Abstract
G protein−coupled receptors (GPCRs) regulate facets of growth, development, and environmental sensing in eukaryotes, including filamentous fungi. The largest predicted GPCR class in these organisms is the Pth11-related, with members similar to a protein required for disease in the plant pathogen Magnaporthe oryzae. However, the Pth11-related class has not been functionally studied in any filamentous fungal species. Here, we analyze phenotypes in available mutants for 36 GPCR genes, including 20 Pth11-related, in the model filamentous fungus Neurospora crassa. We also investigate patterns of gene expression for all 43 predicted GPCR genes in available datasets. A total of 17 mutants (47%) possessed at least one growth or developmental phenotype. We identified 18 mutants (56%) with chemical sensitivity or nutritional phenotypes (11 uniquely), bringing the total number of mutants with at least one defect to 28 (78%), including 15 mutants (75%) in the Pth11-related class. Gene expression trends for GPCR genes correlated with the phenotypes observed for many mutants and also suggested overlapping functions for several groups of co-transcribed genes. Several members of the Pth11-related class have phenotypes and/or are differentially expressed on cellulose, suggesting a possible role for this gene family in plant cell wall sensing or utilization.
Keywords: filamentous fungi, G protein−coupled receptors, functional genomics, signal transduction, Heterotrimeric G proteins
G protein−coupled receptors (GPCRs) are responsible for a diversity of cell functions, including environmental sensing, metabolism, immunity, growth, and development in eukaryotic cells (Bock et al. 2014; Chini et al. 2013). GPCRs are seven alpha-helical transmembrane (TM) proteins anchored in the plasma membrane, with an intracellular carboxyl- and extracellular amino-terminus (Shukla et al. 2014; Chini et al. 2013). The heterotrimeric G protein−signaling cascade initiates with the GPCR sensing the stimulus (ligand) and then transducing this signal to the inside of the cell via heterotrimeric G proteins (Tesmer 2010). Heterotrimeric G proteins are composed of three subunits (α, β, and γ) that are associated with the GPCR in the inactive state. Ligand binding to the GPCR causes a conformational change within the receptor that leads to exchange of GDP for GTP on the Gα subunit and dissociation of the Gα from the Gβγ heterodimer and the GPCR (Jastrzebska 2013; Tesmer 2010). Both the Gα-GTP and Gβγ can then regulate downstream effectors, which include mitogen-activated protein kinase cascades, ion channels, adenylyl cyclases, phosphodiesterases and phopholipases (Chini et al. 2013; Neves et al. 2002; Mende et al. 1998). The Gα subunit has native GTPase activity, hydrolyzing GTP to GDP. In addition, Regulators of G protein Signaling (RGS) proteins, also known as GTPase-Activating Proteins, can accelerate the hydrolysis process by 2000-fold (Ross and Wilkie 2000). Subsequently, the Gα-GDP and Gβγ reassociate to form the inactive heterotrimeric complex at the GPCR on the membrane.
Neurospora crassa has served as a eukaryotic model for human and plant pathogenic fungi and has been the most-studied filamentous fungal species for the past hundred years [reviewed in (Davis and Perkins 2002; Borkovich et al. 2004; Selker 2011)]. N. crassa has a more complex life cycle than budding or fission yeasts and grows through extension, branching, and fusion of tube-like structures called hyphae [reviewed in (Springer 1993; Borkovich et al. 2004; Glass et al. 2004)]. N. crassa hyphae contain incomplete cross-walls (septa) that delineate cell compartments but still allow flow of organelles and cytoplasmic materials throughout the intertwined hyphal structure (mycelium). N. crassa possesses two different asexual sporulation pathways, with one (macroconidiation) under the control of a circadian rhythm, blue light, reactive oxygen species (ROS) and other environmental stimuli (Springer 1993; Greenwald et al. 2010; Baker et al. 2012). N. crassa also has a well-studied sexual cycle, involving two mating types, male and female partners, and production of numerous cell types, culminating in elaboration of the fruiting body that contains the meiotic progeny (Bistis 1981; Raju 1992; Nelson 1996; Kim et al. 2012). N. crassa offers several advantages for genetic analysis, including more than 1000 mapped morphologic mutations and a nearly complete gene knockout collection (Dunlap et al. 2007; Borkovich et al. 2004; Perkins et al. 2001).
Heterotrimeric G proteins and GPCRs have been demonstrated to regulate growth and development in N. crassa (Baasiri et al. 1997; Ivey et al. 1996; Kays and Borkovich 2004; Kays et al. 2000; Kim and Borkovich 2004; Kim et al. 2012; Krystofova and Borkovich 2005, 2006; Li and Borkovich 2006; Won et al. 2012; Li et al. 2007). N. crassa possesses three Gα, one Gβ, and one Gγ subunit(s), and 10 putative GPCRs were originally annotated in the N. crassa genome sequence (Borkovich et al. 2004; Galagan et al. 2003). Later annotation of GPCR genes in the filamentous fungal pathogen Magnaporthe oryzae demonstrated evidence for three additional GPCR classes in filamentous fungi (Kulkarni et al. 2005).
Predicted N. crassa GPCRs fall into 12 of the 13 classes (I-XII) described for Verticillium and Trichoderma fungal species (Zheng et al. 2010; Gruber et al. 2013) (Table 1), with a fourteenth class [Pth11-related; (Kulkarni et al. 2005); see Neurospora crassa GPCR families shared with other eukaryotes] named Class XIV for this study. To date, only five of the 43 predicted N. crassa putative GPCR genes have been functionally characterized. Classes I and II contain the pheromone response receptors PRE-2 and PRE-1 (Kim and Borkovich 2004; Kim et al. 2012). These two GPCRs have been demonstrated to interact with peptide pheromones to regulate female fertility in a mating-type dependent manner (Kim and Borkovich 2004, 2006; Kim et al. 2002, 2012). The N. crassa Class III GPCR is GPR-4, which regulates growth on poor carbon sources (Li and Borkovich 2006). Class V is the cAMP receptor-like (CRL) group containing GPR-1, GPR-2, and GPR-3 (Borkovich et al. 2004; Krystofova and Borkovich 2006), which are similar to chemoattractant GPCRs found in Dictyostelium discoideum (Klein et al. 1988). There is no evidence that fungal CRLs bind cAMP. N. crassa GPR-1 is localized in female reproductive structures and is required for normal formation of perithecial beaks and spore discharge from female fruiting bodies (Krystofova and Borkovich 2006). Class IX includes the microbial opsins NOP-1 and ORP-1. Heterologous experiments showed that NOP-1 is a light-activated retinal-binding protein (Bieszke et al. 1999b). Loss of nop-1 leads to subtle alterations in conidiation and expression of conidiation-specific genes in N. crassa (Bieszke et al. 2007; Bieszke et al. 1999a). However, functions for ORP-1 have not been described.
Table 1. Neurospora crassa G protein−coupled receptor gene families and summary of growth/developmental and chemical sensitivity/nutrition phenotypes.
| Fungal GPCR Class | Description GPCR Class | NCUa | N. crassa Gene(s)b | Saccharomyces cerevisiae Homologc | Aspergillus nidulans Homologd | Linear Growthe | Asexual Developmentf | Sexual Developmentg | Chemical Sensitivityh/ Nutritioni |
|---|---|---|---|---|---|---|---|---|---|
| I | Fungal Pheromone | 05758 | pre-2 | STE2 | gprA | AH | PP, P, Aj | ||
| II | Fungal Pheromone | 00138 | pre-1 | STE3 | gprB | P, Aj | |||
| III | Carbon Sensory | 06312 | gpr-4 | GPR1 | None | AH | |||
| IV | Stm1-like | 00300 | gpr-5 | YPQ1 | AN5720 | C | |||
| IV | Stm1-like | 09195 | gpr-6 | RTC2 | AN5720 | C | |||
| V | cAMP Receptor-Like | 00786 | gpr-1 | None | AN8262 | AH | P | C | |
| V | cAMP Receptor-like | 04626 | gpr-2 | None | AN8262 | AH | P | ||
| V | cAMP Receptor-like | 09427 | gpr-3 | None | AN8262 | AH | P | ||
| VI | GprK-like/RGS Domain | 09883 | gpr-7 | None | AN7795 (gprK) | F | |||
| VII | Rat growth hormone releasing factor receptor-like | 03253 | gpr-8 | None | AN6680 | NAk | |||
| VIII | mPR-Like/PAQR | 03238 | gpr-9 | IZH3 | AN4932 | S | |||
| VIII | mPR-like/PAQR | 04987 | gpr-10 | IZH2 | AN4932 | F | |||
| IX | Microbial Opsin | 10055 | nop-1 | YRO2, MRH1, HSP30 | AN3361 | AH | M | ||
| IX | Microbial Opsin | 01735 | orp-1 | YRO2, MRH1, HSP30 | AN3361 | AH | |||
| X | Lung 7TM Superfamily | 00182 | gpr-11 | PTM1 | AN0063 | YE | |||
| XI | GPCR89/ABA GPCR | 00005 | gpr-12 | YHR078w | None | ||||
| XII | Family C-like | 06629 | gpr-13 | None | AN8601 | ||||
| XIII | DUF300 superfamily/Ps GPR11 | 06987 | gpr-14 | YKR051W | AN2232 | NAk | |||
| XIVl | Pth11-like | 00700 | gpr-15 | None | AN12202 | R | PP, P | ||
| Pth11-like | 02903 | gpr-16 | None | AN0178 | AH | F | |||
| Pth11-like | 04106 | gpr-17 | None | AN7774 | AH | ||||
| Pth11-like | 04931 | gpr-18 | None | AN10886 | NAk | ||||
| Pth11-like | 05101 | gpr-19 | None | AN1930 | NAk | ||||
| Pth11-like | 05187 | gpr-20 | None | AN8661 | NAk | ||||
| Pth11-like | 05189 | gpr-21 | None | AN8661 | |||||
| Pth11-like | 05307 | gpr-22 | None | AN0751 | AH | ||||
| Pth11-like | 05829 | gpr-23 | None | AN0751 | R | AH | C,T | ||
| Pth11-like | 05854 | gpr-24 | None | AN8971 | FL | ||||
| Pth11-like | 06531 | gpr-25 | None | AN5639 | C | ||||
| Pth11-like | 17171 | gpr-26 | None | AN5664 | NAk | ||||
| Pth11-like | 07538 | gpr-27 | None | AN5664 | F | ||||
| Pth11-like | 16721 | gpr-28 | None | AN2249 | NAk | ||||
| Pth11-like | 07649 | gpr-29 | None | AN8943 | R | AH | S,C | ||
| Pth11-like | 07769 | gpr-30 | None | AN4452 | AH | ||||
| Pth11-like | 08429 | gpr-31 | None | None | I | AH | F | ||
| Pth11-like | 08431 | gpr-32 | None | AN5664 | A | ||||
| Pth11-like | 08447 | gpr-33 | None | AN5664 | |||||
| Pth11-like | 08624 | gpr-34 | None | AN0178 | |||||
| Pth11-like | 08718 | gpr-35 | None | AN8951 | |||||
| Pth11-Like | 09022 | gpr-36 | None | AN5664 | A | ||||
| Pth11-like | 09201 | gpr-37 | None | AN5664 | |||||
| Pth11-like | 09796 | gpr-38 | None | AN8328 | R | ||||
| Pth11-like | 09823 | gpr-39 | None | AN1930 | AH | A |
GPCR, G protein−coupled receptor; NCU, N. crassa gene number.
