Abstract
To ensure correct DNA replication, eukaryotes have signaling pathways that respond to replication-associated DNA damage and trigger repair. In both Saccharomyces cerevisiae and Schizosaccharomyces pombe, a complex of proteins, including the cullin protein Rtt101p and two adapter proteins Mms22p and Mms1p, is important for proper response to replication stress. We have investigated this system in Candida albicans. In this pathogen, Mms22p is important for recovery from DNA replication damage induced by agents including methylmethane sulfonate, camptothecin, and ionizing radiation. Although no clear ortholog of Mms1p has been identified in C. albicans, loss of either Mms22p or Rtt101p generates similar damage sensitivity, consistent with a common function. In S. cerevisiae, the Mrc1p−Csm3p−Tof1p complex stabilizes stalled replication forks and activates a replication checkpoint and interacts with Mms22p. A similar complex in S. pombe, consisting of the Tof1p and Csm3p orthologs Swi1p and Swi3p, along with the fission yeast Mrc1p, genetically also interacts with Mms22p. Intriguingly in C. albicans only Mrc1p and Csm3p appear involved in damage repair, and Mms22p is required for responding to DNA damage agents in MRC1 or CSM3 conditional mutants. In C. albicans, although the loss of RAD57 greatly impairs response in the pathogen to many DNA-damaging agents, lethality due to camptothecin damage requires concomitant loss of Rad57p and Mms22p, suggesting that Mms22p is only essential for homologous recombination induced by camptothecin. These results establish that although C. albicans uses conserved cellular modules to respond to DNA damage and replication blocks, the specific details of these modules differ significantly from the S. cerevisiae model.
Keywords: genomic stability, DNA repair, replication fork, homologous recombination, Candida albicans
Accurate transmission of the genome from one generation to the next requires the faithful replication of the DNA. In eukaryotic organisms, the process of DNA replication is challenged by replication stresses, such as dNTP depletion caused by hydroxyurea (HU), alkylated DNA template bases induced by methylmethane sulfonate (MMS), replication fork blockage caused by the topoisomerase I inhibitor camptothecin (CPT) (Vaisica et al. 2011), and single-strand or double-strand breaks due to ionizing radiation (IR) (Ward 1990). To ensure the fidelity and coordinate the progression of DNA replication, this challenging process is regulated by a DNA damage response network that includes S-phase checkpoints that sense stalled replication forks and DNA damage and facilitate DNA repair processes (Harper and Elledge 2007). Mechanisms of DNA repair primarily involve homologous recombination (HR), nonhomologous end-joining, and nucleotide excision repair (Wu and Hickson 2006).
In both the budding yeast Saccharomyces cerevisiae and the fission yeast Schizosaccharomyces pombe, there is an ubiquitin-conjugating complex consisting of orthologs of the budding yeast Rtt101p, Mms22p, and Mms1p that function in the damage repair process. Loss of ScMms22p or SpMms22p increases cellular sensitivity to a range of DNA-damaging drugs that generate lesions specifically in S-phase or that directly impede DNA replication (Chang et al. 2002; Araki et al. 2003; Baldwin et al. 2005; Dovey and Russell 2007; Duro et al. 2008; Vaisica et al. 2011). In S. cerevisiae, genetic epistasis between Mms22p and Mms1p suggests that Mms22p interacts with Rtt101p via Mms1p to form a protein complex (Rtt101p−Mms1p−Mms22p) required to promote recombinational repair at stalled replication forks and that this complex is required for replication of damaged DNA (Ho et al. 2002; Dovey and Russell 2007; Tourriere and Pasero 2007; Duro et al. 2008; Zaidi et al. 2008; Vaisica et al. 2011). ScMms22p is involved in sensing replication intermediates or in the prevention of DNA damage caused by blocked replication forks.
In S. cerevisiae, Mms22p is important for the stable association of the fork-pausing complex (Mrc1p−Tof1p−Csm3p) when replication stress is present (Vaisica et al. 2011). ScMrc1p acts as a primary mediator for transducing replication fork-pausing checkpoint signals and forms a stable complex with Csm3p and Tof1p to promote sister chromatid cohesion after DNA damage (Nedelcheva et al. 2005). S. pombe Mms22p also has been confirmed to interact genetically with components of the replication fork, such as the Swi1p−Swi3p−Mrc1p complex, to restart DNA replication at stalled forks. SpMms22p functions in the stabilization of paused replication forks as well (Dovey and Russell 2007).
HR is a high-fidelity DNA repair pathway. Besides playing a critical role in accurate chromosome segregation during meiosis, HR functions in DNA repair and in the recovery of stalled or broken replication forks to ensure genomic stability. In S. cerevisiae, HR proceeds through either Rad51p-dependent or Rad51p-independent pathways. The Rad51p-dependent pathway of recombination, also requiring Rad52p, Rad55p, Rad57p and Rad54p, is the most efficient pathway for gene conversion and is also required for repair of most double-strand DNA breaks in mitotic cells (Johnson and Symington 1995). The Rad51p-independent pathway depends on Rad59p (Sakofsky et al. 2012). Budding yeast Mms22p is required for HR-mediated repair of stalled or broken DNA replication forks (Duro et al. 2008), whereas S. pombe Mms22p might block the action of HR (Dovey and Russell 2007).
Although the central role of Mms22p in the maintenance of genome integrity is well characterized in S. cerevisiae and in S. pombe (Dovey and Russell 2007; Duro et al. 2008; Vaisica et al. 2011), the orthologous protein in Candida albicans has not been investigated extensively. Here we reported the identification and initial characterization of the MMS22/CR_00390W_A gene in C. albicans as the putative ortholog of the MMS22 gene in S. cerevisiae, and we identified C1_06040W_A, a putative ortholog of ScRTT101 in C. albicans, as the Mms22p-interacting protein in the ubiquitin-conjugating complex. To test the function of Mms22p and its potential partner proteins in the fork-pausing complex in C. albicans, we identified C1_11440C_A (a putative ortholog of ScMRC1) (Shi et al. 2007), C2_06130W_A (a putative ortholog of ScCSM3), and C5_01460W_A (a putative ortholog of ScTOF1) in C. albicans. To further explore the involvement of Mms22p in HR repair, we characterized C2_08110W_A (a putative ortholog of ScRAD57 in C. albicans), as previous studies have revealed that the conserved Rad51p, Rad52p, Rad54p, and Rad59p play important role in the HR in C. albicans (Ciudad et al. 2004; Garcia-Prieto et al. 2010; Hoot et al. 2011). We constructed a set of single-gene and double-gene mutants, including the conditional single gene mutants of PMET3-MMS22, PMET3-MRC1, PMET3-TOF1, and PMET3-CSM3, as well as the double-gene mutants of the aforementioned genes repressed together with MMS22. We also constructed the null mutants of Δrtt101and Δrad57, as well as the double-gene mutants of the genes deleted together with MMS22. Our present study shows that CaMms22p plays a vital role in preserving genome integrity during DNA replication and is important for viability after DNA replication-associated damage.
Materials and Methods
Media and culture conditions
Unless otherwise indicated, all the strains were grown routinely in YPD medium (i.e., 1% yeast extract, 2% peptone, and 2% dextrose) at 30° with shaking overnight, diluted to an OD600 of 0.1−0.2, grown to logarithmic phase, and used for subsequent experiments. As indicated, synthetic complete (SC) medium (0.67% yeast nitrogen base and 2% dextrose) was supplemented with histidine (20 µg/mL), leucine (60 µg/mL), or arginine (40 µg/mL) as appropriate. For repressing conditions for the C. albicans MET3 promoter, mutants were cultured in SC medium with 2.5 mM methionine (Met) and 2.5 mM cysteine (Cys) (SC-Met+/Cys+). To induce the MET3 promoter, mutants were grown in SC medium without Met and Cys (SC-Met−/Cys−) (Care et al. 1999).
