Abstract
Sepsis following hemorrhagic shock is a common clinical condition, in which innate immune system suffers from severe suppression. B and T lymphocyte attenuator (BTLA) is an immune-regulatory co-inhibitory receptor expressed not only on adaptive, but also on innate immune cells. Our previous data showed that BTLA gene deficient mice were protected from septic mortality when compared with wild type control C57BL/6 mice. Here we extended our study by treating C57BL/6 mice with an anti-BTLA monoclonal antibody (clone 6A6; reported to have the ability to neutralize or agonize/potentiate BTLA signaling) in a mouse model of hemorrhagic shock (Hem) followed by sepsis induced by cecal ligation and puncture (CLP); positing initially that if BTLA engagement was neutralized, like gene deficiency, an anti-BTLA mAb would have the similar effects on the inflammatory response/morbidity in these mice after such insults. Here we report that BTLA expression is elevated on innate immune cells after Hem/CLP. However, anti-BTLA antibody treatment increased cytokine (TNF-α, IL-12, IL-10)/ chemokine (KC, MIP-2, MCP-1) levels and inflammatory cells (neutrophils, macrophages, dendritic cells) recruitment in the peritoneal cavity, which in turn aggravated organ injury and elevated these animals’ mortality in Hem/CLP. When compared to the protective effects of our previous study using BTLA gene deficient mice in a model of lethal septic challenge, we further confirmed BTLA’s contribution to enhanced innate cell recruitment, elevated IL-10 levels and reduced survival, and that engagement of antibody with BTLA potentiates/exacerbates the pathophysiology in Hem/sepsis.
Keywords: co-inhibitory receptor, macrophage, dendritic cell, IL-10, recruitment, MODS
Introduction
Hemorrhagic shock (Hem) is commonly present in patients that suffered from trauma, complex surgery, gastrointestinal bleeding, obstetrical bleeding, etc. It leads to a severe deterioration of pro inflammatory cytokine response from macrophages, monocytes and dendritic cells, which is associated with an increased susceptibility to bacterial infections (1–4). The resulting sepsis following hemorrhage is responsible for 60% of the deaths in surgical intensive care units (5). Sepsis is a leading cause of death among critically ill patients and responsible for more than 250,000 deaths in the United States annually (6). Complex immune reactions during sepsis can be conceptualized as an occurrence of a pro-inflammatory along with a concomitant anti-inflammatory response. This persistent anti-inflammatory response is believed to contribute to the profound state of immune paralysis and late septic death (7, 8). With respect to mortality from severe sepsis, suppression of various aspects of the innate immune response has been implicated, including exaggerated anti-inflammatory cytokine production, deficient pro-inflammatory cytokine release capacity, poor pathogen killing, as well as decreased neutrophil apoptosis, etc. (7, 9, 10). Inasmuch, modulation of innate immunosuppression might be a potential therapeutic target of Hem and sepsis (8).
In the past few years, the co-inhibitory molecule family has peaked some investigator’s interests as potential therapeutic targets against sepsis. Huang et al. (11) found that programmed cell death receptor-1 (PD-1) expression by macrophages plays a pathologic role in altering microbial clearance as well as the innate inflammatory response to sepsis. In addition, anti-PD-1 antibody could reverse immune dysfunction and improve survival during sepsis (12). Inoue et al. (13) demonstrated that anti- Cytotoxic T-lymphocyte antigen 4 (CTLA-4) blocking antibody decreased sepsis-induced apoptosis and immunosuppression, and that anti-CTLA-4 antibody treatment could improve septic survival.
B and T lymphocyte attenuator (BTLA), a third co-inhibitory receptor, which shares some general molecular similarities to PD-1 and CTLA-4, is found not only to be expressed on B and T lymphocytes, but also on innate inmmunocytes, including monocytes, macrophages and dendritic cells, etc (14). In its cytoplasmic domain, there is one growth factor receptor-bound protein 2 (Grb-2) association motif related to pro survival function, and two immuno-receptor tyrosine-based inhibition motifs (ITIMs), enabling BTLA to exhibit a largely inhibitory function (15, 16). Considering BTLA’s capacity to interact with the co-stimulatory molecule herpes virus entry mediator (HVEM) (15), which is widely expressed on hematopoietic and non-hematopoietic cells and interacts with four other molecules. HVEM-BTLA engagement not only induces BTLA-mediated phosphatase-dependent inhibitory signaling, but also HVEM-mediated NF-κB activation (important for the induction of pro-inflammatory and cell survival genes) (17). Thus, BTLA is part of a bidirectional signaling complex that balances activation and inhibition during the immune response (18, 19).
Recent studies from our laboratory (20) found that increased BTLA expression contributed to the pathological changes in mice with polymicrobial peritonitis induced-sepsis. BTLA gene deficient mice displayed decreased leukocyte numbers recruited to the peritoneum, decreased IL-10 levels, inhibited neutrophil activation, enhanced bacterial clearance, as well as a reduction in septic mortality and morbidity when compared to wild type (WT) mice. Moreover, a prior study showed that BTLA gene knock out (KO) mice had an increased expression of HVEM on specific leukocyte subpopulations in experimental malaria (21). Therefore, whether the differences between BTLA KO and WT mice that underwent sepsis were caused by BTLA’s function alone or cooperatively in vivo with HVEM, and/or if there is functional redundancy between each molecule, is still not clear. Taken together, this suggests that BTLA has an important impact in sepsis; however, the role of BTLA in pathophysiologic changes in Hem followed by sepsis is not well studied.
Here we investigated the role of BTLA in a mouse model of Hem followed by cecal ligation and puncture (CLP)-induced sepsis using an anti-BTLA monoclonal antibody (clone 6A6), which has been reported to have both aspects of a neutralizing/blocking and an agonistic/potentiating agent for BTLA mediated action/ signaling (22, 23). We initially set out to test the hypothesis that by blocking BTLA signaling, we would reduce the extent of immune response/organ injury/morbidity and mortality seen following the dual insults of Hem followed by CLP (based on data (22) suggesting that anti-BTLA monoclonal [6A6] antibody was a true ‘blocking [signaling inhibiting] agent’). However, what we observe here is that in the Hem/CLP model is when treated with the anti-BTLA monoclonal [6A6] antibody at the dosage of 25 ug/g body weight, there is an increase of immune response/organ injury/morbidity and mortality, supporting the alternate hypothesis that anti-BTLA monoclonal [6A6] antibody agonizes/potentiates BTLA actions.
Materials and methods
Mice
C57BL/6 male mice, 20–25 g body weight, ages 8–12 weeks, were obtained from Jackson Laboratory (Bar Harbor, ME) and used in all experiments following housing (7–10 days) in RI Hospital’s Aldrich building Central Research Facilities (12 h light/12 h dark). All protocols carried out with animals (between 8AM-11AM; no prior fasting) were done according to NIH Guide for Animal Use and Care, and were approved by the Lifespan-Rhode Island Hospital Institutional animal care and use committee (AWC# 0110-13).
Hemorrhagic shock (Hem)
Non-lethal, fixed-pressure hemorrhagic shock was produced as previously described (24) (25) (26). In brief, following randomization to either Sham group or Hem group, mice were anesthetized using isoflurane. Catheters were inserted into both femoral arteries and the wound sites were bathed in lidocaine/bupivacaine during the entire procedure. Blood pressure was continuously monitored through one catheter. When fully awake, the mice were bled from the other catheter to a mean blood pressure of 35± 5mmHg, and kept stable for 90 minutes. Immediately following Hem, mice were resuscitated with Ringers lactate at 4 times drawn blood volume (drawn blood volume was about 0.8ml-1.2ml per mouse; thus, the Ringers lactate used was between 3.2–4.8ml per mouse in total). The resuscitation speed was set at 12 ml/h. For antibody treatment, mice received 2ml Ringers first, followed by 6A6 or IgG at a dose of 25ug/g body weight (27) in 100 µl of saline, then the rest of the Ringers lactate resuscitant as needed. After resuscitation, arteries were ligated, catheters removed, sutured closed and the mice allowed to recover (e.g., righting & re-acquisition of mobility prior to returning them to the vivarium). Sham mice only had their bilateral femoral arteries ligated, but no blood was drawn.
