Abstract
The PAM/Highwire/RPM-1 (PHR) proteins are signaling hubs that function as important regulators of neural development. Loss of function in Caenorhabditis elegans rpm-1 and Drosophila Highwire results in failed axon termination, inappropriate axon targeting, and abnormal synapse formation. Despite broad expression in the nervous system and relatively dramatic defects in synapse formation and axon development, very mild abnormalities in behavior have been found in animals lacking PHR protein function. Therefore, we hypothesized that large defects in behavior might only be detected in scenarios in which evoked, prolonged circuit function is required, or in which behavioral plasticity occurs. Using quantitative approaches in C. elegans, we found that rpm-1 loss-of-function mutants have relatively mild abnormalities in exploratory locomotion, but have large defects in evoked responses to harsh touch and learning associated with tap habituation. We explored the nature of the severe habituation defects in rpm-1 mutants further. To address what part of the habituation circuit was impaired in rpm-1 mutants, we performed rescue analysis with promoters for different neurons. Our findings indicate that RPM-1 function in the mechanosensory neurons affects habituation. Transgenic expression of RPM-1 in adult animals failed to rescue habituation defects, consistent with developmental defects in rpm-1 mutants resulting in impaired habituation. Genetic analysis showed that other regulators of neuronal development that function in the rpm-1 pathway (including glo-4, fsn-1, and dlk-1) also affected habituation. Overall, our findings suggest that developmental defects in rpm-1 mutants manifest most prominently in behaviors that require protracted or plastic circuit function, such as learning.
Keywords: C. elegans, PHR protein, RPM-1, habituation, learning
The PHR proteins, named for the orthologs PAM (MYCBP2) in Homo sapiens, Highwire in Drosophila melanogaster, and RPM-1 in Caenorhabditis elegans, are important regulators of neuronal development (reviewed in Po et al. 2010). PHR proteins function as intracellular signaling hubs that regulate the activity of multiple downstream signaling pathways that include: MAP kinases (Baker et al. 2015; Collins et al. 2006; Lewcock et al. 2007; Nakata et al. 2005; Nix et al. 2011; Yan et al. 2009), the PP2C phosphatase PPM-2 that inhibits the DLK-1 MAP3K in C. elegans (Baker et al. 2014), the tuberous sclerosis complex (Han et al. 2012; Murthy et al. 2004), the microtubule binding protein RAE-1 (Grill et al. 2012; Tian et al. 2011), Rab signaling (Grill et al. 2007), and beta-catenin signaling (Tulgren et al. 2014). In C. elegans, rpm-1 loss-of-function (lf) mutants have strong defects in neuronal development. The mechanosensory neurons that mediate gentle touch responses have axon termination defects (Schaefer et al. 2000), and sensitizing genetic backgrounds have been used to reveal abnormalities in axon guidance (Li et al. 2008). These neurons also have defects in synapse formation with downstream command interneurons (Schaefer et al. 2000). Similar defects in axon termination and synaptogenesis are found in motor neurons (Opperman and Grill 2014; Zhen et al. 2000). Additionally, the interneurons of rpm-1 mutants have postsynaptic defects in which glutamate receptor trafficking is abnormal (Park et al. 2009).
Despite strong developmental abnormalities, very few behavioral phenotypes caused by loss of PHR protein function have been identified in any organism. In adult Drosophila Highwire mutants, mild walking defects have been qualitatively reported (Wan et al. 2000), and altered long-term memory occurs in aversive olfactory conditioning (Huang et al. 2012). In C. elegans, rpm-1 (lf) mutants are mildly hypersensitive to paralysis induced by the acetylcholinesterase inhibitor aldicarb (Vashlishan et al. 2008). Although rpm-1 mutants are reported to move normally, rpm-1 (lf) has a synthetic synaptic phenotype with syd-2 (lf) mutants that causes impaired locomotion (Liao et al. 2004; Noma et al. 2014). The behavioral consequences of impairing Phr1 (the mouse PHR ortholog) have not been tested because Phr1 null mice die shortly after birth, presumably from reduced diaphragm innervation and impaired breathing (Bloom et al. 2007; Burgess et al. 2004; Lewcock et al. 2007).
Understanding how developmental abnormalities caused by loss of PHR protein function impact behavior is critical, if we are to achieve an overall understanding of how PHR proteins function in the nervous system. For more than a decade, we have known that RPM-1 is broadly expressed in the C. elegans nervous system and that rpm-1 (lf) mutants have pronounced defects in neuronal development (Abrams et al. 2008; Schaefer et al. 2000; Zhen et al. 2000). And yet, relatively few behavioral abnormalities have been reported in rpm-1 mutants. Previous studies have assessed spontaneous or baseline behaviors. Thus, we hypothesized that RPM-1 dependent development might be important for behaviors mediated by evoked, protracted, or plastic neuronal function. This prompted us to analyze rpm-1 mutants for three quantifiable C. elegans behaviors that are mediated by neurons that express RPM-1: 1) exploratory locomotion, a spontaneous behavior (Gray et al. 2005; Hills et al. 2004; Pierce-Shimomura et al. 1999; Zhao et al. 2003); 2) harsh touch response, a strongly evoked behavior (Chatzigeorgiou et al. 2010; Li et al. 2011; Way and Chalfie 1989); and 3) tap habituation, a form of short-term learning that results in a decreased response after repeated tap stimulation (Giles and Rankin 2009; Rankin et al. 1990). Although we observed only mild defects in exploratory locomotion in rpm-1 (lf) mutants, defects were much stronger for the harsh touch response and tap habituation. Given the severity of habituation defects in rpm-1 mutants, we explored this phenotype in more detail. We analyzed the role of RPM-1 in different cells of the tap habituation circuit and found that RPM-1 affects habituation by functioning in the mechanosensory neurons. Impaired habituation in rpm-1 mutants was not rescued by expression of RPM-1 in adulthood, which suggests that habituation defects are a developmental consequence of rpm-1 dysfunction. Overall, our findings suggest that loss of rpm-1 function has wide-ranging consequences on behavior, in particular plastic behavior such as short-term learning.
Materials and Methods
Genetics
C. elegans strains/alleles were received from the Caenorhabditis Genetics Center (unless otherwise specified) and maintained by the use of standard protocols (Brenner 1974; Stiernagle 2006). N2 was used as the wild-type reference strain. Mutant alleles were genotyped by polymerase chain reaction and analyzed by product size or digestion with restriction endonucleases. Genotyping was described previously for all alleles with the exception of the following alleles: 1) rpm-1 (ok364) used forward = GAGTTGGGCTGTATGGAGGA, reverse = GACGGAATTTCGTTGGAGAA, internal = TGGAATGGATTCTGGTGGAT primers for wild-type amplicon = 1590 bp and mutant = 753 bp; 2) pha-1 (e2123) used forward = CAATCGACTGGAGCTTCGTG, and reverse = GTTGTCGCGCACTACTGAATC primers and was digested with AccI restriction endonuclease (New England BioLabs) for wild-type product = 934 bp and mutant = 427 bp and 507 bp. Other alleles included: rpm-1 (ju44), dlk-1 (tm4024), glo-4 (ok623), and fsn-1 (hp1).
Transgenics
Transgenic animals were created by injecting mixtures of purified plasmid DNA into the gonad of young adult hermaphrodites (Mello et al. 1991). Injected worms carried the pha-1 temperature-sensitive, lethal mutant allele (e2123) and the injection mixture contained pBX (wild-type pha-1 locus; 20 ng/μL). This allowed positive selection of transgenic progeny using a restrictive temperature (Granato et al. 1994). PHA-1 is only needed early in development, so positive selection was performed at 23° for the first 16 hr after egg-lay. A plasmid carrying Pmyo-2mCherry (1 ng/μL), which induces red fluorescence in the pharynx, also was included in injection mixtures to visually confirm positive transgenic animals. We used the following plasmids: pCZ160 (Prpm-1RPM-1), a gift from Dr. Yishi Jin (Zhen et al. 2000); pSAM13 (Pmec-3RPM-1), a gift from Dr. Michael Nonet (Schaefer et al. 2000); pOR822 (Pglr-1RPM-1), a gift from Dr. Christopher Rongo (Park et al. 2009); pCFJ90 (Pmyo-2mCherry) from Addgene; pBG-GY497 (Pmec-3GFP) and pBG-137 (Punc-25RPM-1) that were previously described (Opperman and Grill 2014). We constructed the following plasmids by using standard techniques including polymerase chain reaction, restriction endonuclease digestion (New England BioLabs), T4 ligase cloning (New England BioLabs), and TOPO-TA and Gateway cloning (Life Technologies): pBG-223 (Punc-129RPM-1) that had a promoter consisting of 4821 bp of genomic DNA upstream of the unc-129 start codon and the rpm-1 coding region and 3′ UTR from pSAM13, pBG-208 (Phsp-16.2RPM-1::GFP) that had a promoter consisting of 392 bp of genomic DNA upstream of the hsp-16.2 start codon and the RPM-1::GFP and 3′ UTR sequence from pBG-46 (Opperman and Grill 2014), and pBG-GY581 (Phsp-16.2GFP) that had the same promoter as pBG-208 and the same GFP coding sequence and unc-54 3′ UTR as pBG-GY497. Plasmids for RPM-1 expression were included in injection mixtures at 10−40 ng/μL, whereas GFP containing plasmids were included at 3−12 ng/μL to ensure comparable molar ratios of plasmids were injected. pBluescript was added to achieve a total DNA concentration of 100 ng/μL in each injection mixture. DNA concentrations were estimated based on fragment intensity after plasmid digestion, gel electrophoresis, and imaging using ethidium bromide. A 1-kb ladder (New England Biolabs) was used as a standard for quantitation.