Strain constructions
The C. albicans strains used in this study are listed in Table 1. The oligonucleotides used in this study are listed in supporting information, Table S1. A detailed version of all the strain constructions is provided as supplementary information (File S1). In summary, to generate the conditional C. albicans MMS22 mutant, one allele was placed under the control of the Met/Cys-repressible MET3 promoter (Care et al. 1999), and the other allele was disrupted with the Candida dubliniensis HIS1 marker (Noble and Johnson 2005). To achieve this, the SAT1-MET3p cassette from plasmid pFA-SAT1-MET3p (Schaub et al. 2006) was amplified by using the primers oLY152 and oLY153 to generate a SAT1-MET3p-MMS22 cassette with 100 bp of homology to the 5′ upstream region of MMS22 and 100 bp of homology to the beginning of the MMS22 open reading frame. The sequence of C. dubliniensis HIS1 from plasmid pSN52 (Noble and Johnson 2005) was fused to flanking homology to the 5′ upstream and 3′ downstream regions of the MMS22 gene to generate a mms22∆::C.d.HIS1 disruption cassette. C. albicans SN152 was then transformed with the mms22∆::C.d.HIS1 cassette to generate strains CaLY8 (MMS22/mms22::C.d.HIS1) and CaLY226 (MET3p-MMS22/mms22::C.d.HIS1).
Table 1. Strains used in this study.
| Strain Name | Strain Ref | Parental Strain | Key Genotype | Reference |
|---|---|---|---|---|
| SN152 | WT | SC5314 | arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434;IRO1/iro1::imm434 | (Noble and Johnson 2005) |
| CaLY8 | SN152 | orf19.7494::HIS1/ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MMS22 | CaLY226 | CaLY8 | orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY219 | SN152 | ORF19.4136/orf19.4136::LEU2 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-TOF1 | CaLY337 | CaLY219 | orf19.4136::LEU2/ARG-MET3p-ORF19.4136 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY220 | SN152 | ORF19.4105/orf19.4105::LEU2 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-CSM3 | CaLY249 | CaLY220 | orf19.4105::LEU2/ARG4-MET3p-ORF19.4105 arg4/arg4; leu2/leu2; his1/his1; URA3/ura3::imm434;IRO1/iro1::imm434 | This study |
| CaLY222 | SN152 | ORF19.658/orf19.658::LEU2 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MRC1 | CaLY316 | CaLY222 | orf19.658::LEU2/ARG4-MET3p-CaORF19.658 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY223 | SN152 | ORF19.2174/orf19.2174::LEU2 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| Δrad57 | CaLY235 | CaLY223 | orf19.2174::LEU2/orf19.2174::ARG4 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY224 | SN152 | ORF19.2440/orf19.2440::LEU2 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| Δrtt101 | CaLY236 | CaLY224 | orf19.2440::LEU2/orf19.2440::ARG4 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY228 | CaLY226 | ORF19.658/orf19.658::LEU2 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MMS22/PMET3-MRC1 | CaLY251 | CaLY228 | orf19.658::LEU2/ARG4-MET3p-CaORF19.658 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY234 | CaLY226 | ORF19.4105/orf19.4105::LEU2 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MMS22/PMET3-CSM3 | CaLY246 | CaLY234 | orf19.4105::LEU2/ARG4-MET3p-ORF19.4105 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY238 | CaLY226 | ORF19.2174/orf19.2174::LEU2 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MMS22/Δrad57 | CaLY242 | CaLY238 | orf19.2174::LEU2/orf19.2174::ARG4 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
| CaLY240 | CaLY226 | ORF19.2440/orf19.2440::LEU2 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study | |
| PMET3-MMS22/Δrtt101 | CaLY244 | CaLY240 | orf19.2440::LEU2/orf19.2440::ARG4 orf19.7494::HIS1/SAT1-MET3p-ORF19.7494 arg4/arg4 leu2/leu2 his1/his1 URA3/ura3::imm434 IRO1/iro1::imm434 | This study |
WT, wild type.
A similar strategy was used to disrupt the TOF1 gene. To summarize, a conditional TOF1 mutant was generated in SN152 by insertion of the MET3 promoter before the ATG start codon of the TOF1 gene. The ARG4-MET3p cassette from plasmid pFA-ARG4-MET3p (Schaub et al. 2006) was amplified by using the primers oLY312 and oLY313 and then fused with the upstream region and the beginning of the TOF1 open reading frame to generate an ARG4-MET3p-TOF1 cassette. The Candida maltosa LEU2 sequence from plasmid pSN40 (Noble and Johnson 2005) was fused to flanking homology to the 5′ upstream and 3′ downstream regions of the TOF1 gene to generate a tof1∆::C.m.LEU2 disruption cassette. C. albicans SN152 was then transformed with the tof1∆::C.m.LEU2 cassette to generate strains CaLY219 (TOF1/tof1::C.m.LEU2) and CaLY337 (MET3p-TOF1/tof1::C.m.LEU2). On the basis of the same logic, the conditional disruptions of CSM3 (CaLY249, MET3p-CSM3/csm3::C.m.LEU2) and MRC1 (CaLY316, MET3p-MRC1/mrc1::C.m.LEU2) were constructed.
The entire encoding sequences of RAD57 and RTT101 were deleted from the wild-type strain SN152 by two-step HR by the use of a fusion−polymerase chain reaction (PCR)-based strategy (Noble and Johnson 2005). To summarize, the two RAD57 alleles were disrupted sequentially with rad57∆::C.m.LEU2 and rad57∆::C.d.ARG4 disruption cassettes to create the homozygous rad57 null mutant (CaLY235). The two RTT101 alleles were replaced sequentially with rtt101∆::C.m.LEU2 and rtt101∆::C.d.ARG4 disruption cassettes to create the homozygous rtt101 null mutant (CaLY236).
The double-gene mutants of PMET3-MMS22/Δcsm3, PMET3-MMS22/Δrad57, and PMET3-MMS22/Δrtt101 were generated by transforming the conditional MMS22 mutant (strain CaLY226) with csm3∆::C.m.LEU2 (creating CaLY234), rtt101∆::C.m.LEU2 (creating CaLY240), or rad57∆::C.m.LEU2 (creating CaLY238), followed by csm3∆::C.d.ARG4 (creating CaLY246), rtt101∆::C.d.ARG4 (creating CaLY244), or rad57∆::C.d.ARG4 (creating CaLY242), respectively. The double mutant of PMET3-MMS22/PMET3-MRC1 was generated by transforming strain CaLY226 with mrc1∆::C.m.LEU2 (creating CaLY228), followed by ARG4-MET3p-MRC1 cassette (creating CaLY251). All transformants were checked for correct genome integration by genomic PCR.
Flow cytometry
C. albicans cells derived from an exponentially growing of culture in SC-Met+/Cys+ medium were arrested with 0.01% MMS or 20 mM HU for 4 hr at 30° with sampling every 2 hr. Cells were then washed to remove MMS or HU, resuspended in SC-Met+/Cys+ medium, and incubated for an additional 4 hr with sampling every 2 hr. Samples of 3 mL were fixed with 70% ethanol overnight at 4°, washed with phosphate-buffered saline (pH = 7.4) and digested with 1 mg/mL RNase A for 1 hr at 37° to remove RNA. Cells were then stained with 50 mg/mL of propidium iodide for at least 4 hr at room temperature. The DNA content of 5 × 104 cells was monitored by fluorescence-activated cell sorting (FACS) analysis using a flow cytometer (FACSCALIBUR; BD Bioscience) and analyzed by Cellquest software (BD Bioscience). The vertical axis is cell counts and the horizontal axis is nuclear fluorescence. Three independent experiments were performed.
