Abstract
Somatic sexual determination and behavior in Drosophila melanogaster are under the control of a genetic cascade initiated by Sex lethal (Sxl). In the female soma, SXL RNA-binding protein regulates the splicing of transformer (tra) transcripts into a female-specific form. The RNA-binding protein TRA and its cofactor TRA2 function in concert in females, whereas SXL, TRA, and TRA2 are thought to not function in males. To better understand sex-specific regulation of gene expression, we analyzed male and female head transcriptome datasets for expression levels and splicing, quantifying sex-biased gene expression via RNA-Seq and qPCR. Our data uncouple the effects of Sxl and tra/tra2 in females in the-sex-biased alternative splicing of head transcripts from the X-linked locus found in neurons (fne), encoding a pan-neuronal RNA-binding protein of the ELAV family. We show that FNE protein levels are downregulated by Sxl in female heads, also independently of tra/tra2. We argue that this regulation may have important sexually dimorphic consequences for the regulation of nervous system development or function.
Keywords: Sex lethal, found in neurons, alternative splicing, sex-specific regulation
The Sxl gene of Drosophila encodes an RNA-binding protein controlling sex determination in the soma. Initiation of Sxl expression in females is dependent on the number of X chromosomes in each cell (Erickson and Quintero 2007), whereas maintenance is by autoregulatory splicing of Sxl pre-mRNA by SXL protein (González et al. 2008). Female-specific Sxl transcripts encode a 354 AA functional protein. Male-specific Sxl transcripts include an additional exon introducing a stop codon in the open reading frame. The male-specific transcript has no known function, but in theory could encode a truncated SXL isoform.
The splicing regulatory cascade activated in XX females by female-specific SXL controls the splicing of Sxl’s own transcripts as well as those of transformer (tra). This latter gene is predicted to produce a protein with 197 AA in females and 36 residues in males, with only 13 AA in common at the N-terminus. tra is crucial for normal female somatic sex determination and behavior, again with no documented functions in males. TRA functions in partnership with TRA2, which also encodes an RNA-binding protein. Alternative TRA2 isoforms of 226 and 264 AA are present in the soma of both females and males (Mattox et al. 1990). As for TRA, no known function in male somatic tissues has been reported for TRA2, although it does function in the male germline (Unni et al. 2003). The absence of either TRA or TRA2 protein eliminates female-specific alternative splicing of doublesex (dsx) and fruitless (fru) transcripts, which encode transcription factors essential for sex-specific development and behavior. SXL also downregulates the expression of male-specific-lethal-2 (msl2) through alternative splicing and translation (Valcárcel et al. 1993; Bashaw and Baker 1995; Kelley et al. 1997; Gebauer et al. 1998; Graindorge et al. 2013). This gene encodes a regulator of chromatin binding factors thought to be functional only in males. MSL2 is key to the normalization of gene expression between the single X chromosome of the males and the two copies of females, a phenomenon referred to as dosage compensation. The prevalent view is that dosage compensation in Drosophila relies on increased X-linked transcription in XY males by protein factors absent in XX females, a key player being msl-2 (Conrad and Akhtar 2012), although alternative mechanisms, including the repression of X-linked genes in females, have been proposed (Kelley et al. 1995; Birchler et al. 2011).
Analyses via microarrays, SAGE (Serial Analysis of Gene Expression), or RNA-Seq suggest that hundreds of genes are differentially expressed in male and female heads and/or are regulated by tra or dsx (Arbeitman et al. 2004; Goldman and Arbeitman 2007; Chang et al. 2011), see Samson and Rabinow 2014 for review, but only a small number of loci have been validated by further experiments.
Sex-specifically expressed genes directly regulated by the somatic sex determination pathway include the yolk protein genes Yp1 and Yp2, as shown via transgenic constructs and DNA-binding experiments with DSX protein (Coschigano and Wensink 1993). Another gene activated by direct DSX-F binding to its promoter region is Fad2/desatF, encoding a fatty acid desaturase essential for pheromone biosynthesis in female oenocytes (Chertemps et al. 2007; Shirangi et al. 2009). Bab1 expression, which regulates abdominal pigmentation, is activated in females by direct binding of DSX-F and the transcription factor ABD-B, whereas it is repressed in males by binding of DSX-M (Williams et al. 2008). Few other targets have been validated for regulation by DSX, TRA, or TRA2. Among those loci is eloF, a gene involved in long-chain hydrocarbon biosynthesis, specifically expressed in female carcasses under tra control (Chertemps et al. 2007) .