Based on the Broad Institute’s Neurospora crassa database (http://www.broadinstitue.org/annotation/genome/neurospora/MultiHome.html).
N. crassa gene names are from the literature or designated during this study.
Yeast orthologs were obtained after a Blastp search of the NCBI database, using a cut-off score of e-05 or less.
A. nidulans orthologs were obtained from a Blastp search of the NCBI database, using a cut-off score of e-05 or less. Only the top hit is shown.
R, reduced growth rate; I, increased growth rate relative to wild type.
Defects in aerial hyphae (AH) or conidial development (C).
Defects in protoperithecial (PP), perithecial (P), and/or ascospore (A) development.
Chemical sensitivity phenotypes are indicated based on sensitivity or resistance to sorbitol (S), cytochalasin A (C), benomyl (B), tert-butyl hydroperoxide (T), menadione (M), FK506 (F), and fludioxonil (FL).
Represented by increased or decreased growth on Avicel (A) or Yeast Extract (Y) compared with wild type.
Mating type dependent.
Not available; see Table S1 for details.
Category XIV named in this study.
A recent study reported functional information for 15 GPCRs corresponding to Classes I-IX in the filamentous fungus Aspergillus flavus (Affeldt et al. 2014). However, as mentioned previously, 38 of the 43 identified putative GPCRs have not been functionally characterized in N. crassa (Table 1). Of these, Class IV contains GPR-5 and GPR-6, similar to Stm1 from the yeast Saccharomyces cerevisiae (Borkovich et al. 2004; Chung et al. 2001). Likewise, classes VI (RGS domain; one gene), VII (similar to rat growth hormone releasing factor receptors; 7tm_1 domain; one gene), VIII (similar to human steroid receptor; HLyIII domain; one gene), X (Lung 7_TM superfamily; one gene), XI (GPCR89/ABA GPCR; one gene), XII (Family C-like; one gene), XIII (DUF300 superfamily; one gene), and XIV (Pth11-related; 25 genes) were previously annotated but have not been functionally studied in N. crassa (Gruber et al. 2013; Li et al. 2007; Lafon et al. 2006; Kulkarni et al. 2005).
In this work, we systematically analyze available N. crassa knockout mutants lacking annotated GPCR genes to determine their growth, developmental, and chemical sensitivity phenotypes. We take advantage of existing messenger RNA (mRNA) profiling datasets to mine expression trends for several GPCR genes during growth and development. We demonstrate phenotypes for many of the uncharacterized mutants, as well as novel chemical phenotypes for some previously studied mutants. Importantly, our study is the first to probe functions for the large family of Pth11-related GPCRs in fungi.
Materials and Methods
Media and strains
Vogel’s minimal medium [VM; (Vogel 1964)] was used to support vegetative growth and development. Sexual differentiation was promoted by culturing on synthetic crossing medium (SCM) plates (Westergaard and Mitchell 1947). Colony formation was facilitated by growth on sorbose-containing medium plates (Davis and Deserres 1970). Where indicated, hygromycin B (Calbiochem, San Diego, CA) was used at a concentration of 200 µg/mL in media. Media were inoculated using conidia propagated on VM agar slants.
From the Fungal Genetics Stock Center (FGSC; Kansas City, MO), we obtained wild-type strains ORS-SL6a (FGSC 4200; mat a) and 74-OR23-IVA (FGSC 2489; mat A). Available GPCR homokaryotic mutants were obtained from the FGSC or created in our laboratory (see Supporting Information, Table S1 for strain information). A total of seven mutants were not available at the time of this study (Table S1). All N. crassa gene numbers are preceded by the prefix “NCU”. Knockouts of NCU03253 (gpr-8), NCU05101 (gpr-19), and NCU05187 (gpr-20) were attempted, but failed, either due to an inability to obtain knockout constructs or N. crassa primary transformants. The gene structures for NCU17171 (gpr-26) and NCU16721 (gpr-28) were reannotated after mutant construction, and the available knockout mutants (former NCU06891 and NCU07591, respectively) are incorrect.
Knockout mutants that were deposited at the FGSC as heterokaryons (NCU09796, NCU00700, and NCU08429) were purified to homokaryons using a genetic cross to wild-type strain FGSC2489, as described (Colot et al. 2006). All putative homokaryons were checked for the presence of the knockout cassette using gene-specific and hph primers in diagnostic polymerase chain reaction as described (Ghosh et al. 2014).
Growth, developmental, chemical sensitivity, and nutritional phenotypes
Phenotypic assays were conducted essentially as previously described (Ghosh et al. 2014; Park et al. 2011b; Turner 2011), except that race tubes were prepared with 25-mL plastic disposable pipets (White and Woodward 1995). Some phenotypic tests used in earlier studies were omitted, including assessment of pigmentation and aerial hyphae formation on yeast extract-containing medium. For quantitative growth and developmental assays (apical extension rate and aerial hyphae height), mutants that exhibited a ±5 mm/d difference relative to wild type were considered significant (Colot et al. 2006; Park et al. 2011b). For detailed analysis of beak formation in the CRL mutant class of GPCR mutants, strains were inoculated on SCM (Westergaard and Mitchell 1947) plates and incubated under constant light for 1 wk at 25°. Cultures were fertilized using conidia from a wild-type strain of opposite mating type at 7 d postinoculation. Formation of perithecia was scored 1 wk after fertilization and beak morphology and ascospore ejection 2 wk after fertilization. Images of perithecia were captured using a SZX9 stereomicroscope (Olympus) and a digital camera. Delayed ascospore ejection and beak formation was checked after an additional week. Wild-type strain (FGSC 4200; mat a) was used as a control.
For chemical sensitivity screens, mutants were inoculated at the edge of 60 × 15-mm VM plates with and without chemical. The following chemicals and concentrations were assayed: sodium chloride (0.35 M), sorbitol (0.8 M), cytochalasin A (40 ng/mL; Sigma, St. Louis, MO), benomyl (92 ng/mL; Fluka, St. Louis, MO), tert-butyl hydroperoxide (0.13 mM; Sigma), Menadione (100 mM; Sigma), FK-506 (50 ng/mL; LC Laboratories, Woburn, MA), and fludioxonil (2.75 ng/mL; a gift from Frank Wong and Allison Tally). Sorbitol and sodium chloride were added to VM agar medium prior to autoclaving, while all other chemicals were added to cooled autoclaved VM agar medium using filter-sterilized concentrated stock solutions. Plates were incubated in the dark for 20−22 hr at 30°. VM plates lacking chemical were used as controls. The percent growth on the chemical was determined by dividing the colony radius for the plate containing the chemical by that of a VM plate. Four biological replicates were used in each of three independent experiments. Student’s t-test (paired, two-tailed) was used to identify mutants with significantly different sensitivity relative to wild type. A mutant was considered different from wild type if P ≤ 0.05 in at least two experiments and ≤0.20 in all three experiments. Mutants with better growth on a chemical or supplement/carbon source relative to wild type were scored as resistant or increased (R or I; Table 2), while slower-growing mutants were scored as sensitive or decreased (S or D; Table 2).
Table 2. G protein−coupled receptor mutants with chemical sensitivity and nutritional phenotypes.
| NCU | N. crassa Gene | Sorbitol | Peroxide | Menadione | FK506 | Cytochalasin A | Fludioxonil | Avicel | Yeast Extract |
|---|---|---|---|---|---|---|---|---|---|
| 00300 | gpr-5 | R (+29%) | |||||||
| 09195 | gpr-6 | R (+25%) | |||||||
| 00786 | gpr-1 | S (-13%) | |||||||
| 09883 | gpr-7 | R (+31%) | |||||||
| 03238 | gpr-9 | R (+11%) | |||||||
| 04987 | gpr-10 | R (+15%) | |||||||
| 10055 | nop-1 | R (+26%) | |||||||
| 00182 | gpr-11 | I (+18%) | |||||||
| 02903 | gpr-16 | R (+18%) | |||||||
| 05829 | gpr-23 | S (-25%) | S (-29%) | ||||||
| 05854 | gpr-24 | S (-29%) | |||||||
| 06531 | gpr-25 | R (+25%) | |||||||
| 07538 | gpr-27 | R (+19%) | |||||||
| 07649 | gpr-29 | R (+21%) | S (-28%) | ||||||
| 08429 | gpr-31 | R (+16%) | |||||||
| 08431 | gpr-32 | I (+39%) | |||||||
| 09022 | gpr-36 | I (+47%) | |||||||
| 09823 | gpr-39 | I (+30%) |
Mutants were considered significantly different than wild type if the results of a T-test yielded P ≤ 0.20 for all three replicates and P ≤ 0.05 for at least two of the three replicates. For chemical sensitivity assays, R = more resistant than wild type and S = more sensitive than wild type. For nutritional phenotypes (yeast extract and avicel), I = increased growth relative to wild type. The percent difference relative to wild type is shown in parentheses for affected mutants. NCU, N. crassa gene number.
The GPCR mutants also were tested for nutritional phenotypes using 60 × 15-mm VM agar plates. Avicel (crystalline cellulose; Sigma) was substituted for sucrose in VM medium at a concentration of 2%. Yeast extract was added to VM medium to a concentration of 2% w/v. Incubation and measurement of plates and identification of mutants that were significantly different than wild type was determined as described for chemical sensitivity screening, above. Mutants with better growth on Avicel or 2% yeast extract relative to wild type were scored as increased (I; Table 2), whereas slower-growing mutants were scored as decreased (D; Table 2).
Clustering of N. crassa GPCR expression data and heatmap generation
Expression data were mined for GPCR genes from four different datasets. Each dataset pertained to a specialized tissue type during development or an environmental condition. RNA-sequencing data were obtained for sexual development (Wang et al. 2014), whereas microarray data were utilized for colony development (Kasuga and Glass 2008) and a time course of conidiation (Greenwald et al. 2010). Lastly, RNA sequencing data for expression using 4 hr liquid cultures grown on either sucrose or the alternative carbon source Avicel (Coradetti et al. 2012) were downloaded from (http://www.ncbi.nlm.nih.gov/geo; Accession number GSE35227). To visualize the expression data, heatmaps were generated with the pheatmap package (V1.0.2); (Kolde 2015) using R (v3.1.1); (R Core Development Team 2014). Expression data for each gene was standardized using the included scaling function in pheatmap.
Phylogenetic analysis of putative Pth11-related proteins
The Pth11 protein sequence from Magnaporthe oryzae and the 25 Pth11-related N. crassa GPCR protein sequences were aligned with T-coffee using default parameters and trimmed using TrimAl with a threshold of 0.7 (Notredame et al. 2000; Capella-Gutierrez et al. 2009). The programs Seqboot, Protpars, and Consense, belonging to the Phylip package were used to generate a consensus parsimony tree using 100 bootstrap replicates (Felsenstein 1989).
Data availability
Strains are available from the FGSC or our laboratory upon request. File S1 contains detailed descriptions of phenotypic data.
