Abstract
We examined the moderating effects of age and cognitive reserve on the relationship between body mass index (BMI) and processing speed, executive function, and working memory based on literature suggesting obese individuals perform more poorly on measures of these abilities. Fifty-six healthy, dementia-free community-dwelling older (mean age 65.72±7.40) and younger (mean age 21.10±2.33) adults completed a neuropsychological battery and reported height and weight. Mixed effects models were used to evaluate the interactive effects of age, education (a proxy for cognitive reserve), and BMI on cognitive scores. Higher education was protective for executive deficits in younger, but not older adults. Age differences in executive functions were reduced at higher education levels but increased in individuals with higher BMI. Results suggest the inter-relationships between cognitive reserve—as measured by education—and BMI differ across age, and that obesity may accelerate the cognitive aging process.
Keywords: Body Mass Index, Executive Function, Cognitive Reserve, Obesity, Cognitive Deficits
Introduction
Obesity is a paramount public health concern facing the United States, and represents a clear health risk in older adults. Approximately one-third (34.9%) of the adult population of the United States is obese and 69.2% are medically overweight, with those age 60+ having the highest obesity rates in any age category (National Center for Health Statistics, 2013). Obesity, often measured by body mass index (BMI), is a risk factor for most cardiovascular health problems (Alpert, Lavie, Agrawal, Aggarwal, & Kumar, 2014) and for metabolic disorders such as diabetes (Zuo, Shi, & Hussain, 2014). Each of these disorders represents an increased risk for cognitive decline (Bordier, Doucet, Boudet, & Bauduceau, 2014; Gifford et al., 2013; Wiesmann, Kiliaan, & Claassen, 2013).
There is evidence that obesity is detrimental not only to physical health, but also to cognitive functioning (Boeka & Lokken, 2008; Wiesmann et al., 2013). Obese individuals showed a fourfold risk of lower cognitive function on speed and executive measures in a recent cross-sectional study (Fergenbaum et al., 2009). Even at an early age, visceral fat has been shown to affect executive functioning (EF)(Schwartz D.H., 2013). Additionally, evidence exists that age affects the relationship between body weight and cognitive functioning. For example, the combination of increased body mass and age predicts poorer EF and processing speed (PS) (Stanek et al., 2013). Longitudinal studies have shown that obesity during midlife predicts higher incidence of dementia later in life (Ravona-Springer, Schnaider-Beeri, & Goldbourt, 2013). Another study found that only obesity in midlife, and not in late life, predicted cognitive deficits in late life (Dahl, 2013).
A common protective factor in many cognitive aging studies is education, which is often used as a proxy measure for cognitive reserve, or the brain's robustness to decline due to aging, injury, and disease (Vance, Roberson, McGuinness, & Fazeli, 2010). Cognitive reserve is thought to reflect high neuroplasticity within an individual (Vance et al., 2010). In addition to possible direct cognitive benefits bestowed by higher education, education may have an indirect effect on cognition by playing a role in the number and frequency of good health behaviors during aging (Vlismas, Stavrinos, & Panagiotakos, 2009). Individuals of higher socioeconomic status and education tend to exercise more often, receive preventative medical care, and eat healthier diets including fresh fruits and vegetables (Barnett, van Sluijs, & Ogilvie, 2012; Darmon & Drewnowski, 2008). Recent work has shown that cognitive reserve, as measured by estimates of premorbid intelligence, protects against the negative association of BMI with attention/executive function, and memory (Galioto, Alosco, Spitznagel, Stanek, & Gunstad, 2013); however, since this work controlled for age, it is unclear whether or not cognitive reserve differentially preserves BMI-related cognitive deficits across the lifespan. Building upon this previous research, the current study was performed to examine the moderating and potentially interactive effects of age, education and BMI on cognitive performance. We hypothesized that there would be significant effects of age and BMI on working memory (WM), EF, and PS, in which those of a greater age or higher BMI would perform worse on cognitive measures. Additionally, we predicted that education would moderate the effect of BMI on cognition, such that those at a higher education level would receive a protective benefit in cognitive performance independent of BMI. Based on the greater impact of obesity on cognition in older age and the large body of literature on cognitive reserve in aging, we expected this effect to be more apparent in older adults than in younger adults.