Sepsis model induced by cecal ligation and puncture (CLP)
Polymicrobial sepsis (CLP) was produced as previously described (24) (25) (26). In brief, 24 hours post Hem (or sham Hem), mice were anesthetized with isoflurane and a midline incision was made in the abdomen. The cecum was isolated and ligated at a point approximately 1 cm from the cecal tip, punctured twice with a 22-gauge needle, then gently squeezed to extrude a small amount of feces from the perforation sites. In the sham CLP mice, the cecum was exposed but neither ligated nor punctured. Then the cecum was placed back into the peritoneal cavity and the incision was sutured closed in 2 layers. Mice were resuscitated with 1ml Ringers lactate by subcutaneous injection and, following recovery returned to the vivarium.
Antibody treatment
Mice received anti-BTLA antibody clone 6A6 (BioXcell, West Lebanon, NH) during Hem resuscitation, and intra-peritoneally right after CLP, 25ug/g body weight per administration. Hamster IgG (BioXcell) was administered as an isotype control. This was done since the model used here produces both a period of leukocyte/ non-immune cell ‘priming’ by exposure to hemorrhagic shock, followed by a “trigger” insult in the form of CLP that precipitates the development of acute lung injury along with other forms of multiple organ injury (24) (25) (26), we wanted to block the contributions of both events in our study.
Survival study
Both 6A6 and hamster IgG treated mice (n=15/group) were subjected to the combined insults of Hem/CLP, and were observed for 10 days for survival. Log-Rank statistical analysis was used to determine if a statistically significant difference in septic mortality was evident between the two groups (20).
Sample collection
At 24 hours post CLP (which was 48 hours after the mice were initially subjected Hem or Sham Hem), mice were euthanized with a CO2 overdose. Blood was collected in a heparinized syringe via cardiac puncture and centrifuged to obtain plasma.
Peritoneal lavage was performed by injecting 2ml sterile PBS into the peritoneum for ex vivo cytokine analysis or to determine the bacterial burden, respectively. In a subsequent set of animals, peritoneal lavage 2X 5ml was done to obtain peritoneal cells to use for the establishment of adherent macrophage cultures as we have previously described in our laboratory (11). For assessment of ex vivo cytokine productive capacity, adherent cell cultures were stimulated for 20 hours with 10 ng LPS/ml, the cell supernatants were harvested/clarified, and retained at −80°C until assay for mouse TNF-α, IL-6, IL-10 or MIP-2 by commercial ELISA kits as we have previously described (11).
Bronchoalveolar lavage fluid was collected to assess protein concentration as an index of lung injury as described by Lomas-Neira et al (26). The trachea was exposed via a midline incision and cannulated with a sterile polypropylene 21-gauge catheter. The lungs were lavaged with 0.6 ml of saline twice. Protein concentration in lavage fluid was assessed. The lung, liver, spleen and kidney were also harvested for Western blot. The ileum and lung were harvested for histological staining or Western blot analysis (20, 26).
Cell culture studies
In an attempt to gain some insight into the nature of 6A6’s effect (establish that it was biologically functional) on myeloid cells, we cultured 1×106/well RAW 264.7 cells (obtained from ATCC [Manassas, VA] and maintained according to their directions) with IgG or 6A6 at a dose of 100 µg/ml for 2 hours followed by LPS stimulation at a dose of 10 ng/ml for 6 hours more. Cells were then harvested, cell lysates produced, protein concentration determined, and stored at −80°C until processing for phosphorylated SHP-1 and SHP-2 expression by Western Blot analysis (28).
Western blot
Protein samples were boiled, separated on 16% denaturing polyacrylamide gels and transferred to Polyvinylidene fluoride membranes (Novex, San Diego, CA). Membranes were incubated with BTLA antibody (T-12), p-SHP-1 or p-SHP-2 antibodies(Santa Cruz Biotechnology, Inc., Dallas, TX) at a concentration of 1:500 overnight and subsequently with donkey anti-goat IgG-HRP (Santa Cruz Biotechnology). After washing, proteins were visualized by enhances chemiluminescence and densitometrically assessed by Alpha-Innotech image analyzer (San Leandro, CA) (28).
Flow cytometry
All the antibodies used for flow cytometric analysis were purchased from eBioscience (San Diego, CA). BTLA expression on innate cells was determined with Phycoerythrin (PE) -labeled anti-BTLA (clone 6F7), Allophyocyanin (APC)-labeled anti-F4/80 (clone 8M8), -CD115 (clone AFS98), or -CD11c (clone N418). Cell populations in the peritoneal lavage were determined with PE-labeled anti-F4/80 (clone BM8), -CD11c (clone N418), or -Gr-1 (clone RB6-8C5). FACSArray flow cytometer (BD Bioscience, San Jose, CA ) and FlowJo software (Tree Star, Ashland, OR) were used for analysis (20).
Measurement of cytokines
Concentrations of tumor necrosis factor-α(TNF-α), interleukin(IL)-6, IL-1β, IL-12, IL-10, keratinocyte chemoattractant (KC), macrophage inflammatory protein-2 (MIP-2), and monocyte chemotactic protein-1(MCP-1) from the peritoneal fluid and plasma were assessed via ELISA according to manufacturer’s protocols (BD Bioscience) (20).
Measurement of bacterial burden
Blood samples and peritoneal lavage fluid were serially diluted in sterile PBS. A 100µl aliquot of each dilution was spread on a tryptic soy blood agar plate (Hardy Diagnostics, Santa Maria, CA). All plates were incubated at 37 °C for 24–48 hours. Colonies were counted and expressed as CFU/100µl samples (11).
Cell apoptosis
Peritoneal leukocytes were stained with APC-labeled Annexin V/propidium iodide (PI) (eBioscience) according to the manufacturer’s guidelines. Briefly, 1*106 cells were washed once in PBS, then once in binding buffer, resuspended in 100 µl of binding buffer with 5 µl of Annexin V and incubated for 10–15 minutes at room temperature. Cells were washed in binding buffer, resuspended in 200 µl of binding buffer containing 5 µl of Propidium Iodide Staining Solution and analyzed via flow cytometry (29).
Measurement of serum creatine and alanine aminotransferase (ALT)
Serum creatine was assayed by QuantiChrom™ Creatine Assay Kit (DICT-500, BioAssay systems, Hayward, CA), and serum ALT was assayed by Alanine Aminotransferase Kit (Biotron Diagnostics Inc, Hemet, CA) following the manufacturer’s instructions (30, 31).
Statistics
Results are expressed as Mean ± SEM. Statistical significance of the results presented were determined by one-way ANOVA (for multiple comparison)with Bonferroni post hoc test, unpaired two-tailed Student’s t-test (for normal distribution data), Mann-Whitney (for nonparametric data) or log-rank test (for survival study) where appropriate. Statistical software used was GraphPad Prism 5 (La Jolla, CA, USA). P < 0.05 was used as a cutoff for significance.