Behavioral recording
Behavioral recordings were taken using a Multi-Worm Tracker (Swierczek et al. 2011) with a 12 Mpixel CMOS sensor camera-link camera (CSC12M25BMP19-01B; Toshiba-Teli), a lens adaptor (F-TAR2), a Rodagon-F 50-mm f/2.8 lens (0703-089-000-24; Qioptiq), and a PCIe-1433 camera-link frame grabber (781169-01; National Instruments). The camera was mounted at a distance above the assay plate so that pixel size was 23.3 µm. A region of interest was set to exclude the area close to the edge of the assay plate where conditions for tracking animals were poor. The frame rate of recordings was ∼13.5 Hz.
Exploratory locomotion
Assay plates were 60 × 15-mm Petri dishes (VWR, 25384-090) filled with nematode growth medium, but without bacteria. C. elegans avoid glycerol, so assay plates were painted with a ring of glycerol around the edge of the agar with a cotton-tipped applicator immediately prior to transferring worms onto the plate. This kept animals from migrating to locations where tracking conditions were poor.
C. elegans were cultivated at 23° and were aged 65−85 hr after egg-lay. Thirty animals were transferred from well-fed plates to assay plates by gently picking them with a platinum wire (without the use of bacteria for adhesion of worms). During the transfer, animals were briefly placed on plain agar and allowed to crawl for a few seconds to remove the majority of bacteria from their body. Transfer of 30 animals typically took 5 min, and then the behavior of all animals on the plate was immediately recorded for the following 5 min by use of the Multi-Worm Tracker (MWT).
Choreography (part of the MWT software package) was used to collect centroid positions for each frame during the final 60 sec of the 5-minute recording. The first 4 min were ignored to avoid potential effects caused by incidental stimulation during transfer and placement into the MWT. Objects that were recorded for less than 10 sec were ignored to avoid imaging artifacts. The “Reoutline” and “Respine” plugins were used in combination with the “segment” argument and the “bias” output argument to identify periods of forward and backward locomotion. Change in position between frames was calculated and then a worm’s average speed was calculated by dividing the sum of changes by the total duration across those frames. The mean of animals on a plate was weighted by the proportion of time an animal was recorded moving in a given direction during the 60-sec measurement period. These weighted means were considered single datum and multiple plates were tested per genotype (6−8). Student’s unpaired t-tests were used for statistical comparisons and reported in the results and figure legends. Statistical analysis was also verified with Mann-Whitney U-tests, which gave comparable findings regarding significance.
Harsh touch response
Harsh touch was assessed with plates similar to those used for exploratory locomotion, but plates were freshly seeded with a thin bacterial lawn. A total of 10−15 L4 animals (cultivated at 23°) were transferred to each assay plate and cultivated at 23° for 12−24 hr. To test harsh touch response, plates were carefully placed under a dissecting microscope and animals that were not near the edge of the lawn and were moving forward were touched with a platinum wire on their anterior body, just behind the pharynx. The number of body bends that the animal moved backward after the stimulus was recorded (rounded to the closest whole number). Twenty animals were tested per day, and the mean response was calculated and considered a single datum. Multiple days of experiments were tested per genotype (4−6). Student’s unpaired t-tests were used for statistical comparisons and reported in the results and figure legends. Statistical analysis was also verified with Mann-Whitney U tests, which gave comparable findings regarding significance.
Tap habituation
The tap stimulus was delivered using a computer controlled linear solenoid as described previously (Swierczek et al. 2011; Timbers et al. 2013), except with a few modifications. We used a different timer/counter card and connection block (National Instruments, PCI-6601 and CB-68LP) and a custom LabView program was written to control the solenoid using these new components.
Habituation experiments were performed similar to previous studies (Swierczek et al. 2011; Timbers et al. 2013). Assay plates were similar to those used for exploratory locomotion except 50 µL of OP50 E. coli cultured in Lysogeny Broth (LB)-Miller medium was spread onto the plate the day before use. Five gravid adults were placed on the assay plates for 3 hr to lay age-synchronized eggs (∼100). More parents were used for mutant strains or transgenic lines that had a lower egg-laying rate or survival rate. The adults were removed and the plates (with eggs) were cultivated at 20° for 86−96 hr. For experiments that included transgenic animals, all groups were cultivated at 23° for the first 16 hr for pha-1−positive selection of transgenes. Then, 30 min before testing, plates were removed from the incubator to acclimatize to room temperature (23°). Plates were secured in the MWT with a lid on the Petri plate. Animals were recorded for 400 sec. Tap stimuli began after the first 100 sec and continued 30 times at a 10-sec interstimulus interval. In some experiments (noted in the figures), 45 stimuli were presented, in which case animals were recorded for 550 sec.
Analysis of the tap response was done using Choreography (MWT analysis software) with custom written scripts (Octave). The “Reoutline” and “Respine” plugins were used in combination with the “segment” argument and the “bias” output argument to process the paths of each animal and the “MeasureReversal” plugin was used to detect reversals within 2 sec of tap stimuli. The probability of reversal in response to tap was estimated by measuring the proportion of animals that reversed within 2 sec of each stimulus. Best-fit exponential curves were fit to the probability vs. stimulus data and the habituation level phenotype was measured by estimating the asymptote as the value on the curve at the final stimulus. This value was calculated for each plate as a single datum and multiple plates were tested per genotype/condition (n = 3−6). We used Student’s t-tests to compare habituation levels between groups and reported these in the results and figure legends. When possible, we also evaluated comparisons with Mann−Whitney U-tests and found comparable significance results. Reported P-values were not corrected for multiple comparisons; however, no more than 5 comparisons were conducted for any group and most significant differences were P < 0.01, so Bonferroni corrections for five comparisons would still be significant at alpha = 0.05. For the few exceptions, we replicated the experiment but did not always show the data (e.g., habituation of glo-4 vs. rpm-1 in Figure 6B was replicated but the replication is not shown).
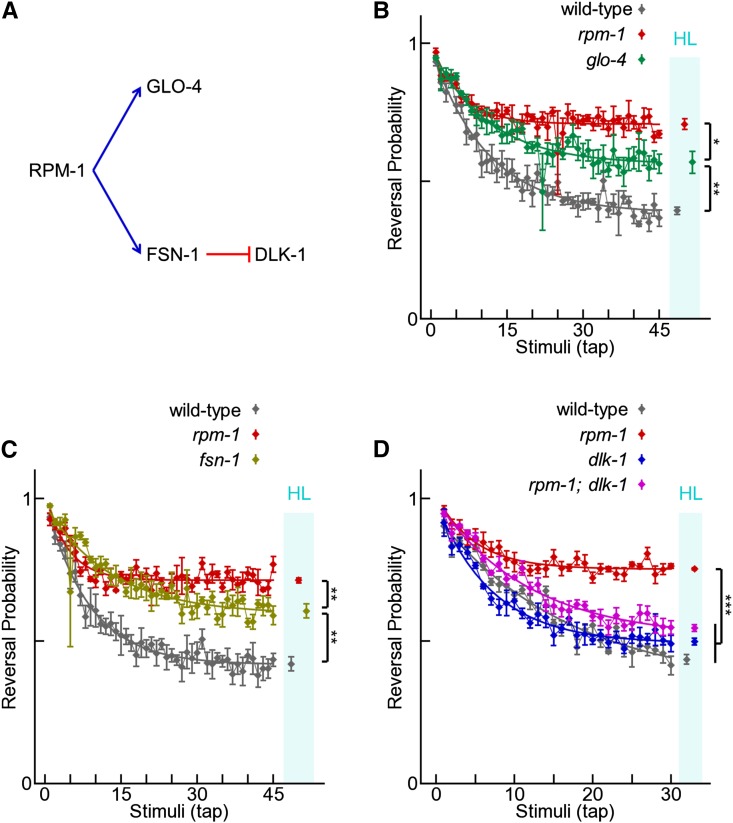
Figure 6.
Molecules that mediate RPM-1 function in neuronal development affect habituation. (A) Known mediators of RPM-1 function in development; blue arrows represent activation and red bars represent inhibition. (B−D) Tap habituation response profiles for the indicated genotypes. Connected points are means ± SEM of tap response (n = 4−6 experiments). Points in cyan bar are habituation levels (HL; asymptotes of fitted curves ± SEM). Note that glo-4 (B) and fsn-1 (C) mutants caused a strong, but partial, habituation defect compared with wild-type and rpm-1 mutants. dlk-1 (lf) strongly suppressed the habituation defect caused by rpm-1 (lf), as indicated by habituation levels in rpm-1; dlk-1 double mutants compared with rpm-1 single mutants (D). *P < 0.05; **P < 0.01; ***P < 0.001 for Student’s unpaired two-tailed t-tests between indicated groups.
Heatshock
Stable transgenic lines were selected initially using pha-1−positive selection. Once lines were isolated, they were maintained at 15° using the visual Pmyo-2mCherry marker so that temperature shifts could be used to control the heat shock promoter transgenes. For adult-specific expression experiments, 200 transgene-positive young adult animals (∼96 hr after egg-lay when cultivated at 15°) were transferred to an assay plate. The plate was placed in a 33° incubator for 2 hr and then moved to room temperature (23°) for 3 hr to recover. Animals were transferred from the heat shock plate to another assay plate (30 per plate, five plates total), allowed to recover for another hour and then tested. For the intense heat shock protocol, the animals received two sessions of 2-hour heat shock with a 30-min rest at 23° in between and animals were tested for habituation 18−24 hr later. For sustained expression throughout development, experiments were conducted as normal except the cultivation temperature was 23° for the entire time and animals were tested at 72 hr after egg-lay to account for the faster development and aging when cultivated at 23° (Byerly et al. 1976). There was no need to visually select transgene-positive animals when animals were grown at 23° because pha-1 selection occurred.
Imaging
For analysis of RPM-1::GFP, live adult animals were anesthetized using Levamisole in M9 buffer and visualized using an epifluorescent microscope (Leica CFR5000) with a 40× magnification oil-immersion lens. Images were acquired using a CCD camera (Leica DFC345 FX). Ten animals per strain were observed across two independent days with representative images shown in Figure 5.