Sex-biased expression of the genes CG11458, yellow-c, CG7433, and Sodh-1 was reported in heads and validated (Fujii and Amrein 2002). In adult neurons, transcripts from the noncoding genes roX-1 and roX-2 (RNA on the X) were also found to be specifically expressed in males (Amrein and Axel 1997). Other examples of sex-specifically expressed or biased transcripts include turn on sex-specificity (tsx) and sex-specific enzyme 1 and 2 (sxe1, sxe2), which are under tra/tra2 control in heads (Fujii and Amrein 2002; Fujii et al. 2008) as well as cpn (calphotin), expressed at higher levels in males and regulated by dsx (Goldman and Arbeitman 2007). The neuropeptide F (npf) gene is expressed at higher levels in male relative to female heads and is negatively regulated by tra (Lee et al. 2006). Finally, sex-specific expression of fit (female-specific independent of transformer) in heads has been reported as independent of tra and tra2 (Fujii and Amrein 2002), but others reported it to be tra-dependent (Evans and Cline 2013).
In addition to sex-specific differences in expression levels, sex-biased splicing has also been reported in fly heads and validated by qPCR (Telonis-Scott et al. 2009; Hartmann et al. 2011; Sturgill et al. 2013). Given that SXL, TRA, and TRA2 encode splicing regulators, some of this sex-specific/biased alternative splicing may be direct, unless it occurs via other RNA-binding proteins regulated by the transcription factors FRU and DSX. Sex-biased/sex-specific expression of alternative transcripts was reported for bcd (bicoid), squid/hrp40, P-element somatic inhibitor (Psi), Hrb27c (Heterogeneous nuclear ribonucleoprotein at 27C), Rbp2 (RNA binding protein 2), and BicC (Bicaudal C), all encoding proteins that bind RNA with potential roles in posttranscriptional regulation (Telonis-Scott et al. 2009; Hartmann et al. 2011). Alternative RNA-binding protein isoforms encoded by these transcripts thus might alter sex-biased splicing of target genes, although whether these differences are under control of the canonical sex determination hierarchy remains unknown.
Additional transcripts showing evidence of sex-biased splicing in heads include those from the J domain containing protein (jdp) gene (Hartmann et al. 2011). Sex-biased/sex-specific alternative splicing for transcripts of jigr1 (jing interacting gene regulatory 1: transcription factor), exba/krasavietz (ribosome binding), CCR4/twin (protein binding/negative regulation of translation), LIMK1 (LIM-protein kinase1), Ubi-p63E (ubiquitin homeostasis), and unc-115 (Zinc finger, actin binding) was also reported.
To gain further insight into regulated sexual dimorphism of gene expression, we used a head RNA-Seq dataset as a starting point (Sturgill et al. 2013). We identified sex-biased events and performed qPCR to determine if sex-biased/sex-specific gene expression was controlled by the regulators of the canonical somatic sex determination pathway tra, tra2, or Sxl. Our results identify the X-linked gene found in neurons (fne) as a target of tra-independent, Sxl-dependent regulation in heads. Direct analysis of FNE protein levels reveals that SXL, but not TRA or TRA2, not only regulates the splicing of fne transcripts but also the level of FNE protein in female heads.
Materials and Methods
Drosophila methods
Flies were maintained on standard yeast/corn meal media at 25°. The isogenic w1118 Canton-S (B) stock was used to generate wild-type RNA (Edwards et al. 2006; Yamamoto et al. 2008). Details on fly husbandry used for the RNA preparations were previously described (Sturgill et al. 2013). Stocks were obtained from the Kyoto Drosophila Genetic Resource Center (KY), Bloomington Drosophila Stock Center (BL), B. Baker (BB), T. Cline (TC), and W. Mattox (WM): Dp(1;Y)BS/wa; st1 tra1/TM2, Ubx130 es (KY), w1118; tra1/TM3, Sb (derived in our lab from wa; tra1/TM2, BL), Dp(1;Ybb−)BS; cn1 tra2B bw/CyO (KY), y w/Dp(1;Y)BS; tra2B/CyO (WM), Df(2R)trix/CyO (BL), y/Yy+; tra21/SM1 (BB), y w SxlM1,fΔ33 ct6 sn3/ Binscy (tc), y cm Sxlf7M1 ct v /Y; P(Sxl+w-)9A/+ (TC). We generated w+; st1 tra1/T(2;3) CyO TM1 and w+; cn1 tra2B bw1/CyO males and crossed them to females from the appropriate stocks to generate sibling XX and XY flies, homozygous and heterozygous mutants for tra and tra2, respectively. More detailed information and the Sxl genotypes are found in the relevant figure legends.