DNA damage sensitivity assays
Mid-log phase cultures were adjusted to 5 × 106 cells/mL, fivefold serially diluted, and spotted onto solid SC-Met+/Cys+ or SC-Met−/Cys− medium, which contains the indicated concentration of MMS, CPT, or HU. Alternatively, serial dilutions of cells were spotted onto solid SC-Met+/Cys+ or SC-Met−/Cys− medium for irradiating with the indicated dose of IR. Growth of cells was detected after a 48-hr incubation period at 30°.
For testing the survival of cells with exposure to MMS or HU, mid-log phase C. albicans cells were cultured in SC-Met+/Cys+ medium containing 0.005% MMS or 20 mM HU for 12 hr. At the indicated time points, samples were pooled, washed, and a range of 500−1000 cells were spread onto solid SC-Met+/Cys+ medium in triplicate. The number of colonies was counted following incubation at 30° for 2 d. The percentage of survival with untreated normalized to 100% at the indicated time points were calculated. Three independent experiments were performed.
Morphogenesis analysis
Mid-log phase C. albicans cells were adjusted to 1 × 103 cells/mL. A total of 100 µL of each strain culture were spread onto solid SC-Met+/Cys+ or SC-Met−/Cys− medium, incubated at 30° for 3−4 d, and photographed. Meanwhile, mid-log cultures adjusted to 1 × 103 cells/mL were grown for another 9 hr in liquid SC-Met+/Cys+ or SC-Met−/Cys− medium with shaking at 30°, then photographed with a EVOS X1 microscope (Life Technologies).
Alignments
We aligned Mms22p, Tof1p, Csm3p, Mrc1p, plus Rad57p primary amino sequences in S. cerevisiae, C. albicans, and S. pombe. Rtt101p sequences were aligned in S. cerevisiae and C. albicans. Multiple protein sequence alignments were performed with the MAFFT web application (http://mafft.cbrc.jp/alignment/server/) and visualized with Jalviewer (Version 2.8). The primary amino sequences of the S. cerevisiae, C. albicans, and S. pombe proteins were downloaded from the Fungal Orthogroups Repository (http://www.broadinstitute.org/cgi-bin/regev/orthogroups) hosted by the Broad Institute, MIT.
Data availability
Strains are available upon request. File S1 contains detailed descriptions of all supplemental files.
Results
Identification of Mms22p and its partner proteins in C. albicans
We used the Fungal Orthogroups Repository (Wapinski et al. 2007) to identify that the C. albicans CR_00390W_A gene is orthologous to both the S. cerevisiae MMS22 gene and the S. pombe mms22 gene. C. albicans CR_00390W_A encodes a protein with 1704 amino acids (molecular weight 196.9 kDa). When this protein is aligned with ScMms22p, it showed 5% identity and 19% similarity (Table 2; Figure S1A). We predicted that CaCR_00390W_A is a functional ortholog of ScMMS22 and classified CaCR_00390W_A as CaMMS22.
Table 2. C. albicans proteins studied in this study and their orthologs in S. cerevisiae and in S. pombe.
| Species | Proteins and Orthologs | ||||||
|---|---|---|---|---|---|---|---|
| C. albicans | Mms22p/CR_00390W_A | Rtt101p/C1_06040W_A | n/a | Mrc1p/C1_11440C_A | Csm3p/C2_06130W_A | Tof1p/C5_01460W_A | Rad57p/C2_08110W_A |
| S. cerevisiae | Mms22p/Ylr320wp | Rtt101p/Yjl047c | Mms1p/Ypr164w | Mrc1p/Ycl061c | Csm3p/Ymr048w | Tof1p/Ynl273w | Rad57p/Ydr004w |
| S. pombe | Mms22p/ SPAC6B12.02c | n/a | Mms1p/SPAC3H8.05c | Mrc1p/SPAC694.06c | Swi3p/SPBC30D10.04 | Swi1p/SPBC216.06c | Rhp57p/SPAC20H4.07 |
| Percentage of Sequence Homology and Identity of C. albicans Proteins Aligned with S. cerevisiae Counterparts | |||||||
| Identity | 5% | 5% | n/a | 7% | 9% | 6% | 5% |
| Similarity | 19% | 18% | n/a | 23% | 22% | 20% | 16% |
n/a, not available.
Similarly, we identified C. albicans C5_01460W_A and C2_06130W_A, as orthologous to ScTOF1 and ScCSM3, as well as Spswi1 and Spswi3, respectively, by using the Fungal Orthogroups Repository. Alignment of ScTOF1 and C. albicans C5_01460W_A indicated 6% identity and 20% similarity over their full-length sequences (Table 2; Figure S1B), and 9 and 22% between ScCSM3 and C. albicans C2_06130W_A (Table 2; Figure S1C), respectively. We named C. albicans C5_01460W_A and C2_06130W_A as CaTOF1 and CaCSM3. We also identified C2_08110W_A/CaRAD57 as the ortholog to ScRAD57 with 5% identity and 16% similarity, and to Sprph57 with 5% identity and 15% similarity (Table 2; Figure S1E). However, we failed to find a gene homologous to ScRAD55 or Sprph55 in C. albicans. To address the function of possible Rtt101p and Mms1p paralogs in C. albicans, the Fungal Orthogroups Repository was used to identify C1_06040W_A as a ortholog to ScRTT101 with 5% identity and 18% similarity (Table 2; Figure S1F), whereas no homologous gene to ScMMS1 was found. C1_11440C_A has previously been reported as orthologous to ScMRC1 (Shi et al. 2007).
Generation of conditional mutants of mms22 and its partner genes in C. albicans
To test the function of MMS22 in C. albicans, the first allele was replaced with the mms22∆::C.d.HIS1 cassette (strain CaLY8) using the fusion−PCR-based strategy in strain SN152 (Noble and Johnson 2005). A conditional MMS22 mutant was constructed from CaLY8 in which the single remaining copy of MMS22 was placed under the control of the MET3 promoter (strain CaLY226; Figure S2). MMS22 mRNA levels in the wild-type SN152 and the PMET3-MMS22 strain grown under nonrepressed and repressed conditions were analyzed by relative quantitative real-time PCR. The transcription level of CaACT1 was used as a standard for normalization. MMS22 mRNA levels in the repressed PMET3-MMS22 strain were 10-fold lower than in the wild-type strain after 12 hr of growth in SC-Met+/Cys+ medium, whereas the transcription level of MMS22 is similar to the wild type strain in SC-Met−/Cys− medium. The conditional expression of the MMS22 mutant allowed us to study the function of this gene in C. albicans.
Since the budding yeast Mms22p and the fission yeast SpMms22p genetically interact with the fork-pausing complex to stabilize the replisome during replication stress, we constructed conditional single-gene mutants of regulated expression of TOF1, MRC1, or CSM3, and the conditional double-gene mutants of PMET3-MMS22/PMET3-MRC1 and PMET3-MMS22/PMET3-CSM3 (Figure S3) to explore a potential link between Mms22p and Tof1p, Mrc1p, or Csm3p in C. albicans. The regulated expression of the target genes was confirmed by quantitative real-time PCR (data not shown).
To probe the function of the RTT101 gene and the relationship between the RTT101 gene and the MMS22 gene in C. albicans, both alleles of RTT101 were deleted in the wild-type SN152 and PMET3-MMS22 strains, to generate ∆rtt101 and PMET3-MMS22/∆rtt101 mutants (Figure S4). Similarly, to explore whether CaMMS22 is involved in HR repair, both alleles of CaRAD57 in either the wild-type SN152 or the PMET3-MMS22 mutant were deleted, respectively, to obtain ∆rad57 and PMET3-MMS22/∆rad57 mutants (Figure S4).