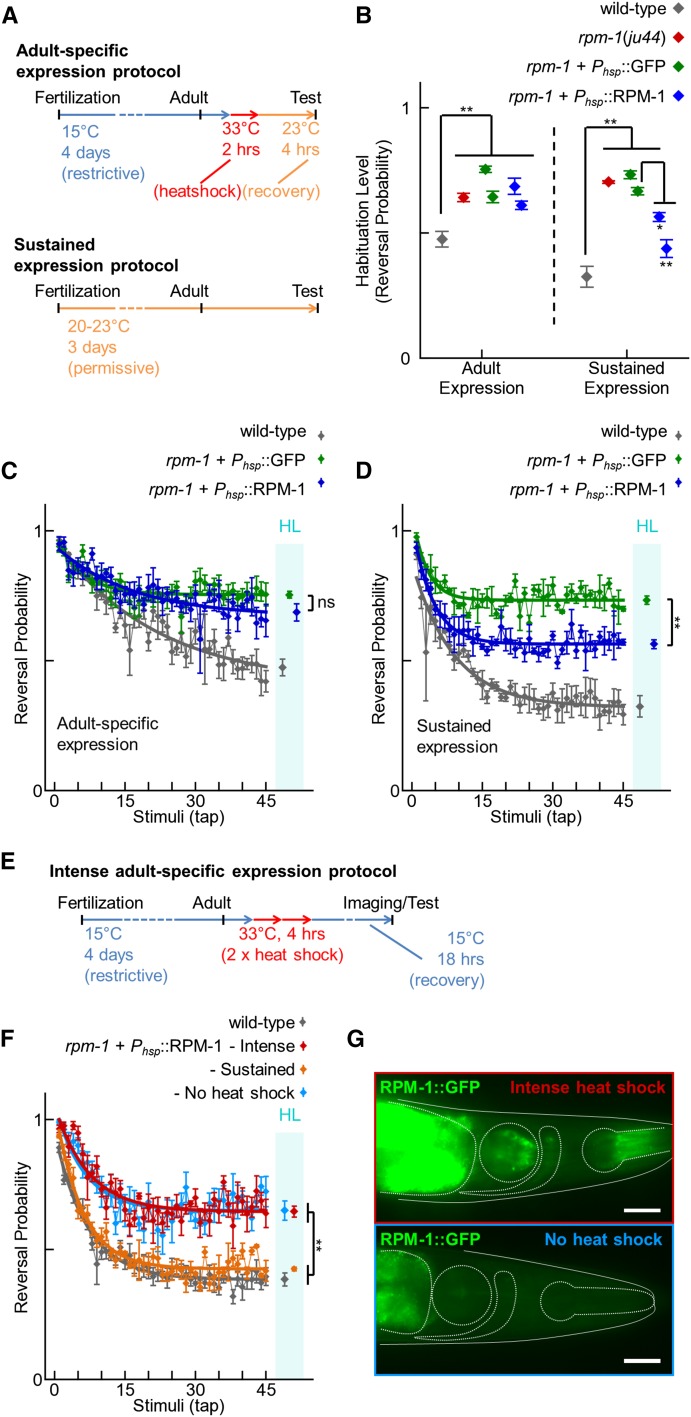
Figure 5.
Adult-specific expression of RPM-1 does not rescue habituation defects in rpm-1 mutants. (A) Schematic of the protocol for induction of adult-specific expression and sustained developmental expression of RPM-1 using the hsp-16.2 heat shock promoter. (B) Habituation of wild-type animals (gray), rpm-1 (lf) mutants (red), and rpm-1 mutants expressing GFP (green) or RPM-1 (blue) using the heat shock promoter under the indicated expression conditions. Note that adult-specific expression of RPM-1 did not rescue the habituation defect in rpm-1 mutants, whereas sustained expression of RPM-1 partially rescued the habituation defect in two independently derived transgenic lines compared with the minimum control line (asterisks). (C−D) Representative examples of complete tap habituation response profiles for the genotypes and conditions in panel B. Connected points represent the mean ± SEM of the tap response for each stimulus (n = 4−6 experiments). Smooth thick lines indicate the best-fit exponential curves. Points in the cyan bar labeled HL indicate the habituation level (asymptote of the curve ± SEM). (E) Schematic of the protocol for induction of intense adult-specific expression using the hsp-16.2 heat shock promoter. (F) Habituation of wild-type animals (gray) and rpm-1 mutants expressing RPM-1::GFP using the heat shock promoter under the indicated expression conditions (various colors). Data are represented as in (C) and (D). (G) Images of the head (solid outline) of Phsp-16.2::RPM-1::GFP positive C. elegans 18 hr after heat shock at 33° for 4 hr (top panel) or not heat shocked (bottom panel). Four internal structures are dash-outlined (from left to right): anterior gut (incomplete shape), posterior pharynx (circle), nerve ring (comma-shaped), and anterior pharynx (extended circle). Only heat shocked animals had visible RPM-1::GFP expression in the nerve ring. Stronger RPM-1::GFP fluorescence was present in the gut and pharynx. Low level autofluorescence is present in gut without heat shock (bottom panel). Scale bar is 25 μm. ns = not significant, *P < 0.05, **P < 0.01 for Student’s unpaired two-tailed t-tests between indicated groups.
Data availability
All experiments should be repeatable using the information described, links to public databases and cited literature. The following data and materials are available upon request: any strains not available from the Caenorhabditis Genetics Center, genotyping reaction details, plasmids.
Results
rpm-1 mutants have defects in exploratory locomotion
RPM-1 is broadly expressed in the nervous system (Schaefer et al. 2000; Zhen et al. 2000). With regard to the locomotion circuit, RPM-1 is expressed in the motor neurons that control body wall muscles (Zhen et al. 2000) and the glutamate-receptor-containing interneurons (Park et al. 2009) that coordinate these motor neurons (Chalfie et al. 1985). Previous work showed that rpm-1 (lf) mutants have disorganized synapses in motor neurons, but gross observation of behavior suggested that locomotion was not impaired in these animals (Nakata et al. 2005; Schaefer et al. 2000; Zhen et al. 2000). Thus, although major deficits in locomotion are not present in rpm-1 mutants, we hypothesized that synapse formation defects in these animals might lead to subtle effects on locomotion.
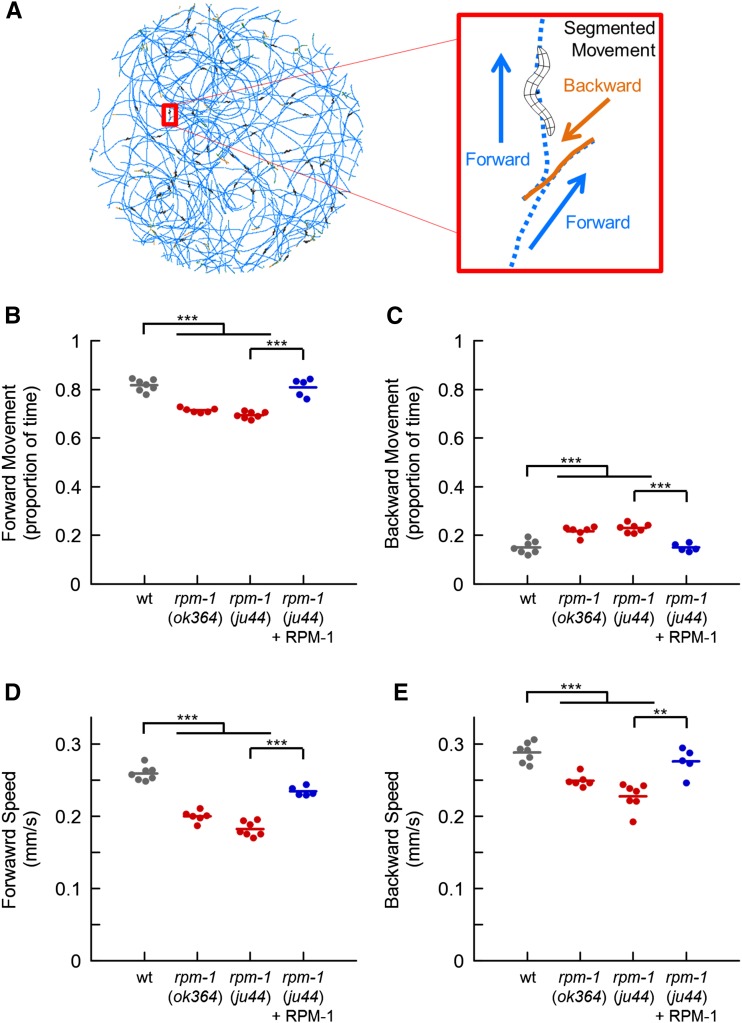
Exploratory locomotion is well characterized in C. elegans (Gray et al. 2005; Hills et al. 2004; Pierce-Shimomura et al. 1999; Zhao et al. 2003). When food is not present, animals are forced to actively explore their environment as opposed to foraging locally. During exploration, predominant forward movement is interspersed with reorientation events known as reversals. Using the MWT (Swierczek et al. 2011), we simultaneously recorded the locomotion of 30 animals on agar plates without food. Animals were analyzed 5−10 min after removal from food, so we were observing their behavior during the local search phase of exploration (Gray et al. 2005). We segmented the animals’ paths into forward and backward movement (Figure 1A). A small portion of locomotion (approximately 5%) was ignored because it could not be characterized as either forward or backward. This included periods when animals were stationary or when animals performed rapid sequential direction changes with large-angle turns that the MWT analysis could not segment properly. As expected, wild-type animals spent most of their time moving forward with small amounts of backward movement during reversals (Figure 1, B and C). We tested two loss-of-function mutants of rpm-1 for defects in locomotion. ok364 has a 2221 bp deletion that causes a frameshift a third of the way through the coding sequence (Park et al. 2009), and ju44 has a missense mutation at a conserved cysteine (Zhen et al. 2000). These alleles are thought to be nulls. Both rpm-1 mutants spent significantly less time moving forward compared with wild-type animals (Figure 1B). The reduction in time spent moving forward was reflected by increased time spent moving backward (Figure 1C), as opposed to staying stationary or performing compound/complex reorientations (data not shown). Analysis of the speed of locomotion showed that rpm-1 mutants moved 15–20% more slowly than wild-type animals during both forward and backward movement (Figure 1, D and E). rpm-1 mutants that were engineered with transgenic extrachromosomal arrays, in which the native rpm-1 promoter was used to drive RPM-1 expression, rescued the change in the proportion of time spent moving forward and backward, as well as the defect in speed (Figure 1). These results demonstrate that RPM-1 plays a role in exploratory locomotion.