Head RNA preparation, RNA-Seq, and quantitative RT-PCR
Methods were previously described (Sturgill et al. 2013). RNAseq data were downloaded from NCBI Sequence Read Archive (GSM928376, GSM928377, GSM928383, GSM928384, GSM928392, and GSM928393). These include data from two biological replicates yielding ≥200 million mapped reads for male and for female head samples, documenting the expression of 17,142 loci in FlyBase release 5.57 (St Pierre et al. 2014). The downloaded sra files were converted to fastq by sratoolkit (2.4.2-1) with the command line: fastq-dump–split-3. Reads that belong to the same biological replicate but different technical replicates were merged first, and then were uniquely mapped back to the genome using TopHat (2.0.10) (Trapnell et al. 2009) and Bowtie2 (2.1.0) (Langmead and Salzberg 2012) with the following settings: -g 1–library-type fr-firststrand –G. The output bam files were then indexed and sorted by samtools (0.1.19) (Li et al. 2009) and used in Spanki (0.4.3) (Sturgill et al. 2013) and MISO (0.5.2) (Katz et al. 2015) for alternative splicing analysis. For Spanki analysis: (1) we ran with the built-in command spankijunc from Spanki with “-m all” option; (2) curated junctions were built from all the junction files made by the first step; (3) the command merge_jtabs was run from Spanki to pools all biological replicates together; (4) splicing events were generated from annotations by Astalavista (Foissac and Sammeth 2007) with the following options: -c asta +ext; (5) the built-in command spankisplice from Spanki was run to make splicing events from junctions with support of the Astalavista output; and (6) the built-in command splicecomp from Spanki was run to compare the alternative splicing between female and male heads. We calculated P values from Fisher’s exact test and used FDR (corrected by Benjamini-Hochberg) <0.05 as the cutoff. More details can be found in the Supporting Information of Sturgill et al. (2013). MISO was used to visualize the alternative splicing pattern for the raw bam files as follows: (1) we merged the bam files with different biological replicates together to make two large bam files for female and male, respectively; (2) we used gtf2gff3.pl (http://genes.mit.edu/burgelab/miso/scripts/gtf2gff3.pl) to convert the gtf annotation to gff3 and then used the built-in command index_gff from miso to index the gff3 annotation; (3) we ran MISO with the merged bam files with the indexed annotation from the previous step and the option:–read-len 76; (4) we obtained the mapped read number from bam files by samtools view –c; (5) we ran the command sashimi_plot from MISO with the following settings: scale of intron and exon = 1:1, ymax = 56, and the total mapped read information from the previous step; and (6) colors and fonts of the sashimi_plot were further modified by Adobe Illustrator (CC 2014) and coding position was guided by Integrative Genomics Viewer (IGV 2.3.46).
Sequencing of fne cDNA
To verify the Flybase model that associates the 5′ alternatively regulated exons in event ASTA0020150 (Supporting Information, File S1) to the fne ORF, we sequenced fne cDNAs from male and female heads. Our data (Genbank accession numbers: KJ815141, KJ815142) support the Flybase fne gene model, both in terms of exon structure, with individual cDNAs including both 5′ exons and the fne ORF, and for the identity of the coding strand (GT… AG intronic splice sites).
qPCR Statistics
Comparisons of stable transcript levels were performed with STATISTICA software (StatSoft, Inc.). Male/female comparisons were performed by t-test, and P values were corrected for multiple testing using the method of Benjamini-Hochberg with InVivoStat software (http://www.bap.org.uk/invivostat.php), a gift from S. Bate (Clark et al. 2012). Transcript levels in multiple genotypes were analyzed by two-way ANOVA, followed by a post-hoc test with the Scheffé method.