Methods
Study Sample
Healthy, community-dwelling adults were recruited in Gainesville, Florida and the surrounding community in Alachua County through flyers on the University of Florida campus, newspaper advertisement, and a public service announcement on the local public news station. Potential participants were screened to exclude individuals with self-reported neurological conditions, dementia, alcohol or substance abuse, or psychotic disorders. This study is part of a larger study that included magnetic resonance imaging (MRI; data not reported here). A subset of participants for the current study completed a second session that included magnetic resonance imaging (MRI) scanning; therefore, we excluded participants with MRI contraindications and only included right-handed individuals. A total of 77 community-dwelling adults were enrolled and completed testing. Twenty-one participants were excluded from the present analyses as a result of missing data (N = 16), comorbid medical conditions (N = 1), narcotic pain management (N = 2), or outlying cognitive data, defined as three standard deviations above or below the sample mean (N = 2). The final sample for the current study consisted of 56 adults age 18-81 years in two age groups (younger mean age=21.10 ±2.33; older mean age=65.72 ±7.40). Excluded participants did not differ demographically from the final sample. Sample characteristics are summarized in Table 1.
Table 1.
Sample characteristics
| Young Adults (N=31) |
Older Adults (N=25) |
Total Sample (N=56) |
|
|---|---|---|---|
| Age (years)*** | 21.10 (±2.33) | 65.72 (±7.40) | 41.02 (±22.98) |
| Education (years) | 14.63 (±1.19) | 15.52 (±2.33) | 15.02 (±1.82) |
| High School Diploma | 3 | 1 | 4 |
| Some College | 21 | 11 | 32 |
| 4-year Degree | 9 | 6 | 15 |
| Graduate Degree | 0 | 5 | 5 |
| Sex (Females) | 19 | 18 | 37 |
| Race (White/Non-White) | 22/9 | 24/1 | 46/10 |
| Body Mass Index** | 23.60 (±4.41) | 27.30 (±5.50) | 23.60 (±4.41) |
| N Obese (%) | 2 (7%) | 4 (16%) | 6 (11%) |
| N Overweight (%) | 5 (16%) | 6 (24%) | 11 (20%) |
| Executive Function | 0.51(±0.52) | −.58(±0.70) | 0.09 (±0.70) |
| Composite*** | |||
| Working Memory Composite | 0.16 (±0.66) | −.13(±0.95) | 0.05 (±0.96) |
| Processing Speed Composite* | .13 (±0.54) | −0.16(±0.50) | −0.01 (±0.54) |
p<0.01
p<.001
Measures
Cognitive assessment
Participants completed a neuropsychological battery that focused on measures of frontal functions, such as attention, EF, and PS. For the purpose of this study, we focused on measures of 1) processing speed (Stroop Word Reading, Stroop Color Naming, Trail Making Test, part A; Armitage, 1946; Stroop, 1935); 2) working memory (Wechsler Adult Intelligence Scale, Third Edition Letter Number Sequencing and Digits Backwards subtests; Wechsler, 1992); and 3) executive functions (Stroop Interference score and Trail Making Test, part B; Armitage, 1946; Stroop, 1935). Composite scores of these three cognitive domains were used in statistical analyses.
BMI
Body mass was calculated from self-reported height in feet and inches and weight in pounds using the National Heart Lung and Blood Institute website calculator (WHO Expert Consultation, 2004). BMI is often underestimated in self-report, particularly in individuals who are attempting to lose weight (Jerome et al., 2014), and under-reporting may increase with age (Magnusson et al., 2014). Nonetheless, clinical useful data can still be gleaned from these data (Kreatsoulas, 2014; Pursey, Burrows, Stanwell, & Collins, 2014), and despite differences in reported and measured BMI, there is a high correlation between reported BMI and actual BMI (r=.99; Pursey et al., 2014). BMI was used as a continuous measure in statistical analyses.