Results
Neurospora crassa GPCR families shared with other eukaryotes
Mammalian GPCRs have been classified using the A-F classification scheme (Attwood and Findlay 1994) and more recently, the GRAFS method (Fredriksson et al. 2003; Schioth and Fredriksson 2005). The A-F classification contains six major superfamilies of GPCRs: Class A (rhodopsin like), Class B (secretin like), Class C (metabotropic glutamate/pheromone), Class D (fungal pheromone), Class E (cAMP receptors), and Class F (Frizzled/Smoothened family). The GRAFS classification grouped the human GPCRs into five families: Glutamate (Class C), Rhodopsin (Class A), Adhesion (Class B), Frizzled/Taste2 (Class F), and Secretin (Class B). A recent analysis provided evidence for four of the five GRAFS families (secretin is absent) in fungi (Krishnan et al. 2012). This work also provided evidence that the fungal cAMP and rhodopsin families share a common ancestor. Other studies have proposed up to 14 classes of GPCRs in fungi (Kulkarni et al. 2005; Zheng et al. 2010; Gruber et al. 2013).
Previous studies have identified 43 putative GPCR genes in the N. crassa genome (Li et al. 2007; Borkovich et al. 2004; Galagan et al. 2003) (Table 1). Of the GPCR families found in mammals, N. crassa possesses one Adhesion family GPCR (GPR-1; (Krystofova and Borkovich 2006) and two cAMP family GPCRs (GPR-2, GPR-3; (Borkovich et al. 2004; Galagan et al. 2003). N. crassa also possesses a mPR class (Class VIII), with homology to seven-helix progestin receptors in mammals (Thomas et al. 2007). These proteins are members of the progesterone and AdipoO receptor (PAQR) group of proteins found in many eukaryotes. Evidence that these proteins can function as bona-fide GPCRs in filamentous fungi is suggested by studies in Sporothrix schenckii, which show that progesterone is a ligand for a PAQR that also interacts with a Gα protein (Gonzalez-Velazquez et al. 2012). The N. crassa genome also predicts one seven-helix protein containing a Lung_7-TM_R domain (Liu et al. 2004) with homology to S. cerevisiae PTM1, a protein of unknown function (Inadome et al. 2005). Finally, N. crassa possesses one protein with weak similarity to Family C-like (metabotropic glutamate/pheromone) GPCRs in chicken (Zheng et al. 2010).
We and others have previously identified proteins similar to predicted N. crassa GPCRs in the yeast S. cerevisiae and the filamentous fungus Aspergillus nidulans (Lafon et al. 2006; Li et al. 2007) (Table 1). The results show that S. cerevisiae possesses proteins similar to 12 of the N. crassa predicted GPCRs, with members in classes I-IV, VIII-XI, and XIII, and an additional member (total of three proteins) in the microbial opsin class (Table 1). A. nidulans and N. crassa share homologs for most predicted N. crassa genes, with the exception of the Class XI GPCR89/ABA related gene gpr-12 and one Pth11-related GPCR, gpr-31 (Table 1).
Pth11 is a seven-helix transmembrane protein required for development of the infectious appressorium structure and pathogenesis in M. oryzae (DeZwaan et al. 1999). It has recently been demonstrated that Pth11, the Gα protein MagA, the RGS Rgs1, and the adenylyl cyclase Mac1 are co-localized on late endosomes during the early stages of pathogenesis, which would enable effective signal transmission (Ramanujam et al. 2013). Sequencing of numerous fungal genomes revealed that filamentous fungi contain a large number of proteins with homology to Pth11 (Kulkarni et al. 2005; Li et al. 2007; Zheng et al. 2010; Gruber et al. 2013), including 25 in N. crassa (Kulkarni et al. 2005; Li et al. 2007). This was particularly surprising in the case of N. crassa, since the wide array of genome defense mechanisms in this species is thought to have limited the number of gene families (Galagan et al. 2003). The presence of a large number of Pth11-related proteins suggests that members of this class serve important functions in N. crassa.
Mutants with phenotypes during asexual growth and development
We had previously produced knockout mutants for 10 predicted GPCR genes in our laboratory [(Kim and Borkovich 2004; Kim et al. 2012; Bieszke et al. 1999a; Krystofova and Borkovich 2006; Li and Borkovich 2006); S. Krystofova, M. Nemcovic, L. Li, A. Michkov and K. Borkovich, unpublished data.]. Mutation of all predicted GPCR genes was attempted by the Neurospora Genome Project (Colot et al. 2006; Dunlap et al. 2007; Park et al. 2011a). Mutants for seven genes were not available (see the section Materials and Methods), leaving us with 36 viable mutants to analyze.
We analyzed the 36 available GPCR knockout mutants for a variety of growth and developmental phenotypes (Table 1; Figure 1; Table S1), first focusing on hyphal growth and asexual sporulation (macroconidiation). The macroconidiation (conidiation) pathway is induced when N. crassa is exposed to oxygen (plate cultures) or to a variety of environmental stresses during growth in submerged culture [reviewed in (Springer 1993; Davis and Perkins 2002; Borkovich et al. 2004)]. The pathway begins with formation of aerial hyphae, tube-like structures that rise up roughly perpendicular to the substratum. Aerial hyphae form constrictions between cell compartments at their tips that eventually lead to formation of mature conidia that can be dispersed by mechanical perturbation or wind currents (Springer 1993).
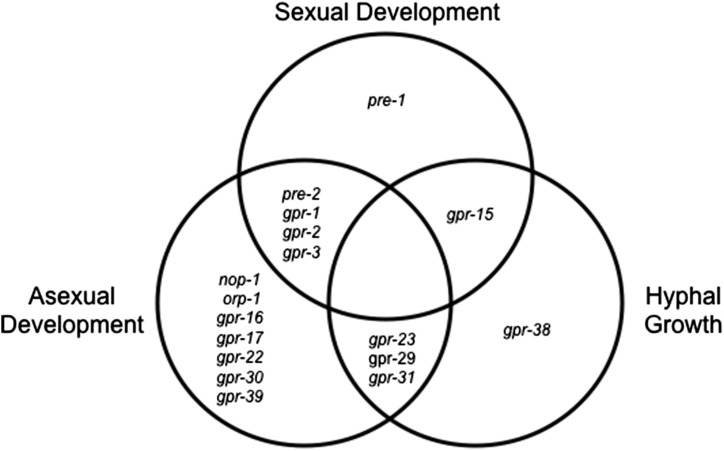
Figure 1.
Venn diagram displaying G protein−coupled receptor mutants with phenotypes in growth and development. From the 36 available mutants, 17 (47%) exhibited a defect in at least one assay for hyphal growth or asexual or sexual development, as shown. Mutants with defects are indicated as gene names for the deleted genes.
We obtained quantitative data for hyphal growth rate and aerial hyphae height and qualitative data for conidia production (Table 1; Table S1). We identified a total of five knockout mutants (14%) with defects in extension of basal hyphae (Figure 1; Table 1; Table S1). Of interest, all five belong to Class XIV (Pth11-like). Δgpr-31 was the only mutant among the group that had an increased growth rate relative to wild type; all others (Δgpr-15, Δgpr-23, Δgpr-29, and Δgpr-38) grew more slowly than wild type.
A total of 14 GPCR mutants (39%) had defects in asexual development, specifically in formation of aerial hyphae (Figure 1; Table 1; Table S1). There were no GPCR mutants with obvious defects in conidia production. Of the mutants with aerial hyphae defects, 57% (8/14) are characterized as Class XIV (Pth11-like). Among the 14 mutants with aerial hyphae defects, half had a reduced phenotype, whereas the other half an increased aerial hyphae phenotype. Finally, of the five mutants with defects in basal hyphal growth, three also exhibited reduced formation of aerial hyphae (Δgpr-23, Δgpr-29, and Δgpr-31).
Mutants with defects in sexual development
Low nitrogen, as found in SCM, induces development of female reproductive structures (protoperithecia) (Westergaard and Mitchell 1947; Raju 1992) approximately 7 d postinoculation. Fertilization occurs when a specialized hyphae (trichogyne) from the protoperithecium fuses with a cell from an opposite mating type cell (male). After nuclear fusion, meiosis is followed by a round of mitosis and sexual spores (ascospores) are then formed within the mature perithecium.
This study analyzed three phases in the sexual cycle: protoperithecia, perithecia, and ascospore formation. A total of six GPCR mutants (17%) showed a defect in sexual development (Figure 1, Table 1; Table S1). As previously reported by our group (Kim and Borkovich 2004, 2006; Kim et al. 2012), the two pheromone receptor mutants displayed defects in sexual development, with pre-2 affecting all three phases (protoperithecia, perithecia, and ascospore formation), and pre-1 affecting perithecia and ascospore formation. However, it is should be noted that the sexual defects of these mutants are mating-type dependent; Δpre-2 is only female-sterile in mat a, and Δpre-1 in mat A (Kim and Borkovich 2004; Kim et al. 2012) (Table 1). The other four GPCR mutants with sexual defects (Δgpr-1, Δgpr-2, Δgpr-3, and Δgpr-15) all exhibited phenotypes in the perithecial phase, with Δgpr-15 having both a protoperithecial and perithecial defect. Despite their perithecial phenotypes, these four mutants still produced ascospores and thus are technically female-fertile (Table 1; Table S1). Thus, allowing for the mating type specificity of the pheromone receptor mutations, the results showed that no single GPCR gene was essential for female fertility in N. crassa.
Mutants with defects in multiple phases of the lifecycle
The aforementioned results revealed that 17 of the 36 predicted GPCR mutants (47%) exhibited at least one growth or developmental phenotype (Table 1). There were no GPCR mutants with defects in all three phenotypic categories, but eight mutants possessed phenotypes in two categories (Figure 1; Table 1). The four mutants with defects in both asexual and sexual development included the Δpre-2 pheromone receptor mutant and those lacking any one of the three CRL receptor genes (gpr-1, gpr-2, and gpr-3). The remaining four mutants with defects in two categories (hyphal growth and either asexual or sexual development) were all in the Pth11-related Group (Figure 1; Table 1). The relatively low number of GPCR mutants with defects in more than one of the analyzed stages suggests either specialization, participation in pathways not uncovered during our study, or gene redundancy in the predicted GPCRs.
The CRL class of predicted GPCRs is required for normal formation of perithecial beaks and ascospore ejection
We were interested to find that all three CRL (Class V) mutants possessed defects in both asexual and sexual development (Table 1). Notably, gpr-1 was reported previously to have an important role in proper formation of perithecial beaks (Krystofova and Borkovich 2006). Beaks are structures formed at the tip of perithecia that are positively phototrophic to blue light and which normally contain an opening (ostiole) from which the mature ascospores are ejected (Harding and Melles 1983; Harris et al. 1975). Δgpr-1 beaks are deformed and lack ostioles, leading to rupture of the perithecium during discharge of ascospores (Krystofova and Borkovich 2006). Based on the phenotype of Δgpr-1 mutants, we decided to explore beak formation in Δgpr-2 and Δgpr-3 single mutants, as well as mutants lacking gpr-1 and various combinations of the other two CRL genes (Figure 2).
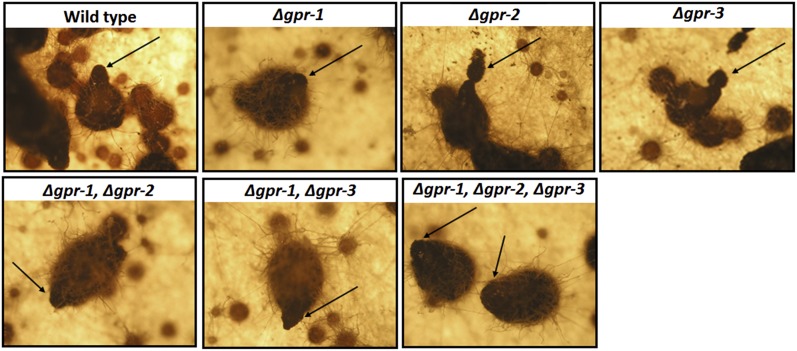
Figure 2.