Statistics for the enrichments
Enrichments are defined as [abundance of transcript form 1 / transcript form 2] in one genotype relative to [abundance of transcript form 1 / transcript form 2] in CS females. We compared [abundance of transcript form 1 / transcript form 2] in one genotype relative to either CS females [abundance of transcript form 1 / transcript form 2] or CS males [abundance of transcript form 1 / transcript form 2]. Raw transcript abundance values were used to calculate C.I.s and P values for those ratios of transcript levels, as in Fay and Gerow (2013). We used their spread sheet and adapted it to our data set. Bonferroni corrections of the P values were implemented when more than one comparison was performed using the data set (Figure 3, Figure 4)
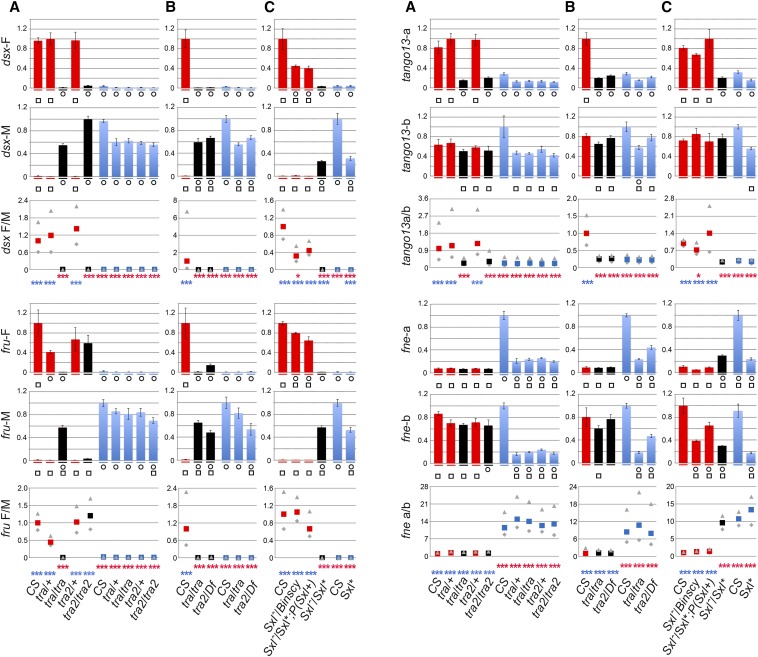
Figure 3.
Impact of tra, tra2, and Sxl mutations on alternative splicing. The bar plots show relative transcript levels as determined by qPCR in females (red), males (blue), and pseudomales represented in black. The primers used for qPCR are specified in Figure 2A and Table S2, and genotypes are indicated below the bar plots. Heterozygous and homozygous flies mutant for tra, tra2, and Sxl, respectively, are siblings. They were respectively obtained from the cross between female w1118; tra1/TM3 Sb and male w+; st tra1/T(2;3) CyO TM1, from the cross between female y w/y w; tra2B/CyO and male w+; cn tra2B bw/CyO. tra21/Df(2R)trix and from the cross between female y/Yy+; tra21/SM1 and male Df(2R)trix/CyO. Siblings of heterozygous and homozygous Sxl mutants are generated from crosses between female y w SxlM1,fΔ33 ct6 sn3/Binscy and male y cm Sxlf7M1 ct v/Y; P(Sxl+w-)9A/+ or +/+. Conclusions of the ANOVA (two-way, followed by post hoc test): [ANOVA: F(32, 86.415) = 33.076, P ≤ 1E-5] for the experiment in (A); [F(16, 22) = 26.695, P ≤ 1E-5] for the experiment in (B); and [F(40, 42.025) = 56.186, P ≤ 1E-5] for the experiment in (C) are provided under each set of bar plots. Circles (squares) denote statistically significant departure from CS female (male) expression (as detailed in File S2). Enrichments are defined and analyzed as specified in the legend of Figure 2. The conclusions of the statistical analysis (after Bonferroni correction, see File S3) are shown in red for comparisons with CS females and in blue for comparisons with CS males. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
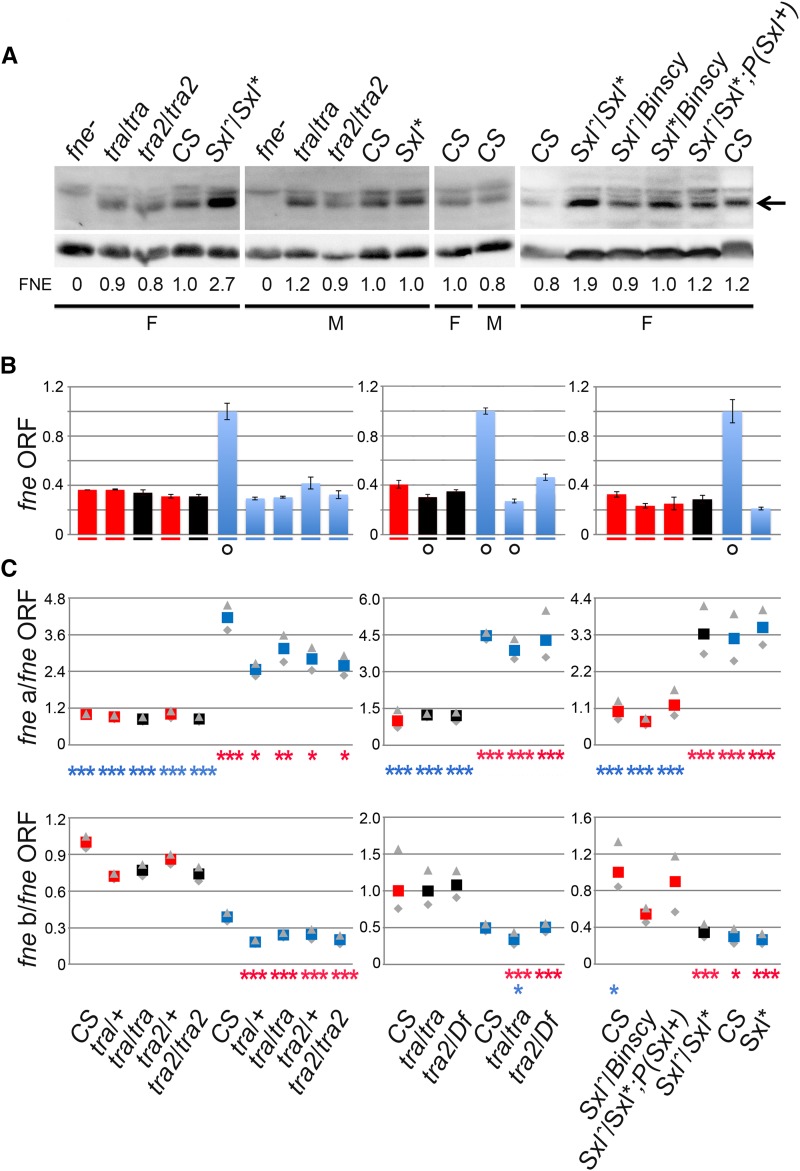
Figure 4.