Cognitive reserve
Cognitive reserve was quantified using self-reported years of completed education, a common method for transposing the far more complex concept of cognitive reserve into a simple numerical measure (Mitchell, Shaughnessy, Shirk, Yang, & Atri, 2012; Reed et al., 2010; Vance et al., 2010). Education was a continuous measure in statistical analyses.
Questionnaires
Self-report questionnaires included the Center for Epidemiological Studies Depression Scale (CES-D), a 20-item measure of the presence and severity of depressive symptoms over the last week (Radloff, 1977), and the State Trait Anxiety Inventory state form, a measure of current situational anxiety (Spielberger, 1983).
Vascular Covariates
A vascular risk score was computed by summing self-reported presence of and medication usage for hypertension, hypercholesterolemia, and diabetes. Data were coded as 0 (no) or 1 (yes) for each condition if the individual reported a diagnosis or medication use for the condition, for a total vascular risk score ranging from 0 to 3.
Statistical Methods
Separate composite scores were calculated for PS, WM and EF by averaging the z-scores for each test within the domain using within sample performance. Before averaging, z-scores for the Trailmaking Test were reoriented so that higher scores represented better performance. The distributions of the composite scores were examined for skewness using the z-skew statistic (skewness statistic divided by the standard error) and were found to be normally distributed. Individuals with outlying cognitive data, defined as three standard deviations above or below the sample mean, were removed from the respective analyses. Separate mixed effects models were performed for each cognitive composite using the PROC GLM procedure in SAS 9.4 (Cary, NC). Education, BMI, and age were entered as independent variables, as well as their 2-way and 3-way interactions. Initially CES-D scores, state anxiety scores, vascular risk score, and sex were included as covariates, but none were included in the final models as they did not contribute significant variance in any model. Non-significant interactions were removed to obtain the most parsimonious model for each composite.
Results
Statistical results for all models are presented in Table 2. Analysis of the EF composite showed a significant age effect, F(1,52)=5.83; p=.0199, with older adults performing more poorly than young adults. Both education and BMI impacted this relationship, as revealed by significant education × age, F(1,52)=5.68 , p=.0214, and BMI × age, F(1,52)=5.29, p=.0261, effects. Age differences in EF were reduced at higher education levels but increased in individuals with higher BMI, and older adults showed greater BMI-related deficits. An age group × education × BMI effect was also observed, F(1,52)=5.50 , p=.0235 (Figure 1). In older adults, scores on the EF composite were lower as a function of higher BMI independent of education level, thus, education did not protect against executive deficits. In contrast, in younger adults, higher BMI was associated with reduced scores only in those with lower education.
Table 2.
Model Statistics for Most Parsimonious Models in Cognitive Composite Analyses
| Executive Function (R2 = .55) |
Working Memory (R2 = .20) |
Processing Speed (R2 = .21) |
||||
|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | |
| Intercept | −10.31 | 5.66 | −1.36 | 1.09 | −0.33 | 0.72 |
| Education | 0.70 | 0.37 | 0.12 | .06 | −0.02 | 0.04 |
| BMI | 0.37 | 0.21 | −0.02 | 0.02 | 0.02 | 0.01 |
| Age Group | 32.19* | 13.33 | 0.26 | 0.24 | 0.36* | 0.15 |
| BMI × Age Group | −1.32* | 0.58 | -- | -- | -- | -- |
| Education × BMI | −0.03 | 0.01 | -- | -- | -- | -- |
| Education × Age Group | −2.12* | 0.89 | -- | -- | -- | -- |
| Education × BMI × Age Group | 0.09* | 0.04 | -- | -- | -- | -- |
Note: BMI= Body Mass Index. A backwards elimination procedure was employed to eliminate non-significant interaction terms.
p<0.05
Figure 1.