Figure 1.
Loss of RPM-1 affects exploratory locomotion. (A) Multi-Worm Tracker was used to quantitatively analyze the path of 30 wild-type C. elegans during 5 min of exploratory behavior on an agar plate with no food (left); paths were segmented into forward and backward movement (right). The majority of locomotion is forward, with relatively infrequent backward movement. (B−C) Proportion of time animals spent moving forward (B) and backward (C) during the final minute of observation. Shown are wild-type animals (wt; gray), rpm-1 (lf) mutants (red), and rpm-1 mutants with a transgene that uses the native rpm-1 promoter to express RPM-1 (blue). (D−E) Average speed during the final minute of observation of forward (D) and backward (E) movement for wild-type animals (wt; gray), rpm-1 mutants (red), and rpm-1 mutants with a transgene that uses the native rpm-1 promoter to express RPM-1 (blue). Points represent the mean of 30 animals for a single independent trial. Lines represent the mean of the points for each genotype. For transgenic rescue (blue), each point represents an independently derived transgenic line. **P < 0.01; ***P < 0.001 for Student’s unpaired two-tailed t-test between indicated groups.
The harsh touch response is impaired in rpm-1 mutants
The defect in exploratory locomotion caused by loss of rpm-1 function that we uncovered was relatively mild compared with the developmental defects described previously. Therefore, we postulated that more vigorous or complex behavior (requiring stronger or protracted activation of the nervous system) might be needed to detect more pronounced behavioral defects in rpm-1 mutants. To test this, we examined a stimulus-evoked behavior, the harsh touch response.
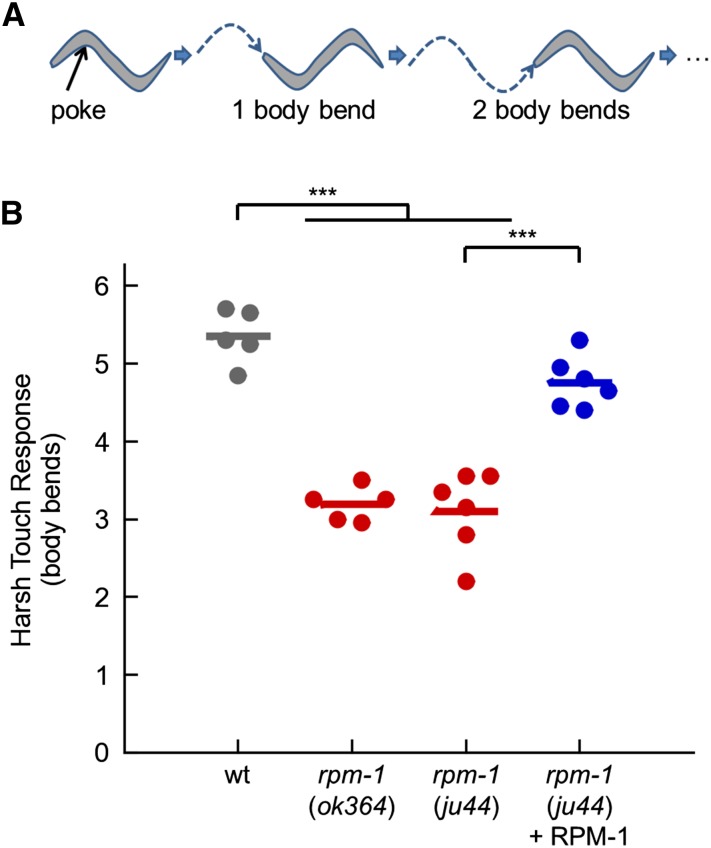
In C. elegans, harsh mechanical touch stimulates robust and extended reverse movement away from the stimulus (Chatzigeorgiou et al. 2010; Li et al. 2011; Way and Chalfie 1989). To quantitatively analyze locomotion following harsh touch, we counted the number of reverse body bends that occurred following a harsh stimulus to the anterior of the animal with a platinum wire (Figure 2A). In wild-type animals, harsh touch resulted in robust reverse movement (Figure 2B). In contrast, while all rpm-1 mutants responded to the harsh touch stimulus (n = 300), we observed a 40% reduction in reverse locomotion after harsh touch (Figure 2B). This defect was rescued with transgenic extrachromosomal arrays that express RPM-1 using the native rpm-1 promoter. These results show that RPM-1 is required for the response to harsh touch.
Figure 2.
RPM-1 is required for the harsh touch response. (A) Schematic of C. elegans harsh touch response. (B) Harsh touch responses of wild-type animals (wt; gray), rpm-1 (lf) mutants (red), and rpm-1 mutants with a transgene that uses the native rpm-1 promoter to express RPM-1 (blue). Response was measured as the number of reverse body bends an animal makes following a harsh touch stimulus to the head. Points represent the mean response of 20 animals on different experimental days. Lines represent the mean of all points within each genotype. For transgenic rescue (blue), each point represents an independently derived transgenic line. ***P < 0.001 for Student’s unpaired two-tailed t-test between indicated groups.
Loss of RPM-1 affects habituation, a simple form of learning
Behaviors that require the nervous system to be plastic and change with experience, such as learning, rely on protracted and efficient neuron and circuit function, and therefore might reveal rpm-1 defects. C. elegans is capable of a number of simple learning tasks (Ardiel and Rankin 2010; Sasakura and Mori 2013). We chose to test rpm-1 mutants for defects in habituation, a form of learning in which animals learn to ignore repeated irrelevant stimuli.
Previous work has shown that the tap stimulus, a brief knock against the side of a Petri plate that is a nonlocalized version of the gentle touch response, is ideal for assessing habituation (Giles and Rankin 2009; Rankin et al. 1990). The neural circuit that mediates the tap response has been characterized, and the neurons that form the circuit include the mechanosensory neurons, interneurons and motor neurons (Wicks and Rankin 1995). Previous work has shown that each of these types of neurons that make up the tap circuit have developmental defects in rpm-1 mutants. Thus, tap habituation was a plausible behavioral readout to test for deficits in rpm-1 mutants. Although tap activates both anterior and posterior mechanosensory neurons, which mediate reversal and acceleration respectively, the neural circuit that integrates tap input in adult animals is biased toward reversal (Wicks and Rankin 1995). As a result, 90% of naïve animals reverse in response to tap, and 10% accelerate or do not respond. An advantage of using the tap stimulus is that many worms can be stimulated at once, and the MWT can be used to record the behavior of a population of animals and detect the number of reversals in response to tap (Swierczek et al. 2011) (Figure 3A). Consistent with previous studies that examined gentle touch (Bounoutas et al. 2009; Schaefer et al. 2000), we found that rpm-1 (lf) mutants did not have a defect in the initial response to tap stimulus. In fact, we found a very mild, but statistically significant, increase in the reversal probability in response to tap (Figure 3C, initial data point mean ± SEM: wild-type = 0.916 ± 0.008, n = 6; and rpm-1(ju44) = 0.960 ± 0.014, n = 4; P = 0.042). This effect was only detected in 7 of 15 replicate experiments that had a similar statistical design (i.e., n = 3−6; data not shown). Analysis across replicates, where each n-value was the mean of one experiment, increased statistical power and provided further confidence that rpm-1 mutants had a mild, but significant, increase in reversal probability to tap compared with wild-type animals (wild-type = 0.890 ± 0.008 and rpm-1 (ju44) = 0.954 ± 0.003; n = 15 experiments with 3−6 trials per experiment; P < 10−6). In contrast, the magnitude of the tap response, measured by the duration of the response and the speed of the response, was not distinguishable from wild-type (duration mean ± SEM: wild-type = 2.74 ± 0.06 s and rpm-1 (ju44) = 2.69 ± 0.03 s, n = 15, P = 0.41; speed mean ± SEM: wild-type = 0.282 ± 0.005 mm/s and rpm-1(ju44) = 0.279 ± 0.006 mm/s, n = 15, P = 0.88). These results show that rpm-1 mutants have no deficits in registering or responding to a single tap stimulus.
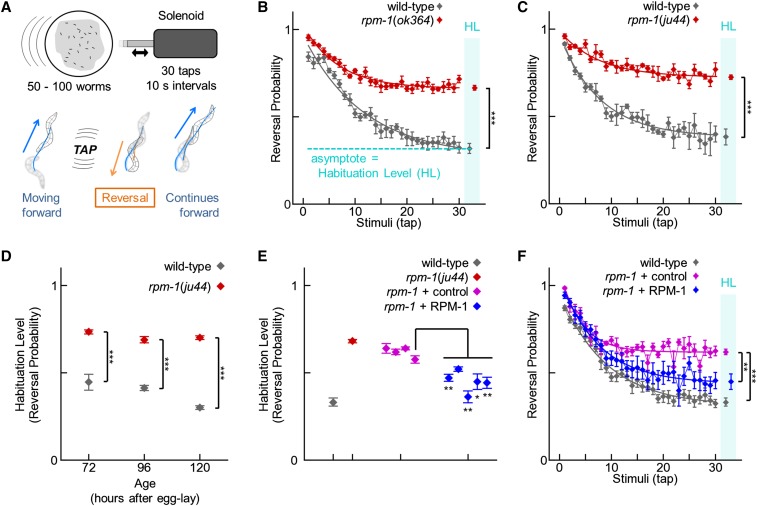
Figure 3.