Mms22p is important for the recovery from a disturbed DNA replication in C. albicans
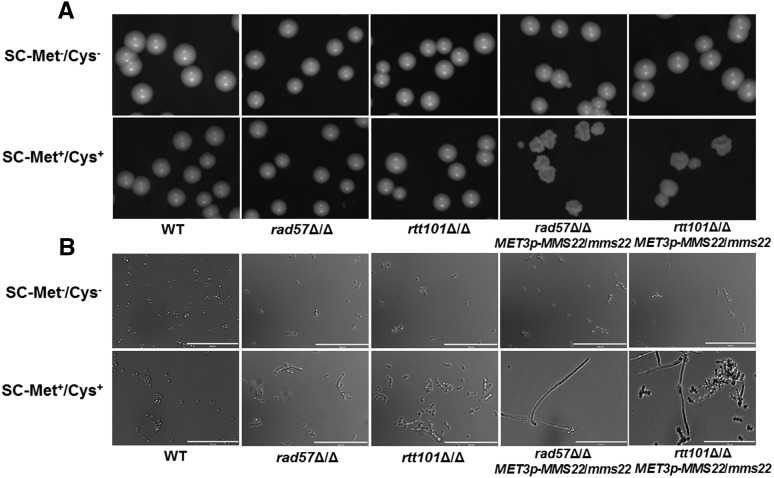
In contrast to the wild-type strain, in which yeast cells formed smooth, domed colonies and separated readily after cytokinesis, we found that the PMET3-MMS22 strain grew normally under nonrepressing conditions but formed rough, flattened colonies (Figure 1A) and elongated cells (Figure 1B) when the MET3 promoter was repressed in SC-Met+/Cys+ medium, even in the absence of any genotoxic stress. In general, in response to cell-cycle arrest in C. albicans, a filamentous cell type with characteristics of both pseudohyphae and true hyphae appears (Berman 2006). The elongated cells suggested that the repressed MMS22 mutant could be defective in DNA replication or were unable to repair DNA breaks appearing spontaneously during replication.
Figure 1.
Colony and single-cell morphology of the parental strain SN152 (wild type; WT) and the PMET3-MMS22 mutant strains. (A) Colony morphology after 3 d of growth on solid SC-Met−/Cys− and SC-Met+/Cys+ media at 30° are shown. (B) Cells from an overnight liquid SC-Met−/Cys− and SC-Met+/Cys+ culture at 30° were examined under microscope. Bar = 100 μm.
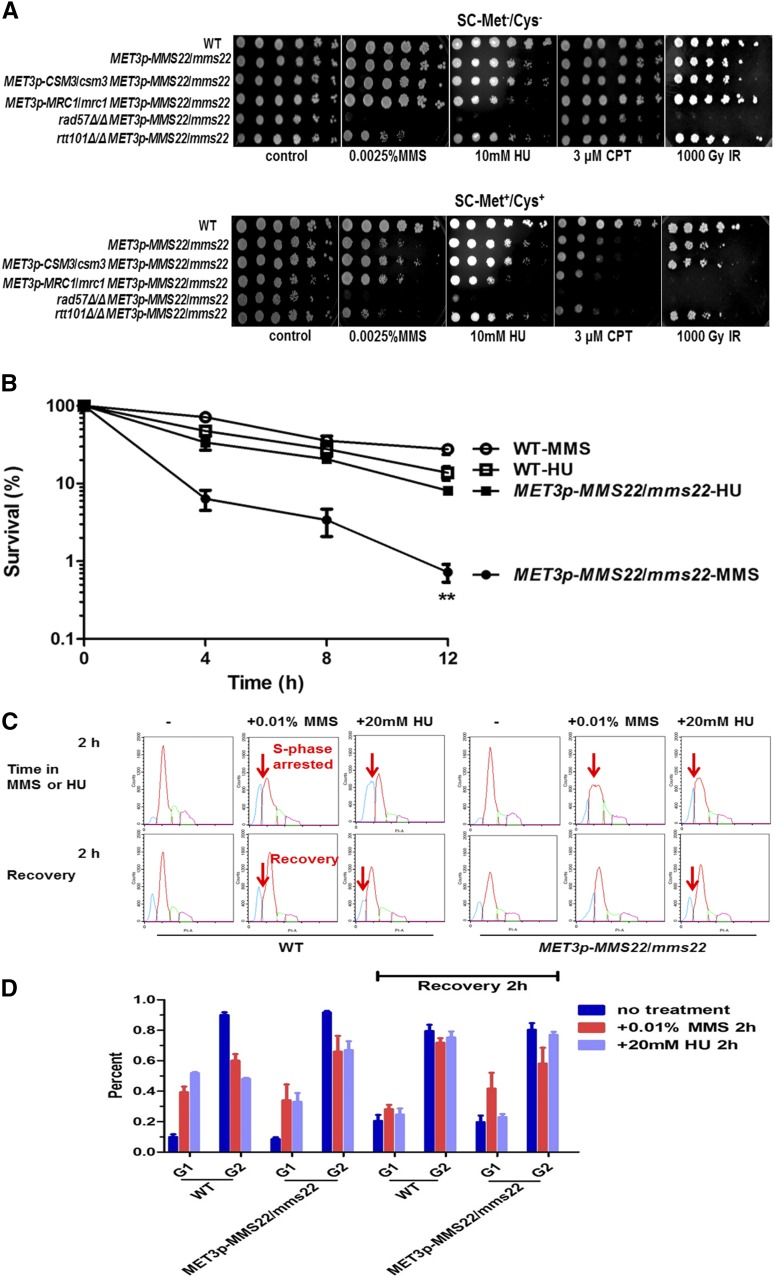
S. cerevisiae mms22Δ and S. pombe mms22Δ mutants are sensitive to MMS, HU, and CPT but less sensitive (or resistant) to IR (Table 3) (Chang et al. 2002; Araki et al. 2003; Dovey and Russell 2007). We assessed the sensitivity of the PMET3-MMS22 mutant strain to various DNA damaging agents. The PMET3-MMS22 strain showed increased sensitivity to MMS and CPT, and intriguingly showed enhanced sensitivity to IR, but not to HU, when the gene is shut off by growth in SC-Met+/Cys+ medium (Figure 2A). To further confirm the differential sensitivity to MMS and HU in the PMET3-MMS22 strain in the repressed condition, we checked the viability of the strains during a 12-hr period of MMS or HU exposure (Figure 2B). MMS began to significantly inhibit growth of the PMET3-MMS22 strain relative to wild type after 4 hr of treatment, whereas HU affected viability in both the wild-type and PMET3-MMS22 strains similarly. These results indicated that the MMS22 gene is required for MMS resistance even during short-term MMS exposure in C. albicans, but is not needed for HU resistance, in contrast to S. cerevisiae MMS22 and S. pombe mms22, which are required for both MMS and HU resistance (Bennett et al. 2001; Dovey and Russell 2007).