Impact of tra, tra2, and Sxl mutations on the expression of FNE protein and fne ORF-containing transcripts. (A) Immunoblot analysis of head protein extracts with anti-FNE antibodies. Tubulin is used as a loading control. Genotypes are as in Figure 2 and Figure 3. Head protein extracts were prepared from sibling flies born from the same parents and collected at the same time as the flies used for the head RNA preparations. An arrow indicates the position of FNE protein on the blots. The quantification of relative amounts of FNE is shown below the blots. (B) Quantitation of the amount of fne ORF. Genotypes and RNA batches used in this experiment are the same as those used for Figure 3. Each set of bar plots shows the female (red), pseudomale (black), and male (blue) relative transcript levels (determined by qPCR). Symbols summarizing the conclusions of ANOVA [F(4, 22)=107.85, P ≤ 1E-5 for the experiment with tra and tra2 mutants, F(2, 18)=217.66, P ≤ 1E-5 for the experiment with tra, tra2, and Df(2R)trix, F(5, 18)=108.39, P ≤ 1E-5 for Sxl as detailed in File S2] followed by post hoc test are as in Figure 3. (C) Enrichments (defined in Figure 2) of fne-a (and, respectively, fne-b) relative to ORF-containing fne transcripts are shown below the bar plots using the same symbols as in Figure 3, and as detailed in File S3.
Head protein preparations and FNE immunodetection
Frozen fly heads (30–100) were homogenized on ice in 40 μl of freshly prepared 1X PBS, 0.5% IGEPAL CA-630, 1 mM EGTA pH 8, 0.5 μg/μl leupeptin, 1 μg/μl peptastin, and 0.2 mM PMSF. Samples were spun for 10 min, 10,000 rpm, at RT and the soluble proteins were recovered in a fresh tube. Protein extracts (four head-equivalents per lane) were resolved on 10% SDS-polvacrylamide and transferred to a PVDF membrane (0.45 μm; Millipore Corporation). Immunodetection was performed at room temperature as follows: (1) blocking in 5% skim milk, TBST (50 mM Tris; 0.15 M NaCl; 0.05% Tween-20; pH 7.6) 30 min, 37°; (2) incubation with 1:1000 rat polyclonal anti-FNE (Zanini et al. 2012) in TBST for 30 min; (3) three washes in TBST, 10 min each; (4) incubation with 1:5000 anti-rat IgG (Invitrogen) in BST for 30 min; and (5) three washes in TBST, 10 min each. Detection was performed with chemiluminescent reagents (Immobilon Western, Millipore) and quantification was on a Carestream (In-Vivo FPRO) using the Carestream MI software.
Data availability
Figure S1 is a graphic representation of sex-biased alternative splicing for key splice events. File S1 is the complete Spanki analysis of the RNA-Seq data. File S2 and File S3 are detailed statistical analyses, respectively for qPCR results and for enrichments. File S4 is a supplemental discussion. Table S1 contains the Spanki analysis of the RNA-Seq data and qPCR validation for key splice events. Table S2 provides details on the primers. Accession numbers for sequence and gene expression data are specified in the Materials and Methods.