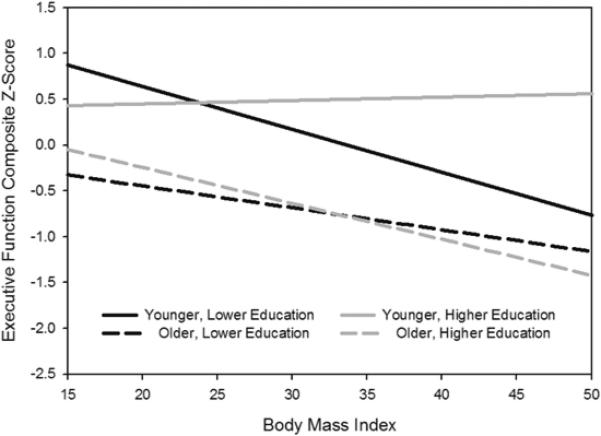
Education × BMI × Age Group effect for executive functions. The education groups are based on a median split and for graphical purposes only, as education was used as a continuous measure in all analyses
PS was slower in older adults than in young adults (age effect for PS composite, F(1,52)=5.25; p=.0262), but there were no effects of education or BMI on PS. There were no significant effects in the analysis of the WM composite.
Discussion
We examined the interactive effects of age, BMI and education—a proxy for cognitive reserve—on cognitive functioning. This is an important area of study due to the pervasive nature of the obesity epidemic. Our results were partially consistent with our hypotheses and showed that education protects against executive deficits at higher BMI in younger adults, but not in older adults.
We expected the protective effect of education to be more salient in older adults compared to young adults, but instead found that education protected against BMI-related executive dysfunction only in young adults. The cognitive reserve literature predominantly focuses on cognitive function in older adults, but there are investigations of the impact of cognitive reserve in younger adults with neurological conditions such as traumatic brain injury and multiple sclerosis, which reveal benefits of cognitive reserve in young adults, consistent with our results (Schneider et al., 2014). Findings are also consistent with a recent study which found that cognitive reserve, as measured by a premorbid IQ estimate, protects against the negative effects of BMI on attention/EF and memory function in a group of young to older adults (Galioto et al., 2013). However, our results extend the previous findings by examining age differences and demonstrating that the benefit of education in the BMI-executive relationship may be specific to younger individuals. The reasons for the age differences are unclear. Contrary to our findings, there is evidence that cognitive reserve protects against the development of dementia in older adults, and neuroimaging studies have documented overlapping neural substrates related to cognitive reserve in young and older adults (Duff et al., 2013; Y. Stern, 2012; Y. Stern, Habek, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J., Flynn, J., Saceim, H., & van Heertum, R., 2005; Y. Stern et al., 2008). However, investigations of neural reserve also reveal more efficient recruitment of reserve-related neural networks in young adults (Y. Stern et al., 2008), which may in part explain the age difference in our study. It is also possible that the cumulative effect of age and higher body weight, which both preferentially impact EF, confers a greater negative impact than can be mitigated by higher education. Indeed, this possibility is supported by our finding that age differences in EF decreased at higher education levels, with highly educated older adults performing more like younger adults. This suggests that education did impact EF in older adults, but higher BMI counteracted that benefit.