Table 3. Morphology and sensitivity to DNA damage agents of the mutants in the three species.
| Species | PMET3-MMS22 | RTT101Δ/Δ | PMET3-MMS22 RTT101Δ/Δ | PMET3-MRC1 | PMET3-MMS22 PMET3-MRC1 | PMET3-CSM3 | PMET3-MMS22 PMET3-CSM3 | PMET3-TOF1 | RAD57Δ/Δ | PMET3-MMS22 RAD57Δ/Δ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans | |||||||||||
| Morphology | Elongated | Wild type | Elongated | Elongated | Elongated | Elongated | Elongated | Wild type | Wild type | Elongated | |
| MMS sensitivity | Increased | Increased | More increased | Increased | Less increased | Increased | Less increased | No change | Increased | Increased | |
| HU sensitivity | No change | No change | No change | Less increased | Less increased | Less increased | Less increased | No change | Increased | Increased | |
| CPT sensitivity | Increased | Increased | Increased | Increased | More increased | Increased | More increased | No change | Moderate increased | More increased | |
| IR sensitivity | Increased | Increased | Increased | Increased | More increased | Increased | More increased | No change | Increased | Increased | |
| S. cerevisiae | mms22Δ | ||||||||||
| MMS sensitivity | Increased | ||||||||||
| HU sensitivity | Increased | ||||||||||
| CPT sensitivity | Increased | ||||||||||
| IR sensitivity | Moderate increased | ||||||||||
| S. pombe | mms22Δ | mrc1Δ | mms22Δ mrc1Δ | swi3Δ | mms22Δ swi3Δ | swi1Δ | mms22Δ swi1Δ | rhp57Δ | mms22Δrhp57Δ | ||
| Morphology | Elongated | ||||||||||
| MMS sensitivity | Increased | No change | More increased | No change | Less increased | No change | Less increased | Increased | More increased | ||
| HU sensitivity | Increased | Increased | More increased | Increased | Less increased | Increased | Less increased | Increased | More increased | ||
| CPT sensitivity | Increased | No change | More increased | No change | Less increased | No change | Less increased | Increased | More increased | ||
| IR sensitivity | Resistant | Increased | More increased |
Figure 2.
Sensitivity of the mutant strains to DNA-damaging agents. (A) Spot assays comparing the sensitivity of the wild-type SN152 (WT) and the mutant strains. Cells were grown as described in the section Materials and Methods, diluted, and spotted onto plates with the indicated concentrations of MMS, HU, CPT, and indicated dose of IR. The strains were cultured on solid SC-Met−/Cys− and SC-Met+/Cys+ media independently, and photographed after 2 d growth at 30°. (B) Survival curves of MET3p-MMS22/mms22 mutant exposed to 0.01% MMS or 20 mM HU for 12 hr. A total of 500–1000 cells were plated on SC-Met+/Cys+ agar in triplicate. Colony numbers were counted following incubation at 30° for 2 d. The percentage of survival with untreated normalized to 100% at the indicated time points are shown. The mean and the standard deviation of three independent experiments are plotted. (C) DNA content analyzed by flow cytometry. Cells derived from an exponentially culture in SC-Met+/Cys+ medium were arrested with 0.01% MMS or 20 mM HU for 4 hr, and then washed and released into SC-Met+/Cys+ medium without MMS or HU for another 4 hr. Samples were collected every 2 hr. One represented cell-cycle progress in the mutants is shown. The vertical axis is cell counts and the horizontal axis is nuclear fluorescence. Blue and red curves indicate cells in G1 and G2 phases, respectively. Green and purple curves indicate DNA contents of aggregated cells which have not been analyzed. Arrows indicate the S-phase arrested and the recovery points. (D) Percentage of G1/G1+G2 and G2/G1+G2 of three independent experiments are shown.
We then used flow cytometry (FACS) to examine the changes in cell-cycle progress in the PMET3-MMS22 strain during treatment with MMS or HU for 4 hr, and during a following 4-hr recovery period. The wild-type and PMET3-MMS22 strains showed similar behavior during the whole cell cycle with the treatment of MMS or HU, and arrested in S phase (Figure 2C, S-phase arrested arrows). After removal of either agent followed by culturing in fresh media, the wild-type strain progressed through the cell cycle within 2 hr, as evidenced by the re-emergence of cells in the G2 phase (Figure 2C, recovery arrows) and the increase in the percentage of G2 cells (Figure 2D). By contrast, the PMET3-MMS22 strain treated with MMS remained in S phase with only one peak and did not proceed into the cell cycle within 2 hr (Figure 2C, no recovery arrow) and even 4 hr (data not shown) without the increased percentage of G2 cells (Figure 2D). However, the PMET3-MMS22 strain treated with HU re-entered the cell cycle 2 hr upon removal of the HU, as evidenced by the re-emergence of G2-phase peak and the increase in the percentage of G2 cells, similar to the wild-type strain (Figure 2C, recovery arrows; Figure 2D). These results suggested that during decreased expression of MMS22, cells were unable to recover from arrest triggered by MMS. Thus, our data suggest that C. albicans Mms22p is essential for recovery from the DNA replication damage induced by MMS (and potentially CPT and IR) and that the repression of MMS22 caused an abnormal cell cycle after recovery from replication stress.
Mms22p is required for responding to DNA damage agents in MRC1 or CSM3 conditional mutants’ fork-pausing complex
To explore a potential link between Mms22p and Tof1p (S. pombe Swi1p), Mrc1p or Csm3p (S. pombe Swi3p) in C. albicans, the conditional single-gene mutants permitting regulated expression of TOF1, MRC1, or CSM3, and the conditional double-gene mutants of PMET3-MMS22/PMET3-MRC1, PMET3-MMS22/PMET3-CSM3 were constructed.
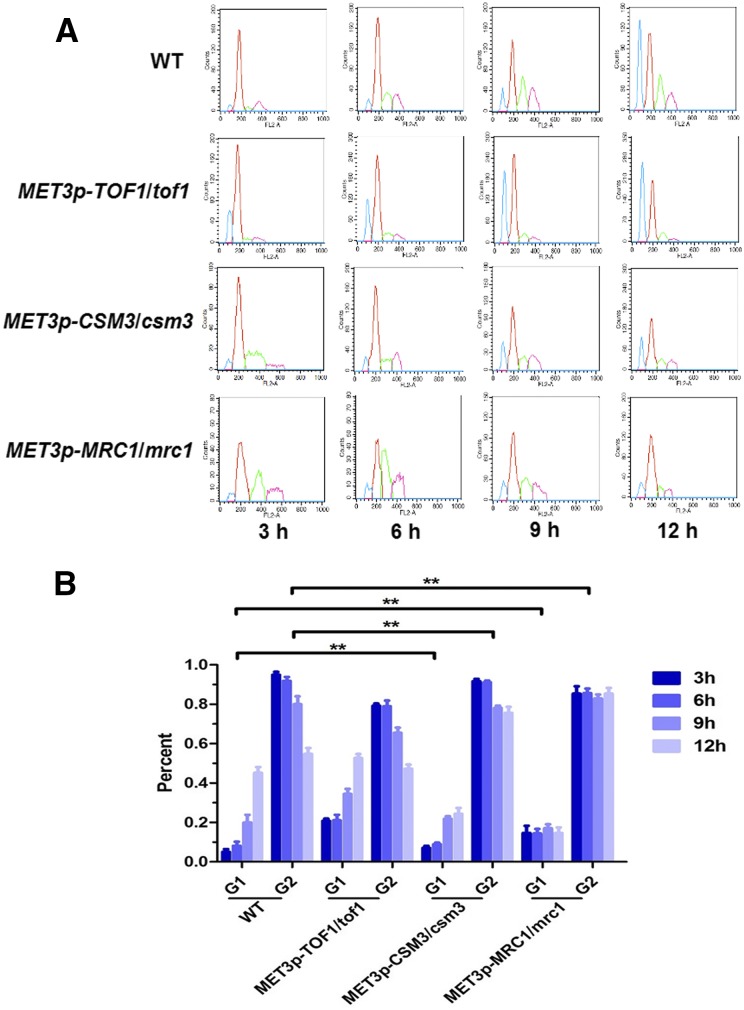
In the absence of any genotoxic stresses, repression of C. albicans CSM3 or MRC1 produced viable colonies that were rough, flatted, and small in size compared with the wild-type colonies (Figure 3A). The single cells also displayed an elongated phenotype (Figure 3C). These were consistent with the FACS results, which revealed an accumulation of PMET3-MRC1 or PMET3-CSM3 mutants arrested in the G2 phase that can’t complete mitosis within 12 hr in the repressive conditions (Figure 4). By contrast, the repression of TOF1 generated similar colony and cellular morphology to the wild type (Figure 3, A and C), and cells progressed through the cell cycle and completed mitosis within 12 hr normally (Figure 4). Strikingly, combined repression of both MMS22 and MRC1 led to significantly smaller colonies with wrinkled edges (Figure 3B) and generated elongated cells (Figure 3D), whereas combined repression of both MMS22 and CSM3 generated similar morphological phenotypes to those in each independent shut off (Figure 3, B and D). Because the filamentous cells can be indicative of DNA replication defects, our results suggest that the loss of MRC1 or CSM3 may result in defects in either DNA replication or in the repair of DNA breaks that arise spontaneously during DNA replication. The absence of MMS22 exacerbated the defect in the mrc1 mutant in C. albicans, but interestingly not in the csm3 mutant.