Results
Identification of genes whose alternative splicing patterns differ between female and male heads
Analysis of RNA-Seq replicates from female and male heads prepared from an isogenic w1118 Canton-S (B) stock (Edwards et al. 2006; Yamamoto et al. 2008), referred to as CS throughout this article, was previously reported (Sturgill et al. 2013). We remapped the same reads to the current genome annotation (FlyBase 5.57) (St Pierre et al. 2014) with updated versions of RNAseq analysis software (see Materials and Methods) that provide candidate gene lists for further study (File S1).
Among the genes identified were Sxl, dsx, and fru, which produce alternatively spliced components of the sex determination pathway (Figure S1). A new sex-biased splicing event was identified in fne (Figure 1). The analysis of splicing by RNA-Seq has many caveats of statistical power, and there are still serious alignment errors that make accurate assessment of splice junctions problematic (Sturgill et al. 2013). For instance, in our analyses, the transcripts of tango13 apparently display sex-specific splicing but their computed sex-biased expression is not deemed statistically significant (Figure S1). However, theses transcripts were independently identified as sex-specifically spliced in a microarray analysis (McIntyre et al. 2006). Therefore, we also decided to further examine the splicing of tango13 transcripts.
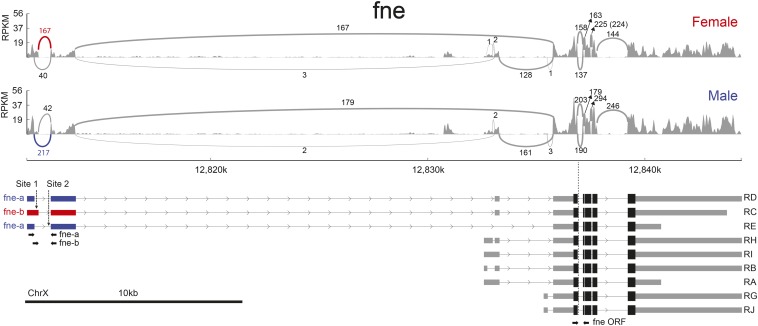
Figure 1.
Graphic representation of sex-biased alternative splicing for fne in the RNA-Seq analysis. RPKM for each alignment track are shown as sashimi plots (Katz et al. 2015). Arcs denote splice junctions, quantified in spanning reads, as specified near each exon junction. Exons and splice junctions relevant to the sex-biased splice event are in red (female-enriched junction) or blue (male-enriched junction). The nine transcripts predicted by the FlyBase (5.57) gene model are shown at the bottom. We have sequenced cDNAs that link the 5′ alternatively regulated exons to the fne ORF (see Materials and Methods). X chromosome coordinates are indicated and arrows in introns represent the direction of transcription. The structure of the RT-PCR amplicons specific for fne-a, fne-b, and fne ORF, respectively, are shown with converging arrows below the transcript diagrams. The position of the stretches UUUUUUAUCUCUUUUU (site 1) and UUUUUUUU (site 2) mentioned in File S4 are shown with vertical arrows.
We used qPCR to explore these alternative events and confirmed significant sex differential splicing for all of them (Figure 2, Table S1). Although we measured individual transcript levels, we focused on the changes in the relative enrichment of one alternative splice form vs. the other form when comparing genotypes, as detailed in the legend of Figure 2. The major advantage of using enrichment in the analysis of splicing is that it frees the data from the potential impact of different backgrounds on transcript expression levels, facilitating comparisons between genotypes.
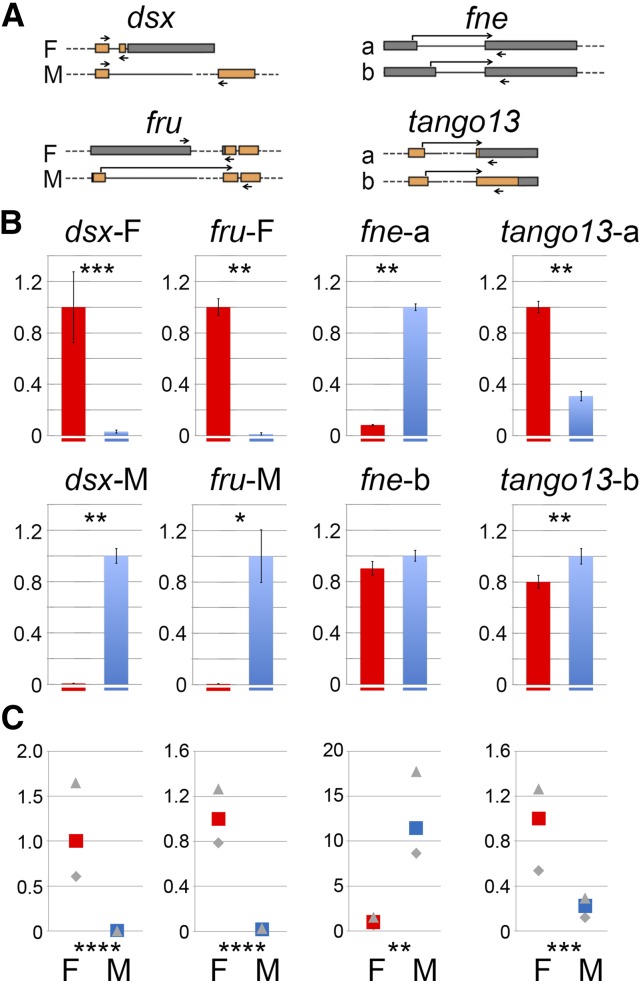
Figure 2.