Results
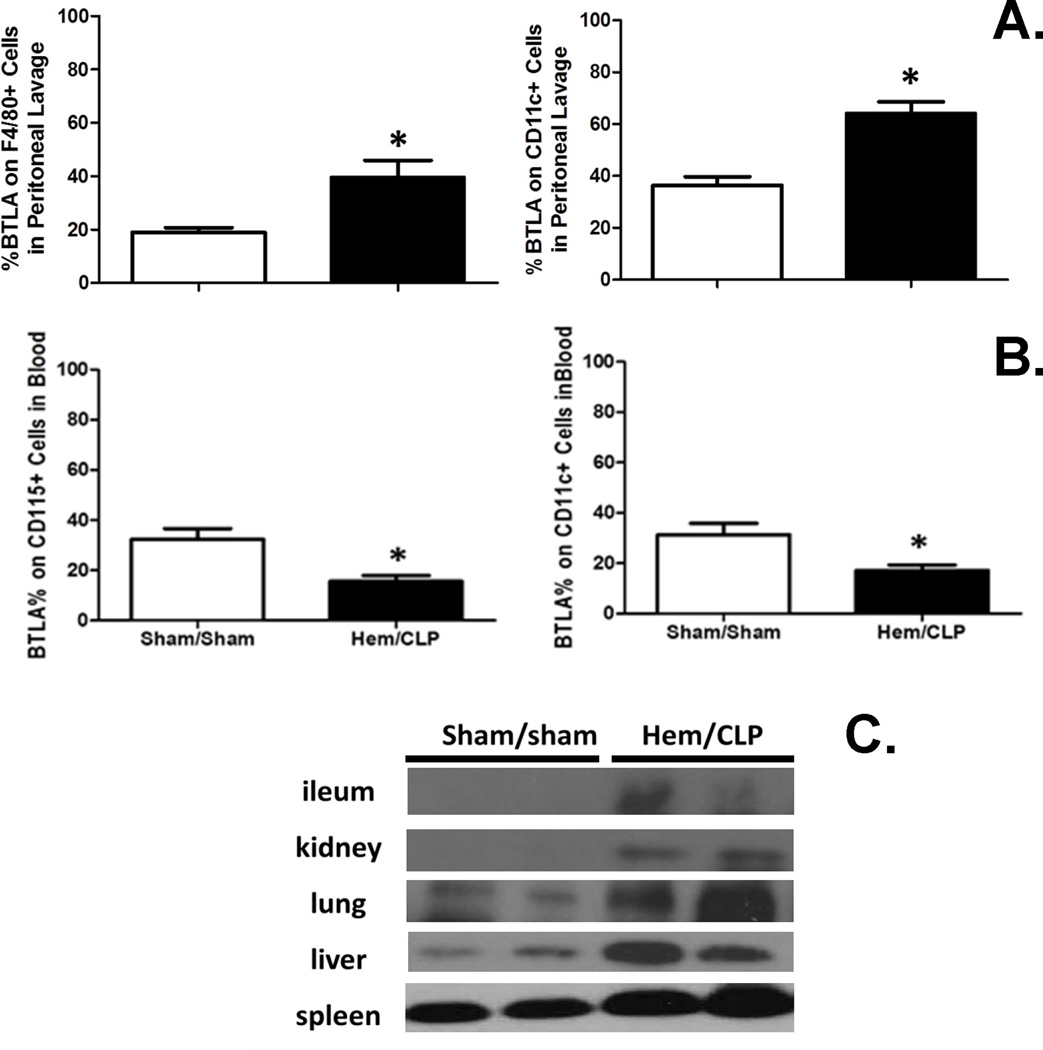
BTLA expression on innate immune cells is elevated at the site of infection (peritoneum) after Hem/CLP
Since BTLA was reported to be expressed on monocytes/macrophages and dendritic cells during experimental sepsis (20), we first examined innate inflammatory cell populations for changes in BTLA expression during hemorrhagic shock followed by sepsis. We found that 24 hours after Hem/CLP, BTLA expression was elevated significantly on peritoneal F4/80+ cells (macrophages) and CD11c+ cells (dendritic cells) (Fig. 1A), while it was markedly decreased on blood CD115+ (monocytes) and CD11c+ cells (Fig. 1B), when compared to sham surgery mice. We also found that BTLA expression increased in ileum, kidney, lung, liver, and spleen tissues via Western blot analysis (Fig. 1C). Together, this indicates that BTLA might play roles not only at the site of infection, but also in the systemic inflammatory responses that develop following the sequential challenges of Hem and sepsis.
Figure 1. The changes of BTLA expression 24 hours after Hem/CLP.
(A) BTLA expression elevated on F4/80+ and CD11c+ cells in peritoneal cavity. N=5–9/group. (B) BTLA expression decreased on CD115+ and CD11c+ cells in blood. N=5–9/group. (C) BTLA expression increased in ileum, kidney, lung, liver and spleen tissues. The data are provided as the mean±SEM, and significant difference between groups is indicated as *P<0.05 by unpaired two-tailed Student’s t-test.
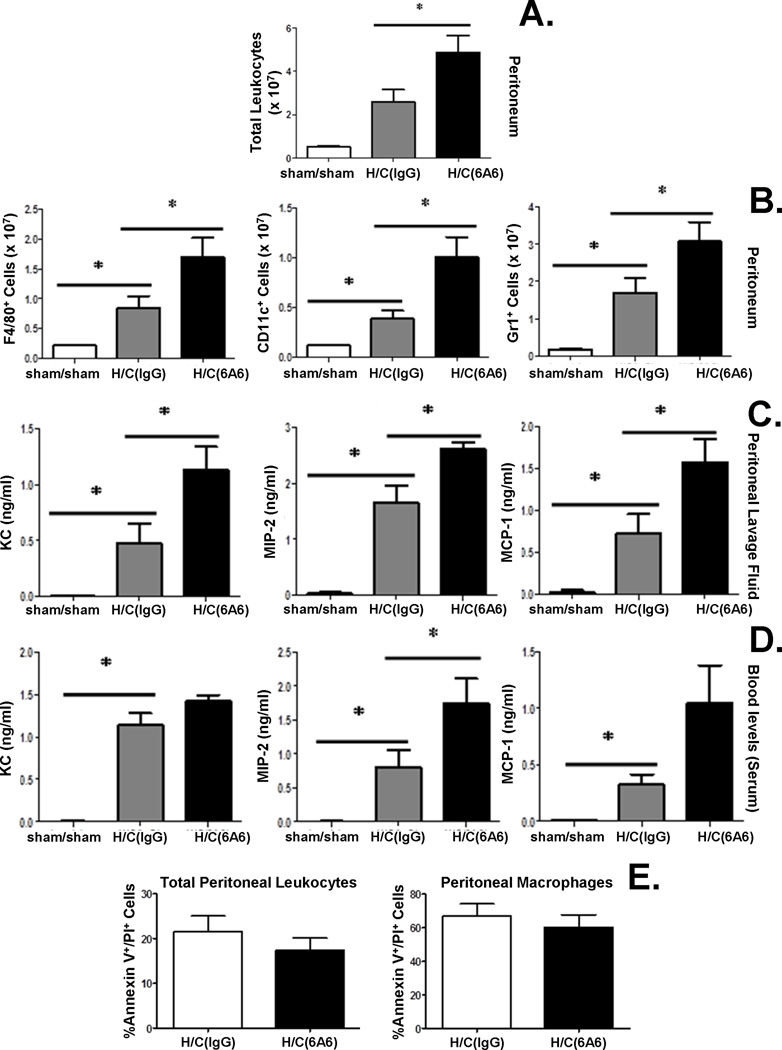
Anti-BTLA antibody treatment induces higher chemokine levels and enhanced leukocyte recruitment to peritoneum after Hem/CLP, but has no effect on the development of increased innate immune cell apoptosis
Previous studies (20) have determined that BTLA KO mice exhibited a decreased capacity to recruit innate cells to the peritoneum following CLP. Here we assessed the cell numbers of innate inflammatory cells recruited to the peritoneum following Hem/CLP, theorizing that engagement of BTLA blocking antibody, like gene deficient KO mice, would have a similar affect in cell recruitment in the peritoneum. We found that an increased number of total leukocytes recruited to the peritoneum after Hem/CLP when compared to sham/sham mice (Fig. 2A). Interestingly, there is a further increase in leukocyte recruitment in Hem/CLP mice treated with 6A6 monoclonal antibody (Fig. 2A), irrespective of whether they were neutrophils, macrophages or dendritic cells when compared to IgG treated Hem/CLP mice (Fig. 2B).