Figure 3.
Colony and single cell morphology of the wild-type SN152 (WT) and the mutant strains. (A, B) Colony morphology after 2 d of growth on solid SC-Met−/Cys− or SC-Met+/Cys+ medium at 30° were shown. (C, D) Cells from an overnight liquid SC-Met−/Cys− or SC-Met+/Cys+ culture at 30° were examined under microscope. Bar = 100 μm.
Figure 4.
DNA content analyzed by flow cytometry. Cells derived from an exponentially growing of culture in SC-Met+/Cys+ medium for 12 hr. Samples were collected every 3 hr. (A) One represented cell-cycle progress in the mutants is shown. The vertical axis is cell counts and the horizontal axis is nuclear fluorescence. Blue and red curves indicate cells in G1 and G2 phases, respectively. Green and purple curves indicate DNA contents of aggregated cells which have not been analyzed. (B) Percentage of G1/G1+G2 and G2/G1+G2 of three independent experiments are shown. **P < 0.01 when compared with the counterpart phase of the wild type.
Repression of either MRC1 or CSM3 caused increased sensitivity to MMS, CPT, IR, and less sensitivity to HU, whereas the repression of TOF1 did not affect cellular sensitivity to these agents (Figure 5; Table 3). These data suggest that these three proteins might have different function in checkpoint control or in DNA replication. By contrast, Mrc1p is essential in fork-pausing in S. cerevisiae but dispensable in S. pombe (Bennett et al. 2001; Calzada et al. 2005; Dovey and Russell 2007). Moreover, the repression of MMS22 led to a partial rescue of the sensitivity of the PMET3-CSM3 or PMET3-MRC1 mutant to MMS and HU, whereas the repression of MMS22 caused increased the sensitivity to CPT and IR of the PMET3-MRC1 mutant (Figure 2A; Table 3), supporting the idea that Mms22p is required for responding to paused replication forks.
Figure 5.
Sensitivity of the mutant strains to DNA-damaging agents. Spot assays comparing the sensitivity of the wild-type SN152 (WT) and the mutant strains. Cells were grown as described in the section Materials and Methods, diluted, and spotted onto plates with the indicated concentrations of MMS, HU, CPT, and indicated dose of IR. The strains were cultured on solid SC-Met−/Cys− (A) and SC-Met+/Cys+ (B) media independently and photographed after 2 d of growth at 30°.
Mms22p is required for responding to CPT in the rad57-null mutants
The S. cerevisiae Rad55p-Rad57p complex and the S. pombe Rhp55p-Rhp57p complex have unique nonredundant functions in recombination, and mutations in any one of these components can lead to recombination defects, chromosomal instability, sensitivity to DNA damage, and meiotic defects (Khasanov et al. 2008). Because S. pombe mms22 is indispensable for replication-associated DNA damage that is repaired by HR, and the mms22Δ/rph57Δ double mutant displayed additive growth deficiencies and DNA damage sensitivites (Table 3) (Dovey and Russell 2007; Yokoyama et al. 2007), we investigated a similar interaction of CaMMS22 with the HR genes in C. albicans.
The rad57∆/∆ cells formed wild-type colonies on solid media. As well, they grew as yeast cells in liquid media (Figure 6). The rad57 null mutant was highly sensitive to the presence of DNA damaging agents such as MMS, HU, and IR (Figure 7, Figure 2A in SC-Met−/Cys− medium). Intriguingly, the mutant showed only a slight sensitivity to CPT in comparison with the wild type, but the PMET3-MMS22/∆rad57 strain showed high sensitivity to CPT after MMS22 promoter shut-off (Figure 2A in SC-Met+/Cys+ medium; Table 3). These data suggested that RAD57 is critical for responding to MMS, HU, or IR damage in C. albicans but is only essential for CPT damage repair in the absence of MMS22. This requirement of Rad57p for DNA repair in either the PMET3-MMS22 mutant or the wild type strain indicated that in the absence or the presence of MMS22, cells could experience DNA damage that is repaired by HR.
Figure 6.
Colony and single-cell morphology of the parental strain SN152 (WT) and the indicated mutant strains. (A) Colony morphology after 2 d of growth on solid SC-Met−/Cys− and SC-Met+/Cys+ media are shown. (B) Cells from an overnight liquid SC-Met−/Cys− and SC-Met+/Cys+ culture at 30° were examined under a microscope. Bar = 100 μm.
Figure 7.
Sensitivity of the mutant strains to DNA-damaging agents. Spot assays comparing the sensitivity of the wild-type SN152 (WT) and the mutant strains. Cells were grown as described in the section Materials and Methods, diluted, and spotted onto plates with the indicated concentrations of MMS, HU, CPT, and indicated dose of IR. The strains were cultured on solid SC-Met−/Cys− medium and photographed after 2 d growth at 30°.
Mms22p and Rtt101p promote replication through damaged DNA
In budding yeast, Mms22p interacts with Rtt101p, bridged by the DNA repair protein Mms1p, and is recruited to ubiquitinate a currently unidentified substrate (or substrates) in the DNA repair process (Zaidi et al. 2008). Cells lacking MMS22, RTT101, or MMS1 showed similarly increased sensitivities to MMS and HU. The importance of the Mms22p-Mms1p module in stabilizing the replisome during replication stress is conserved in both budding yeast and fission yeast (Dovey and Russell 2007; Zaidi et al. 2008; Vaisica et al. 2011). However, no clear ortholog to ScRTT101 has been identified in S. pombe.
The C. albicans rtt101 null mutant displayed wild-type colony growth on both solid media and liquid media, which was similar to the rad57-null mutant (Figure 6). The rtt101-null mutant was sensitive to MMS but not to HU, CPT, or IR (Figure 7; Figure 2A in SC-Met−/Cys− medium). Compared with the PMET3-MMS22 single mutant, the PMET3-MMS22/Δrtt101 strain exhibited enhanced sensitivity to MMS, similar sensitivity to CPT and IR, and unchanged sensitivity to HU (Figure 2A; Table 3), suggesting that Rtt101p might work together with Mms22p in the same pathway in response to MMS.
Discussion
In this study, we identified and characterized a DNA-repair protein, Mms22p, in C. albicans. In untreated cells, repression of the MMS22 gene resulted in elongated and deformed cells. Shut off of the PMET3-MMS22 mutant on solid SC-Met+/Cys+ medium caused hypersensitivity to the chemical agents MMS and CPT as well as IR. Moreover, after transient exposure to MMS, PMET3-MMS22 mutants were unable to complete mitosis in a timely fashion and showed decreased viability, accumulating with an elongated morphology and arresting in S phase. In C. albicans, Mms22p likely participates in the DNA repair pathway that is important for the recovery from S-phase-specific DNA damage caused by MMS, CPT or IR. As well, Mms22p is required for normal cell cycle progression after recovery from replication stress.
