve contrast agent must be designed to cross the tight junctions of the blood nerve barrier, a crucial requirement for systemic injection. Thus, in addition to being fluorescent, the contrast agent should show high selectivity to a nerve target as well as exhibit properties of small molecules. The targeting moiety has to be inherently fluorescent because conjugating the targeting component to a fluorescent dye would significantly increase its molecular weight beyond the desirable range.
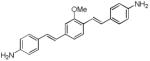
The styryl dye, BMB, (1,4-bis(p-aminostyryl)-2-methoxybenzene), developed to be a PET tracer for imaging myelin absorbs in the near-ultraviolet range and emits in the blue region.14 While it bound with high affinity to myelin extracts from brain, BMB suffered from poor aqueous solubility. Moreover, intraoperative imaging at wavelengths optimal for BMB produced high background in non-target tissue because tissue autofluorescence was high in this region. Using BMB as a starting point, we sought to explore whether chemical modifications applied to bis-styryl dyes15,16 would allow us to elaborate them into dyes having optical and biological properties suitable for their use in an intraoperative setting.
We have since reported on bis-styryl dyes (d1, d7 and d9) for selectively targeting myelin basic protein (MBP), a major component of nerves,17-19 along with compact optical imaging instruments for open and minimal access surgeries.20,21 Following intravenous injection, these dyes visualized central and peripheral nerves in vivo with high contrast relative to the surrounding muscle tissue, despite absorbing and emitting in the visible region. The affinity for MBP was maintained provided certain structural features were conserved.22 We have shown that both the absorption and the emission maxima can be red-shifted significantly by employing through-π system conjugated donor-acceptor (D-A) group, producing dyes with large Stokes shifts, thus minimizing the impact of autofluorescence in the visible region.
Herein, we report the screening and optical characterization of a series of molecules (d1-d14) with the aim of creating dyes with red-shifted fluorescence, large Stokes shifts and high fluorescence yields while maintaining binding to nerves and improved aqueous solubility. Furthermore, we elaborate on the unusual photophysical properties of our previously reported fluorophores, which were observed for both in-vitro (in organic solvent) and in-vivo (in mouse nerves).
MATERIALS AND METHODS
Synthesis of dyes
The dyes shown in Table 1 were synthesized in step-wise procedures as illustrated in the Schemes below. Details of the synthetic methodology can be found in the Supplemental Material. Compounds were purified by medium performance liquid chromatography on silica gel (ISCO Companion with RediSep Gold silica columns). Those selected for advancement into in vivo imaging studies were further purified by high performance liquid chromatography (HPLC). For normal phase HPLC, a Shimadzu LC-8A equipped with a SIL-10AP autosampler, an SPD-M20A diode array detector and an FRC-10 A fraction collector were used, together with a Waters Sunfire preparative silica OBD column, 5 μm, either 19×150 mm or 50×100 mm, and a hexanes/ ethyl acetate gradient. For reverse phase HPLC, a GE AKTA Avant system was used, with a Waters XTerra PrepMS C18 column, 5 μm, 30×100 mm and water − 0.1% formic acid/ acetonitrile/ 0.1% formic acid gradient.
Table 1.
Styryl and bis-styryl fluorophores in solution
| Namea | Chemical Structure | λAbsb, nm | λEm
c, nm (Intensity)d |
|---|---|---|---|
|
d1
(BMB)a |
|
408 | 495 (106,800) |
| d2 |
|
362 | 414 (246,900) |
| d3 |
|
383 | 448 (84,300) |
| d4 |
|
400 | 575 (200) |
| d5 |
|
415 | 511 (93,300) |
| d6 |
|
385 | 522 (2,800) |
|
d7
(GE3082)a |
|
417 | 491, 621*
(1,700; 2,520*) |
| d8 |
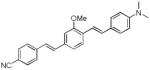
|
412 | 518 (264) |
|
d9
(GE3111)a |
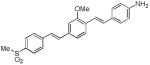
|
412 | 480, 624*
(2,250; 4,700*) |
|
d10
(GE3126)a |
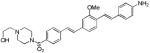
|
414 | 510, 618*
(2,100; 3,970*) |
| d11 |
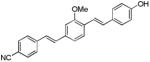
|
396 | 532 (12,640) |
| d12 |
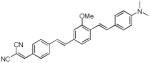
|
467 | 582 (180) |
| d13 |
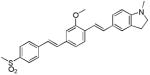
|
419 | 533 (2,900) |
| d14 |
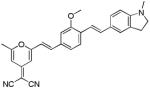
|
482 | 649 (150) |
The d-numbering scheme for each dye is used for simplicity. For previously published fluorophores, the original designations are shown in parenthesis.
Absorbance maximum wavelength measured in DMSO
Emission maximum wavelength measured in DMSO, the relative fluorescence intensity at the maximum wavelength shown in parenthesis
Relative emission intensity values at λEm RFU. For dual emissions, the emission intensities in parentheses correspond to the λEm shown, respectively.
Dual emission (TICT) in polar solvents.
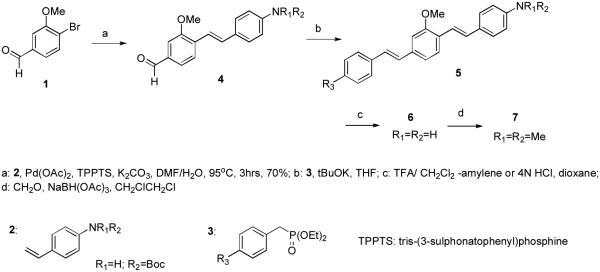
Bis-styryl dyes having three ring structures similar to that of d7 (vide infra) have been synthesized following the methodology previously reported.18 Essentially, this consists of a tandem Heck coupling of the appropriately functionalized aminostyrene with the correspondingly substituted bromo arylaldehyde, followed by a Horner-Wittig olefination with the appropriately substituted benzyldiethyl phosphonate (Scheme 1).
Scheme 1.
Synthesis of D-A bis-styryl dyes d7-d10 and their analogs d7: R1=R2=H, R3=CN; d8: R1=R2=Me, R3=CN; d9: R1=R2=H, R3=MeSO2; d10: R1=R2=H, R3=N’-(2-hydroxyethyl)-N-piperazinylsulfonyl.
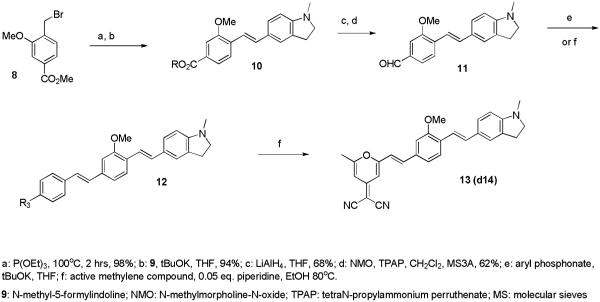
Compounds d5 and d8 were prepared via reductive amination of d1 and d7, respectively. Compound d11 was prepared as illustrated in Scheme 2.
Scheme 2.
Synthesis of compound d11
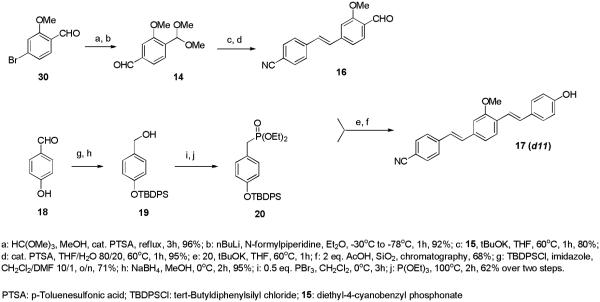
Compounds d12 and d14, containing multiple electron withdrawing groups were prepared via a Knoevenagel condensation of the corresponding styryl aldehyde with the active methylene compounds, as described previously23 and illustrated in Scheme 3 for compound d14 and detailed in the experimental sections for compounds d12-d14.