Loss of RPM-1 disrupts habituation, a simple form of learning. (A) Schematic of the tap stimulus and habituation protocol (top), and the reversal response that follows a tap stimulus (bottom). (B−C) Reversal probability after each tap stimulus of wild-type animals (gray) and rpm-1 (lf) mutants (red). Connected points represent the mean ± SEM of the tap response for each stimulus (n = 3−6 experiments; each experiment consists of 50−100 animals). Smooth thick lines indicate the best-fit exponential curves. Habituation level was measured as the asymptote of the curve (plotted inside the cyan column labeled HL with ± SEM). (D) Habituation level (asymptote) of wild-type animals (gray) and rpm-1 mutants at different ages. rpm-1 mutants have a strong habituation defect at all ages tested. (E) Habituation level (asymptote) of wild-type animals (gray), rpm-1 mutants (red), four independent transgenic lines of rpm-1 mutants expressing transgenic co-injection markers (control; magenta), and five independent transgenic lines of rpm-1 mutants in which the native rpm-1 promoter is used to express RPM-1 (blue). All RPM-1−expressing lines were significantly lower than the median control line and 4/5 RPM-1 lines were significantly less than the minimum control line (asterisks). (F) Representative example of a complete tap habituation response profile for one of the transgenic lines summarized in panel E (median transgenic lines are shown). *P < 0.05; **P < 0.01; ***P < 0.001 for Student’s unpaired two-tailed t-test between indicated groups.
When presented with repeated tap stimuli (30 taps with 10 sec interstimulus intervals), wild-type C. elegans habituate (Figure 3, A and B). This is detected as an exponential decrease in the probability of response to approximately 0.4 following multiple taps. We designated the habituation level as the asymptote of the best-fit curve for a population’s responses (Figure 3B). Both rpm-1 (lf) mutants, ok364 and ju44, had a significantly higher habituation level than wild-type animals (Figure 3, B and C). The failure of rpm-1 mutants to decrease their response to tap over time indicated these animals had a defect in habituation.
The habituation level of wild-type animals decreases with age (Timbers et al. 2013). Therefore, a mutant that develops or ages slowly might appear to have a habituation defect if the animals are not staged carefully. We found that rpm-1 mutants developed and aged at a similar rate to wild-type animals as assessed by the onset of the L4 larval stage and egg-laying in synchronized populations (data not shown). To confirm that habituation defects in rpm-1 animals were not due to altered rates of aging, we tested wild-type animals and rpm-1 mutants 24 hr before and after the age at which we normally assess habituation (96 hr). We found that rpm-1 mutants had a significant defect in habituation at all ages tested (Figure 3D). This eliminates the possibility that habituation defects caused by rpm-1 (lf) were due to delayed development or aging.
Next, we tested whether the lesions in rpm-1 caused defects in habituation by performing transgenic rescues. rpm-1 (lf) mutants were engineered with transgenic extrachromosomal arrays in which RPM-1 was expressed using the endogenous rpm-1 promoter. Transgenic animals were generated using a pha-1(e2123) temperature-sensitive lethal mutant background to allow for rapid positive selection (Granato et al. 1994). This was a valuable approach when assessing hundreds of transgenic animals simultaneously using the MWT. Transgenic arrays also contained Pmyo-2mCherry, which was used to visually confirm transgenic animals. As a control, we created transgenic rpm-1 mutants that had the pha-1 mutation and transgenically expressed both PHA-1 and mCherry for selection, but lacked the rpm-1 transgene. Transgenic expression of markers alone had a mild effect on the habituation defect in rpm-1 mutants because three of four control lines had habituation levels slightly, but significantly, less than non-transgenic rpm-1 mutants (Figure 3E, not indicated on graph; P < 0.05). However, we found that the RPM-1 expressing transgenic lines significantly rescued the habituation defect beyond the level of control lines (Figure 3, E and F). These results show that tap habituation is strongly impaired in animals lacking RPM-1.
RPM-1 functions in the mechanosensory neurons to affect habituation
The neural circuit that mediates the tap response in C. elegans has been well characterized (Wicks and Rankin 1995). It is composed of mechanosensory neurons, glutamate receptor-containing interneurons, and both cholinergic and GABAergic motor neurons (Figure 4A). RPM-1 is expressed in all the neurons in this circuit (Park et al. 2009; Schaefer et al. 2000; Zhen et al. 2000). To identify the portion of the tap circuit in which RPM-1 function affects habituation, we performed transgenic rescue experiments using neuron-specific promoters that restrict expression to a subset of neurons in the tap circuit (Figure 4A). We used the mec-3 promoter for the sensory neurons (Way and Chalfie 1989), the glr-1 promoter for the interneurons (Park et al. 2009), the unc-129 promoter for the cholinergic motor neurons (Colavita et al. 1998), and the unc-25 promoter for the GABAergic motor neurons (Jin et al. 1999). We tested five independently derived transgenic lines for each promoter. As a control, we analyzed rpm-1; pha-1 animals that expressed the PHA-1 and mCherry transgenic selection markers but not RPM-1. Only expression of RPM-1 in the sensory neurons significantly rescued the habituation defect in rpm-1 mutants (Figure 4, B−F). To control for possible effects caused by the mec-3 promoter, we generated five independent transgenic lines in which rpm-1 mutants expressed GFP using the mec-3 promoter. The habituation levels in these mec-3 promoter control lines were compared with five additional independently-derived transgenic lines that expressed RPM-1 using the mec-3 promoter. As shown in Figure 4, G and H, RPM-1 expression significantly rescued the habituation defect compared with GFP expression. Taken together, our findings suggest that RPM-1 function in the mechanosensory neurons is required for normal tap habituation. However, our results do not definitively rule out the possibility that RPM-1 might function in other parts of the tap habituation circuit.
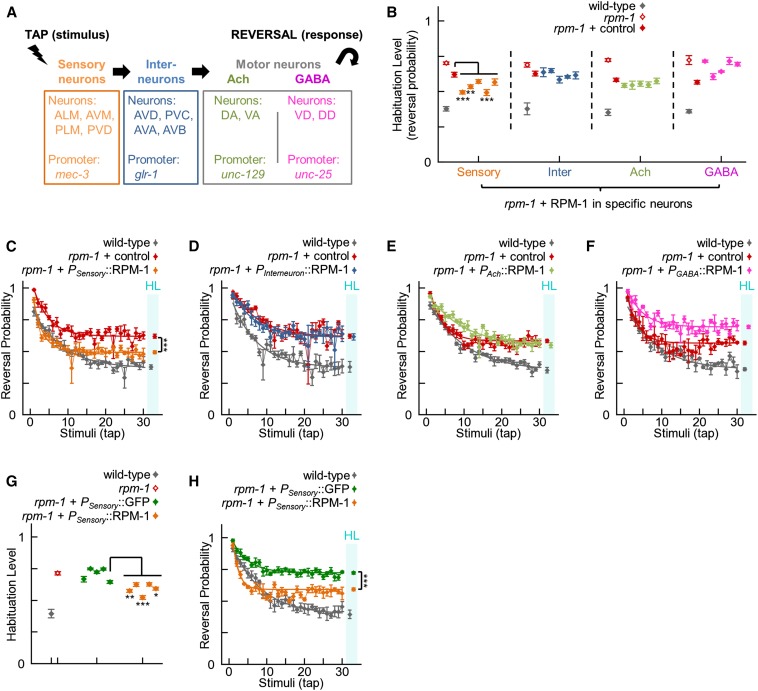
Figure 4.
RPM-1 affects habituation to tap by functioning in the sensory neurons. (A) Summary of the neural circuit that mediates the tap withdrawal response, and promoters used to drive transgene expression in different parts of the circuit. (B) Habituation level of wild-type animals (gray), rpm-1 (lf) mutants (open red), rpm-1 mutants expressing transgenic coinjection markers (control; red), and five transgenic lines of rpm-1 mutants in which RPM-1 is expressed in different neurons of the tap habituation circuit (various colors). Only expression of RPM-1 in the sensory neurons significantly rescued the mutant defect compared with the paired control line (3/5 lines, asterisks; orange vs. red). (C−F) Representative examples of complete tap habituation response profiles for the genotypes summarized in (B). Connected points represent the mean ± SEM of the tap response for each stimulus (n = 4−6 experiments). Smooth thick lines indicate the best-fit exponential curves. Points in the cyan bar labeled HL indicate the habituation level (asymptote of the curve ± SEM). Expression in the sensory neurons partially rescued the rpm-1 mutant defect (C). (G) Habituation of wild-type animals (gray), rpm-1 mutants (open red), and five transgenic lines of rpm-1 mutants using the sensory neuron promoter to express GFP (green) or RPM-1 (orange). All transgenic lines expressing RPM-1 significantly rescued habituation defects compared with the median control line, and 3/5 RPM-1 lines were significantly rescued compared with the minimum control line (asterisks). Therefore, expression of RPM-1, and not the presence of the sensory neuron promoter in transgenic arrays, was responsible for rescuing habituation defects in rpm-1 mutants. (H) Representative examples of complete tap habituation response profiles for the genotypes in panel G. For all panels, the rpm-1 allele used was ju44 and asterisks indicate transgenic lines that were significantly lower than the indicated control using Student’s unpaired two-tailed t-tests (*P < 0.05; **P < 0.01; ***P < 0.001). Note that different, independently derived transgenic lines were used for B and G, thus collectively 6/10 sensory neuron lines showed significant rescue.
RPM-1 is required throughout development for normal habituation
Although RPM-1 is an important player in neuronal development, RPM-1 continues to be expressed into adulthood, where it plays a role in axon regeneration(Hammarlund et al. 2009; Nix et al. 2011). Orthologs of RPM-1 in mouse and fly also function postdevelopmentally to regulate axon degeneration, axon regeneration, and aversive long-term memory (Babetto et al. 2013; Huang et al. 2012; Xiong et al. 2010, 2012). Although our initial hypothesis was that habituation defects in rpm-1 mutants result from abnormalities in neuronal development, it is possible RPM-1 might function in adulthood to regulate habituation. To address this, we tested whether adult-specific expression of RPM-1 could rescue the habituation defect in rpm-1 mutants.