This cumulative effect of age and BMI may also explain our finding that older adults showed greater deficits in EF at higher BMI than younger adults. This is similar to previous findings showing age effects on the relationship between BMI and cognitive function, with greater deficits due to BMI in older adults (Stanek et al., 2013). The greater disadvantage due to higher BMI in older adults is not surprising when considering the neurobiological changes related to age and body weight. The fronto-subcortical pattern of deficits related to obesity has been primarily attributed to disruption in white matter circuits connecting the subcortical nuclei and frontal cortical areas via vascular pathology such as endothelial dysfunction, dysregulation of vasodilation and constriction, arteriosclerosis and inflammation. There is considerable evidence that similar vascular changes occur with age (Seals, Kaplon, Gioscia-Ryan, & LaRocca, 2014). Although our measure of cardiovascular health was not significant in our models, it is possible that older adults in our sample had undiagnosed, subclinical vascular symptoms which were not captured by our measure but may have contributed to our results. Interestingly, the age × BMI effect for the EF composite also reflected a tendency for younger individuals with lower education and high BMI to perform more similarly to older adults than to than to other younger adults. This highlights the significant impact of high BMI on cognitive functioning, even at a young age. This result also suggests that overweight or obese young adults who also have a lower education level are particularly at risk for executive deficits.
Our findings were specific to EF and did not extend to WM and PS. This was unexpected in light of the well-documented age effects on these cognitive processes, as well as evidence that obesity can affect WM and PS (Stanek et al., 2013; Yau, Kang, Javier, & Convit, 2014). However, executive functions are disproportionately affected by both age and body weight compared to WM and PS, which may explain the greater sensitivity of EF to age and BMI effects in our study. Additionally, the generally healthy nature of our sample may contribute to the differences in our findings. We excluded major medical conditions, only 14% reported diagnosis or treatment of hypertension, hypercholesterolemia, and diabetes status, and only 31% were in the overweight or obese categories, which is lower than national averages. This is in contrast to other studies that used a sample with higher average BMI, or that included individuals with more health comorbidities such as diabetes/ insulin resistance (Sanz et al., 2013; Stanek et al., 2013).
In addition to health status, other characteristics of our sample may have contributed to our findings, including the relatively high education level and the lack of ethnic/racial diversity in the older group. The present results should be interpreted in the context of these characteristics. The use of education as a measure for cognitive reserve may also be seen as a weakness of the present study. It has been argued that because some younger adults are still in school, their educational attainment is not complete and therefore education is not the best proxy for reserve (Y. Stern, Habek, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J., Flynn, J., Saceim, H., & van Heertum, R., 2005). Thus, it is possible that other measures may have provided more sensitive measures of cognitive reserve in our sample, particularly given the relatively restricted range of years of education. Nonetheless, years of education has been shown to correlate well with other measures of cognitive reserve (Mitchell et al., 2012; Reed et al., 2010; Y. Stern, Habek, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J., Flynn, J., Saceim, H., & van Heertum, R., 2005), and is therefore generally accepted and widely used as an indirect measure of cognitive reserve. The lack of information regarding socioeconomic status and physical activity level of our participants is an additional limitation, as these confounds could be differentially related to age and may be associated with level of education. Physical activity is a particularly important confound given its link to brain structure and function, cognitive functioning, and vascular health (Bherer, Erickson, & Liu-Ambrose, 2013). Despite these limitations, our results contribute to a small but growing literature on the inter-relationships between cognitive reserve, body weight, age, and cognitive functioning.
This research provides evidence that cognitive reserve, as measured by years of education, protects against BMI-related executive dysfunction in younger adults, but not in older adults. These results suggest that the cumulative effect of age-related cognitive decline and increased body weight may minimize or negate any protective benefit of cognitive reserve on executive functioning. Findings highlight the importance of older adults maintaining body weight to reduce the risk of cognitive dysfunction.
Acknowledgements
This work was supported by an Age Related Memory Loss award from the McKnight Brain Research Foundation (VMD), and by the National High Magnetic Field Laboratory. Dr. Dotson is partially supported by the UF Claude D. Pepper Center (NIA P30 AG028740-01). This project was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida. The authors thank Christopher Sozda, Ph.D. and Sarah Syzmkowicz, M.S. for their assistance with data collection.
Footnotes
There are no conflicts of interest to be disclosed.