Scheme 3.
Synthesis of dyes bearing multiple electron-withdrawing groups
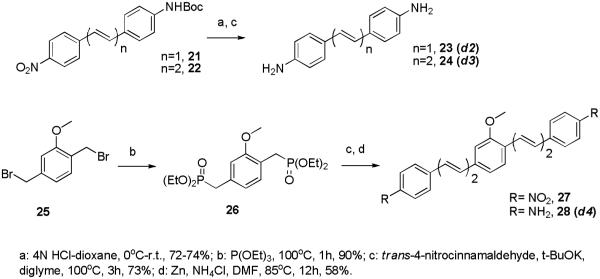
The styryl compounds d2-d4 have been prepared via straightforward transformations depicted in Scheme 4. Compound d2 has been previously reported 16 and has been included here for comparison within the polyene series. Updated reviews of the general methodologies described above have been published recently.24-26
Scheme 4.
Synthesis of styryl and polyene dyes d2-d4
Characterization of nerve labeling dyes
Methods for spectroscopic and physicochemical characterizations were as previously described.18 Briefly, absorbance spectra were taken using a Lambda 20 UV/Vis spectrometer (Perkin Elmer, Waltham, MA). The wavelength of maximum absorbance was then used as the excitation wavelength for the collection of the fluorescence emission spectra on a steady state fluorimeter (Photon Technology International, Birmingham, NJ). A 10 mM stock solution of each dye in dimethylsulfoxide (DMSO) was used in the preparation of 10 µM solutions of the dye in DMSO, unless indicated otherwise.
Ex vivo and in-vivo imaging
Ex vivo nerve tissue staining on each of the dye was performed as described.17 A buffer only control (no dye) was performed using exactly the same procedure to determine autofluorescence under identical imaging settings.
In vivo nerve imaging studies on selected dyes were described previously.18,27 All procedures were approved by the Institutional Animal Care and Use Committee at GE Global Research. Briefly, CD- 1 mice ranging in body weight from 25 to 30 g, and Sprague-Dawley rats ranging in body weight from 250 to 300 g, were purchased from Charles River Laboratories (Wilmington, MA). On the day of the experiment, mice and rats were anesthetized using 2%– 4% isofluorane and given a single tail vein injection each of 50 mg/kg of d7 (GE3082), 8 mg/kg of d9 (GE3111), or 12.2 mg/kg of d10 (GE3126). The animals were then returned to the home cage until the designated time-point for imaging (3 h post-injection for d7 and d9; 1 h post-injection for d10). The nerves were exposed and multispectral imaging was initially performed using a fluorescence stereomicroscope (SteREO Lumar V12, Carl Zeiss Inc., Thornwood, NY) equipped with a multispectral imaging camera (Nuance, CRI, Woburn, MA). Imaging using our custom laparoscopic instrument was as described.27 Imaging of control animals (with no dye injected) was performed under identical conditions.
Spectral measurements of tissue fluorescence were done using a spectroscopic probe. The probe consisted of illumination (405 nm laser, which was the same as the laparoscopic instrument illumination, or a 447 nm laser) coupled to a fiber, a premium-grade reflection ferrule (Ocean Optics, 6 illumination fibers, 1 600-micron read fiber, 3-inch long ferrule), a collimator with a holder for excitation rejection filter, and a spectrometer (Ocean Optics, USB2000+UV-VIS). The 405 nm excitation spectra were measured with BLP01-405R-25 rejection filter, while the 447 nm excitation spectrum was measured with LP03-458RU long-pass filter.
RESULTS & DISCUSSION
We investigated the absorption and emission properties of dyes d1-d14 in non-polar (toluene) and polar (DMSO, MeOH) solvents. The dyes d1-d14, shown in Table 1, can be classified into two categories, where dyes d1-d5 have conjugated amine based electron donating “push-push” end groups, while d6-d14 are based on electron-donating and electron-withdrawing “push-pull” end groups in conjugation. The purpose of screening these individual categories was to see which one of these gave the longest wavelength emission and highest fluorescence yields while maintaining nerve binding.
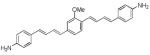
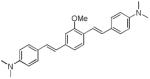
Since we had previously shown that BMB (d1) can bind and visualize nerves in vivo,19 we utilized this molecule as a starting point to develop dyes with improved optical properties. The absorbance and fluorescence emission maxima in DMSO for each synthesized dye are shown in Table 1. Compounds d2 and d3 lacking a central arene group (Scheme 4) displayed a hypsochromic shift in the absorption and emission while retaining high emission intensity as compared to d1. Compound d4, in which the central arene was preserved while incorporating an extra alkene on each side of the arene, showed a reasonable bathochromic shift of the emission compared to d1, however its fluorescence intensity was reduced dramatically (fluorescence emission intensity: d1=106,800 vs. d4=200). Alkylation of the amine groups (d5) helped in further bathochromic shift of absorption and emission with respect to d1 while maintaining sufficient fluorescent intensity.
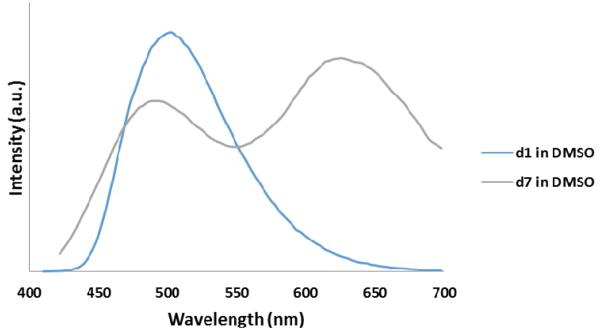
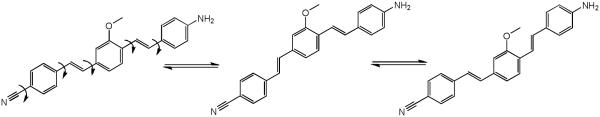
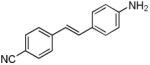
In the push-pull dye system category, d6 was used as a model compound which displayed solvent dependent behavior; In the push-pull dye systems category, d6 was used as a model compound which displayed solvent dependent behavior; absorption and emission maxima of 368 nm and 432 nm respectively in non-polar solvents (toluene). In polar solvent (DMSO), d6 displayed an absorption maximum at 385 nm and emission maximum at 522 nm which we assign to a Twisted Intramolecular Charge Transfer (TICT) state .28 Extending the conjugation of this system by adding an arene middle ring (d7) resulted in a bathochromic shift of ∼30 nm in absorption and a dual emission in DMSO at 491 nm and 621 nm. The solvent dependent behavior of d7 relative to the analogous non push-pull system (d1) is shown in Figure 1.
Figure 1.