To induce adult-specific expression of RPM-1, we used a heat shock promoter (Phsp-16.2) that allows temperature dependent induction of gene expression. Phsp-16.2 has been used previously in a variety of contexts to temporally regulate gene expression in the C. elegans nervous system (Adler et al. 2006; Flames and Hobert 2009; Kurup et al. 2015; Stringham et al. 1992). Notably, Phsp-16.2 was used to induce adult-specific expression in the mechanosensory neurons (where RPM-1 regulates habituation) and was previously used to assess the role of a gene involved in learning and memory (Ikeda et al. 2008; Xu et al. 2001). Thus, temporal control of RPM-1 expression using Phsp-16.2 was a suitable way to determine whether RPM-1 regulates habituation by functioning in adulthood.
For adult-specific expression, we cultivated transgenic animals at 15° (a restrictive temperature for Phsp-16.2) until the young adult stage and then shifted to 33° (a strongly permissive temperature) for 2 hr (Figure 5A). The studies mentioned above successfully induced Phsp-16.2 with various durations of heat shock ranging from 15 min to 4 hr. We chose 2 hr because it was the most commonly used duration and was used previously to rescue a learning phenotype (Ikeda et al. 2008). After heat shock, animals were allowed to recover for 4 hr at 20−23° and were then tested for habituation. rpm-1 (lf) mutants were engineered with extrachromosomal arrays that used Phsp-16.2 to express RPM-1 or GFP (negative control). rpm-1 mutants that expressed RPM-1 in adulthood did not rescue habituation defects compared with mutants expressing GFP (Figure 5, B and C). In contrast, when transgenic animals were cultivated at 20−23° (a mildly permissive temperature for Phsp-16.2) for their entire lifespan prior to testing, we found that animals expressing heat shock promoter driven RPM-1 had reduced defects in habituation compared with animals that expressed GFP (Figure 5, B and D). Wild-type animals habituated to tap differently when cultivated at 15° vs. 23° (Figure 5, B−D). The habituation level was slightly greater at 15° and the habituation rate (the half-life of the exponential curve) was longer at 15°. This could be the result of slight differences in aging that occur with different cultivation temperatures as habituation is sensitive to age (Timbers et al. 2013). Although we attempted to age match animals from different cultivation temperatures based on the well characterized timing of developmental and aging landmarks at various cultivation temperatures (Byerly et al. 1976), slight differences in age could still have occurred. Alternatively, the heat shock promoter could affect endogenous heat shock protein levels and modestly impair habituation. To ensure differences were not simply a failure to reach maximal habituation levels, we habituated animals to 45 stimuli instead of 30. We also confirmed that statistical power was sufficient for adult temperature shift experiments to detect a rescue proportional to the rescue observed with cultivation at 23°.
Our results suggest that adult-specific expression of RPM-1 is not sufficient to rescue the habituation defects in rpm-1 mutants. However, it was possible sufficient RPM-1::GFP expression was not occurring with our heat shock induction protocol. Therefore, we induced RPM-1::GFP expression in the stronger of our two rescuing lines with a more intense heat shock protocol (4 hr total: two sessions at 33° for 2 hr separated by a 30-min rest; Figure 5E) (Stringham et al. 1992). Even with intense heat shock induction of RPM-1::GFP, we observed no rescue of habituation defects in rpm-1 mutants (Figure 5F). Expression of RPM-1::GFP was detected in the nervous system after intense heat shock (Figure 5G). Therefore, it is unlikely that adult-specific expression of RPM-1 failed to rescue habituation defects in rpm-1 mutants because of technical issues with heat shock induced expression. Instead, it is likely that the tap habituation defect in rpm-1 mutants is a consequence of the developmental abnormalities in these animals.
Molecules that mediate RPM-1 function in development also affect habituation
Several downstream proteins that mediate the function of RPM-1 in neuronal development have been identified (Baker et al. 2014, 2015; Grill et al. 2007, 2012; Liao et al. 2004; Nakata et al. 2005; Tulgren et al. 2014; Yan et al. 2009). For example, RPM-1 positively activates GLO-4 to regulate the GLO-1 Rab GTPase (Figure 6A) (Grill et al. 2007). RPM-1 also functions in a complex with the F-box protein, FSN-1, to negatively regulate the DLK-1 MAP3K (Figure 6A) (Grill et al. 2007; Liao et al. 2004; Nakata et al. 2005). If the developmental abnormalities in rpm-1 mutants result in habituation defects, we would expect habituation to also be affected by molecules that mediate RPM-1 function in development.
RPM-1 function is mediated in part by GLO-4 and FSN-1 (Grill et al. 2007). As a result, animals that have a loss-of-function mutation in either glo-4 or fsn-1 have mild developmental defects compared with rpm-1 mutants. We tested habituation of glo-4 (ok623) and fsn-1 (hp1) and found that glo-4 mutants and fsn-1 mutants each had significantly higher habituation levels than wild-type animals, but lower levels than rpm-1 mutants (Figure 6, B and C). These results are consistent with GLO-4 and FSN-1 mediating RPM-1 function in habituation.
dlk-1 (lf) mutations strongly, but partially, suppress developmental defects in rpm-1 mutants, such as impaired axon termination and synapse formation (Grill et al. 2007; Nakata et al. 2005). We tested whether this genetic relationship also existed for habituation. dlk-1 (tm4024) mutants had similar habituation to wild-type animals, and rpm-1; dlk-1 double mutants had suppressed habituation defects compared with rpm-1 single mutants (Figure 6D). Thus, dlk-1 and rpm-1 show similar genetic relationships in the context of both habituation and neuronal development. These findings support the proposition that habituation defects in rpm-1 mutants arise from defects in neuronal development.
Discussion
After more than a decade of research, the PHR proteins have emerged as key, conserved regulators of neuronal development and axon degeneration (Conforti et al. 2014; Po et al. 2010). Numerous downstream effector proteins and pathways that mediate PHR protein function have also been discovered (Baker et al. 2014, 2015; Collins et al. 2006; Grill et al. 2007, 2012; Liao et al. 2004; Murthy et al. 2004; Nakata et al. 2005; Tian et al. 2011; Tulgren et al. 2014; Xiong et al. 2012; Yan et al. 2009). Despite this important progress, a lingering question has remained: how does the function of PHR proteins during development impact behavior? To address this question, we used a broad, quantitative approach by testing three diverse behaviors mediated by neurons known to express RPM-1: spontaneous exploratory locomotion, evoked harsh touch response, and tap habituation (a simple but critical form of short-term learning).
Using the MWT to quantitatively analyze exploratory locomotion, we observed defects in rpm-1 (lf) mutants (Figure 1). rpm-1 mutants spend slightly more time performing reversals and their speed is 15–20% slower than wild-type animals. Previous studies in Drosophila hinted at this possibility, as qualitative analysis of Highwire mutants described a mild walking defect (Wan et al. 2000) and rpm-1 mutants were previously shown to trend toward decreased locomotion (Noma et al. 2014). Our observation, combined with previous findings, suggests that loss of PHR protein function in invertebrates leads to relatively mild defects in locomotion. The role of PHR proteins in vertebrate locomotion has not been explored due to early larval lethality in fish (Odenthal et al. 1996), and death shortly after birth in mice lacking Phr1 (Burgess et al. 2004; Lewcock et al. 2007; Bloom et al. 2007). However, the motor neurons of Phr1 mutant and knockout mice have axon extension and synapse formation defects, which suggests that locomotion is likely to be impaired if Phr1 were specifically ablated in these neurons.
Given that rpm-1 mutants have abnormalities in synapse formation, but not complete loss of synaptic connections (Schaefer et al. 2000; Zhen et al. 2000), we postulated that behaviors requiring intense, protracted, or complex activity of the nervous system might be more sensitive to rpm-1 dysfunction. Consistent with this idea, rpm-1 mutants had relatively severe defects in their response to harsh touch, which requires coordinated and protracted circuit function (Figure 2). To expand on this idea, we investigated how rpm-1 mutants habituate to tap, a form of short-term learning that takes place over several minutes of repeated stimulation. We found that rpm-1 mutants had slight hypersensitivity to the initial tap stimulus, but extremely strong defects in habituation to tap (Figure 3). Overall, our results suggest that the more protracted or complex a given behavior, the more it will be impaired with loss of rpm-1 function.
Habituation defects in rpm-1 mutants were particularly pronounced. To our knowledge, rpm-1 mutants have some of the strongest defects in habituation detected to date. Importantly, a converging series of observations supported our finding that RPM-1 affects habituation including: multiple alleles of rpm-1 having habituation defects (Figure 3), rescue of habituation defects by transgenic expression of RPM-1 (Figure 3), and known mediators of RPM-1 signaling (i.e., GLO-4 and FSN-1) affecting habituation (Figure 6). Transgenic rescue using drivers that are specific for subsets of neurons in the tap circuit showed that RPM-1 affects habituation by functioning in the mechanosensory neurons (Figure 4). It should be noted that because we only performed rescues with single drivers at a time, our results do not rule out the possibility that RPM-1 also functions in a combination of other neuron types in the tap circuit. rpm-1 mutants also have synapse formation defects in the chemosensory neurons (Crump et al. 2001), which mediate simple forms of behavioral plasticity such as adaptation (Colbert and Bargmann 1995), habituation (Wen et al. 1997), and associative learning (Saeki et al. 2001; Wen et al. 1997). Thus, the effect of RPM-1 on learning might generalize beyond the tap response circuit to chemosensory responses.