Perithecial development in Δgpr-1, Δgpr-2, and Δgpr-3 mutants lacking CRL class G protein−coupled receptorr. Synthetic crossing medium cultures of the indicated strains containing protoperithecia were fertilized using opposite mating type conidia from a wild-type strain. Perithecia were photographed 10 d later. The arrows indicate normal perithecial beaks (wild type), perithecia with beaks that lack ostioles (Δgpr-1) or perithecia with beaks that bend downward or are torn during ascospore ejection (single, double or triple mutants lacking gpr-2 or gpr-3).
As shown previously, Δgpr-1 beaks do not form ostioles and therefore cannot eject ascospores (Figure 2; data not shown). Loss of gpr-2 or gpr-3 leads to formation of beaks that bend downward and/or are torn during ascospore ejection (Figure 2). Similarly, mutants lacking gpr-1 in combination with gpr-2 and/or gpr-3 form beaks that are not positively phototrophic and do not contain ostioles (Figure 2). Thus, all three CRL genes influence beak morphology and ascospore discharge in N. crassa.
Chemical sensitivity and nutritional screens reveal additional GPCR mutants with phenotypes
As shown in our previous studies with N. crassa protein kinase and phosphatase mutants (Park et al. 2011b; Ghosh et al. 2014) and in work with the yeast S. cerevisiae (Hillenmeyer et al. 2008), chemical sensitivity and nutritional screening is a useful tool to uncover functions for mutants that lack observable growth or developmental defects. With this in mind, we conducted chemical/nutritional screens on the 36 GPCR mutants, using a total of 10 chemicals. Mutants were grown on agar medium with and without the added chemical, the percent growth determined and compared to wild type (see the section Materials and Methods). Mutants with relative growth rates statistically greater than or less than wild type were categorized as resistant or sensitive, respectively (Table 2). The chemicals used induced osmotic stress [sorbitol and NaCl; (Ivey et al. 1996)], oxidative stress [menadione and tert-butyl hydrogen peroxide; (Belden et al. 2007; Kato et al. 2004)], cytoskeletal disruption [cytochalasin A and benomyl; (Cooper 1987; Willhite 1983)], inhibition of the Ca2+-calmodulin dependent phosphatase calcineurin [FK506; (Prokisch et al. 1997)], fungicidal activity [fludioxonil; (Ochiai et al. 2001)], and nutritional substitution or supplementation (Avicel and yeast extract).
No mutants were significantly different than wild type during growth on NaCl or benomyl, and therefore these two agents are not shown in Table 2. A total of 18 GPCR mutants exhibited at least one phenotype using the other eight chemicals in the screen and 11 of these mutants did not exhibit a growth or developmental phenotype. More than half (10/18; 56%) of the 18 mutants with chemical phenotypes are classified as Pth11-like receptors.
The chemical that yielded the largest number of mutants with phenotypes was cytochalasin A, with six affected strains (Table 2). Cytochalasin A interferes with actin function by inhibiting polymerization of monomers (Cooper 1987). Three mutants (Δgpr-5, Δgpr-6, and Δgpr-25) were found to be resistant to cytochalasin A, while Δgpr-1, Δgpr-23, and Δgpr-29 strains were sensitive. GPR-5 and GPR-6 are similar to Stm1p, a PQ-loop family protein originally implicated in nitrogen sensing upstream of the Gα protein Gpa2 in S. pombe (Chung et al. 2001), with more recent evidence suggesting that the S. cerevisiae homolog YPQ1 may function as a vacuolar amino acid transporter (Sekito et al. 2014). The finding that two Stm1p-related proteins confer resistance to cytochalasin A in N. crassa suggests a connection between actin polymerization and G protein signaling or vacuolar amino acid transport. GPR-1 is a CRL, whereas gpr-23, gpr-25, and gpr-29 encode Pth11-related proteins. Of these, GPR-25 is the closest N. crassa homolog to M. oryzae Pth11 (Figure 6). The cytochalasin A phenotype for Δgpr-25 mutants may point to a role for GPR-25 in actin dynamics.
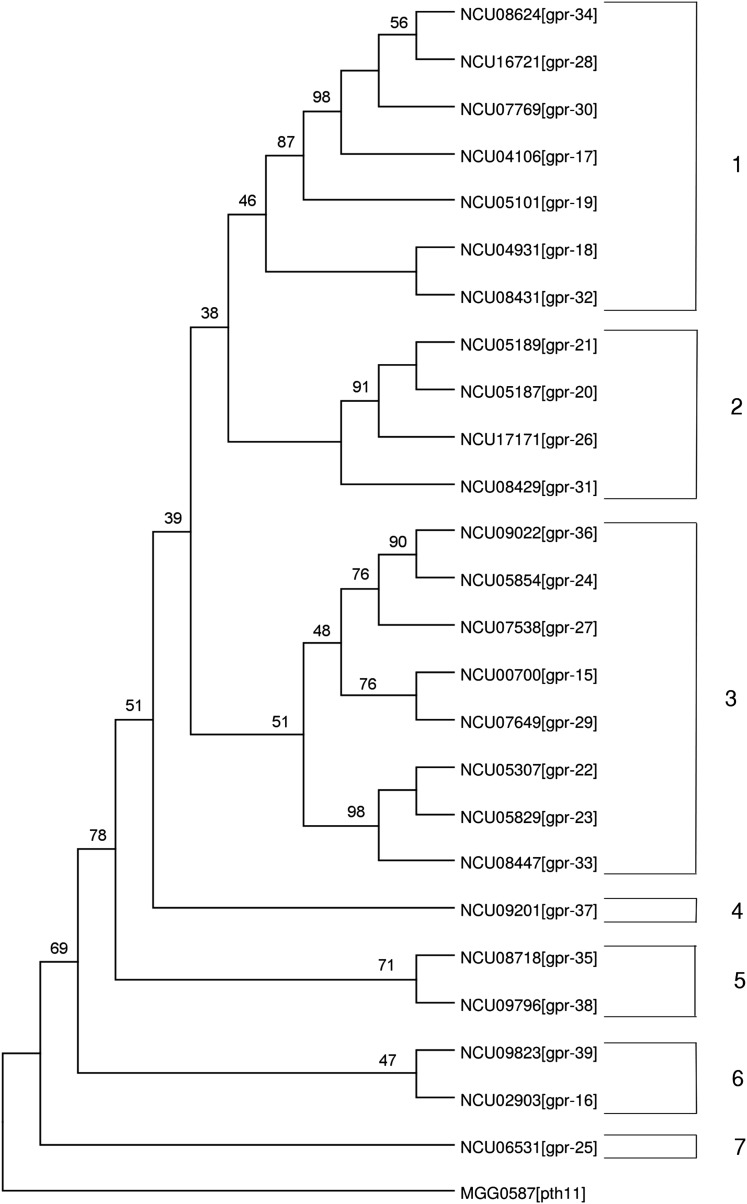
Figure 6.
Phylogenetic analysis of Pth11-related proteins in N. crassa. The protein sequences for the 25 N. crassa Pth11-related proteins from Li et al. (2007) (Table 1) and M. oryzae Pth11 (outgroup) were aligned using T-coffee and trimmed using TrimAl. The consensus parsimony tree was produced using 100 bootstrap replicates. Branch lengths are indicated on the left side of the figure, whereas groups of related proteins (1−7) are indicated on the right. See the section Materials and Methods for details.
FK506 inhibits the phosphatase calcineurin, which is regulated by Ca2+-calmodulin. Five GPCR mutants, Δgpr-7, Δgpr-10, Δgpr-16, Δgpr-27, and Δgpr-31, were all resistant to FK506, suggesting a possible role in regulation of the phosphatase or modulating calcium signaling. Of interest, the closest yeast homolog for N. crassa GPR-10 is Izh2p (Table 1). Izh2p and three other proteins in yeast each have four metal ion binding sites and have been variously implicated in zinc homeostasis, regulation of membrane sterol content and a signaling pathway upstream of the Zap1 transcription factor (Lyons et al. 2004). Izh2p is of particular interest, since it has recently been shown to function as a receptor for plant PR-5 proteins and is required for the response to polyene antifungal drugs (Narasimhan et al. 2005; Villa et al. 2011). Our finding that the five N. crassa mutants, including Δgpr-10, are resistant to FK506 suggests that the calcineurin phosphatase may be a component of the their downstream pathways.
Two GPCR mutants, Δgpr-9 and Δgpr-29, showed increased resistance to osmotic stress induced by sorbitol. GPR-9 is most homologous to the S. cerevisiae protein Izh3p, a mPR-like PAQR class protein similar to Izh2p, whereas GPR-29 is a Pth11-related receptor. Izh3p dosage affects resistance to polyene drugs in yeast, similar to Izh2p (Villa et al. 2011).
Peroxide, menadione, and fludioxonil testing each yielded one GPCR with a phenotype (Δgpr-23, Δnop-1, and Δgpr-24). We observed that Δnop-1 mutants are resistant to the ROS generating compound menadione. The only phenotypes that had previously been noted for Δnop-1 mutants were effects on colony morphology (Bieszke et al. 1999a) and conidiation-regulated gene expression (Bieszke et al. 2007). The menadione phenotype is of particular interest since a link between conidiation and ROS has been determined for N. crassa, with elevated ROS correlating with aerial hyphae and conidia development (Hansberg et al. 1993). Our demonstration that Δnop-1 mutants are more resistant to menadione correlates with the slightly increased formation of aerial hyphae in these mutants in plate cultures and with elevated expression of certain conidiation specific genes at specific times during asexual development (Bieszke et al. 2007).
We also explored growth of the mutants in medium supplemented with yeast extract or with crystalline cellulose (Avicel) as an alternative carbon source. Yeast extract is rich in vitamins, amino acids and peptides and we have previously used this supplement to recover knockout mutants with defects in amino acid biosynthetic pathways (Colot et al. 2006). Δgpr-11 mutants grew better than wild type in medium containing yeast extract (Table 2). This was the only phenotype noted for this Lung7_TM superfamily protein during our study. Three Pth11 class mutants, Δgpr-32, Δgpr-36, and Δgpr-39, grew better than wild type on medium containing 2% Avicel as an alternative to sucrose (30–47% greater; Table 2). This finding suggests that these Pth11 class proteins may participate in sensing plant-related carbohydrates.