Overlay of emission of d1 and d7 in Toluene (A) and in DMSO (B)
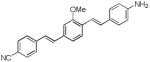
N-methylated dye system d8 resulted in a slight (5 nm) hypsochromic shift of the absorption with a maximum at 412 nm and a modest 27 nm shift in emission, with respect to the short wavelength emission of the unalkylated amine dye system d7. To our surprise this system failed to show dual emission in polar solvents. Replacing the electron withdrawing cyano group with other electron withdrawing groups −SO2Me, −SO2-piperazine (d9 and d10) showed similar absorption trends and values and large Stokes shifts, with emission maxima around 620 nm. Replacing the electron donating system −NH2by a hydroxy (−OH) group in d11 resulted in a hypsochromic shift in absorption and emission with respect to d6. Similar to compounds d7, d9 and d10 also showed dual a emission in polar solvents.. Using the indoline or N,N-dimethyl amino groups as electron donating groups with a variety of electron withdrawing moieties such as dicyanovinyl, methylsulfonyl, dicyanopyranyl (d12-d14), resulted in bathochromic shifts in absorption (467, 419, and 482nm, respectively), and reasonable Stokes shifts albeit low emission yields.
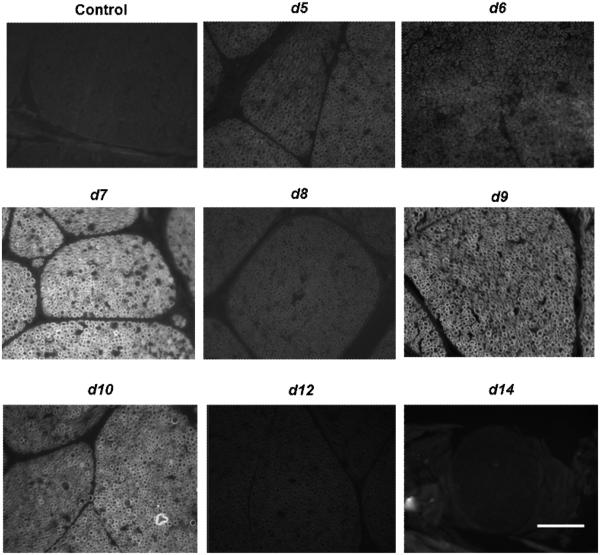
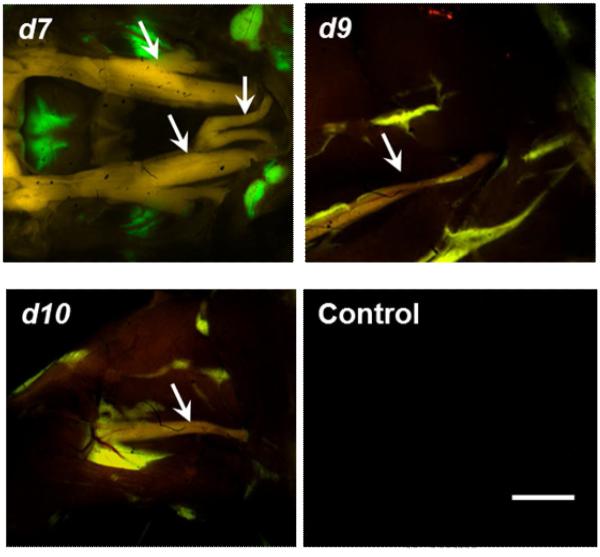
To identify the most promising nerve labeling dyes, a qualitative ex vivo tissue staining assay was performed using each of the dye with detectable fluorescence. Figure 2 shows examples of microscopic images of fluorescently labeled rat nerve tissue sections. Here, compounds d7, d9, and d10 exhibited the most robust fluorescent staining of nerve tissue sections. These dyes were subsequently selected for in vivo fluorescence imaging studies in rodents- using (1) the small animal multispectral imaging instrument shown in Figure 3; and (2) the dual-mode laparoscopic instrument developed in-house shown in Figure 4.28
Figure 2.
Qualitative ex vivo nerve labeling screening of several fluorophores.10 μM of each dye was incubated with rat nerve tissue sections to assess their ability to stain the myelin bundles by fluorescence microscopy. The control tissue was incubated with buffer only. Scale bar ∼ 50 μm.
Figure 3.
In vivo multispectral imaging of mice injected with d7 (GE3082), d9 (GE3111), or d10 (GE3126). The control animal received injection buffer only, and imaged under the same conditions as the dye-treated mice. Arrows are pointing to the trigeminal and optic nerves (d7) and to the sciatic nerve (d9, d10). Scale bar ∼1 mm.
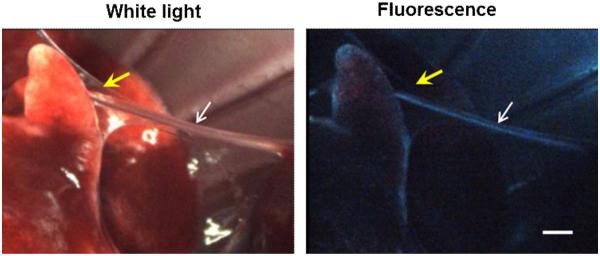
Figure 4.
Simultaneous dual-mode (white light and fluorescence laparoscopic imaging of the thoracic cavity of a rat injected with d7 (GE3082). The white arrows are pointing to the phrenic nerve, and yellow arrows are pointing to a layer of fascia surrounding the nerve. Scale bar ∼1 mm.
Figure 3 shows examples of multispectral imaging in mice. In general, the nerves appeared red-orange and the surrounding muscle tissue was dark. Because the dyes are lipophilic, there was some non-specific partitioning to adipose tissue which appeared as yellow-green fluorescence under the multispectral camera.
Figure 4 shows the dual-mode laparoscopic imaging of a rat injected with d7. Note the thin layer of fascia obscuring the nerve under white light imaging, but not under fluorescence visualization. More detailed in vivo dosing and kinetics results for these three dyes were described elsewhere (d717,19,28; d918; manuscript submitted for d10;).
We have also developed a spectroscopic probe for exact spectral measurements of tissue fluorescence in-situ. The probe allows precise local measurement to be taken at a tissue of interest at a high-resolution (1 nm) and relatively small integration times, typically 1-2 sec for nerve, 0.5 sec for adipose tissue, and 2-5 sec for muscle. This spectral probe used the same illumination as the dual-mode laparoscopic instrument (405 nm excitation). Figure 5 shows spectral measurements of the nerve, adipose tissue, and muscle in a dissected mouse treated with of d10. The probe was pointed at the relevant tissue inside the mouse. The shape and intensity of the spectrum for nerve, adipose tissue, and muscle was different from one another. The emission spectrum of the nerve was reminiscent of the dual emission wavelength observed in DMSO (Table 1),
Figure 5.
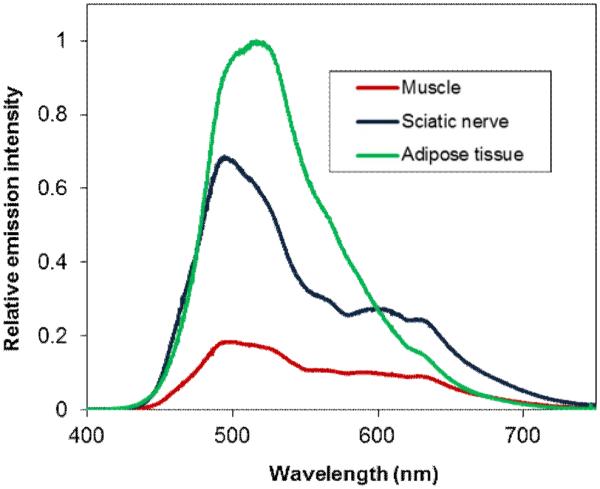
Fluorescence emission spectra of nerve, adipose tissue, and muscle of a mouse injected with d10 (GE3126).