Several of our findings support the conclusion that impaired habituation in rpm-1 mutants is likely to be the consequence of developmental defects in these animals. First, adult-specific expression of RPM-1 was unable to rescue habituation defects in rpm-1 (lf) mutants, whereas expression of RPM-1 throughout development rescued habituation defects (Figure 5). Second, mediators of RPM-1 function in development (i.e., GLO-4, FSN-1, and DLK-1) also affected habituation (Figure 6). Thus, our findings suggest that RPM-1 function in neuronal development is critical for normal habituation. In contrast to our results, a previous study found that Highwire, the Drosophila ortholog of RPM-1, functions in adulthood to properly gate long-term aversive memory (Huang et al. 2012). Taken together, these observations suggest two distinct roles for PHR proteins in learning and memory: 1) a role in the development of neurons, which has a critical consequence on short-term learning later in adulthood, and 2) a role in regulating the consolidation of long-term memories specifically in adulthood.
How might disrupted neuron development in rpm-1 (lf) mutants lead to defects in habituation? rpm-1 mutants have two developmental defects in their mechanosensory neurons, abnormal synapse formation and overextended axons as a result of failed axon termination (Grill et al. 2007; Schaefer et al. 2000). The chemical synapses of the mechanosensory neurons are not critical for the tap response itself, which is likely mediated by electrical synapses (Chalfie et al. 1985; Wicks and Rankin 1995). This finding is consistent with our observation that rpm-1 mutants had intact tap responses. However, one cellular mechanism of habituation is chemical synapse plasticity (Bailey and Chen 1988; Castellucci et al. 1970; Castellucci and Kandel 1974; Das et al. 2011; Davis et al. 1982; Gover et al. 2002; Krasne 1969; Lingenhohl and Friauf 1994; Rankin and Wicks 2000; Simons-Weidenmaier et al. 2006). Therefore, it is possible that impaired formation of chemical synapses in rpm-1 mutants might explain the defects in habituation we have uncovered in these animals. Another cellular mechanism of habituation is the modulation of neuron excitability (Kindt et al. 2007). Mechanosensory axons contain voltage-gated calcium channels that propagate the signal from sensory transduction (Frokjaer-Jensen et al. 2006; Goodman 2006; O’Hagan and Chalfie 2006; Suzuki et al. 2003). Therefore, it is possible that the abnormally long mechanosensory axons in rpm-1 mutants might alter neuron excitability thereby disrupting habituation. At this point, it remains difficult to determine which of these possibilities is more likely. It is also possible the dramatic habituation defects in rpm-1 mutants could stem from a combination of both developmental abnormalities.
Abnormalities in short-term learning are often associated with neurodevelopmental disorders. Specifically, habituation defects have been observed in patients with schizophrenia (Akdag et al. 2003; Bolino et al. 1992; Braff et al. 1992; Ludewig et al. 2003; Meincke et al. 2004; Parwani et al. 2000; Taiminen et al. 2000), autism (Green et al. 2015; Guiraud et al. 2011; Kleinhans et al. 2009; Lombardo et al. 2009), and fragile X syndrome (Bruno et al. 2014; Van der Molen et al. 2012). Several molecules in PHR protein signaling pathways also are associated with schizophrenia and autism, including the Tuberous Sclerosis Complex (Han et al. 2012; Murthy et al. 2004), FSN-1/Fbxo45 (Sagar et al. 2013; Wang et al. 2014), and ESS-2/DGCR14 (Noma et al. 2014). Our finding that RPM-1 and FSN-1 affect habituation, most likely through roles in neuronal development, provides behavioral evidence from a model system to support the growing genetic associations between PHR protein signaling and neurodevelopmental disorders.
Acknowledgments
We thank Drs. Michael Nonet and Chris Rongo for sharing reagents (pSAM13 and pOR822, respectively). We thank Dr. Melissa Borgen for valuable discussions regarding Highwire mutant behavior. The Caenorhabditis Genetics Center (NIH Office of Research Infrastructure Programs, P40 OD010440) provided strains, and the C. elegans Knockout Consortium generated several alleles used in this study. Wormbase was used to view C. elegans genome sequence. Our work was supported by the US National Institutes of Health (NIH; R01 NS072129 to B.G.) and the US National Science Foundation (IOS-1121095 to B.G.).
Footnotes
Communicating editor: J. K. Kim
Literature Cited
- Abrams B., Grill B., Huang X., Jin Y., 2008. Cellular and molecular determinants targeting the Caenorhabditis elegans PHR protein RPM-1 to perisynaptic regions. Dev. Dyn. 237: 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler C. E., Fetter R. D., Bargmann C. I., 2006. UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdag S. J., Nestor P. G., O’Donnell B. F., Niznikiewicz M. A., Shenton M. E., et al. , 2003. The startle reflex in schizophrenia: habituation and personality correlates. Schizophr. Res. 64: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel E. L., Rankin C. H., 2010. An elegant mind: learning and memory in Caenorhabditis elegans. Learn. Mem. 17: 191–201. [DOI] [PubMed] [Google Scholar]
- Babetto E., Beirowski B., Russler E. V., Milbrandt J., DiAntonio A., 2013. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Reports 3: 1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Chen M., 1988. Morphological basis of short-term habituation in Aplysia. J. Neurosci. 8: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. T., Opperman K. J., Tulgren E. D., Turgeon S. M., Bienvenut W., et al. , 2014. RPM-1 uses both ubiquitin ligase and phosphatase-based mechanisms to regulate DLK-1 during neuronal development. PLoS Genet. 10: e1004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. T., Turgeon S. M., Tulgren E. D., Wigant J., Rahimi O., et al. , 2015. Neuronal development in Caenorhabditis elegans is regulated by inhibition of an MLK MAP kinase pathway. Genetics 199: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom A. J., Miller B. R., Sanes J. R., DiAntonio A., 2007. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev. 21: 2593–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolino F., Manna V., Di Cicco L., Di Michele V., Daneluzzo E., et al. , 1992. Startle reflex habituation in functional psychoses: a controlled study. Neurosci. Lett. 145: 126–128. [DOI] [PubMed] [Google Scholar]
- Bounoutas A., Zheng Q., Nonet M. L., Chalfie M., 2009. mec-15 encodes an F-box protein required for touch receptor neuron mechanosensation, synapse formation and development. Genetics 183: 607–617, 1SI-4SI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff D. L., Grillon C., Geyer M. A., 1992. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry 49: 206–215. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno J. L., Garrett A. S., Quintin E. M., Mazaika P. K., Reiss A. L., 2014. Aberrant face and gaze habituation in fragile x syndrome. Am. J. Psychiatry 171: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. W., Peterson K. A., Johnson M. J., Roix J. J., Welsh I. C., et al. , 2004. Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice. Mol. Cell. Biol. 24: 1096–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Cassada R. C., Russell R. L., 1976. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev. Biol. 51: 23–33. [DOI] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R., 1970. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167: 1745–1748. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., 1974. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc. Natl. Acad. Sci. USA 71: 5004–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., et al. , 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M., Yoo S., Watson J. D., Lee W. H., Spencer W. C., et al. , 2010. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat. Neurosci. 13: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita A., Krishna S., Zheng H., Padgett R. W., Culotti J. G., 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281: 706–709. [DOI] [PubMed] [Google Scholar]
- Colbert H. A., Bargmann C. I., 1995. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14: 803–812. [DOI] [PubMed] [Google Scholar]
- Collins C. A., Wairkar Y. P., Johnson S. L., DiAntonio A., 2006. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51: 57–69. [DOI] [PubMed] [Google Scholar]
- Conforti L., Gilley J., Coleman M. P., 2014. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat. Rev. Neurosci. 15: 394–409. [DOI] [PubMed] [Google Scholar]
- Crump J. G., Zhen M., Jin Y., Bargmann C. I., 2001. The SAD-1 kinase regulates presynaptic vesicle clustering and axon termination. Neuron 29: 115–129. [DOI] [PubMed] [Google Scholar]
- Das S., Sadanandappa M. K., Dervan A., Larkin A., Lee J. A., et al. , 2011. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc. Natl. Acad. Sci. USA 108: E646–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Parisi T., Gendelman D. S., Tischler M., Kehne J. H., 1982. Habituation and sensitization of startle reflexes elicited electrically from the brain-stem. Science 218: 688–690. [DOI] [PubMed] [Google Scholar]
- Flames N., Hobert O., 2009. Gene regulatory logic of dopamine neuron differentiation. Nature 458: 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C., Kindt K. S., Kerr R. A., Suzuki H., Melnik-Martinez K., et al. , 2006. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J. Neurobiol. 66: 1125–1139. [DOI] [PubMed] [Google Scholar]
- Giles A. C., Rankin C. H., 2009. Behavioral and genetic characterization of habituation using Caenorhabditis elegans. Neurobiol. Learn. Mem. 92: 139–146. [DOI] [PubMed] [Google Scholar]
- Goodman, M. B., 2006 Mechanosensation (January 6, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.62.1, http//www.wormbook.org 1–14.