Predicted GPCR genes are differentially expressed during growth and development
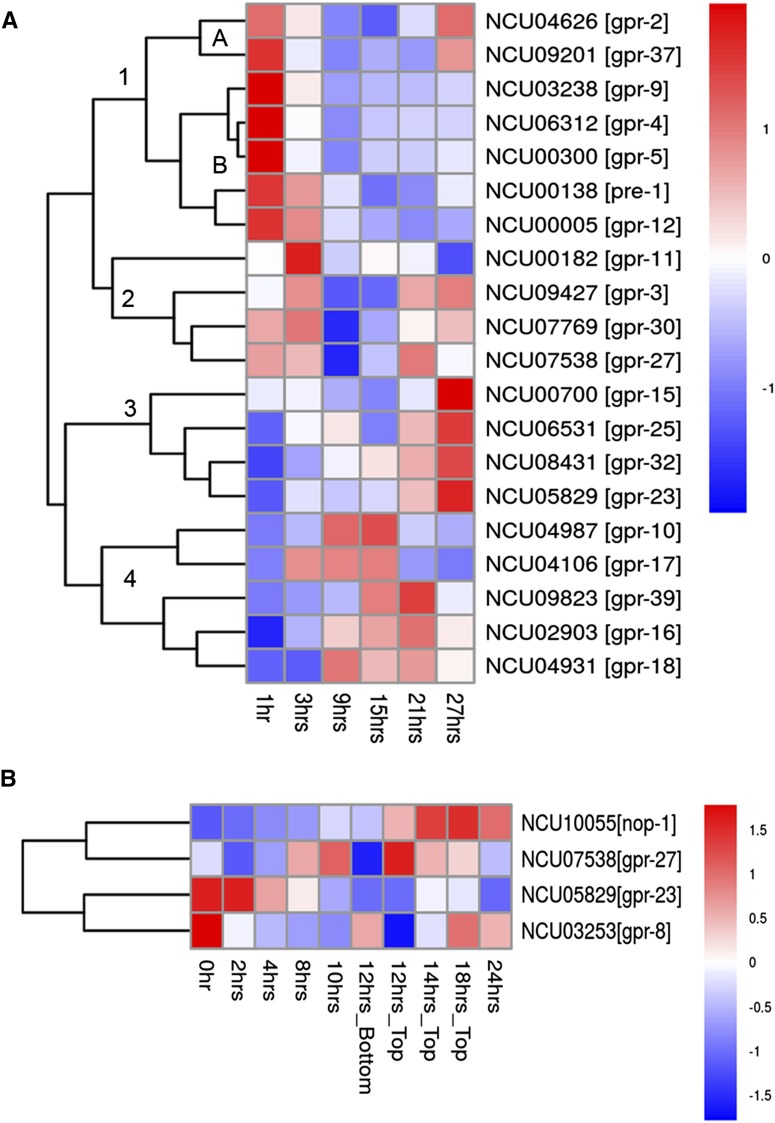
RNA expression data for GPCRs was mined from publically available datasets. Experiments included those performed under different environmental conditions and time-courses for specialized tissue types (Kasuga and Glass 2008; Greenwald et al. 2010; Wang et al. 2014; Coradetti et al. 2012; Nordberg et al. 2014). In all instances, data were analyzed and visualized using pheatmap (V1.0.2) (Kolde 2015) (Figure 3, Figure 4, and Figure 5).
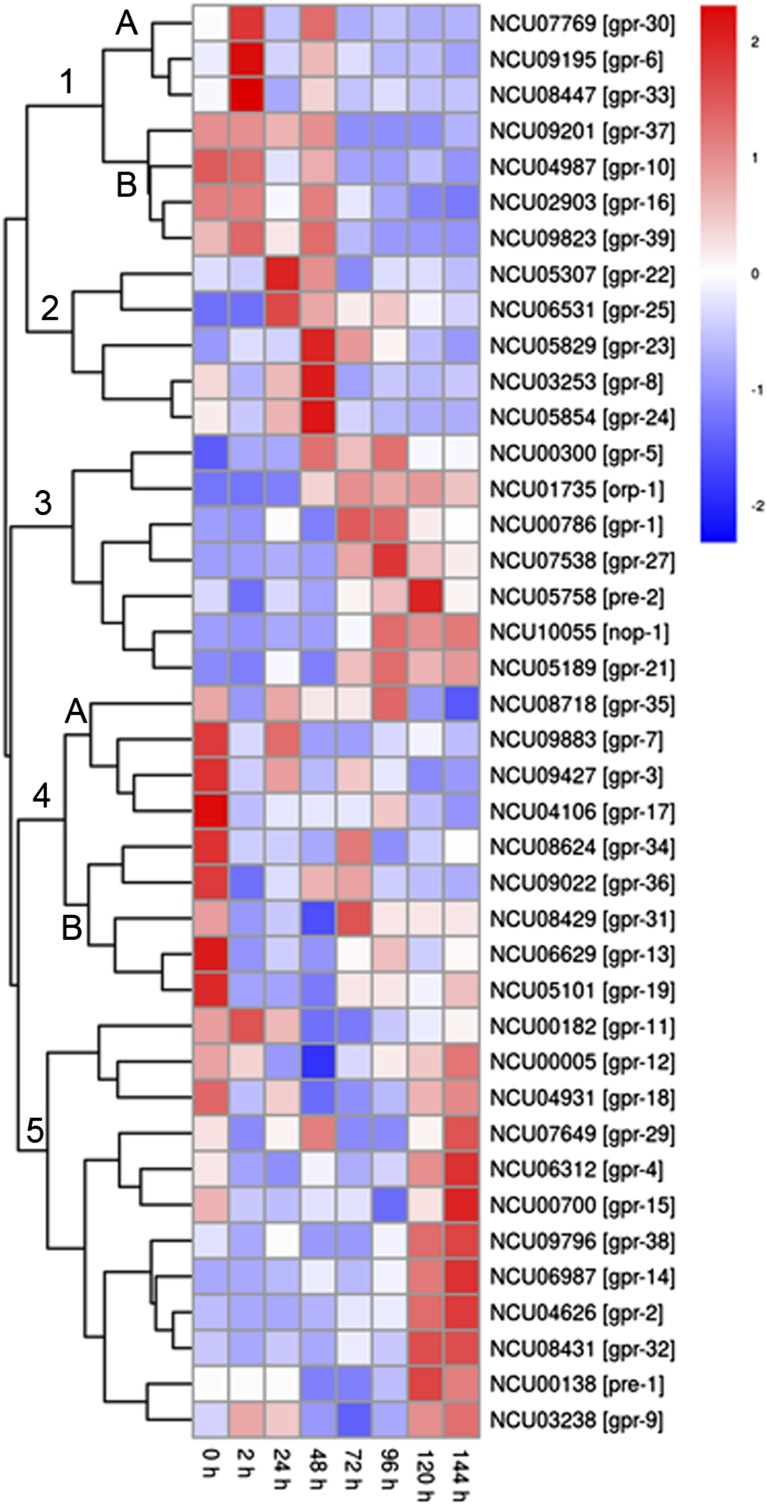
Figure 3.
Clustering and heatmap generation of mRNA expression data for N. crassa G protein−coupled receptor (GPCR) genes during a time course of sexual development. RNAseq data were mined from (Wang et al. 2014). Expression data for 37 of the 43 predicted GPCR genes was contained in the dataset and a heat map prepared as described in the section Materials and Methods. Red color denotes greater levels of expression, whereas blue corresponds to lower expression.
Figure 4.
Clustering and heatmap generation of mRNA expression data for N. crassa G protein−coupled receptor (GPCR) genes during time courses of colony growth and asexual development (conidiation). Data were taken from the indicated sources and heat maps generated as described in the section Materials and Methods. Red color denotes greater levels of expression, whereas blue corresponds to lower expression. (A) Gene expression during colony growth. Microarray data were obtained from (Kasuga and Glass 2008) for 20 predicted GPCR genes expressed during colony growth. (B) Gene expression during conidiation. Microarray data were mined from (Greenwald et al. 2010) for four predicted GPCR genes expressed during asexual development (conidiation).
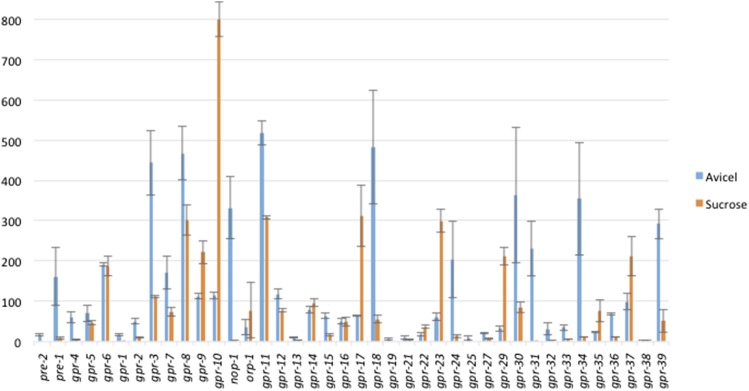
Figure 5.
Expression patterns for 40 predicted G protein−coupled receptor genes on two different carbon sources. RNAseq data were obtained from Coradetti et al. (2012) for genes expressed in tissue grown for 4 hr in liquid medium containing either sucrose (orange bars) or Avicel (crystalline cellulose; blue bars) as a carbon source. Y-axis is reads per kilobase per million mapped reads, whereas x-axis indicates genes.
We first analyzed an RNAseq dataset for sexually differentiating cultures that included eight time-points (0, 2, 24, 48, 72, 98, 120, and 144 hr after fertilization) (Wang et al. 2014). At time 0 hr, cultures contain vegetative hyphae and unfertilized female structures (protoperithecia). Within 24 hr after fertilization, perithecia can be observed. Croziers, structures that will develop into asci containing the meiotic progeny (ascospores) become apparent between 48 and 72 hr after fertilization (Raju 1992; Read and Beckett 1996). After 96 hr, asci become detectable and at 120−144 hr perithecia produce beaks, a structure required to eject ascospores into the environment.
The dataset for sexual development contained expression data for 37 of the 43 predicted GPCR genes, with the genes falling into five major groups (Figure 3). Group 1 genes are all down-regulated after 72 hr, at the time when the synchronization of nuclei is occurring in the croziers. Group 1 can be subdivided into subgroups 1A and 1B, which differ in their patterns of expression before 72 hr. The three genes in Subgroup 1A (gpr-6 and two Pth11-related genes) are up-regulated at 2 hr after fertilization, with expression diminishing at 24 hr, rising again at 48 hr. Cluster 1B genes (gpr-10 and three Pth11-related GPCRs) are highly expressed at 0 and 2 hr after fertilization, but then resemble Subgroup 1A for the rest of the time course.
Group 2 genes (gpr-8 and four Pth11-related GPCRs) exhibited the greatest levels of expression at 24 or 48 hr after fertilization (the time when perithecia first become visible), with relatively lower levels at 72−144 hr. Group 3 genes (seven total) have relatively low levels of expression until 48−72 hr, corresponding to the emergence of croziers. In general, Group 3 gene expression remains high throughout the rest of perithecial development. Three Group 3 genes have been previously examined for expression during at least one phase of sexual development using northern analysis: pre-2, gpr-1, and nop-1. In congruence with the RNAseq data, expression of the pheromone receptor pre-2 is low in mat A protoperithecia (0 hr), but increases after fertilization with mat a conidia (Kim et al. 2012). Northern analysis demonstrated that The CRL GPCR gpr-1 is highly expressed in protoperithecia, with even greater levels in perithecia (Krystofova and Borkovich 2006) and the RNAseq results showed that mRNA levels are greater during the time of perithecial development than in protoperithecia (Figure 3). Finally, although the microbial opsin nop-1 is highly expressed in protoperithecia, levels in perithecia have not been investigated using northern analysis (Bieszke et al. 1999a), precluding direct comparison to the RNAseq results.
Group 4 (nine genes) is divided into Subgroups 4A and 4B (Figure 3). In general, genes in both subgroups exhibit their highest levels of expression at 0 hr, prior to fertilization. Group 4A genes have dropped to their lowest levels of expression by 120−144 hr, whereas expression of most Group 4B genes rises again later during perithecial development. The authors of the RNAseq study noted that due to the difficulty in obtaining pure reproductive structures, vegetative hyphae is likely present in the samples before 48 hr postfertilization (Wang et al. 2014). With this in mind, it is important to note that mutants lacking three of the genes in Group 4 had a phenotype during growth or sexual or asexual development. Δgpr-3 has defects in asexual and sexual development; Δgpr-31 in asexual development and linear growth; and Δgpr-17 only in asexual development (Table 1, Figure 1).