Our approach to improving the photophysical properties from our initial model system BMB (d1) focused on increasing conjugation of the push-push based dye system, and investigating the effect of a series of push-pull based Donor-Acceptor (D-A) groups, conjugated across a variable length π-system, followed by down-selection and biological screening. Of particular interest was the number of connecting double bonds, the number of aromatic rings, and the strength of the acceptor group, including the effect of multiple acceptor groups. Additionally, we were presented with the challenge of having to determine what molecular characteristics were necessary to retain in order to maintain the binding affinity to our target of interest - myelin basic protein.17 In order to obtain reasonable solubility in the three ring system and attenuate lipophilicity we incorporated a methoxy- group in the middle aryl ring.29
We sought to understand the influence of conjugation length in this structural space by extending the π-system via the addition of double bonds, an aim most readily achieved, from a synthetic point of view, in symmetrical D-π-D systems. Extending the conjugation of the simple 2-ring diaminostilbene d2 by one double bond provided the expected, modest bathochromic (red) shift to both absorption and fluorescence emission. The same transformation applied to the 3-ring BMB system (compound d1) led to compound d4, in which the absorbance displayed a slight hypsochromic (blue) shift and the fluorescence was essentially suppressed. In agreement with previous reports on diene chromophores30,31 we are compelled to conclude that extended alkene systems such as d4, despite the addition of only one double bond on each side of a rigidifying middle ring, possess too flexible a backbone. Thus the fluorescence quantum yields and lifetimes in these systems are dependent on mechanisms that compete with non-radiative decay which do not lead to photoisomerization and might not involve large amplitude torsional motions. Through NMR and LC-MS analysis we established that isomerization to cis-products does not occur upon irradiation of our lead candidate dyes, d7, d9 and d10. Similarly, in the trans-diphenylbutadiene system, Saltiel et. al30 have observed radiationless decay pathways that do not lead to photoisomerization resulting in the reduction of fluorescence quantum yields and lifetimes in contrast to the corresponding trans-stilbene derivatives.
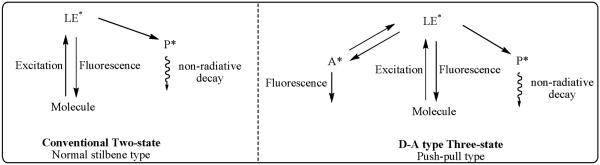
Electronic Donor-Acceptor (push-pull) D-π-A systems, where D = −NH2, −N(CH3)2, −OH and A= −CN, −NO2 etc., have been studied extensively for their tendency to exhibit large stokes shifts and a solvent dependent dual fluorescence behavior.32 These observations have been explained using a three-state model as opposed to a conventional two-state model as seen in Figure 6, adapted from the work reported by Lapouyade and coworkers33,34 wherein LE* represents a highly polar, locally excited fluorescent state with planar geometry; A* represents a highly polar, TICT fluorescent state with a twisted group, and P* represents a weakly polar, non-fluorescent funnel to the ground state with twisting about the stilbene double bond. The dynamic transformation process between LE* and A* has been hypothesized to go through an energy barrier that has been shown to be sensitive to the polarity of solvents and the concentration of the dye.33
Figure 6.
Interpretation of photophysical behavior of push-pull stilbenes (Left) Conventional Two-State and (Right) Proposed Three-State Kinetic Scheme.
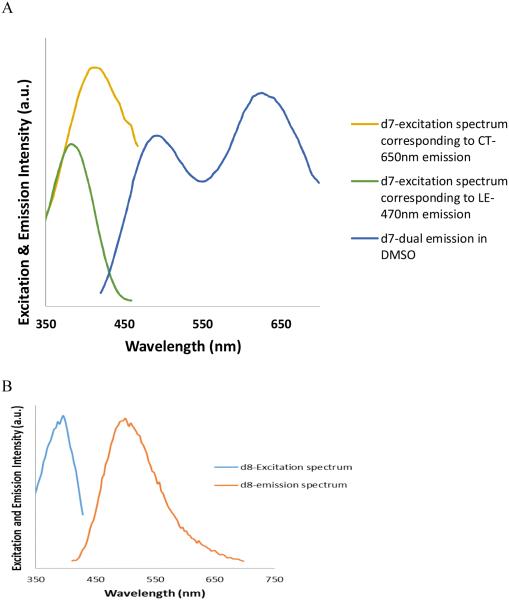
We sought to exploit this effect in the bis-styryl system. Substitution of an electron-withdrawing cyano moiety for one of the amines in the two-ring stilbene (d2) led to a large stokes shift as a result of accessing the ICT (A*) state.35 Applying this same substitution to the three-ring BMB system (comparison of compounds d7 vs. d1) led to a modest 9 nm bathochromic shift in the absorbance, a primary emission band at 491nm attributed to the LE* state and an additional long wavelength emission attributed to the A* ICT state at 621 nm. Substitution with alternative electron withdrawing groups methylsulfonyl or sulfonamide, introduced to improve pharmacokinetics and pharmacology properties, (compounds d9 and d10) resulted in similar shifts in absorption and emission. The long wavelength emission is only observed in polar solvents such as DMSO, Figure 7A, and providing further evidence in support of formation of an ICT state.
Figure 7.
Excitation and emission spectra of compounds d7 (A) and d8 (B). d7 shows two distinct excitation peaks corresponding to LE and TICT state emission in DMSO, while and compound d8 shows only one excitation and emission corresponding to the LE state.
Alkylation of the amine in the 3-ring, D-π-D BMB system (d1) led to compound d5 with a 7 nm bathochromic shift in absorption and a 16 nm shift in emission, as might be expected; however, the emission intensity was significantly reduced. Similar N-alkylation in the push-pull D-π-A system (d8 & d12) led to complete suppression of the long wavelength emission. This observation is contrary to the behavior reported in the well-studied DMABN system in which aminobenzonitrile displayed emission only from the LE* state and N,N-dimethyl aminobenzonitrile displayed a long wavelength emission from the A* (ICT) state, in turn attributed to twisting about the aniline bond.35 Gruen & Gorner also reported lack of dual emission in the 2-ring N,N-dimethylamino-4-cyanostilbene.36 We hypothesize that the dissimilar emission behavior in the alkylated vs. non-alkylated 3-ring system may be due to a combination of twisting about the stilbene bonds and the presence of an electron-donating methoxy substituent in the central ring though more extensive photophysical studies are required to explain the behavior of this unique system
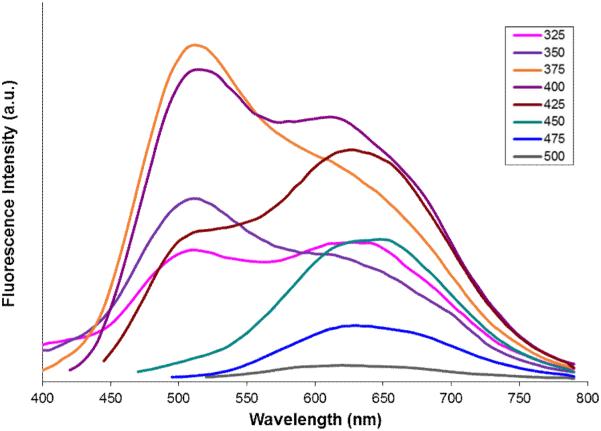
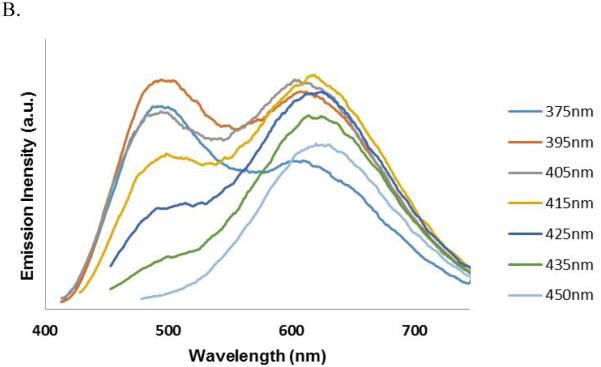
Further investigation of the four systems (d7-d10) uncovered an additional phenomenon which is unprecedented, at least in the case of the three-ring, push-pull stilbene systems. Compound d7 showed a dual emission in a polar solvent (DMSO) similar to that of 4-amino-4’-cyanostilbene (ACS).37 However, to our surprise, this dual emission displayed two distinct excitation bands at 380 and 420 nm respectively for the 491 nm (LE) and 621 nm (CT) emissions, yet only a single excitation and emission in a non-polar solvent (Figure 7). The wavelength dependent emission of compound d7 is further illustrated by exciting from 300 nm-500 nm in 5 nm intervals and collecting the corresponding emission spectra in DMSO (Figure 8). The observed solvent dependence and large Stokes shift associated with the long-wavelength emission strongly support the assignment of dual emission peaks at 491 and 621 nm to LE and CT states respectively. The wavelength dependence and distinct excitation bands would suggest the excitation of two different species in polar media. We hypothesize that these may represent ground-state conformers that differ through twisting about one of the stilbene single-bonds and that one of these conformers has an arrangement that facilitates formation of the ICT state.