In S. cerevisiae, Mms22p has been proposed to be a substrate-specific adaptor of a DNA repair−specific Rtt101p-based cullin complex that is stimulated by MMS, works in an Mms1p-dependent manner, and is involved in the processing of stalled replication forks (Zaidi et al. 2008). Cullins are a family of proteins that act as scaffolds for the assembly of multisubunit ubiquitin ligases. Protein ubiquitination involves three enzymes: E1, E2, and a ubiquitin ligase E3,which can directly recognize specific substrates to perform different functions (Mellon et al. 1987; Dovey and Russell 2007; Daulny and Tansey 2009; Fujii et al. 2009). Rtt101p is a cullin-based protein that forms part of an E3 ubiquitin ligase complex required for replication fork progression through DNA lesions and naturally occurring pause sites in yeast (Luke et al. 2006). In response to DNA damage, Rtt101p is recruited to chromatin, in a process that depends on the histone H3 lysine-56 acetyltransferase Rtt109p and the BRCA1 C terminus repeat-containing protein Rtt107p (Roberts et al. 2008).
Each component of the Rtt101p−Mms1p−Mms22p complex is important for the stable association of the replisome with replication forks during replication stress (Vaisica et al. 2011). Moreover, an evolutionary conserved Mms1p−Mms22p module also is required for replication of damaged DNA in fission yeast (Dovey et al. 2009). Although no clear ortholog of Mms1p has been identified in C. albicans, the Δrtt101 mutant displayed similar sensitivities to the chemical agents and IR, as did the PMET3-MMS22 mutant, which suggests that Mms22p and Rtt101p may function in the same pathway in the presence of replication-associated DNA damage and is consistent with the Rtt101p−Mms22p complex (either without Mms1p or with a structurally highly divergent Mms1p) also being essential for the stabilization of the replisome during replication stress in C. albicans.
During DNA synthesis, replication forks are exposed to various types of stress. Csm3p, Tof1p, and Mrc1p have been identified as checkpoint-specific mediators in budding yeast, and they have the overlapping role during activation the replication checkpoint (Foss 2001; Osborn and Elledge 2003; Tong et al. 2004). Recent studies suggested that Mrc1p was required to maintain the normal rate of replication fork progression, whereas Tof1p was critical for DNA replication forks to pause at diverse chromosomal sites where non-nucleosomal proteins bind very tightly to DNA (Bando et al. 2009). Swi1p and Swi3p of S. pombe, the homologs of S. cerevisiae Tof1p and Csm3p, form a complex and play important roles in the stabilization of stalled replication forks and activation of the DNA replication checkpoint (Noguchi et al. 2004). Our study suggests that although Mrc1p and Csm3p are involved in DNA replication and repair in C. albicans, Tof1p is apparently not required for these processes. In the absence of exogenous DNA damaging agents, when either MRC1 or CSM3 was repressed, the cells exhibited a mitotic delay and were arrested in the G2 phase with a constitutively pseudohyphal morphology. Furthermore, these mutants had increased sensitivity to the agents MMS, HU, CPT, and IR, resulting in reduced viability compared with the wild-type strain; this occurred whether Mms22p was repressed or not. Usually, pseudohyphae and true hyphae emerge in response to cell-cycle arrest in C. albicans (Berman 2006). We speculate that the delayed cell cycle in either mutants or cells treated with reagents that alter cell-cycle progression can cause cell elongation in C. albicans. This point is consistent with the view that cell polarity during hyphal morphogenesis is regulated by a change in the cell cycle (Ahn et al. 1999; Loeb et al. 1999). Our results suggest that the mutants in which the replisome components Mrc1p or Csm3p were repressed were unable to recover from DNA damage, supporting an important role for Mrc1p and Csm3p in DNA repair in the fungal pathogen.
Mms22p, together with Mms1p, is indispensable for the stabilization of the S. cerevisiae Mrc1p−Csm3p−Tof1p component under conditions of replication stress. The deletion of MMS22 reduces either Mrc1p or Csm3p localization to stalled replication forks (Dovey and Russell 2007; Vaisica et al. 2011). In contrast, S. pombe has a negative relationship between Mms22p and Swi1p or Swi3p. The deletion of either swi1 or swi3 rescues the phenotypes in the mms22 mutant (Table 3) (Dovey and Russell 2007). Similarly to the situation in S. pombe, in this study, we observed that the repression of either CSM3 or MRC1 led to a partial rescue of the sensitivity of the PMET3-MMS22 mutant to MMS, whereas the repression of MRC1 caused increased the sensitivity to CPT and IR of the PMET3-MMS22 mutant. This finding suggests that Mms22p is required for responding to DNA damage agents in MRC1 or CSM3 conditional mutants.
In the budding yeast, Rad51p-mediated HR plays a central role in promoting repair of double-strand breaks generated during replication (Heyer et al. 2010; Holthausen et al. 2010). HR is initiated at regions of single-strand DNA that become coated by the evolutionarily conserved Rad51p recombinase to form nucleoprotein filaments. These filaments, assisted by Rad52p and Rad55p-Rad57p, facilitate the search for homologous sequences in an intact duplex that acts as a template for repair synthesis (Paques and Haber 1999; Herzberg et al. 2006; Wu and Hickson 2006). In this study, the Δrad57 mutant was more sensitive to MMS and IR and especially to HU compared with PMET3-MMS22 mutant. The Δrad57 mutant was hypersensitive to CPT in the absence of MMS22. Our results suggest that Mms22p is only essential for HR induced by CPT. This finding is in contrast to the requirement of Mms22p in budding yeast for HR-mediated repair (Duro et al. 2008), or the blockage action of S. pombe Mms22p for HR repair pathway (Dovey and Russell 2007).
In conclusion, our results show that although C. albicans orthologs of S. cerevisiae and S. pombe DNA damage repair pathway members are involved in DNA damage repair in the fungal pathogen, the details of their function show distinct characteristics. In the pathogen Mms22p has little role in protecting against HU-mediated damage, whereas Tof1p appears unimportant in response to any damage investigated, in sharp contrast to their importance in these roles in S. cerevisiae. Overall, in the pathogen it appears that Mms22p plays a critical role in preserving genome integrity during DNA replication; perhaps Mms22 functions to maintain genomic integrity by HR through coordination of DNA synthesis by interacting with Rtt101p in the rescue of paused replication forks after they confront a block.