Group 5 is the largest, with 12 genes (Figure 3). Most of the genes in this group are up-regulated 120−144 hr after fertilization, when mature perithecia are present. However, only the CRL gpr-2 and the pheromone receptor pre-1 play obvious roles during sexual development (Table 1). The finding that the other 10 genes have no obvious sexual cycle phenotype suggests at least some of them may be functionally redundant. Finally, the expression trend for pre-1 is supported by previous northern analysis revealing increased expression of pre-1 in mat A protoperithecia after fertilization with mat a conidia (Kim and Borkovich 2004).
We next mined microarray expression data from six time points during vegetative colony development (1, 3, 9, 15, 21, and 27 hr) (Kasuga and Glass 2008). Colony establishment consists of multiple events: hyphal extension, branching, anastomosis, and asexual sporulation (Kasuga and Glass 2008). Early features of colony establishment can be seen in the 1-hr time point at the leading hyphal edge. This “periphery growth zone” is rich with organelles, such as endoplasmic reticulum, Golgi apparatus, polysomes, and mitochondria. Asexual development involves aerial hyphae growing upward from the colony, followed by production of conidiophores and mature conidia from the tips of aerial hyphae. Asexual development structures were first seen at 15 hr and mature conidia at 27 hr (Kasuga and Glass 2008).
We mapped expression for 20 GPCRs, present in four groups (Figure 4A). Group 1 genes are highly expressed in leading basal hyphae and tend to decrease in expression in older parts of the colony (Figure 4A). The only exceptions are gpr-2 and gpr-37, which exhibit elevated expression at 27 hr. Δgpr-2 mutants have defects in aerial hyphae development (Table 1), consistent with increased expression of gpr-2 later during colony development. Group 2 (four genes) has a pattern of high expression early and late during colony development (Figure 4A). This group includes two genes (gpr-3 and gpr-30) that are required for normal aerial hyphae development. The expression of Group 3 genes peaked late during asexual sporulation (27 hr) at the time when conidia are mature. Of these four genes, mutants lacking gpr-23 have defects in aerial hyphae development (Table 1; also see below). Lastly, Group 4 genes have low levels of expression early during colony development, with most increasing from 3−21 hr and then falling again at 27 hr (Figure 4A). This group includes two genes (gpr-16 and gpr-39) that are required for normal aerial hyphae development (Table 1).
Expression data for a time course of asexual development was mined from microarray data (Greenwald et al. 2010) (Figure 4B). RNA was extracted from the entire colony at 0, 2, 4, 8, 10, and 24 hr. At 12 hr, the basal hyphae (bottom) were collected separately from the upper portion of the colony containing aerial hyphae and conidia. This was done to detect expression of genes in the cell types that give rise to aerial hyphae and mature conidia. Only the top of the colony was analyzed at 14 and 18 hr, to focus on genes expressed in aerial hyphae and conidia. We were able to detect expression of four GPCR genes (gpr-8, gpr-23, gpr-17, and nop-1) in this dataset (Figure 4B).
Mutants lacking gpr-23 have a defect in both hyphal growth and asexual development (Table 1). Therefore, it is of interest that gpr-23 expression is highest during the early stages of colony establishment from 0 to 4 hr, the time during which aerial hyphae production is initiated. As supported by previous work (Bieszke et al. 2007), nop-1 is a late-stage conidiation gene with high transcript levels from 12 to 24 hr in aerial hyphae tissue (Greenwald et al. 2010). Δnop-1 mutants have defects in aerial hyphae development (Table 1).
We next analyzed RNAseq expression data for strains grown in two different carbon sources: sucrose and crystalline cellulose (Avicel) (Figure 5) (Coradetti et al. 2012). Sucrose is the carbon source used for VM medium and, with the exception of testing growth on Avicel (see below), was present in all media used for phenotypic analysis in our study. We detected expression for 40 GPCR genes in the dataset (Figure 5). Since we had tested available mutants for increased or decreased growth relative to wild type on Avicel (Table 2), we were able to compare growth phenotypes and gene expression for numerous GPCR genes.
The majority of GPCR genes (24) were expressed to greater levels on Avicel relative to sucrose (Figure 5). Among these, 10 genes (nop-1, gpr-3, gpr-8, gpr-11, gpr-18, gpr-24, gpr-30, gpr-31, gpr-34, and gpr-39) had mean expression levels greater than 200 reads per kilobase per million mapped reads on Avicel (Figure 5). From this highly expressed group, nop-1 had the greatest fold difference of 166, whereas gpr-34 was second, with a 32-fold difference comparing Avicel vs. sucrose. Of interest, 60% of the genes with the greatest expression levels on Avicel are annotated as Pth11-related. As described previously, our analysis revealed that three GPCR mutants (Δgpr-32, Δgpr-36, and Δgpr-39; all Pth11-related class) had a phenotype during growth on Avicel (Table 2). mRNA levels for all three of these genes are greater on Avicel than sucrose (Figure 5), with gpr-39 levels increased sixfold on Avicel vs. sucrose). These observations suggest that the other GPCRs that are highly expressed on Avicel may be required for growth on this carbon source, but that gene redundancy is masking phenotypes in single mutants. This is of particular interest for the Pth11-related group, as 14 out of the 22 detected genes are expressed to higher levels on Avicel than sucrose (Figure 5) and this class of GPCRs has the most members that are required for normal growth on Avicel.
There are nine genes (gpr-9, gpr-10, orp-1, gpr-17, gpr-22, gpr-23, gpr-29, gpr-35, and gpr-37) for which the mRNA amount is at least twofold greater during growth on sucrose vs. Avicel (Figure 5). This set includes the two mPR-like GPCRs in N. crassa (gpr-9 and gpr-10; Table 1). In particular, when cultured on sucrose, gpr-10 is the most highly expressed GPCR in the dataset. Δgpr-10 (and Δgpr-9) mutants had no growth or developmental phenotypes on VM medium, perhaps reflecting gene redundancy between these two mPR-like GPCRs in N. crassa.
Phylogenetic analysis of N. crassa Pth11-related GPCRs
The 25 N. crassa Pth11-related GPCR protein sequences were aligned with the M. oryzae Pth11 protein sequence (MG05871) (see the section Materials and Methods). The alignment was adjusted manually to account for conservation of the CFEM domains present in N. crassa GPR-25 and M. oryzae Pth11. The CFEM domain consists of a conserved sequence containing eight cysteines that is proposed to play an important role in fungal pathogenesis (Kulkarni et al. 2003). GPR-25 is the only N. crassa GPCR with a CFEM domain and is the protein most similar to M. oryzae Pth11 in N. crassa (Figure 6).
A phylogenetic tree was generated from the alignment data (Figure 6). A parsimony tree was built in the original M. oryzae-N. crassa analysis (Kulkarni et al. 2005), so a similar approach was taken with the N. crassa sequences in this study. The tree revealed seven distinct groups among the N. crassa Pth11-related proteins. In Group 1, three Pth11-related genes were highly expressed on Avicel (expression greater than 200 FPKM; gpr-18, gpr-30, and gpr-34; Figure 5). A mutant was not available for gpr-18, and the Δgpr-30 was normal, but the Δgpr-32 strain had a phenotype when grown on Avicel. Therefore, not only does Group 1 have protein-based homology, but its members may function in processing Avicel.
The tree revealed eight clusters, each containing two closely related proteins. Inspection of the characteristics of several of these pairs revealed shared patterns of gene expression and/or phenotypes that suggest overlapping functions. For example, gpr-18 and gpr-32 cluster together in Group 1. Both genes are more highly expressed on Avicel relative to sucrose (Figure 5); are similarly expressed during sexual development (Group 5; Figure 3); and are in neighboring expression clusters for colony development (Groups 3 and 4; Figure 4A). Although the mutant for gpr-18 was not available, Δgpr-32 mutants have a phenotype on Avicel (Table 2).
gpr-36 and gpr-24 are related proteins in Group 3. Both genes are more highly expressed on Avicel than sucrose (Figure 5) but have differing patterns of expression during sexual development (Figure 3). Δgpr-36 has a phenotype on Avicel, whereas Δgpr-24 has a chemical phenotype on fludioxonil (Table 1). gpr-15 and gpr-29 branch together in Group 3. These two genes have similar patterns of expression during sexual development (Figure 3) but exhibit opposite trends during growth on sucrose vs. Avicel (Figure 5). Importantly, although Δgpr-15 and Δgpr-29 mutants both have reduced linear growth rates, Δgpr-15 uniquely possesses defects in sexual development and Δgpr-29 in aerial hyphae extension (Table 1). These results may indicate that these two genes have overlapping functions not only during linear hyphal growth, but also possibly asexual and sexual development.
gpr-22 and gpr-23 are another closely related pair in Group 3. Both genes are more highly expressed on sucrose than Avicel (Figure 5), are in the same expression group during sexual development (Group 2; Figure 3), and share phenotypic defects in aerial hyphae development (Table 1). Expression of gpr-23, but not gpr-22, was detected during the time course of asexual development in two microarray experiments (Figure 4, A and B). These results strongly suggest a role for these two genes in asexual development and Avicel utilization.
gpr-16 and gpr-39 comprise Group 6. These genes are coexpressed during the time courses for sexual development and colony growth (Figure 3; Figure 4A). Both mutants have defects in aerial hyphae development, coinciding with their expression later during colony development (Table 1; Figure 4A). Of interest, these two proteins are the most closely related to GPR-25, the Pth11 homolog in N. crassa. As mentioned previously, Δgpr-25 mutants are resistant to the actin depolymerizing agent cytochalasin A. The observation of functions for gpr-16 and gpr-39 in aerial hyphae development may indicate similarities in regulation of this process and the infectious appressorium in M. oryzae by G protein signaling. Furthermore, the phenotype of Δgpr-25 mutants may indicate a role for actin polymerization in this process.
Our phylogenetic results have some similarities but also differ from those of Kulkarni et al. 2005, who performed analysis using all M. oryzae and N. crassa Pth11-related proteins. As mentioned previously, GPR-25 is the closest N. crassa homolog to M. oryzae Pth11 in both studies (Kulkarni et al. 2005) (Figure 6). In addition, GPR-16 (NCU02903) and GPR-39 (NCU09823) are the nearest N. crassa relatives to N. crassa GPR-25 (Figure 6). However, there are several instances of closely related pairs of N. crassa proteins that did not cluster together in the Kulkarni study (e.g., GPR-18 and GPR-32; GPR-24 and GPR-36), possibly due to the absence of the M. oryzae proteins in our analysis or the different method used to produce the tree.
Discussion
We have analyzed available mutants for annotated GPCR genes in N. crassa. Of the 36 available mutants, 29 (81%) exhibited at least one phenotypic defect, and 15 of these are members of the Pth11-related class. In addition, 59% (10/17) of the GPCR genes with a phenotype in one the three major growth/developmental pathways analyzed were Pth11-like. Specifically, 17% (1/6) of sexual development, 57% (8/14) of aerial hyphae and 100% (5/5) of hyphal growth mutants lacked Pth11-related genes, revealing the functional importance of this group, particularly for hyphal growth and aerial hyphae development.