Figure 8.
Wavelength dependent dual-emission spectrum of compound d7 (GE3082) showing the presence of two distinct conformers in the ground state with different population levels.
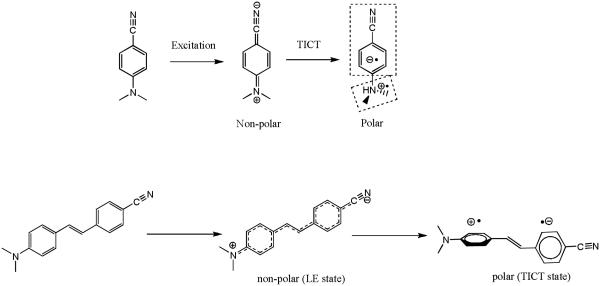
Recently, Atsbeha et al.38 and Catalan et al.39 reported that a similar trend was seen for DMABN in polar solvents. Catalan hypothesized that a dipolar solvent forms species with charge transfer properties in its ground electronic state and such forms can be directly excited to a TICT structure. However the anomalous behavior with the systems under our study stems from the fact that while the amino derivative d7 showed dual emission in a polar medium, the N,N-dimethyl derivative d8 did not display the expected long-wavelength TICT emission. Compound d8 showed a single LE emission maximum at 460 nm in non-polar medium (toluene) and a single emission peak maximum at 500 nm in polar medium (DMSO) as shown in Figure 7. Unlike DMABN where twisting of N,N-dialkyl group is responsible for TICT emission (Scheme 5), the push pull stilbene based system is known to undergo aniline moiety twisting which could also thought be responsible for dual emission characteristics.40 Unlike 4-cyano-4’-amino stilbene systems in which twisting about the aniline bond represents the only degree of rotational freedom (Scheme 5), multiple ground state conformers that may exist in the three ring stilbene-type systems as shown in Scheme 6.
Scheme 5.
Access of TICT state in polar medium via Intramolecular N,N-dimethyl- group twisting of DMABN in polar medium and anilino- group twisting in push-pull stilbenes.
Scheme 6.
Possibility of multiple ground state conformations (rotamers) due to single bond rotations in push-pull tri ring systems.
Thus, it was not surprising that compound d7, with a less bulky amino group, could still show a dual emission behavior in a polar solvent, as the dual emission in the stilbene type system does not depend on amine bond twisting. However, one would have expected the corresponding N,N-dimethyl system d8 to still show at least a similar dual emission behavior, even if not a more pronounced effect. We believe that the methoxy- substitution has an important role to play for the dual emission behavior of d7 and absence of the same in d8. Attempts to cross check if the methoxy- substituent is indeed responsible for the above emission behavior in d7 and d8 by making the parent tri-ring stilbene system without the methoxy- susbstituent was not pursued because this molecule was shown to be insoluble in polar solvents such as MeOH and DMSO.41 Recently Singh et al.42 reported a similar observation in the 1,2-diarylethene system having a linear acceptor group such as −CN on one of the phenyl rings and two electron donor groups such as −OMe on the other phenyl group, giving systems capable of solvent polarity-dependent dual fluorescence. The short wavelength fluorescence band was assigned to LE state, which is electronically delocalized with a planar geometry. The long wavelength fluorescence band has been attributed to a TICT state having a dipolar character. The authors reported that they couldn’t determine the exact site of excited state bond twist leading to the formation of TICT species.
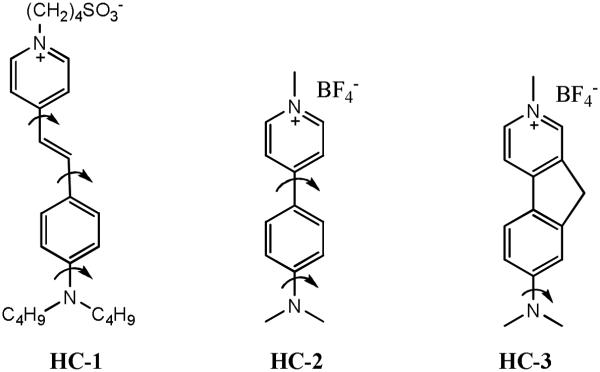
There is literature precedence for molecular structures that possess N,N-dimethyl substituted push-pull dyes which show no TICT emission. Since double bond rotation trans→cis is excluded in these systems, we hypothesized that the deactivation from the singlet-excited state is mainly achieved through an internal conversion mechanism in polar solvents. Fromherz, Heilmann and others reported that N,N-dimethyl substituted hemicyanine undergoes internal twisting and a yields a twisted internal charge transfer (TICT) state on photoexcitation [Scheme 7].43,44 However, no dual emission was observed in this molecule (HC-3) and the authors hypothesized a non-emissive TICT state. Due to multiple bond rotations producing rotamers in hemicyanine dyes, the ICT states are deactivated. Strehmel and co-workers performed quantum mechanical calculations on various TICT conformations of hemicyanine dyes, and ruled out the possibility that deactivation of TICT state is due to twisting of diamino group, since this charge transfer state is approx. 1.5 eV higher in energy than that of the Frank–Condon state.45 The authors attributed twisting of single bonds adjacent to the central double bond to the formation of CT states comparable in energy to that of the FC state and causing the singlet-excited state twisting of the aniline ring to become barrierless in polar solvents. Fromherz also studied two other dye systems, (dimethy1amino)methylazafluorenium with a rigid fluorene group [HC-3] and [(dimethylamino)phenyl]methylpyridinium [HC-2] with a freely rotatable biphenyl frame.43 The quantum yield of the more rigid dye is around 50%, while the quantum yield of the twistable homolog is about 0.05% in solvents of high polarity and fluidity. The difference in the fluorescence behavior is attributed to the formation of a TICT state with efficient radiationless deactivation.