Figure 2. Anti-BTLA antibody treatment contributes to higher chemokine levels and enhanced leukocyte recruitment to peritoneum after Hem/CLP, but has no effect on peritoneal cell apoptosis 24 hours after Hem/CLP.
Total peritoneal leukocyte (A.), macrophage (F4/80) dendritic cell(CD11c) and neutrophil (Gr1) numbers elevated in 6A6 treated group compared to IgG treated group (B.) (N=7 in H/C group, N=4 in Sham/sham group). *P<0.05, by Mann-Whitney (for nonparametric data). Chemokine (KC, MIP-2, MCP-1) levels were elevated significantly in peritoneal lavage fluid (C.) and for MIP-2 in the blood (serum) (D.) 24hs after Hem/CLP in 6A6 treated group compared to IgG treated group. No significant difference in cell apoptosis was observed in peritoneal leukocytes (N=7/group) (E.). The data are provided as the mean±SEM, and significant difference between groups is indicated as *P<0.05, by Bonferroni’s post hoc-test following one-way ANOVA.
To determine the extent that this increased cell recruitment was due to altered chemotactic gradient, we assessed chemokine levels in the peritoneum and plasma. We found that chemokine, including KC, MIP-2, and MCP-1, levels were all elevated significantly in the peritoneal lavage fluid from the 6A6 monoclonal antibody treated when compared to IgG treated group 24 hours after Hem/CLP (Fig. 2C). In peripheral blood, although there was not a statistically significant increase in KC and MCP-1 levels, MIP-2 levels were found to be significantly increased in the 6A6 monoclonal antibody treatment group when compared to IgG treated Hem/CLP group (Fig. 2D).
Since changes in leukocyte cell numbers in septic mice have been reported to be affected by the induction of co-inhibitory molecule PD-1 (11), we also attempted to determine if the frequency of apoptosis in total peritoneal leukocytes and in peritoneal macrophages was affected by BTLA. We found that there was no significant difference between 6A6 monoclonal antibody vs. IgG treated Hem/CLP mice (Fig. 2E), suggesting that BTLA ligation did not change the rate of leukocytes undergoing apoptosis in Hem/CLP mice. Therefore, the increased number of local leukocytes seen is likely due to elevated chemokine levels during recruitment.
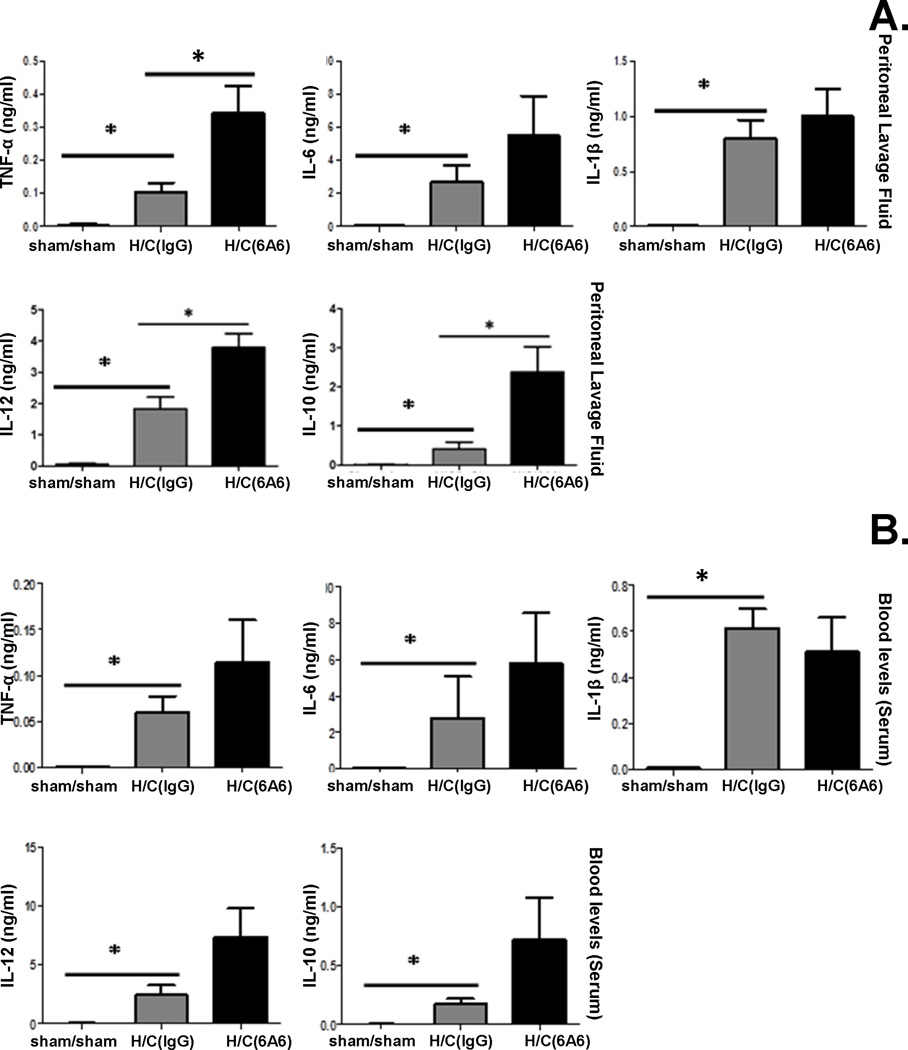
Anti-BTLA antibody treatment leads to higher cytokine levels in peritoneal cavity after Hem/CLP
Cytokine levels are important indices of inflammation. Kobayashi et al. (27) showed that BTLA expression inhibited pro-inflammatory cytokine production by bone marrow derived macrophages and dendritic cells in a LPS-induced endotoxemia model. However, in the CLP mouse model (20) while BTLA gene deficiency decreased IL-10 levels, it had no effect on pro-inflammatory cytokine levels. Inasmuch, we attempted to determine the extent to which anti-BTLA monoclonal antibody (6A6) treatment affected cytokine production. We found that Hem/CLP induced an increase in all cytokines (TNF-α, IL-6, IL-1β, IL-10 and IL-12) we tested when compared to sham/sham mice 24 hours after surgery. However, pro-inflammatory cytokines, TNF-α and IL-12, as well as anti-inflammatory cytokine IL-10 levels elevated significantly, and there were trends for an increase in IL-6 and IL-1β levels as well in the peritoneal lavage fluid from 6A6 monoclonal antibody treated group when compared to IgG treated Hem/CLP mice (Fig. 3A). Similarly, in peripheral blood, trends toward an increase in TNF-α, IL-6, IL-12, IL-10 levels were found in treatment groups when compared to IgG treated Hem/CLP groups (Fig. 3B). This indicates that 6A6 monoclonal antibody treatment promotes local as well as systemic inflammatory responses.
Figure 3. Anti-BTLA antibody treatment leads to higher cytokine levels in the peritoneum and blood 24 hours after Hem/CLP.