Sex-dependent alternative splicing of dsx, fru, fne, and tango13. (A) Diagrams of the qPCR tested sex-biased alternative splicing events. Exons are represented by boxes; orange indicates coding regions and gray indicates untranslated regions (UTRs). Introns are represented by lines (in scale) and dashes (out of scale). The positions of the primers used for qPCR analysis are indicated by arrows joining two exons when a primer overlaps an exon junction. (B) qPCR analysis of four alternative splicing events. The bar plots show the female (red) vs. male (blue) relative transcript levels as determined by qPCR. P values (t-test) for the comparison of transcript abundance in female vs. male are indicated on each panel. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 (see File S2). (C) As a convenient way to compare splicing between two genotypes, we use enrichment values. The average enrichments are computed from our qPCR quantifications as [abundance of transcript form a / transcript form b] in one genotype relative to [abundance of transcript form a / transcript form b] in CS females. By definition, the enrichment is 1 in CS female. Maximum and minimum enrichments calculated from our qPCR quantifications are indicated in gray. The complete statistical analysis of enrichments is in File S3 (Fay and Gerow 2013). P values for the comparisons of enrichments are indicated below the bar plots (*, **, ***, and **** as above).
Importantly, both RNA-Seq and qPCR identified and quantified the sex-specific alternative dsx and fru transcript isoforms (Figure 2 and Figure S1), demonstrating that known alternative splicing events were captured in the datasets. We also quantified with qPCR the sex-biased alternative splicing events in fne, obtaining congruence between RNA-Seq and qPCR results where two different 5′ donor sites and a fixed 3′ acceptor site in the 5′ UTR are used differentially in males and females (Figure 2, Table S1): RNA-Seq computes a 21-fold enrichment in the relative amounts of fne-a vs. fne-b in males compared to females, whereas qPCR measures a 9- to 18-fold increase (P ≤ 0.01) (Figure 2). In the case of tango13, qPCR, but not RNA-Seq (Spanki analysis), measured significant gene-level sex-biased expression (Table S1). Analysis of the tango13 transcripts reveals the use of alternative 5′ splicing donor sites predicted to shift the reading frame. As a result, two forms of TANGO13 with alternative carboxy termini and different sizes would be produced. Transcripts encoding the predicted 346 AA isoform were predominant in females, whereas those encoding the 499 AA isoform were predominant in males (Figure 2). qPCR measures up to a five-fold increase (P ≤ 0.001) (Figure 2) in the enrichment of tango13-b vs. tango13-a in males compared to levels observed females.
Differential impact of tra21 and tra2B alleles on the splicing of fru but not of dsx
To better understand the modalities that govern sex-specific splicing, we quantified the potential dependence of the sex determination pathway on the sex-biased splicing events. We used the following alleles: tra1, which deletes approximately 1 kb of the gene, including the entire ORF (Yuan and Belote 1995); tra21, which is a spontaneous allele with an unknown lesion; and tra2B, which introduces a premature stop codon and is predicted to produce a truncated polypeptide missing a portion of the RNA recognition motif (RRM) and the RS2 domain essential for TRA2 function (Mattox and Baker 1991). However, suppression of the stop codon cannot be excluded because it is a common occurrence in Drosophila (Jungreis et al. 2011). XX females homozygous for tra1 or tra2B or that are tra21/Df(2R)trix exhibit a full cuticular transformation of female to male-like (pseudomales).