A recent study of GPCR knockout mutants in the filamentous fungal pathogen Aspergillus flavus analyzed 15 mutants in Groups I-IX (Affeldt et al. 2014). N. crassa has 14 genes in these groups. The A. flavus study did not include Groups X-XIII or the Pth11-related class (named class XIV in our study), corresponding to 31 genes in N. crassa and the majority of predicted GPCRs in filamentous fungi. Although N. crassa mutants for groups VII and XIII were not available for our study, we are the first to systematically analyze mutants in the Pth11-related class of predicted GPCR genes in a filamentous fungus. Only a small subset of the phenotypic analyses addressed in the A. flavus study overlapped with those in our experiments, including hyphal extension on sucrose-containing medium and sensitivity to hydrogen peroxide and sodium chloride (Affeldt et al. 2014). Inspection of the data showed there was no overlap in the three phenotypes for the corresponding A. flavus and N. crassa mutants. It is difficult to draw strong conclusions from such a small set of phenotypes, and further experimentation is necessary to investigate evolutionarily conserved functions for predicted GPCRs in the two species.
Our results with GPCR genes contrast with those obtained for analysis of other N. crassa gene groups implicated in early steps in signal transduction in eukaryotes: serine-threonine protein kinases and serine-threonine and tyrosine protein phosphatases (Park et al. 2011b; Ghosh et al. 2014). The number of predicted GPCRs (43) is intermediate between serine-threonine protein kinases (77) and protein phosphatases (30). The fraction of GPCR mutants with a growth rate phenotype (14%) is much lower than that observed for kinase (42%) and phosphatase (50%) mutants. This result suggests either that GPCRs are not critical for growth rate regulation or the presence of gene redundancy. Likewise, no single GPCR mutant was absolutely required for female fertility in N. crassa in both mating types, whereas complete female sterility was observed for 39 of the kinase and four of the phosphatase mutants. In contrast to the results for growth rate and sexual cycle phenotypes, the proportion of predicted GPCR mutants with defects in asexual development (39%) is similar to that observed for protein kinase mutants (40%), but less than that for protein phosphatase (58%) mutants. In terms of the fraction of mutants with at least one growth, developmental or chemical/nutritional phenotype, GPCRs are intermediate (81%) between the phosphatases (100%) and kinases (71%). Closer inspection of phenotype classes indicates that in comparison with GPCRs, a larger proportion of kinase and phosphatase mutants are multiply defective in the three major growth/developmental pathways. This result may reflect greater gene redundancy in the GPCRs and/or their involvement in functions that were not assayed during our investigations.
We used existing RNA profiling data from three datasets to mine expression trends for GPCR genes during perithecial development, colony growth, conidiation and growth on Avicel. We were able to analyze 37 of 43 genes (86%) from the sexual development time course, 20 of 43 (47%) in the colony establishment dataset, 4 of 43 (9%) during the conidiation time course, and 40 of 43 genes during expression on Avicel vs. sucrose (93%). We observed several instances in which the expression and phenotypic data appeared to overlap. From the colony development time course data, we noted that the CRL GPCR gpr-2 is expressed in the older parts of the colony (Figure 4A). Consistent with this observation, Δgpr-2 mutants have defects in aerial hyphae development (Table 1). During the conidiation time course, we saw that mRNA levels for the Pth11-like GPCR gene gpr-23 are greatest during the time aerial hyphae production is initiated (Figure 4B), and mutants lacking this gene have defects in basal hyphae extension and aerial hyphae development (Table 1).
We also noted instances in which only one gene in a larger group of coexpressed genes yielded a phenotype for that process. For example, during the sexual development time course, although the majority of Group 5 genes are highly expressed at the time of perithecial maturation (Figure 3), only two genes in this group, the CRL gpr-2 and the pheromone receptor pre-1, have sexual development defects (Table 1). The finding that the other 10 genes have no obvious sexual cycle phenotype suggests that at least some of them may be functionally redundant.
As previously mentioned, 20 genes were available for expression analysis on Avicel and sucrose. Of interest, 14 of the 20 available (70%) were Pth11-class genes that were expressed to higher levels on Avicel than sucrose. This observation, along with the fact that all three of the mutants (Δgpr-32, Δgpr-36, and Δgpr-39) with defects during growth on Avicel were Pth11-related genes, suggests that this group of predicted GPCRs may function in sensing or utilization of plant-derived biomass. In the phylogenetic tree (Figure 6) Group 1 was particularly noteworthy, as three of the genes (gpr-34, gpr-30, and gpr-18) were highly expressed on Avicel and one member (not highly expressed; gpr-32) had a phenotype on Avicel. We hypothesize that construction and analysis of strains carrying multiple knockout mutations in Group 1 genes may reveal additional genes with phenotypes on Avicel.
The identification of GPCR mutants with resistance to cytochalasin A may be related to the requirement for actin during endocytic recycling of GPCRs [reviewed in (Engqvist-Goldstein and Drubin 2003)]. Presumably, loss of a GPCR that is actively endocytosed during hyphal growth in wild type would result in greater tolerance to an actin destabilizing agent, such as cytochalasin A. Alternatively, these GPCRs may be involved in signaling pathways that direct the relative amount or positioning of actin cables or patches during hyphal growth (Berepiki et al. 2011).
In this study we examined the expression and functions of predicted GPCR genes during growth and development in N. crassa. Although none of the four transcriptomic data sets included data for every predicted GPCR, all contained data for the gene in at least one unavailable mutant. We obtained expression data for many GPCR genes that did not yield a phenotype when mutated. In several cases, closely related genes shared expression patterns, but only one mutant had a phenotype, suggesting overlapping functions for the two genes. These findings can be addressed in the future through construction of strains carrying multiple GPCR gene knockouts. Special interest was taken in the Pth11-related class due to its influence on asexual development and hyphal growth. This study has expanded knowledge of a gene family related to a pathogenicity locus in a fungal plant pathogen using an easily tractable non-pathogenic fungal species.
Supplementary Material
Acknowledgments
L.M. Davidson, A.H. Barsoum, J. Cao, R. Castillo, W.-C. Chen, A. Dinkchian, S. Kim, S.M. Kitada, T.H. Lai, A. Mach, C. Malekyan, T.R. Moua, C. Rojas Torres, and A. Yamamoto were undergraduates who performed this research as students during the Experimental Microbiology (MCBL125) course at the University of California Riverside. We thank members of the Borkovich laboratory, Jason Stajich, and Gloria Turner for many helpful discussions. We acknowledge Michael Plamann, Kevin McCluskey, and Aric Wiest at the Fungal Genetics Stock Center for maintenance of N. crassa knockout strains. Fludioxonil was a gift from Frank Wong and Allison Tally. This work was partially supported by GM068087 and GM086565 to K.A.B.
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.020974/-/DC1
Communicating editor: J. C. Dunlap
Literature Cited
- Affeldt K. J., Carrig J., Amare M., Keller N. P., 2014. Global survey of canonical Aspergillus flavus G protein-coupled receptors. MBio 5: e01501–e01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood T. K., Findlay J. B., 1994. Fingerprinting G-protein-coupled receptors. Protein Eng. 7: 195–203. [DOI] [PubMed] [Google Scholar]
- Baasiri R. A., Lu X., Rowley P. S., Turner G. E., Borkovich K. A., 1997. Overlapping functions for two G protein alpha subunits in Neurospora crassa. Genetics 147: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. L., Loros J. J., Dunlap J. C., 2012. The circadian clock of Neurospora crassa. FEMS Microbiol. Rev. 36: 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden W. J., Larrondo L. F., Froehlich A. C., Shi M., Chen C. H., et al. , 2007. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 21: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berepiki A., Lichius A., Read N. D., 2011. Actin organization and dynamics in filamentous fungi. Nat. Rev. Microbiol. 9: 876–887. [DOI] [PubMed] [Google Scholar]
- Bieszke J., Li L., Borkovich K., 2007. The fungal opsin gene nop-1 is negatively-regulated by a component of the blue light sensing pathway and influences conidiation-specific gene expression in Neurospora crassa. Curr. Genet. 52: 149–157. [DOI] [PubMed] [Google Scholar]
- Bieszke J. A., Braun E. L., Bean L. E., Kang S., Natvig D. O., et al. , 1999a The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc. Natl. Acad. Sci. USA 96: 8034–8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszke J. A., Spudich E. N., Scott K. L., Borkovich K. A., Spudich J. L., 1999b A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry 38: 14138–14145. [DOI] [PubMed] [Google Scholar]
- Bistis G. N., 1981. Chemotropic Interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73: 959–975. [Google Scholar]
- Bock A., Kostenis E., Trankle C., Lohse M. J., Mohr K., 2014. Pilot the pulse: controlling the multiplicity of receptor dynamics. Trends Pharmacol. Sci. 35: 630–638. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Alex L. A., Yarden O., Freitag M., Turner G. E., et al. , 2004. Lessons from the genome sequence of Neurospora crassa: Tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68: 1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutierrez S., Silla-Martinez J. M., Gabaldon T., 2009. TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B., Parenti M., Poyner D. R., Wheatley M., 2013. G-protein-coupled receptors: from structural insights to functional mechanisms. Biochem. Soc. Trans. 41: 135–136. [DOI] [PubMed] [Google Scholar]
- Chung K. S., Won M., Lee S. B., Jang Y. J., Hoe K. L., et al. , 2001. Isolation of a novel gene from Schizosaccharomyces pombe: stm1+ encoding a seven-transmembrane loop protein that may couple with the heterotrimeric Galpha 2 protein, Gpa2. J. Biol. Chem. 276: 40190–40201. [DOI] [PubMed] [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., 1987. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 105: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradetti S. T., Craig J. P., Xiong Y., Shock T., Tian C., et al. , 2012. Conserved and essential transcription factors for cellulase gene expression in ascomycete fungi. Proc. Natl. Acad. Sci. USA 109: 7397–7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., deSerres F. J., 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 71A: 79–143. [Google Scholar]
- Davis R. H., Perkins D. D., 2002. Timeline: Neurospora: a model of model microbes. Nat. Rev. Genet. 3: 397–403. [DOI] [PubMed] [Google Scholar]
- DeZwaan T. M., Carroll A. M., Valent B., Sweigard J. A., 1999. Magnaporthe grisea Pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell 11: 2013–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J. C., Borkovich K. A., Henn M. R., Turner G. E., Sachs M. S., et al. , 2007. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv. Genet. 57: 49–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E., Drubin D. G., 2003. Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19: 287–332. [DOI] [PubMed] [Google Scholar]
- Felsenstein J., 1989. PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics 5: 164–166. [Google Scholar]
- Fredriksson R., Lagerstrom M. C., Lundin L. G., Schioth H. B., 2003. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63: 1256–1272. [DOI] [PubMed] [Google Scholar]
- Galagan J. E., Calvo S. E., Borkovich K. A., Selker E. U., Read N. D., et al. , 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422: 859–868. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Servin J. A., Park G., Borkovich K. A., 2014. Global analysis of serine/threonine and tyrosine protein phosphatase catalytic subunit genes in Neurospora crassa reveals interplay between phosphatases and the p38 Mitogen-activated protein kinase. G3 (Bethesda) 4: 349–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass N. L., Rasmussen C., Roca M. G., Read N. D., 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12: 135–141. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Velazquez W., Gonzalez-Mendez R., Rodriguez-del Valle N., 2012. Characterization and ligand identification of a membrane progesterone receptor in fungi: existence of a novel PAQR in Sporothrix schenckii. BMC Microbiol. 12: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald C. J., Kasuga T., Glass N. L., Shaw B. D., Ebbole D. J., et al. , 2010. Temporal and spatial regulation of gene expression during asexual development of Neurospora crassa. Genetics 186: 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber S., Omann M., Zeilinger S., 2013. Comparative analysis of the repertoire of G protein-coupled receptors of three species of the fungal genus Trichoderma. BMC Microbiol. 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansberg W., de Groot H., Sies H., 1993. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic. Biol. Med. 14: 287–293. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Melles S., 1983. Genetic Analysis of phototropism of Neurospora crassa perithecial beaks using white collar and albino mutants. Plant Physiol. 72: 996–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. L., Howe H. B., Jr, Roth I. L., 1975. Scanning electron microscopy of surface and internal features of developing perithecia of Neurospora crassa. J. Bacteriol. 122: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer M. E., Fung E., Wildenhain J., Pierce S. E., Hoon S., et al. , 2008. The chemical genomic portrait of yeast: Uncovering a phenotype for all genes. Science 320: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inadome H., Noda Y., Adachi H., Yoda K., 2005. Immunoisolaton of the yeast Golgi subcompartments and characterization of a novel membrane protein, Svp26, discovered in the Sed5-containing compartments. Mol. Cell. Biol. 25: 7696–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey F. D., Hodge P. N., Turner G. E., Borkovich K. A., 1996. The G alphai homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7: 1283–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrzebska B., 2013. GPCR: G protein complexes–the fundamental signaling assembly. Amino Acids 45: 1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga T., Glass N. L., 2008. Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot. Cell 7: 1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Akamatsu Y., Sakuraba Y., Inoue H., 2004. The Neurospora crassa mus-19 gene is identical to the qde-3 gene, which encodes a RecQ homologue and is involved in recombination repair and postreplication repair. Curr. Genet. 45: 37–44. [DOI] [PubMed] [Google Scholar]
- Kays A. M., Borkovich K. A., 2004. Severe impairment of growth and differentiation in a Neurospora crassa mutant lacking all heterotrimeric Galpha proteins. Genetics 166: 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays A. M., Rowley P. S., Baasiri R. A., Borkovich K. A., 2000. Regulation of conidiation and adenylyl cyclase levels by the Galpha protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20: 7693–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Borkovich K. A., 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52: 1781–1798. [DOI] [PubMed] [Google Scholar]
- Kim H., Borkovich K. A., 2006. Pheromones are essential for male fertility and sufficient to direct chemotropic polarized growth of trichogynes during mating in Neurospora crassa. Eukaryot. Cell 5: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Metzenberg R. L., Nelson M. A., 2002. Multiple functions of mfa-1, a putative pheromone precursor gene of Neurospora crassa. Eukaryot. Cell 1: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Wright S. J., Park G., Ouyang S., Krystofova S., et al. , 2012. Roles for receptors, pheromones, G proteins, and mating type genes during sexual reproduction in Neurospora crassa. Genetics 190: 1389–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., et al. , 1988. A chemoattractant receptor controls development in Dictyostelium discoideum. Science 241: 1467–1472. [DOI] [PubMed] [Google Scholar]
- Kolde, R., 2015 pheatmap: Pretty Heatmaps. Available at: https://cran.r-project.org/web/packages/pheatmap/index.html. Accessed October 22, 2015.