Supplementary Material
Acknowledgments
L.Y. is supported by Natural Science Foundation of China (31000079, 81470158). Y.J. is supported by China National 973 Program (2013CB531602), Natural Science Foundation of China (81330083), and Translational Medicine project of the Second Military Medical University (2014-01). H.L. is supported by Natural Science Foundation of China (81401552). M.W. and P.C. were supported in part by Canadian Institutes of Health Research grant MOP-42516.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.021840/-/DC1
Communicating editor: B. J. Andrews
Literature Cited
- Ahn S. H., Acurio A., Kron S. J., 1999. Regulation of G2/M progression by the STE mitogen-activated protein kinase pathway in budding yeast filamentous growth. Mol. Biol. Cell 10: 3301–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Kawasaki Y., Sasanuma H., Tye B. K., Sugino A., 2003. Budding yeast mcm10/dna43 mutant requires a novel repair pathway for viability. Genes Cells 8: 465–480. [DOI] [PubMed] [Google Scholar]
- Baldwin E. L., Berger A. C., Corbett A. H., Osheroff N., 2005. Mms22p protects Saccharomyces cerevisiae from DNA damage induced by topoisomerase II. Nucleic Acids Res. 33: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando M., Katou Y., Komata M., Tanaka H., Itoh T., et al. , 2009. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J. Biol. Chem. 284: 34355–34365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. B., Lewis L. K., Karthikeyan G., Lobachev K. S., Jin Y. H., et al. , 2001. Genes required for ionizing radiation resistance in yeast. Nat. Genet. 29: 426–434. [DOI] [PubMed] [Google Scholar]
- Berman J., 2006. Morphogenesis and cell cycle progression in Candida albicans. Curr. Opin. Microbiol. 9: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K., 2005. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 19: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care R. S., Trevethick J., Binley K. M., Sudbery P. E., 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34: 792–798. [DOI] [PubMed] [Google Scholar]
- Chang M., Bellaoui M., Boone C., Brown G. W., 2002. A genome-wide screen for methyl methanesulfonate-sensitive mutants reveals genes required for S phase progression in the presence of DNA damage. Proc. Natl. Acad. Sci. USA 99: 16934–16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciudad T., Andaluz E., Steinberg-Neifach O., Lue N. F., Gow N. A., et al. , 2004. Homologous recombination in Candida albicans: role of CaRad52p in DNA repair, integration of linear DNA fragments and telomere length. Mol. Microbiol. 53: 1177–1194. [DOI] [PubMed] [Google Scholar]
- Daulny A., Tansey W. P., 2009. Damage control: DNA repair, transcription, and the ubiquitin-proteasome system. DNA Repair (Amst.) 8: 444–448. [DOI] [PubMed] [Google Scholar]
- Dovey C. L., Aslanian A., Sofueva S., Yates J. R., 3rd, Russell P., 2009. Mms1-Mms22 complex protects genome integrity in Schizosaccharomyces pombe. DNA Repair (Amst.) 8: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey C. L., Russell P., 2007. Mms22 preserves genomic integrity during DNA replication in Schizosaccharomyces pombe. Genetics 177: 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duro E., Vaisica J. A., Brown G. W., Rouse J., 2008. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair (Amst.) 7: 811–818. [DOI] [PubMed] [Google Scholar]
- Foss E. J., 2001. Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae. Genetics 157: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Kitabatake M., Sakata T., Miyata A., Ohno M., 2009. A role for ubiquitin in the clearance of nonfunctional rRNAs. Genes Dev. 23: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Prieto F., Gomez-Raja J., Andaluz E., Calderone R., Larriba G., 2010. Role of the homologous recombination genes RAD51 and RAD59 in the resistance of Candida albicans to UV light, radiomimetic and anti-tumor compounds and oxidizing agents. Fungal Genet. Biol. 47: 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Elledge S. J., 2007. The DNA damage response: ten years after. Mol. Cell 28: 739–745. [DOI] [PubMed] [Google Scholar]
- Herzberg K., Bashkirov V. I., Rolfsmeier M., Haghnazari E., McDonald W. H., et al. , 2006. Phosphorylation of Rad55 on serines 2, 8, and 14 is required for efficient homologous recombination in the recovery of stalled replication forks. Mol. Cell. Biol. 26: 8396–8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer W. D., Ehmsen K. T., Liu J., 2010. Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 44: 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y., Gruhler A., Heilbut A., Bader G. D., Moore L., et al. , 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415: 180–183. [DOI] [PubMed] [Google Scholar]
- Holthausen J. T., Wyman C., Kanaar R., 2010. Regulation of DNA strand exchange in homologous recombination. DNA Repair (Amst.) 9: 1264–1272. [DOI] [PubMed] [Google Scholar]
- Hoot S. J., Zheng X., Potenski C. J., White T. C., Klein H. L., 2011. The role of Candida albicans homologous recombination factors Rad54 and Rdh54 in DNA damage sensitivity. BMC Microbiol. 11: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. D., Symington L. S., 1995. Functional differences and interactions among the putative RecA homologs Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15: 4843–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasanov F. K., Salakhova A. F., Khasanova O. S., Grishchuk A. L., Chepurnaja O. V., et al. , 2008. Genetic analysis reveals different roles of Schizosaccharomyces pombe sfr1/dds20 in meiotic and mitotic DNA recombination and repair. Curr. Genet. 54: 197–211. [DOI] [PubMed] [Google Scholar]
- Loeb J. D., Kerentseva T. A., Pan T., Sepulveda-Becerra M., Liu H., 1999. Saccharomyces cerevisiae G1 cyclins are differentially involved in invasive and pseudohyphal growth independent of the filamentation mitogen-activated protein kinase pathway. Genetics 153: 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B., Versini G., Jaquenoud M., Zaidi I. W., Kurz T., et al. , 2006. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 16: 786–792. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C., 1987. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell 51: 241–249. [DOI] [PubMed] [Google Scholar]
- Nedelcheva M. N., Roguev A., Dolapchiev L. B., Shevchenko A., Taskov H. B., et al. , 2005. Uncoupling of unwinding from DNA synthesis implies regulation of MCM helicase by Tof1/Mrc1/Csm3 checkpoint complex. J. Mol. Biol. 347: 509–521. [DOI] [PubMed] [Google Scholar]
- Noble S. M., Johnson A. D., 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell 4: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi E., Noguchi C., McDonald W. H., Yates J. R., 3rd, Russell P., 2004. Swi1 and Swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 24: 8342–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn A. J., Elledge S. J., 2003. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 17: 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques F., Haber J. E., 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63: 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T. M., Zaidi I. W., Vaisica J. A., Peter M., Brown G. W., 2008. Regulation of rtt107 recruitment to stalled DNA replication forks by the cullin rtt101 and the rtt109 acetyltransferase. Mol. Biol. Cell 19: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakofsky C. J., Ayyar S., Malkova A., 2012. Break-induced replication and genome stability. Biomolecules 2: 483–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub Y., Dunkler A., Walther A., Wendland J., 2006. New pFA-cassettes for PCR-based gene manipulation in Candida albicans. J. Basic Microbiol. 46: 416–429. [DOI] [PubMed] [Google Scholar]
- Shi Q. M., Wang Y. M., Zheng X. D., Lee R. T., Wang Y., 2007. Critical role of DNA checkpoints in mediating genotoxic-stress-induced filamentous growth in Candida albicans. Mol. Biol. Cell 18: 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., et al. , 2004. Global mapping of the yeast genetic interaction network. Science 303: 808–813. [DOI] [PubMed] [Google Scholar]
- Tourriere H., Pasero P., 2007. Maintenance of fork integrity at damaged DNA and natural pause sites. DNA Repair (Amst.) 6: 900–913. [DOI] [PubMed] [Google Scholar]
- Vaisica J. A., Baryshnikova A., Costanzo M., Boone C., Brown G. W., 2011. Mms1 and Mms22 stabilize the replisome during replication stress. Mol. Biol. Cell 22: 2396–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski I., Pfeffer A., Friedman N., Regev A., 2007. Automatic genome-wide reconstruction of phylogenetic gene trees. Bioinformatics 23: i549–i558. [DOI] [PubMed] [Google Scholar]
- Ward J. F., 1990. The yield of DNA double-strand breaks produced intracellularly by ionizing radiation: a review. Int. J. Radiat. Biol. 57: 1141–1150. [DOI] [PubMed] [Google Scholar]
- Wu L., Hickson I. D., 2006. DNA helicases required for homologous recombination and repair of damaged replication forks. Annu. Rev. Genet. 40: 279–306. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Inoue H., Ishii C., Murakami Y., 2007. The novel gene mus7(+) is involved in the repair of replication-associated DNA damage in fission yeast. DNA Repair (Amst.) 6: 770–780. [DOI] [PubMed] [Google Scholar]
- Zaidi I. W., Rabut G., Poveda A., Scheel H., Malmstrom J., et al. , 2008. Rtt101 and Mms1 in budding yeast form a CUL4(DDB1)-like ubiquitin ligase that promotes replication through damaged DNA. EMBO Rep. 9: 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. File S1 contains detailed descriptions of all supplemental files.