Cytokine (TNF-α, IL-12, and IL-10) levels elevated significantly in peritoneal lavage fluid 24hs after Hem/CLP in 6A6 treated group compared to IgG treated group (A.). 6A6 treatment led to a trend of increase in TNF-α, IL-12, IL-10 and MCP-1 levels in serum (B.). All cytokines/chemokines were low or non-detected in sham mice. N=7–12 in H/C group, N=4 in Sham/sham group. The data are provided as the mean±SEM, and significant difference between groups is indicated as *P<0.05, by Bonferroni’s post-test following one-way ANOVA.
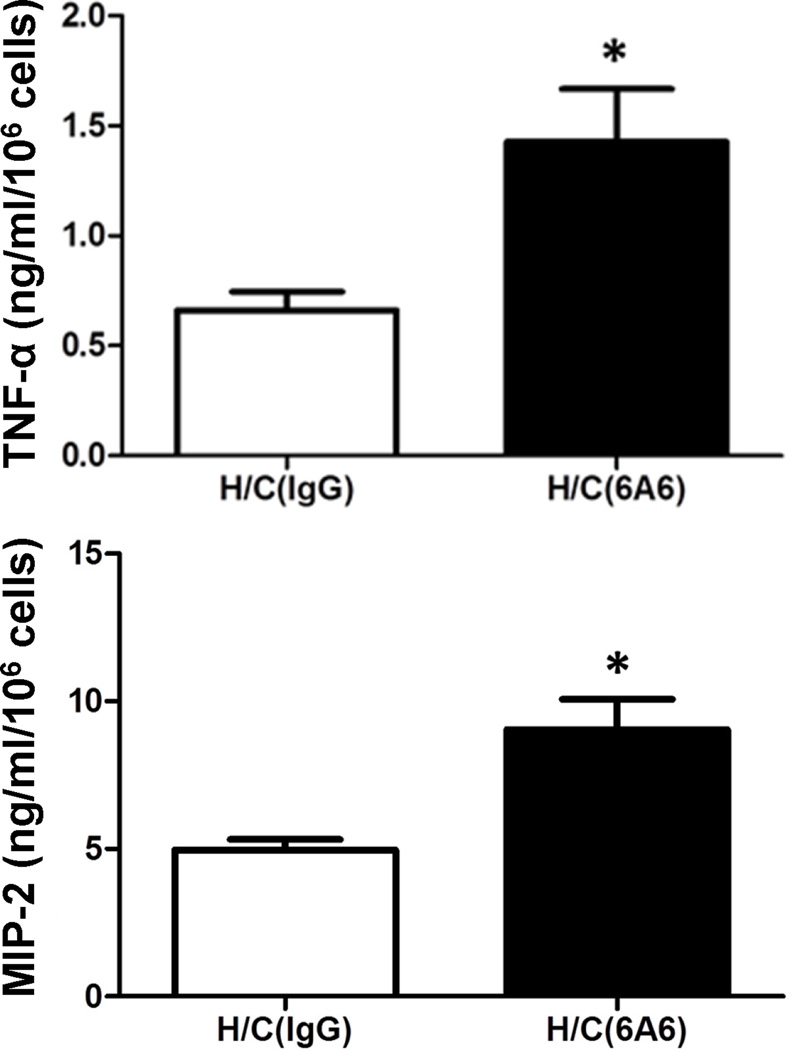
Anti-BTLA antibody treatment increases LPS-induced cytokine and chemokine production capability of peritoneal macrophages after Hem/CLP
Cytokines and chemokines are produced by many kinds of cells and are maintained by various regulatory factors. To determine whether 6A6 monoclonal antibody treatment would alter innate immune cells’ cytokine and chemokine production ex vivo, we collected peritoneal macrophages from mice that had received either 6A6 monoclonal antibody or IgG treatment 24 hours after HEM/CLP. The macrophages were cultured in 6-well plates, stimulated with LPS (10 ng/ml) for 20 hours, and the supernatant was collected for ELISA. We found that peritoneal macrophages in 6A6 monoclonal antibody treated group had the capability of producing higher levels of TNF-α and MIP-2 compared to the IgG treated group (Fig. 4), suggesting that anti-BTLA antibody treatment was having a potentiating effect on innate cells through activation of the cytokine pathway.
Figure 4. Effects of anti-BTLA antibody on cytokine/chemokine production in macrophages ex vivo.
Peritoneal macrophages taken from 6A6 or IgG treated mice 24 hours after Hem/CLP were collected and stimulated with 10 ng LPS for 20 hours and cultured supernatants were analyzed by ELISA. When compared to IgG treatment, anti- BTLA antibody treatment increases the cytokine and chemokine production capability of peritoneal macrophages stimulated with LPS after Hem/CLP. N=3–5/group. The data are provided as the mean±SEM, and significant difference between groups is indicated as *P<0.05 by unpaired two-tailed Student’s t-test.
Of note to the extent that 6A6 monoclonal antibody anti-BTLA treatment was altering macrophage intracellular signaling (altering BTLA-mediated SHP-1 and/or SHP-2 phosphorylation/activation) (15, 16), we noted that pre-treatment of the LPS stimulated mouse cell line, RAW 264.7, with 6A6 activated SHP-1 and SHP-2 phosphorylation as compared to IgG control (see supplemental, Fig. 1).
Effects of anti-BTLA antibody treatment on bacterial burden after Hem/CLP
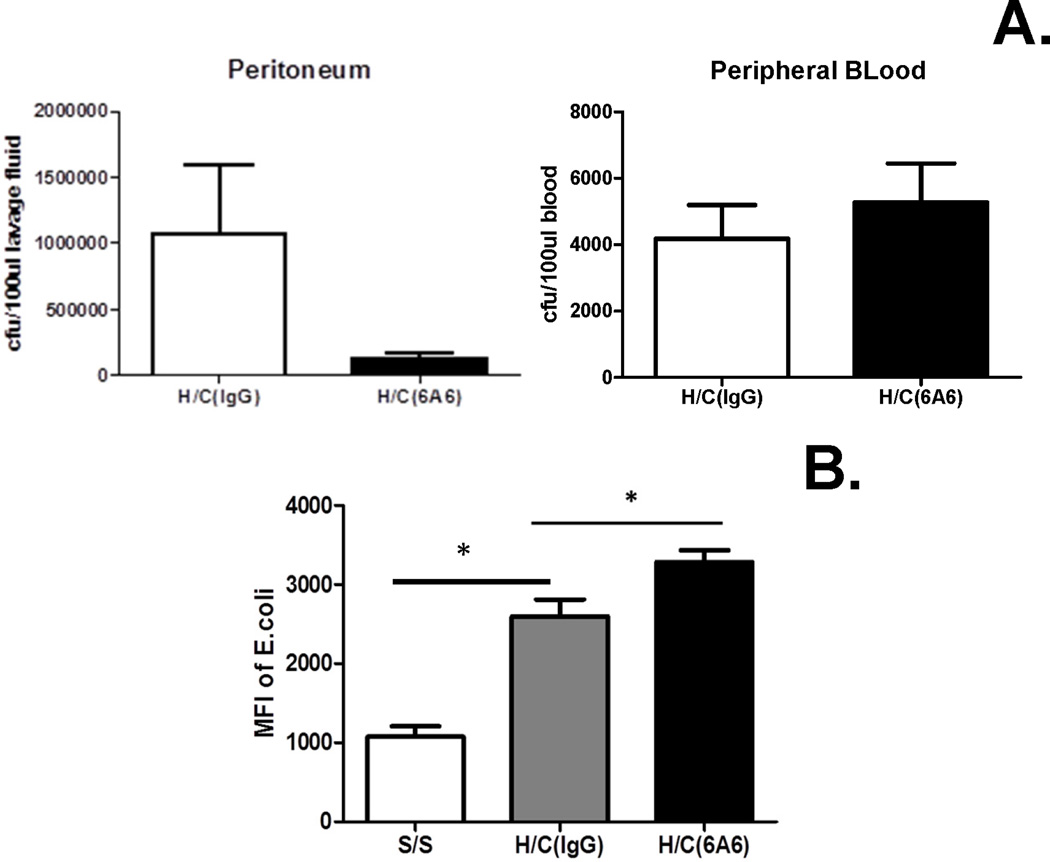
Pro inflammatory mediators and increased pro-inflammatory cytokine levels seem to be helpful for bacterial clearance (32–34). However, BTLA gene expression has been shown to be detrimental in the clearance of L. monocytogenes and Plasmodium, suggesting a role for BTLA in inhibiting innate phagocytic cell function (21, 35). To this end, we attempted to determine whether anti-BTLA antibody treatment could alter the bacterial burden in Hem/CLP. While there was a decrease in local peritoneal bacterial burden in the 6A6 monoclonal antibody treated group as compared with IgG group, the finding was not statistically significant (Fig. 5). No changes in bacterial burden were noted in the peripheral blood between the 6A6 antibody and IgG treated Hem/CLP groups.