Scheme 7.
Dependence of bond rotations on TICT emission resulting in non-Fluorescent TICT (HC-1); weakly fluorescent (HC-2) and strongly fluorescent (HC-3).
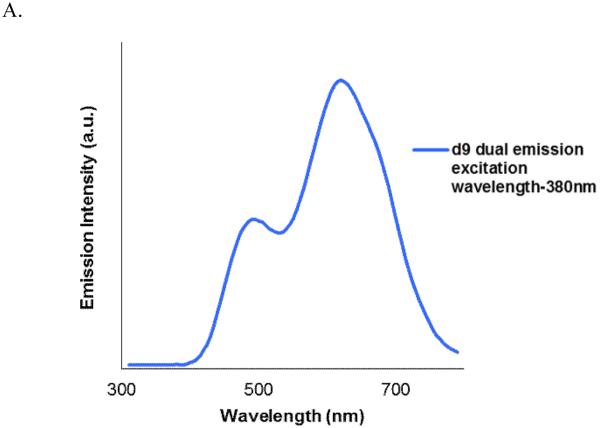
We investigated additional systems to verify that the emission behavior of compound d7 and d8 was neither a single anomaly nor an artifact. Compounds d9 and d10 are similar to Compound d7 but the electron accepting –cyano group replaced by another electron accepting groups methylsulfonyl and piperazinylsulfonyl respectively. Compound d9 and d10 still showed similar LE and TICT dual emissions similar to that of d7 and exhibited two ground state conformers that could be individually excited. Dual emission of compound d9 in DMSO obtained using excitation wavelength 380 nm is shown in Figure 9.
Figure 9.
A. Dual-emission seen in compound d9. Emission obtained using excitation wavelength-380 nm. B. Wavelength dependent dual-emission spectrum of compound d10 showing presence of two distinct conformers in the ground state with different population.
A phenol replacing the amino group proved to be a much poorer donating group (compound d11) even under pH conditions which ensured complete deprotonation to the respective phenoxide resulting in hypsochromic shift by ∼20 nm and reduced stokes shift compared to d7. Locking the tertiary amine moiety in a planar conformation in the indoline system (compounds d13-d14) resulted in different effects depending on the system it was implemented. Compound d13 showed reasonable red-shifted absorption characteristics and modest fluorescence. Increasing the strength of the acceptor group by using dicyanomethylene-pyranone, as in compound d14, resulted in a significant bathochromic shift in absorbance (Δ = 63 nm in compound d14 vs. compound d13) accompanied by the same large fluorescence shift but had very poor emission.
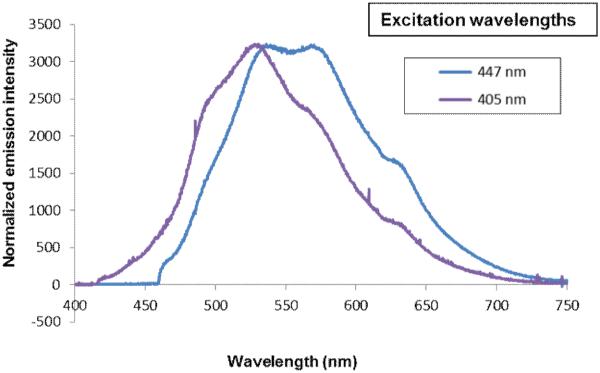
Interestingly, when compound d7 was injected into a mouse and the dye bound to nerves were excited by either 405 nm or 447 nm laser, the wavelength dependent emission described above was observed (Figure 10). Excitation at longer wavelength (447 nm) showed a more pronounced red-shifted second peak. There were subtle differences between the in vivo (in mouse nerve) and in vitro (in DMSO solution) spectra of d7, which was expected because one medium is in solution while the other is in rigid tissue environment.
Figure 10.
Wavelength dependent emission spectrum of compound d7 (GE3082) in the sciatic nerve of a mouse injected with d7.
Conclusion
We have successfully screened a series of styryl- and distyryl dye systems (d1-d14) and identified three promising dyes (d7, d9 and d10) that exhibit long wavelength emission, large Stokes shifts and reasonable fluorescence intensities while maintaining adequate solubility and high affinity to myelin. We observed an unusual wavelength-dependent dual emission behavior in which two distinct ground state conformers could individually be excited to favor emission from the locally excited (LE) or twisted intramolecular charge transfer (TICT) excited states in push-pull dye systems d7, d9 and d10. Additionally, we observed an anomalous emission behavior of dye systems d7and d8 under our study conditions, wherein the amino- derivative d7 showed dual emission characteristic in polar medium, and the N,N-dimethyl derivative d8 and the other N-alkylated derivatives d12-d14 did not show any TICT emission at all in either ground state or excited state. We envision that results presented here will elicit other research groups to elaborate on these photophysical observations.
The in vivo fluorescence imaging studies in rodents injected with selected dyes showed that visualization of nerves against a dark surrounding muscle tissue was possible. Because of their lipophilicity, the dyes also showed some non-specific uptake in adipose tissue, which appeared a different color than that of the nerves under multispectral imaging. These nerves were detectable while surrounded by fascia under fluorescence guidance.
Despite operating in the visible spectrum, we demonstrated that intraoperative fluorescence imaging in this optical window is a viable solution under certain conditions. For example, the large Stokes shift helps to minimize the impact of autofluorescence, similar to the approach taken in near-infrared imaging. Our future work on this class of dyes includes the development of derivatives that are less lipophilic, while maintaining their ability to cross the blood-nerve-barrier, in conjunction with applying novel strategies for differentiating the fluorescence of nerves from that of adipose tissue.
Supplementary Material
Highlights.
Synthesized and investigated photophysical properties of several substituted styryl type dyes d1-d15.
Three dyes d7, d9 and d10 showed good fluorescent properties and maintained drug-like binding property.
Observed an unusual dual emission behavior with two distinct ground state conformers that could individually be excited.
Observed an anomalous emission behavior on dye systems d7 and d8 the amino- derivative d7 showed a dual emission characteristic in the polar medium, while the N,N-dimethyl derivative d8 did not.
Interestingly, when compound d7 was injected into a mouse and the dye bound to nerves were excited by either 405 nm or 447 nm laser, a wavelength dependent emission described above was observed similar to fluorescence in DMSO.
in-vivo fluorescence imaging studies in rodents using small animal multispectral imaging instrument and the dual-mode laparoscopic instrument developed in-house.
ACKNOWLEDGMENTS
We thank Bruce Johnson, Eric Williams, Jorge Ramos, Joseph Greene, Nicole LaPlante, Daniel Gray, and Kenneth Fish for technical assistance. This work is supported by NIH grant EB022872.
Biographies

Tiberiu Siclovan obtained his early education in chemical engineering from Timisoara Polytechnic, Romania. In 1992 he moved to the United States, and in 1996 received his Ph.D. in organic chemistry from Iowa State University under the direction of professor George A. Kraus. That same year he accepted a position at GE Global Research, where he is currently a senior scientist. His research interests include various aspects of biomedical and imaging technologies.

Rong Zhang received her B.S in chemistry from Nanjing University in China and her MS in organic chemistry from Worcester Polytechnic Institute. Prior to joining GE Global Research in 2005, she was a medicinal chemist at Abbott Laboratories. At GE Global Research, Rong is a lead chemist working on developing technologies and agents to enable diagnostic medical imaging.