References
- Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Translational Research. 2014;164(4):345–356. doi: 10.1016/j.trsl.2014.04.010. doi: 10.1016/j.trsl.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychology Monographs. 1946;60:1–47. [Google Scholar]
- Barnett I, van Sluijs EM, Ogilvie D. Physical activity and transitioning to retirement: a systematic review. American Journal of Preventive Medicine. 2012;43(3):329–336. doi: 10.1016/j.amepre.2012.05.026. doi: 10.1016/j.amepre.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508. doi: 10.1155/2013/657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeka AG, Lokken KL. Neuropsychological performance of a clinical sample of extremely obese individuals. Arch Clin Neuropsychol. 2008;23(4):467–474. doi: 10.1016/j.acn.2008.03.003. doi: 10.1016/j.acn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes & Metabolism. 2014 doi: 10.1016/j.diabet.2014.02.002. doi: 10.1016/j.diabet.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, Pedersen NL. Being Overweight in Midlife Is Associated With Lower Cognitive Ability and Steeper Cognitive Decline in Late Life. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;65A(1):57–62. doi: 10.1093/gerona/glp035. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon N, Drewnowski A. Does social class predict diet quality? American Journal of Clinical Nutrition. 2008;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- Duff K, Foster NL, Dennett K, Hammers DB, Zollinger LV, Christian PE, Hoffman JM. Amyloid deposition and cognition in older adults: the effects of premorbid intellect. Arch Clin Neuropsychol. 2013;28(7):665–671. doi: 10.1093/arclin/act047. doi: 10.1093/arclin/act047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergenbaum JH, Bruce S, Lou W, Hanley AJ, Greenwood C, Young TK. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity (Silver Spring) 2009;17(10):1957–1963. doi: 10.1038/oby.2009.161. doi: 10.1038/oby.2009.161. [DOI] [PubMed] [Google Scholar]
- Galioto RM, Alosco ML, Spitznagel MB, Stanek KM, Gunstad J. Cognitive reserve preserves cognitive function in obese individuals. Neuropsychology, Development, and Cognition. Section B: Aging, Neuropsychology and Cognition. 2013;20(6):684–699. doi: 10.1080/13825585.2012.762972. doi: 10.1080/13825585.2012.762972. [DOI] [PubMed] [Google Scholar]
- Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Jefferson AL. Blood pressure and cognition among older adults: a meta-analysis. Arch Clin Neuropsychol. 2013;28(7):649–664. doi: 10.1093/arclin/act046. doi: 10.1093/arclin/act046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, United States . 2012: With Special Feature on Emergency Care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- Jerome GJ, Dalcin A, Coughlin JW, Fitzpatrick S, Wang NY, Durkin N, Appel LJ. Longitudinal accuracy of web-based self-reported weights: results from the Hopkins POWER Trial. J Med Internet Res. 2014;16(7):e173. doi: 10.2196/jmir.3332. doi: 10.2196/jmir.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreatsoulas C, Hassan A, Subramanian SV, Fleegler EW. Accuracy of Self-Reported Height and Weight to Determine Body Mass Index Among Youth. Journal of Child and Adolescent Behaviour. 2014 doi: 10.4172/jcalb.1000126. [Google Scholar]
- Magnusson K, Haugen IK, Osteras N, Nordsletten L, Natvig B, Hagen KB. The validity of self-reported body mass index in a population-based osteoarthritis study. Bmc Musculoskeletal Disorders. 2014;15 doi: 10.1186/1471-2474-15-442. doi: Artn 442 Doi 10.1186/1471-2474-15-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MB, Shaughnessy LW, Shirk SD, Yang FM, Atri A. Neuropsychological test performance and cognitive reserve in healthy aging and the Alzheimer's disease spectrum: a theoretically driven factor analysis. Journal of the International Neuropsychological Society. 