Figure 5. Effects of anti-BTLA antibody treatment on the bacterial burden 24 hours after Hem/CLP.
6A6 treatment led to a decreasing trend in peritoneum bacterial burden (N=11/group), but no change in blood (N=8/group). The data are provided as the mean±SEM, unpaired two-tailed Student’s t-test (for normal distribution data).
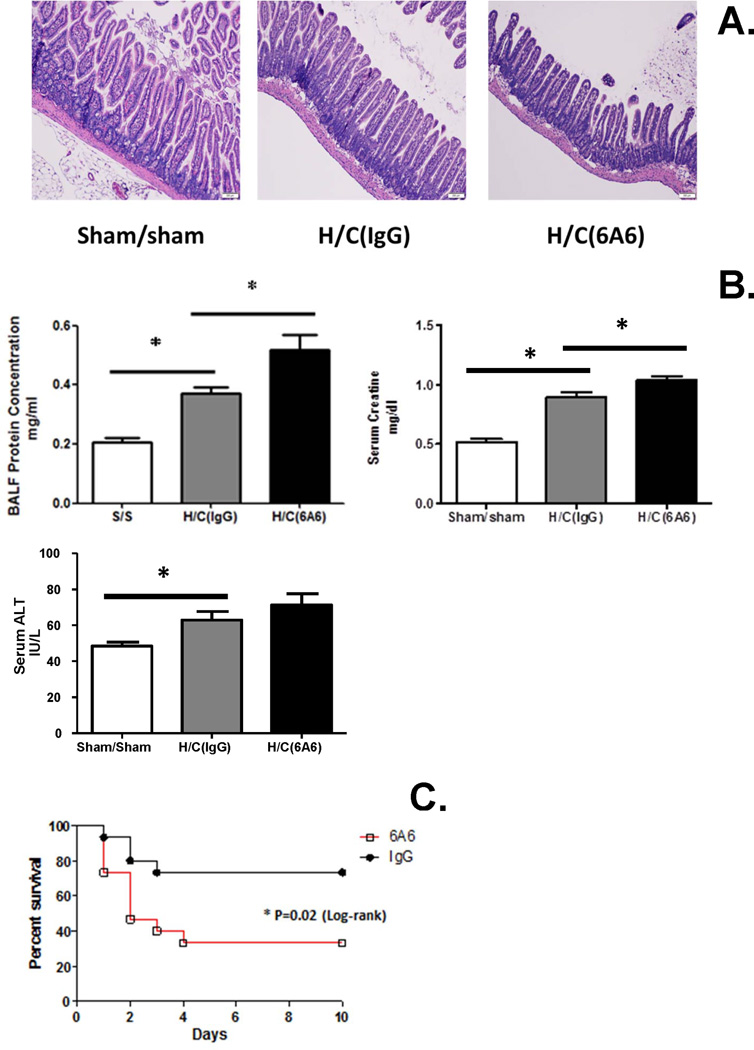
Effects of anti-BTLA antibody treatment on morbidity and mortality following Hem/CLP in mice
Multiple organ dysfunction is the main cause of death in patients with severe sepsis (36). Here we assessed some indices of target organ injuries and survival of 6A6 or IgG treatment after Hem/CLP. There was increased protein concentrations in broncho-alveolar lavage fluid (which was supported by increased septal wall thickening and increased cellularity [Supplemental Figure 2]) and serum creatine levels (Fig. 6B), which are indices of lung and kidney injury, respectively, as well as villus blunting and widening (via histological H&E staining, Fig. 6A [or switch 6A and 6B panels]) – an indicator of ileum injury, in 6A6 monoclonal antibody treated group when compared to IgG treated group. In addition, we observed that there was a rising trend in serum glutamic-pyruvic transaminase (GPT) levels (Fig. 6 B), which is an index of liver injury. Finally, anti-BTLA antibody treatment significantly decreased survival when compared to IgG treated mice after Hem/CLP (Fig. 6C).
Figure 6. Anti-BTLA antibody treatment aggravated organs injury (24 hours) and increased mortality of Hem/CLP mice.
(A) Blunting and widening villus was observed in ileum with 6A6 treatment. (B) BALF protein concentration and serum creatine levels elevated significantly, but there is no change in serum ALT level (N=8 in H/C group, N=6 in Sham/sham group). The data are provided as the mean±SEM, and significant difference between groups is indicated as *P<0.05, by Bonferroni’s post-test following one-way ANOVA. (C) 6A6 treatment significantly increased the mortality of Hem/CLP mice (N=15/group). *P<0.05, Log-rank test.
Discussion
Although a few studies have examined the potential role of BTLA in sepsis, published studies have shown that it appears to be important in both the innate and adaptive immune response to septic insult (20). BTLA gene deficient mice showed significantly improved septic survival and BTLA may have a role as a potential biomarker as well as a mediator of sepsis-induced immunosuppression in septic patients (37). However, much remains to be understood about BTLA’s molecular mechanism(s) of action in sepsis. Since BTLA’s ligand HVEM has another four receptors/ligands, the interactions among them can not only affect each other (14), but this complex interactive network potentially brings difficulties to the understanding of what BTLA’s role is. Inasmuch; in this study, we chose a blocking and/or agonistic anti-BTLA antibody (6A6) treatment (22, 23) in a Hem/CLP model to compare with BTLA KO mice in a septic CLP model (20), and to further clarify BTLA’s patho-physiological role in Hem/CLP.
We initially observed that BTLA expression changed in different compartments in distinct ways after Hem/CLP compared to sham animals. At the site of infection (the peritoneum), BTLA expression was significantly increased on innate immune cells, while systemically, the percentage of BTLA positive monocytes and dendritic cells notably decreased. This could indicate that BTLA expression on innate immunocytes may be related to their evolving functions at the inflammatory site following Hem/CLP. Interestingly, BTLA expression in major organs was up-regulated and associated with organ injury after Hem/CLP. This is consistent with our previous study (20) where we observed that organ injury was reduced in BTLA gene deficient mice when compared to WT mice after sepsis alone. This suggests that BTLA not only plays a role in the local response to infection, but also has effects on the systemic reaction in the combination of hemorrhagic shock and septic insults.