Victoria Cotero received her B.S in chemistry from Fairleigh Dickinson University and her Ph.D. in pharmacology and human physiology from New Jersey Medical School, now Rutgers Medical School, in 2009. She then served as a postdoctoral fellow in neuroendocrinology/behavioral neuroscience at SUNY-Albany. At GE Global Research, Victoria is a lead biologist working on medical technologies for imaging and preclinical applications.

Anshika Bajaj obtained a B.S. in Pharmacy and M.S. in Biological sciences from BITS, Pilani, India in 2000, after which she moved to the United States to pursue a Ph.D. in Biochemistry from the University of Rochester. In 2006 she started her post-doctoral work at Albany Medical College and focused on studying signaling mechanisms involved in angiogenesis. She joined GE Global Research in 2010 where she contributed to and led biology tasks on multiple neuro, cell therapy, and immunotherapy projects. In 2015 she joined Immune Design a clinical stage immunotherapy company where she is responsible for in vitro characterization of lenti-

Dmitry V. Dylov received his B.S.and M.S. degree in applied physics and mathematics from the Moscow Institute of Physics and Technology and Ph.D. from Princeton University in 2010. His research interests include theoretical aspects of imaging, spectroscopy and biophotonics.

Siavash Yazdanfar received his BS from Boston University and MS and PhD from Case Western Reserve University in biomedical engineering. Prior to joining GE in 2005, he was a postdoctoral fellow at the Massachusetts Institute of Technology. He has published 38 peer reviewed journal articles, 5 book chapters, and 22 issued US patents, primarily in the area of biomedical optical imaging and instrumentation.

Randall L. Carter received his B.S. in chemistry from the Pennsylvania State University and Ph.D. from Yale University in 1993. He served as an NIH post-doctoral fellow and visiting assistant professor at Duke University before joining GE Global Research in 1996. His is currently managing the Translational Sciences Laboratory with a research focus on the development of technologies and agents to enable diagnostic medical imaging.

Cristina Tan Hehir received her bachelor’s degree in chemistry from the University of the Philippines Diliman. She obtained her Ph.D. in Chemistry/Biochemistry from Boston College and was a visiting associate at the Laboratory of Cell Biology at NHLBI/NIH. At GE Global Research, Tina is a senior scientist and project leader working on technologies for fluorescence guided surgery and development of imaging probes for neuroscience. She is also an NIH panel reviewer for Clinical Molecular Imaging Probe Development.

Arunkumar Natarajan obtained his B.S. degree in 1997 from Loyola College, Chennai, India and obtained his M.S. from the Indian Institute of Technology (IIT), Mumbai, India in 1999. Then he moved to the United States, and in 2005 obtained his Ph.D. from Tulane University. He continued his postdoctoral studies at UCLA from 2005-07. He joined GE Global Research in 2008, where he currently holds a lead scientist position. He has over 42 peer reviewed publications including 7 book chapters and 24-filed and 15-issued US patents. His scientific interests include photochemistry, polymers and materials chemistry for various technology applications including biological imaging, holography and additive manufacturing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL MATERIAL
See Supplemental Material for detailed synthetic procedures for dyes d1-d11 and the corresponding characterization of the compounds using NMR and other relevant spectroscopic analysis.
References
- [1].Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU International. 2004;94(7):1071–1076. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- [2].Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of Complications of Prostate Cancer Treatment. CA: A Cancer Journal for Clinicians. 2008;58(4):196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walz J, Graefen M, Huland H. Basic principles of anatomy for optimal surgical treatment of prostate cancer. World Journal of Urology. 2007;25(1):31–38. doi: 10.1007/s00345-007-0159-6. [DOI] [PubMed] [Google Scholar]
- [4].Aluffi P, Policarpo M, Cherovac C, Olina M, Dosdegani R, Pia F. Post-thyroidectomy superior laryngeal nerve injury. European Archives of Oto-Rhino-Laryngology. 2001;258(9):451–454. doi: 10.1007/s004050100382. [DOI] [PubMed] [Google Scholar]
- [5].Cooper DS. Thyroxine Monotherapy After Thyroidectomy. JAMA: The Journal of the American Medical Association. 2008 Feb 20;299(7):817–819. doi: 10.1001/jama.299.7.817. 2008. [DOI] [PubMed] [Google Scholar]
- [6].Baker D, Conley J. Avoiding facial nerve injuries in rhytidectomy. Anatomical variations and pitfalls. Plast Reconstr Surg. 1979;64(6):781–795. doi: 10.1097/00006534-197912000-00005. [DOI] [PubMed] [Google Scholar]
- [7].Warmuth M, Bowen G, Prosnitz L, et al. Complications of axillary lymph node dissection for carcinoma of the breast. Cancer. 1998;83(7):1362–1368. doi: 10.1002/(sici)1097-0142(19981001)83:7<1362::aid-cncr13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [8].Thawait SK, Wang K, Subhawong TK, et al. Peripheral Nerve Surgery: The Role of High-Resolution MR Neurography. American Journal of Neuroradiology. 2012 Feb 1;33(2):203–210. doi: 10.3174/ajnr.A2465. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kau T, Rabitsch E, Celedin S, et al. Feasibility and potential value of flat-panel detector–based computed tomography in myelography after spinal surgery. Journal of Neurosurgery: Spine. 2009;10(1):66–72. doi: 10.3171/2008.10.SPI08627. [DOI] [PubMed] [Google Scholar]
- [10].Bartels RHMA, Meulstee J, Verhagen WIM. Luttikhuis TTMC-O. Ultrasound imaging of the ulnar nerve: Correlation of preoperative and intraoperative dimensions. Clinical Neurology and Neurosurgery. 2008;110(7):687–690. doi: 10.1016/j.clineuro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [11].Zhang T, Jiang W, Li Y, Li B, Yamakawa T. Perioperative Approach in the Surgical Management of Carotid Body Tumors. Annals of Vascular Surgery. 2012;26(6):775–782. doi: 10.1016/j.avsg.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [12].Rais-Bahrami S, Levinson AW, Fried NM, et al. Optical Coherence Tomography of Cavernous Nerves: A Step Toward Real-Time Intraoperative Imaging During Nerve-Sparing Radical Prostatectomy. Urology. 2007;72(1):198–204. doi: 10.1016/j.urology.2007.11.084. [DOI] [PubMed] [Google Scholar]
- [13].Schols R, Bouvy N, van Dam R, Stassen LS. Advanced intraoperative imaging methods for laparoscopic anatomy navigation: an overview. Surg Endosc. 2013 Jun 01;27(6):1851–1859. doi: 10.1007/s00464-012-2701-x. 2013. [DOI] [PubMed] [Google Scholar]