- Krishnan A., Almen M. S., Fredriksson R., Schioth H. B., 2012. The origin of GPCRs: identification of mammalian like Rhodopsin, Adhesion, Glutamate and Frizzled GPCRs in fungi. PLoS One 7: e29817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystofova S., Borkovich K. A., 2005. The heterotrimeric G-protein subunits GNG-1 and GNB-1 form a Gbetagamma dimer required for normal female fertility, asexual development, and Galpha protein levels in Neurospora crassa. Eukaryot. Cell 4: 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystofova S., Borkovich K. A., 2006. The predicted G-protein-coupled receptor GPR-1 is required for female sexual development in the multicellular fungus Neurospora crassa. Eukaryot. Cell 5: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. D., Kelkar H. S., Dean R. A., 2003. An eight-cysteine-containing CFEM domain unique to a group of fungal membrane proteins. Trends Biochem. Sci. 28: 118–121. [DOI] [PubMed] [Google Scholar]
- Kulkarni R. D., Thon M. R., Pan H., Dean R. A., 2005. Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol. 6: R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon A., Han K. H., Seo J. A., Yu J. H., d’Enfert C., 2006. G-protein and cAMP-mediated signaling in aspergilli: a genomic perspective. Fungal Genet. Biol. 43: 490–502. [DOI] [PubMed] [Google Scholar]
- Li L., Borkovich K. A., 2006. GPR-4 is a predicted G-protein-coupled receptor required for carbon source-dependent asexual growth and development in Neurospora crassa. Eukaryot. Cell 5: 1287–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Wright S. J., Krystofova S., Park G., Borkovich K. A., 2007. Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61: 423–452. [DOI] [PubMed] [Google Scholar]
- Liu W., Xu D., Yang H., Xu H., Shi Z., et al. , 2004. Functional identification of three receptor activator of NF-kappa B cytoplasmic motifs mediating osteoclast differentiation and function. J. Biol. Chem. 279: 54759–54769. [DOI] [PubMed] [Google Scholar]
- Lyons T. J., Villa N. Y., Regalla L. M., Kupchak B. R., Vagstad A., et al. , 2004. Metalloregulation of yeast membrane steroid receptor homologs. Proc. Natl. Acad. Sci. USA 101: 5506–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende U., Zagrovic B., Cohen A., Li Y., Valenzuela D., et al. , 1998. Effect of deletion of the major brain G-protein alpha subunit alphao on coordination of G-protein subunits and on adenylyl cyclase activity. J. Neurosci. Res. 54: 263–272. [DOI] [PubMed] [Google Scholar]
- Narasimhan M. L., Coca M. A., Jin J., Yamauchi T., Ito Y., et al. , 2005. Osmotin is a homolog of mammalian adiponectin and controls apoptosis in yeast through a homolog of mammalian adiponectin receptor. Mol. Cell 17: 171–180. [DOI] [PubMed] [Google Scholar]
- Nelson M. A., 1996. Mating systems in ascomycetes: a romp in the sac. Trends Genet. 12: 69–74. [DOI] [PubMed] [Google Scholar]
- Neves S. R., Ram P. T., Iyengar R., 2002. G protein pathways. Science 296: 1636–1639. [DOI] [PubMed] [Google Scholar]
- Nordberg H., Cantor M., Dusheyko S., Hua S., Poliakov A., et al. , 2014. The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res. 42(Database issue): D26–D31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notredame C., Higgins D. G., Heringa J., 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302: 205–217. [DOI] [PubMed] [Google Scholar]
- Ochiai N., Fujimura M., Motoyama T., Ichiishi A., Usami R., et al. , 2001. Characterization of mutations in the two-component histidine kinase gene that confer fludioxonil resistance and osmotic sensitivity in the os-1 mutants of Neurospora crassa. Pest Manag. Sci. 57: 437–442. [DOI] [PubMed] [Google Scholar]
- Park G., Colot H. V., Collopy P. D., Krystofova S., Crew C., et al. , 2011a High-throughput production of gene replacement mutants in Neurospora crassa. Methods Mol. Biol. 722: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park G., Servin J. A., Turner G. E., Altamirano L., Colot H. V., et al. , 2011b Global analysis of serine-threonine protein kinase genes in Neurospora crassa. Eukaryot. Cell 10: 1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Sachs M. S., 2001. The Neurospora Compendium. Academic Press, San Diego, CA. [Google Scholar]
- Prokisch H., Yarden O., Dieminger M., Tropschug M., Barthelmess I. B., 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256: 104–114. [DOI] [PubMed] [Google Scholar]
- Raju N. B., 1992. Genetic control of the sexual cycle in Neurospora. Mycol. Res. 96: 241–262. [Google Scholar]
- Ramanujam R., Calvert M. E., Selvaraj P., Naqvi N. I., 2013. The late endosomal HOPS complex anchors active G-protein signaling essential for pathogenesis in Magnaporthe oryzae. PLoS Pathog. 9: e1003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Development Team , 2014. R: A Language and Environment for Statistical Computing. R Development Core Team, Vienna, Austria. [Google Scholar]
- Read N. D., Beckett A., 1996. Ascus and ascospore morphogenesis. Mycol. Res. 100: 1281–1314. [Google Scholar]
- Ross E. M., Wilkie T. M., 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69: 795–827. [DOI] [PubMed] [Google Scholar]
- Schioth H. B., Fredriksson R., 2005. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen. Comp. Endocrinol. 142: 94–101. [DOI] [PubMed] [Google Scholar]
- Sekito T., Nakamura K., Manabe K., Tone J., Sato Y., et al. , 2014. Loss of ATP-dependent lysine uptake in the vacuolar membrane vesicles of Saccharomyces cerevisiae ypq1 mutant. Biosci. Biotechnol. Biochem. 78: 1199–1202. [DOI] [PubMed] [Google Scholar]
- Selker E. U., 2011. Neurospora. Curr. Biol. 21: R139–R140. [DOI] [PubMed] [Google Scholar]
- Shukla A. K., Singh G., Ghosh E., 2014. Emerging structural insights into biased GPCR signaling. Trends Biochem. Sci. 39: 594–602. [DOI] [PubMed] [Google Scholar]
- Springer M. L., 1993. Genetic control of fungal differentiation: The three sporulation pathways of Neurospora crassa. BioEssays 15: 365–374. [DOI] [PubMed] [Google Scholar]
- Tesmer J. J., 2010. The quest to understand heterotrimeric G protein signaling. Nat. Struct. Mol. Biol. 17: 650–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Pang Y., Dong J., Groenen P., Kelder J., et al. , 2007. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology 148: 705–718. [DOI] [PubMed] [Google Scholar]
- Turner G. E., 2011. Phenotypic analysis of Neurospora crassa gene deletion strains. Methods Mol. Biol. 722: 191–198. [DOI] [PubMed] [Google Scholar]
- Villa N. Y., Moussatche P., Chamberlin S. G., Kumar A., Lyons T. J., 2011. Phylogenetic and preliminary phenotypic analysis of yeast PAQR receptors: potential antifungal targets. J. Mol. Evol. 73: 134–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J., 1964. Distribution of lysine pathways among fungi: Evolutionary implications. Am. Nat. 98: 435–446. [Google Scholar]
- Wang Z., Lopez-Giraldez F., Lehr N., Farre M., Common R., et al. , 2014. Global gene expression and focused knockout analysis reveals genes associated with fungal fruiting body development in Neurospora crassa. Eukaryot. Cell 13: 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard M., Mitchell H. K., 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34: 573–577. [Google Scholar]
- White B., Woodward D., 1995. A simple method for making disposable race tubes. Fungal Genet. Newsl. 42: 79. [Google Scholar]
- Willhite C. C., 1983. Benomyl. J. Appl. Toxicol. 3: 261–264. [DOI] [PubMed] [Google Scholar]
- Won S., Michkov A. V., Krystofova S., Garud A. V., Borkovich K. A., 2012. Genetic and physical interactions between Galpha subunits and components of the Gbetagamma dimer of heterotrimeric G proteins in Neurospora crassa. Eukaryot. Cell 11: 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Zhou L., Dou T., Han X., Cai Y., et al. , 2010. Genome-wide prediction of G protein-coupled receptors in Verticillium spp. Fungal Biol. 114: 359–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available from the FGSC or our laboratory upon request. File S1 contains detailed descriptions of phenotypic data.