With respect to inflammatory cell recruitment, we found a significant increase not only in the total number of leukocytes, but also throughout the neutrophil, macrophage and dendritic cell subpopulations, respectively. This is also consistent with the impaired innate cell recruitment reported in septic BTLA gene deficient mice (20). Inasmuch, we feel that BTLA engagement appears to affect inflammatory cell recruitment directly, independent from HVEM signaling. With respect to this increased BTLA-mediated leukocyte recruitment, we found higher chemokine levels, including KC, MIP-2, MCP-1 in mice treated with the BTLA-antibody. Unfortunately, it is undetermined whether the increase in cell numbers leads to additional chemokine production, or if higher chemokine levels served to recruit more inflammatory cells, or whether there might be some other explanation. Since engagement of BTLA induces ITIMs to recruit SHP-1 or SHP-2 (and as supported by our in vitro RAW 264.7 cell line assessment documenting 6A6 antibody-induced activation of both phosphorylated SHP-1 and SHP-2 [see supplemental data]), which can effect cell chemotaxis and motility (38, 39), further investigation is needed to determine the role of BTLA signaling in enhanced cell recruitment and its relation to the activation of SHP-1 or SHP-2.
As mentioned previously, there is a Grb-2 related motif in BTLA’s intracellular domain, which is relevant to its pro-survival function. BTLA activity has been shown to have a role in sustaining CD4+ T cell survival under the conditions of chronic stimulation in a non-irradiated parental-into-F1 graft-versus-host disease model (18). BTLA ligation was also reported to inhibit apoptosis in macrophages infected with hepatitis virus 3 in a mouse model (40). In our study, we found that there was no significant difference in the frequency of cell apoptosis between 6A6 monoclonal antibody and IgG treated Hem/CLP mice. This indicates that BTLA engagement does not change innate leukocyte apoptosis in a hemorrhage plus bacterial infection model and therefore, the increased number of local leukocytes was not related to changes in cell apoptosis or increased cell survival in any particular subpopulations. Whether there are other possible reasons for the cell number changes, like cell emigration to lymph nodes, or other mechanisms, needs further investigation. This, however, is presently outside the scope of this study.
We reported an increase in TNF-α and IL-12 in the peritoneum in the 6A6 treated group. There is no excitatory/ITAM motif in BTLA so far as we know, and an earlier study had demonstrated an inhibitory function of 6A6 (as an agonistic antibody) on LPS-induced pro-inflammatory cytokine production via cultured innate immunocytes (27). However, in contrast to these previous reported results (27) our data showed that 6A6 treatment enhanced cytoine/chemokine production in an ex-vivo LPS stimulation assay. One possible explanation may be related to the increase in pro-inflammatory cell numbers, although we are not sure about the cytokine production capability of single cell. We noted in our previous study that the decrease of local immunocytes did not change pro-inflammatory cytokine levels between WT and BTLA gene deficient mice after CLP (20). Hence, the cytokine levels seen in vivo could be the result of changes in cell number and cytokine production capability of single cells. Secondly, a prior study (21) showed that malaria infected BTLA KO mice also had an increased expression of HVEM on their leukocytes. Thus, the effects we have seen here may, in part, be a result of increased HVEM:LIGHT interaction, which can increase proinflammatory signaling (41). To address this concern, future studies are needed to determine how increased expression of HVEM is regulated after 6A6 monoclonal antibody treatment during Hem/CLP. Finally, the differences in results between the Kobayashi study (27) and ours could be attributed to the differences between a LPS-induced toxemia model vs. the Hem/CLP model used in this study.
As for the anti-inflammatory cytokine IL-10, we found that treatment with anti-BTLA antibody led to higher IL-10 levels at the site of infection, which is consistent with the lower IL-10 level in septic BTLA gene deficient mice (20). The data provided here confirmed that BTLA expression affects IL-10 induction. BTLA has been shown to inhibit CD4+ T cell polarization into Th1 cells (42). However, further studies are needed to evaluate whether BTLA also effects macrophage polarization in Hem/CLP mice.
Recently, BTLA has been shown to be detrimental in the clearance of L. monocytogenes and Plasmodium, suggesting a role for BTLA in inhibiting innate phagocytic cell activity (21, 35). In a polymicrobial sepsis model, BTLA gene deficient mice were significantly protected from the CLP-induced rise in bacterial burden in the peritoneum as compared with WT mice (20). In this study with 6A6 monoclonal antibody administration, we also observed a decrease in bacterial burden in the peritoneum. Besides BTLA, there are many other factors which may influence the final bacterial burden in these animals. For example, we did not study the change in lymphocyte function with or without 6A6 treatment in the 2-hit model, as these cell populations have had significant numbers of BTLA positive cells that could affect the removal of pathogens; nor did we examine the impact of neutrophil expression of HVEM, which may also be involved in the process of bacterial clearance.
Finally, here we report 6A6 monoclonal antibody administration increased septic mortality and led to an increase in organ injury. While BTLA gene deficient mice were protected from kidney injury and septic mortality (20), our data suggests that anti-BTLA monoclonal antibody [6A6] contributes to acute septic morbidity and mortality. In this study, elevated cytokine levels proved to be a double-edged sword. Although increased levels can help the host to activate cells to eliminate bacteria, they also hold the potential to damage organs and surrounding tissues.
Collectively, the data we present here shows that anti-BTLA 6A6 monoclonal antibody treatment leads to increased cytokine/chemokine production, increased innate immunocyte recruitment into the peritoneum, enhanced peritoneal macrophage phagocytic capacity, increased organ injury, and decreased survival. Through the comparison with the data from BTLA gene deficient mice, we further confirmed BTLA’s contribution in enhancing innate cell recruitment, elevated IL-10 levels, and that BTLA’s engagement serves to exacerbate the pathophysiology of Hem/CLP. Nonetheless, we recognize BTLA protein abrogation as created by gene deficiency in KO mice may produce distinct effects to that seen in BTLA binding/ ligation that would be encountered in antibody treated mice. However, more extensive studies examining the effects of 6A6 binding are needed to too truly clarify its actions on BTLA.
In closing, while we initially set out to test the hypothesis that by “blocking” BTLA signaling we should have reduced the extent of immune responses/organ injury/morbidity and mortality seen following the dual insults of Hem followed by CLP (based on the data from Lepenies (22) and Crawford (23), suggesting that anti-BTLA monoclonal [6A6] antibody was a true ‘blocking [signaling inhibiting] agent’), our results showed the contrary. What we report here is that in this model,treatment with the anti-BTLA monoclonal [6A6] antibody at a dose of 25ug/g body weight actually appears to support the alternate hypothesis that 6A6 antibody agonizes/potentiates BTLA’s actions by demonstrating a negative effect of BTLA signaling on shock/sepsis induced morbidity/mortality after Hem/CLP. While the duration and durability of anti-BTLA monoclonal [6A6] antibody’s agonistic activity need to be further vetted at different dosages and with alter timing of this antibody’s delivery following insult, this documents some of the challenges that can be encountered in trying to take advantage of BTLA as a potential therapeutic target.
Supplementary Material
While an increased septal wall thickening and cellularity were seen after HEM/CLP as compared to sham mice, 6A6 antibody treated mice showed increased injury when compared to IgG treated Hem/CLP mice. Original magnifications, X200.
1×106/well RAW 264.7 cells were cultured with IgG or 6A6 at a dose of 100mg/ml for 2 hours. Then LPS stimulation at a dose of 100ng/ml was performed for 6 hours. 6A6 antibody pre-treatment activated SHP-1 and SHP-2 activation in LPS stimulated RAW cells as compared to IgG control.
Acknowledgments
This work was funded by NIH R01-GM46354, NIH R01-GM53209 and NIH R01-GM107149 (A.A.). The authors thank Mr. Paul Monfils (Core Research Laboratories, Rhode Island Hospital) for assistance with histology.