- [14].Stankoff B, Wang Y, Bottlaender M, et al. Imaging of CNS myelin by positron-emission tomography. Proc Natl Acad of Sci. 2006 Jun 13;103(24):9304–9309. doi: 10.1073/pnas.0600769103. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu C, Tian D, Feng Y, et al. A Novel Fluorescent Probe That Is Brain Permeable and Selectively Binds to {alpha}Myelin. J. Histochem. Cytochem. 2006 Sep 1;54(9):997–1004. doi: 10.1369/jhc.5A6901.2006. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu C, Wei J, Tian D, Feng Y, Miller RH, Wang Y. Molecular Probes for Imaging Myelinated White Matter in CNS. Journal of Medicinal Chemistry. 2008;51(21):6682–6688. doi: 10.1021/jm8003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bajaj A, LaPlante NE, Cotero VE, et al. Identification of the Protein Target of Myelin-Binding Ligands by Immunohistochemistry and Biochemical Analyses. J Histochem Cytochem. 2013 Jan 1;61(1):19–30. doi: 10.1369/0022155412467353. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cotero V, Siclovan T, Zhang R, et al. Intraoperative Fluorescence Imaging of Peripheral and Central Nerves Through a Myelin-Selective Contrast Agent. Mol Imaging Biol. 2012;14(6):708–717. doi: 10.1007/s11307-012-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gibbs-Strauss S, Nasr K, Fish K, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging. 2011;10(2):91–101. [PMC free article] [PubMed] [Google Scholar]
- [20].Gray D, Kim J, Cotero V, et al. Dual-mode laparoscopic fluorescence image-guided surgery using a single camera. Biomedical Optics Express. 2012;3(8):1880–1890. doi: 10.1364/BOE.3.001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang X, Bhaumik S, Li Q, Staudinger V, Yazdanfar S. Compact instrument for fluorescence image-guided surgery. J. Biomed. Opt. 2010 Apr 12; doi: 10.1117/1.3378128. [DOI] [PubMed] [Google Scholar]
- [22].Gibbs SL, Xie Y, Goodwill HL, et al. Structure-Activity Relationship of Nerve-Highlighting Fluorophores. PLOS One. 2013;8(9):e73493. doi: 10.1371/journal.pone.0073493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang Y, Chow T. Highly efficient red fluorescent dyes for organic light-emitting diodes. J Mat Chem. 2011;21(9):3091–3099. [Google Scholar]
- [24].Jana R, Pathak TP, Sigman MS. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chemical Reviews. 2011 Mar 09;111(3):1417–1492. doi: 10.1021/cr100327p. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Molnár Á . Efficient, Selective, and Recyclable Palladium Catalysts in Carbon–Carbon Coupling Reactions. Chemical Reviews. 2011 Mar 09;111(3):2251–2320. doi: 10.1021/cr100355b. 2011. [DOI] [PubMed] [Google Scholar]
- [26].Li J. A Collection of Detailed Mechanisms and Synthetic Applications. Fourth Expanded Springer-Verlag; Berlin Heidelberg: 2009. Name Reactions. [Google Scholar]
- [27].Gray DC, Kim EM, Cotero VE, et al. Dual-mode laparoscopic fluorescence image-guided surgery using a single camera. Biomed Opt Express. 2012;3(8):1880–1890. doi: 10.1364/BOE.3.001880. doi: 1810.1364/BOE. 1883.001880. Epub 002012 Jul 001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gray D, Kim E, Cotero V, Staudinger P, Yazdanfar S, Tan Hehir C. Compact fluorescence and white light imaging system for intraoperative visualization of nerves. Proc SPIE. 8207 doi: 10.1117/12.905354. Photonic Therapeutics and Diagnostics VIII2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ulf S, Heiner D. Synthesis and electronic spectra of substituted p-distyrylbenzenes for the use in light-emitting diodes. Journal fuer Praktische Chemie. 2000;342(1):10–16. [Google Scholar]
- [30].Saltiel J, Krishna T, Laohhasurayotin K, et al. Medium Effects on the Direct Cis-Trans Photoisomerization of 1,4-Diphenyl-1,3-butadiene in Solution. Journal of Physical Chemistry A. 2011;115(11):2120–2129. doi: 10.1021/jp111482m. [DOI] [PubMed] [Google Scholar]
- [31].Velsko S, Fleming G. Chem Phys. 1982;76:3553–3562. [Google Scholar]
- [32].Meier H, Gerold J, Kolshorn H, Muhling B. Extension of Conjugation Leading to Bathochromic or Hypsochromic Effects in OPV Series. Chem. Eur. J. 2004;10:360–370. doi: 10.1002/chem.200305447. [DOI] [PubMed] [Google Scholar]
- [33].Lapouyade R, Czeschka K, Majenz W, Rettig W, Gilabert E, Rullie‘re C. Photophysics of donor-acceptor substituted stilbenes. A time-resolved fluorescence study using selectively bridged dimethylamino cyano model compounds. Journal of Physical Chemistry. 1992;96:9643–9650. [Google Scholar]
- [34].Letard J, Lapouyade R, Rettig W. J. Am. Chem. Soc. 1993;115:2441–2447. [Google Scholar]
- [35].Grabowski Z, Rotkiewicz K, Rettig W. Structural changes accompanying intramolecular electron transfer: focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003;103:3899–4031. doi: 10.1021/cr940745l. [DOI] [PubMed] [Google Scholar]
- [36].Gruen H, Görner H. Fluorescence of Trans-Cyano-4'-dimethylaminostilbene; No Evidence for a TICT State. Z Naturforsch. 1983;38 a:928–936. [Google Scholar]
- [37].Pines D, Pines E, Rettig W. ual fluorescence and excited state structural relaxations in donor-acceptor-stilbenes. J. Phys. Chem. A. 2003;107:236–242. [Google Scholar]
- [38].Atsbeha T, Mohammed A, Redi-Abshiro M. Excitation Wavelength Dependence of Dual Fluorescence of DMABN in Polar Solvents. J Fluoresc. 2010 Nov 01;20(6):1241–1248. doi: 10.1007/s10895-010-0675-4. 2010. [DOI] [PubMed] [Google Scholar]
- [39].Catalan J. Can the dipolarity of the medium induce the formation of charge transfer structures? An unexpected finding in the photophysics of DMABN. Physical Chemistry Chemical Physics. 2014;16(17):7734–7740. doi: 10.1039/c4cp00177j. [DOI] [PubMed] [Google Scholar]
- [40].Druzhinin SI, Demeter A, Galievsky VA, Yoshihara T, Zachariasse KA. Thermally Activated Internal Conversion with 4-(Dimethylamino)benzonitrile, 4-(Methylamino)benzonitrile, and 4-Aminobenzonitrile in Alkane Solvents. No Correlation with Intramolecular Charge Transfer†. The Journal of Physical Chemistry A. 2003 Oct 01;107(40):8075–8085. 2003. [Google Scholar]
- [41].Koch F, Heitz W. Soluble poly(1,4-phenylenevinylene)s and poly(1,4-phenyleneethynylene)s via suzuki coupling. Macromolecular Chemistry and Physics. 1997;198(5):1531–1544. [Google Scholar]
- [42].Singh AK, Kanvah S. Photophysical studies of substituted 1,2-diarylethenes: twisted intramolecular charge transfer fluorescence in dimethoxycyano-substituted 1,2-diarylethene. Journal of the Chemical Society, Perkin Transactions 2. 2001;(3):395–401. [Google Scholar]
- [43].Fromherz P, Heilemann A. Twisted internal charge transfer in (aminophenyl)pyridinium. The Journal of Physical Chemistry. 1992 Aug 01;96(17):6864–6866. 1992. [Google Scholar]
- [44].Nag A, Kundu T, Bhattacharyya K. Effect of solvent polarity on the yield of twisted intramolecular charge transfer (TICT) emission. Competition between formation and nonradiative decay of the TICT state. Chemical Physics Letters. 1989;160(3):257–260. [Google Scholar]
- [45].Strehmel B, Seifert H, Rettig W. Photophysical Properties of Fluorescence Probes. 2. A Model of Multiple Fluorescence for Stilbazolium Dyes Studied by Global Analysis and Quantum Chemical Calculations†. The Journal of Physical Chemistry B. 1997 Mar 01;101(12):2232–2243. 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.