- Gover T. D., Jiang X. Y., Abrams T. W., 2002. Persistent, exocytosis-independent silencing of release sites underlies homosynaptic depression at sensory synapses in Aplysia. J. Neurosci. 22: 1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M., Schnabel H., Schnabel R., 1994. pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res. 22: 1762–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. M., Hill J. J., Bargmann C. I., 2005. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102: 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A., Hernandez L., Tottenham N., Krasileva K., Bookheimer S. Y., et al. , 2015. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry 72: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill B., Bienvenut W. V., Brown H. M., Ackley B. D., Quadroni M., et al. , 2007. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron 55: 587–601. [DOI] [PubMed] [Google Scholar]
- Grill B., Chen L., Tulgren E. D., Baker S. T., Bienvenut W., et al. , 2012. RAE-1, a novel PHR binding protein, is required for axon termination and synapse formation in Caenorhabditis elegans. J. Neurosci. 32: 2628–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud J. A., Kushnerenko E., Tomalski P., Davies K., Ribeiro H., et al. , 2011. Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport 22: 845–849. [DOI] [PubMed] [Google Scholar]
- Hammarlund M., Nix P., Hauth L., Jorgensen E. M., Bastiani M., 2009. Axon regeneration requires a conserved MAP kinase pathway. Science 323: 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Kim S., Bahl S., Li L., Burande C. F., et al. , 2012. The E3 ubiquitin ligase protein associated with Myc (Pam) regulates mammalian/mechanistic target of rapamycin complex 1 (mTORC1) signaling in vivo through N- and C-terminal domains. J. Biol. Chem. 287: 30063–30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T., Brockie P. J., Maricq A. V., 2004. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 24: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Zheng X., Zhao H., Li M., Wang P., et al. , 2012. A permissive role of mushroom body alpha/beta core neurons in long-term memory consolidation in Drosophila. Curr. Biol. 22: 1981–1989. [DOI] [PubMed] [Google Scholar]
- Ikeda D. D., Duan Y., Matsuki M., Kunitomo H., Hutter H., et al. , 2008. CASY-1, an ortholog of calsyntenins/alcadeins, is essential for learning in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105: 5260–5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., Horvitz H. R., 1999. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt K. S., Quast K. B., Giles A. C., De S., Hendrey D., et al. , 2007. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676. [DOI] [PubMed] [Google Scholar]
- Kleinhans N. M., Johnson L. C., Richards T., Mahurin R., Greenson J., et al. , 2009. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am. J. Psychiatry 166: 467–475. [DOI] [PubMed] [Google Scholar]
- Krasne F. B., 1969. Excitation and habituation of the crayfish escape reflex: the depolarizing response in lateral giant fibres of the isolated abdomen. J. Exp. Biol. 50: 29–46. [DOI] [PubMed] [Google Scholar]
- Kurup N., Yan D., Goncharov A., Jin Y., 2015. Dynamic microtubules drive circuit rewiring in the absence of neurite remodeling. Curr. Biol. 25: 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock J. W., Genoud N., Lettieri K., Pfaff S. L., 2007. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56: 604–620. [DOI] [PubMed] [Google Scholar]
- Li H., Kulkarni G., Wadsworth W. G., 2008. RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity. J. Neurosci. 28: 3595–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kang L., Piggott B. J., Feng Z., Xu X. Z., 2011. The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nat. Commun. 2: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao E. H., Hung W., Abrams B., Zhen M., 2004. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 430: 345–350. [DOI] [PubMed] [Google Scholar]
- Lingenhohl K., Friauf E., 1994. Giant-neurons in the rat reticular-formation—a sensorimotor interface in the elementary acoustic startle circuit. J. Neurosci. 14: 1176–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M. V., Chakrabarti B., Baron-Cohen S., 2009. The amygdala in autism: not adapting to faces? Am. J. Psychiatry 166: 395–397. [DOI] [PubMed] [Google Scholar]
- Ludewig K., Geyer M. A., Vollenweider F. X., 2003. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol. Psychiatry 54: 121–128. [DOI] [PubMed] [Google Scholar]
- Meincke U., Light G. A., Geyer M. A., Braff D. L., Gouzoulis-Mayfrank E., 2004. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Res. 126: 51–61. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V., Han S., Beauchamp R. L., Smith N., Haddad L. A., et al. , 2004. Pam and its ortholog highwire interact with and may negatively regulate the TSC1.TSC2 complex. J. Biol. Chem. 279: 1351–1358. [DOI] [PubMed] [Google Scholar]
- Nakata K., Abrams B., Grill B., Goncharov A., Huang X., et al. , 2005. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120: 407–420. [DOI] [PubMed] [Google Scholar]
- Nix P., Hisamoto N., Matsumoto K., Bastiani M., 2011. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc. Natl. Acad. Sci. USA 108: 10738–10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K., Goncharov A., Jin Y., 2014. Systematic analyses of rpm-1 suppressors reveal roles for ESS-2 in mRNA splicing in Caenorhabditis elegans. Genetics 198: 1101–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R., Chalfie M., 2006. Mechanosensation in Caenorhabditis elegans. Int. Rev. Neurobiol. 69: 169–203. [DOI] [PubMed] [Google Scholar]
- Odenthal J., Rossnagel K., Haffter P., Kelsh R. N., Vogelsang E., et al. , 1996. Mutations affecting xanthophore pigmentation in the zebrafish, Danio rerio. Development 123: 391–398. [DOI] [PubMed] [Google Scholar]
- Opperman K. J., Grill B., 2014. RPM-1 is localized to distinct subcellular compartments and regulates axon length in GABAergic motor neurons. Neural Dev. 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. C., Glodowski D. R., Rongo C., 2009. The ubiquitin ligase RPM-1 and the p38 MAPK PMK-3 regulate AMPA receptor trafficking. PLoS One 4: e4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwani A., Duncan E. J., Bartlett E., Madonick S. H., Efferen T. R., et al. , 2000. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol. Psychiatry 47: 662–669. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura J. T., Morse T. M., Lockery S. R., 1999. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J. Neurosci. 19: 9557–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Po M. D., Hwang C., Zhen M., 2010. PHRs: bridging axon guidance, outgrowth and synapse development. Curr. Opin. Neurobiol. 20: 100–107. [DOI] [PubMed] [Google Scholar]
- Rankin C. H., Wicks S. R., 2000. Mutations of the caenorhabditis elegans brain-specific inorganic phosphate transporter eat-4 affect habituation of the tap-withdrawal response without affecting the response itself. J. Neurosci. 20: 4337–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C. H., Beck C. D., Chiba C. M., 1990. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav. Brain Res. 37: 89–92. [DOI] [PubMed] [Google Scholar]
- Saeki S., Yamamoto M., Iino Y., 2001. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J. Exp. Biol. 204: 1757–1764. [DOI] [PubMed] [Google Scholar]
- Sagar A., Bishop J. R., Tessman D. C., Guter S., Martin C. L., et al. , 2013. Co-occurrence of autism, childhood psychosis, and intellectual disability associated with a de novo 3q29 microdeletion. Am. J. Med. Genet. A. 161A: 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H., Mori I., 2013. Behavioral plasticity, learning, and memory in C. elegans. Curr. Opin. Neurobiol. 23: 92–99. [DOI] [PubMed] [Google Scholar]
- Schaefer A. M., Hadwiger G. D., Nonet M. L., 2000. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26: 345–356. [DOI] [PubMed] [Google Scholar]
- Simons-Weidenmaier N. S., Weber M., Plappert C. F., Pilz P. K., Schmid S., 2006. Synaptic depression and short-term habituation are located in the sensory part of the mammalian startle pathway. BMC Neurosci. 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle, T., 2006 Maintenance of C. elegans (February 11, 2006), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.101.1, http//www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Stringham E. G., Dixon D. K., Jones D., Candido E. P., 1992. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol. Biol. Cell 3: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kerr R., Bianchi L., Frokjaer-Jensen C., Slone D., et al. , 2003. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Swierczek N. A., Giles A. C., Rankin C. H., Kerr R. A., 2011. High-throughput behavioral analysis in C. elegans. Nat. Methods 8: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiminen T., Jaaskelainen S., Ilonen T., Meyer H., Karlsson H., et al. , 2000. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophr. Res. 44: 69–79. [DOI] [PubMed] [Google Scholar]
- Tian X., Li J., Valakh V., DiAntonio A., Wu C., 2011. Drosophila Rae1 controls the abundance of the ubiquitin ligase Highwire in post-mitotic neurons. Nat. Neurosci. 14: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbers T. A., Giles A. C., Ardiel E. L., Kerr R. A., Rankin C. H., 2013. Intensity discrimination deficits cause habituation changes in middle-aged Caenorhabditis elegans. Neurobiol. Aging 34: 621–631. [DOI] [PubMed] [Google Scholar]
- Tulgren E. D., Turgeon S. M., Opperman K. J., Grill B., 2014. The Nesprin family member ANC-1 regulates synapse formation and axon termination by functioning in a pathway with RPM-1 and β-Catenin. PLoS Genet. 10: e1004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Molen M. J., Van der Molen M. W., Ridderinkhof K. R., Hamel B. C., Curfs L. M., et al. , 2012. Auditory change detection in fragile X syndrome males: a brain potential study. Clin. Neurophysiol. 123: 1309–1318. [DOI] [PubMed] [Google Scholar]
- Vashlishan A. B., Madison J. M., Dybbs M., Bai J., Sieburth D., et al. , 2008. An RNAi screen identifies genes that regulate GABA synapses. Neuron 58: 346–361. [DOI] [PubMed] [Google Scholar]
- Wan H. I., DiAntonio A., Fetter R. D., Bergstrom K., Strauss R., et al. , 2000. Highwire regulates synaptic growth in Drosophila. Neuron 26: 313–329. [DOI] [PubMed] [Google Scholar]
- Wang C., Koide T., Kimura H., Kunimoto S., Yoshimi A., et al. , 2014. Novel rare variants in F-box protein 45 (FBXO45) in schizophrenia. Schizophr. Res. 157: 149–156. [DOI] [PubMed] [Google Scholar]
- Way J. C., Chalfie M., 1989. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 3: 1823–1833. [DOI] [PubMed] [Google Scholar]
- Wen J. Y., Kumar N., Morrison G., Rambaldini G., Runciman S., et al. , 1997. Mutations that prevent associative learning in C. elegans. Behav. Neurosci. 111: 354–368. [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Rankin C. H., 1995. Integration of mechanosensory stimuli in Caenorhabditis elegans. J. Neurosci. 15: 2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Wang X., Ewanek R., Bhat P., Diantonio A., et al. , 2010. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J. Cell. Biol. 191: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Hao Y., Sun K., Li J., Li X., et al. , 2012. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 10: e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Tavernarakis N., Driscoll M., 2001. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron 31: 957–971. [DOI] [PubMed] [Google Scholar]
- Yan D., Wu Z., Chisholm A. D., Jin Y., 2009. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138: 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Khare P., Feldman L., Dent J. A., 2003. Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J. Neurosci. 23: 5319–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen M., Huang X., Bamber B., Jin Y., 2000. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 26: 331–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All experiments should be repeatable using the information described, links to public databases and cited literature. The following data and materials are available upon request: any strains not available from the Caenorhabditis Genetics Center, genotyping reaction details, plasmids.