Footnotes
Portions of this work were presented in an abstract as a part of the 28th Annual Conference of Shock, June 8th, 2005 in Denver, CO
References Cited
- 1.Ertel W, Morrison MH, Ayala A, Dean RE, Chaudry IH. Interferon-gamma attenuates hemorrhage-induced suppression of macrophage and splenocyte functions and decreases susceptibility to sepsis. Surgery. 1992;111:177–187. [PubMed] [Google Scholar]
- 2.Zervos EE, Kramer AA, Salhab KF, Norman JG, Carey LC, Rosemurgy AS. Sublethal hemorrhage blunts the inflammatory cytokine response to endotoxin in a rat model. J Trauma. 1999;46:145–149. doi: 10.1097/00005373-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Flohé S, Ackermann M, Reuter M, Nast-Kolb D, Schade FU. Sublethal hemorrhagic shock reduces tumor necrosis factor-alpha-producing capacity in different cell compartments. Eur Cytokine Netw. 2000;11:420–426. [PubMed] [Google Scholar]
- 4.Schröder J, Kahlke V, Fändrich F, Zabel P, Kremer B. Tumor necrosis factor-alpha hyporesponsiveness of rat intestinal mononuclear cells and whole portal venous blood after hemorrhagic shock. Crit Care Med. 1998;26:526–532. doi: 10.1097/00003246-199803000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Goris RJ. Multiple organ failure: whole body inflammation? Schweiz Med Wochenschr. 1989;119:347–353. [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamayo E, Gómez E, Bustamante J, Gómez-Herreras JI, Fonteriz R, Bobillo F, Bermejo-Martín JF, Castrodeza J, Heredia M, Fierro I, Álvarez FJ. Evolution of neutrophil apoptosis in septic shock survivors and nonsurvivors. J Crit Care. 2012;27:415. doi: 10.1016/j.jcrc.2011.09.001. e1–11. [DOI] [PubMed] [Google Scholar]
- 10.Drifte G, Dunn-Siegrist I, Tissières P, Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung C-S, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 15.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 16.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236–1243. doi: 10.1016/j.bbrc.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 17.Shui J-W, Steinberg MW, Kronenberg M. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. J Leukoc Biol. 2011;89:517–523. doi: 10.1189/jlb.0910528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurchla MA, Sedy JR, Murphy KM. Unexpected role of B and T lymphocyte attenuator in sustaining cell survival during chronic allostimulation. J Immunol. 2007;178:6073–6082. doi: 10.4049/jimmunol.178.10.6073. [DOI] [PubMed] [Google Scholar]
- 19.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D’Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shubin NJ, Chung CS, Heffernan DS, Irwin LR, Monaghan SF, Ayala A. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler G, Steeg C, Pfeffer K, Murphy TL, Murphy KM, Langhorne J, Jacobs T. B and T lymphocyte attenuator restricts the protective immune response against experimental malaria. J Immunol. 2011;187:5310–5319. doi: 10.4049/jimmunol.1101456. [DOI] [PubMed] [Google Scholar]
- 22.Lepenies B, Pfeffer K, Hurchla MA, Murphy TL, Murphy KM, Oetzel J, Fleischer B, Jacobs T. Ligation of B and T lymphocyte attenuator prevents the genesis of experimental cerebral malaria. J Immunol. 2007;179:4093–4100. doi: 10.4049/jimmunol.179.6.4093. [DOI] [PubMed] [Google Scholar]
- 23.Crawford A, Wherry EJ. Editorial: Therapeutic potential of targeting BTLA. J Leukoc Biol. 2009;86:5–8. doi: 10.1189/JLB.0209076. [DOI] [PubMed] [Google Scholar]
- 24.Tang L, Bai J, Chung C-S, Lomas-Neira J, Chen Y, Huang X, Ayala A. Programmed cell death receptor ligand 1 modulates the regulatory T cells’ capacity to repress shock/sepsis-induced indirect acute lung injury by recruiting phosphatase SRC homology region 2 domain-containing phosphatase 1. Shock. 2015;43:47–54. doi: 10.1097/SHK.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai J, Tang L, Lomas-Neira J, Chen Y, McLeish KR, Uriarte SM, Chung C-S, Ayala A. TAT-SNAP-23 treatment inhibits the priming of neutrophil functions contributing to shock and/or sepsis-induced extra-pulmonary acute lung injury. Innate Immun. 2015;21:42–54. doi: 10.1177/1753425913516524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lomas-Neira JL, Chung C-S, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004;76:58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi Y, Iwata A, Suzuki K, Suto A, Kawashima S, Saito Y, Owada T, Kobayashi M, Watanabe N, Nakajima H. B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells. Proc Natl Acad Sci U S A. 2013;110:5121–5126. doi: 10.1073/pnas.1222093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perl M, Chung C-S, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermes I, Haanen C, Steffens-Nakken H, Reutelings perger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 30.Davalos-Misslitz ACM, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, Förster R. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur J Immunol. 2007;37:613–622. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- 31.Young DS, Pestaner LC, Gibberman V. Effects of drugs on clinical laboratory tests. Clin Chem. 1975;21:1D–432D. [PubMed] [Google Scholar]
- 32.Jin L, Batra S, Douda DN, Palaniyar N, Jeyaseelan S. CXCL1 contributes to host defense in polymicrobial sepsis via modulating T cell and neutrophil functions. J Immunol. 2014;193:3549–3558. doi: 10.4049/jimmunol.1401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber GF, Swirski FK. Immunopathogenesis of abdominal sepsis. Langenbecks Arch Surg. 2014;399:1–9. doi: 10.1007/s00423-013-1129-7. [DOI] [PubMed] [Google Scholar]
- 34.Naiki Y, Michelsen KS, Schröder NWJ, Alsabeh R, Slepenkin A, Zhang W, Chen S, Wei B, Bulut Y, Wong MH, Peterson EM, Arditi M. MyD88 is pivotal for the early inflammatory response and subsequent bacterial clearance and survival in a mouse model of Chlamydia pneumoniae pneumonia. J Biol Chem. 2005;280:29242–29249. doi: 10.1074/jbc.M503225200. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Brown NK, Ruddy MJ, Miller ML, Lee Y, Wang Y, Murphy KM, Pfeffer K, Chen L, Kaye J, Fu Y-X. B and T lymphocyte attenuator tempers early infection immunity. J Immunol. 2009;183:1946–1951. doi: 10.4049/jimmunol.0801866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent J-L, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med. 2011;39:1050–1055. doi: 10.1097/CCM.0b013e31820eda29. [DOI] [PubMed] [Google Scholar]
- 37.Shubin NJ, Monaghan SF, Heffernan DS, Chung C-S, Ayala A. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 2013;17:R276. doi: 10.1186/cc13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mañes S, Mira E, Gómez-Mouton C, Zhao ZJ, Lacalle RA, Martínez-A C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma N, Everingham S, Ramdas B, Kapur R, Craig AWB. SHP2 phosphatase promotes mast cell chemotaxis toward stem cell factor via enhancing activation of the Lyn/Vav/Rac signaling axis. J Immunol. 2014;192:4859–4866. doi: 10.4049/jimmunol.1301155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang C, Chen Y, Guo G, Li H, Cao D, Xu H, Guo S, Fei L, Yan W, Ning Q, Zheng L, Wu Y. Expression of B and T lymphocyte attenuator (BTLA) in macrophages contributes to the fulminant hepatitis caused by murine hepatitis virus strain-3. Gut. 2013;62:1204–1213. doi: 10.1136/gutjnl-2012-302239. [DOI] [PubMed] [Google Scholar]
- 41.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
While an increased septal wall thickening and cellularity were seen after HEM/CLP as compared to sham mice, 6A6 antibody treated mice showed increased injury when compared to IgG treated Hem/CLP mice. Original magnifications, X200.
1×106/well RAW 264.7 cells were cultured with IgG or 6A6 at a dose of 100mg/ml for 2 hours. Then LPS stimulation at a dose of 100ng/ml was performed for 6 hours. 6A6 antibody pre-treatment activated SHP-1 and SHP-2 activation in LPS stimulated RAW cells as compared to IgG control.