2012;18(6):1071–1080. doi: 10.1017/S1355617712000859. doi: 10.1017/S1355617712000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey K, Burrows TL, Stanwell P, Collins CE. How Accurate is Web-Based Self-Reported Height, Weight, and Body Mass Index in Young Adults? J Med Internet Res. 2014;16(1) doi: 10.2196/jmir.2909. doi: ARTN e4 DOI 10.2196/jmir.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ravona-Springer R, Schnaider-Beeri M, Goldbourt U. Body weight variability in midlife and risk for dementia in old age. Neurology. 2013;80(18):1677–1683. doi: 10.1212/WNL.0b013e3182904cee. doi: 10.1212/WNL.0b013e3182904cee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, DeCarli C. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain. 2010;133(Pt 8):2196–2209. doi: 10.1093/brain/awq154. doi: 10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz CM, Ruidavets JB, Bongard V, Marquie JC, Hanaire H, Ferrieres J, Andrieu S. Relationship Between Markers of Insulin Resistance, Markers of Adiposity, HbA(1c), and Cognitive Functions in a Middle-Aged Population-Based Sample: the MONA LISA Study. Diabetes Care. 2013;36(6):1512–1521. doi: 10.2337/dc12-1017. doi: Doi 10.2337/Dc12-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider EB, Sur S, Raymont V, Duckworth J, Kowalski RG, Efron DT, Stevens RD. Functional recovery after moderate/severe traumatic brain injury: a role for cognitive reserve? Neurology. 2014;82(18):1636–1642. doi: 10.1212/WNL.0000000000000379. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.H. LG, Perron M, Richer L, Syme C, Veillette S, Pausova Z, Paus T. Visceral fat is associated with lower executive functioning in adolescents. International Journal of Obesity. 2013;37(10):1336–1343. doi: 10.1038/ijo.2013.104. doi: 10.1038/ijo.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Kaplon RE, Gioscia-Ryan RA, LaRocca TJ. You're Only as Old as Your Arteries: Translational Strategies for Preserving Vascular Endothelial Function with Aging. Physiology (Bethesda) 2014;29(4):250–264. doi: 10.1152/physiol.00059.2013. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press Inc; 1983. [Google Scholar]
- Stanek KM, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Gunstad J. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27(2):141–151. doi: 10.1037/a0031988. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurology. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habek C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Saceim H, van Heertum R. Brain Networks Associated with Cognitive Reserve in Healthy Young and Old Adults. Cerebral Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Zarahn E, Habeck C, Holtzer R, Rakitin BC, Kumar A, Brown T. A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cerebral Cortex. 2008;18(4):959–967. doi: 10.1093/cercor/bhm134. doi: 10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–662. doi: 10.1037/h0054651. [Google Scholar]
- Vance DE, Roberson AJ, McGuinness TM, Fazeli PL. How neuroplasticity and cognitive reserve protect cognitive functioning. Journal of Psychosocial Nursing and Mental Health Services. 2010;48(4):23–30. doi: 10.3928/02793695-20100302-01. doi: 10.3928/02793695-20100302-01. [DOI] [PubMed] [Google Scholar]
- Vlismas K, Stavrinos V, Panagiotakos DB. Socio-economic status, dietary habits and health-related outcomes in various parts of the world: a review. Central European Journal of Public Health. 2009;17(2):55–63. doi: 10.21101/cejph.a3475. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. Psychological Corporation; San Antonio, TX: 1992. [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA. Vascular aspects of cognitive impairment and dementia. Journal of Cerebral Blood Flow and Metabolism. 2013;33(11):1696–1706. doi: 10.1038/jcbfm.2013.159. doi: 10.1038/jcbfm.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22(8):1865–1871. doi: 10.1002/oby.20801. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: A systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2014;104(1):63–72. doi: 10.1016/j.diabres.2014.01.002. doi: 10.1016/j.diabres.2014.01.002. [DOI] [PubMed] [Google Scholar]