Consistent with the current sex determination model, we found that in these three types of XX pseudomales the major dsx transcript form is male-specific dsx-M and not female-specific dsx-F (P ≤ 1E-6) (Figure 3). Based on the qPCR, dsx-F enrichment relative to dsx-M in these XX pseudomales is reduced at least 600 compared to CS females (P ≤ 1E-3) (Figure 3), not significantly different from the level observed in CS males (P ≥ 0.05) (Figure 3). Interestingly, although in tra1/tra1 and tra21/Df(2R)trix XX pseudomales fru is alternatively spliced in a male mode (P ≤ 1E-3), tra2B/tra2B pseudomales produce a major fru-F isoform similar to CS females (P > 0.05) (Figure 3): fru-M enrichment relative to fru-F in tra2B/tra2B pseudomales is 0.6- to 1.2-fold the levels observed in CS females, but it is at least 40-fold higher in the other pseudomales. We conclude that in the context of limited tra2 function (in tra2B mutant XX flies transformed from females to pseudomales) dsx transcripts are spliced in a male mode but fru transcripts are spliced in female mode. This suggests that the effect of the homozygous tra2B mutations on fru splicing is weaker than the effect of homozygous tra1/tra1 and tra21/Df(2R)trix. Although we cannot exclude that the tra2B background is responsible for unexpected splicing of the fru alternative transcripts, we suggest that these observations may reveal distinct dosage requirements for TRA2 in the regulation of dsx and fru splicing, respectively.
Regulation of tango13 alternative splicing events in the heads of tra, tra2, and Sxl mutant females
Because sex-biased expression, and perhaps alternative splicing, is expected to be regulated by the canonical sex determination pathway, we addressed the level of control of tango13 within the sex determination network by examining its splicing via qPCR in XX females, XX pseudomales, and XY males (Figure 3). Specifically, we used tra1, tra2B, tra21/Df(2R)trix and also a heteroallelic combination of the hypomorphic alleles SxlM1,fΔ33 and Sxlf7M1 (Evans and Cline 2013). SxlM1,fΔ33 / Sxlf7M1 females are phenotypically masculinized to the same degree as other reported Sxl mutant combinations but are more viable and longer lived (Evans and Cline 2013). To ascertain that splicing changes were specifically due to Sxl, we compared these pseudomales to sibling female SxlM1,fΔ33 / Sxlf7M1, P(Sxl+w)9A, where the Sxl mutation is rescued with one copy of an autosomal Sxl+ minigene. As expected, we found that dsx-M and fru-M become the major transcript forms in the Sxl- XX pseudomales. Based on qPCR, dsx-F enrichment relative to dsx-M in Sxl XX pseudomales is decreased at least 300-fold compared to CS or SxlM1,fΔ33 / Sxlf7M1, P(Sxl+w)9A females (P ≤ 0.0006) (Figure 3C). Similarly, fru-F enrichment relative to fru-M in Sxl- XX pseudomales is lowered at least 1800-fold compared to levels observed in CS females or in SxlM1,fΔ33 / Sxlf7M1, P(Sxl+w)9A females (P ≤ 0.0006) (Figure 3C), and is close to the levels observed in CS males (P ≥ 0.05) (Figure 3C). This is consistent with SXL regulating these two genes in female heads.
Using this approach, we found evidence for the misregulation of tango13 in tra, in tra2, and in Sxl- XX pseudomales, where tango13 is spliced in a male mode (Figure 3). Compared to CS females, the enrichment of tango13-b vs. tango13-a transcript levels increased up to seven-fold in XX homozygous tra1 and tra2B and in XX tra21/Df(2R)trix and SxlM1,fΔ33 / Sxlf7M1 pseudomales (P ≤ 0.0006) (Figure 3), close to male levels (P > 0.05). Noticeably, this happens while the level of tango13-b remains constant (P ≥ 0.18). This is an unexpected outcome for a pair of mutually exclusive transcripts whose expression was anticipated to be modified in a reciprocal manner. These data are consistent with tango13 sex-biased regulation of gene expression by tra/tra2 at a level distinct from alternative splicing, perhaps via DSX and/or FRU or further downstream in the pathway.
Regulation of fne alternative splicing in Sxl but not in tra or tra2 mutant heads
Northern blot analyses of fne transcription patterns resolve at least four tissue-regulated and developmentally regulated transcripts (Samson and Chalvet 2003). In heads, these analyses identified two (groups of) transcripts approximately 4.4 kb long, and two more approximately 7.5 kb. The Flybase gene model predicts nine transcripts produced from two major transcription initiation sites, compatible with the sizes determined by Northern blot analysis (Figure 1). The 199 nt size difference between the fne-a and fne-b forms generated by alternative splicing in the upstream 5′ UTR is not sufficient for discrimination by Northern blot analyses. The optional sex-neutral extension of the first coding exon (six nucleotides) (Figure 1) generates two alternative fne transcript forms that, in conjugation with the use of three alternative 3′ UTRs (Hilgers et al. 2011), have the potential to generate six alternative forms of fne-a and fne-b, respectively. In head samples, the number of splice junction reads in the 5′ UTR of fne-a and fne-b (167+40 = 207 in females and 217+42 = 257 in males) (Figure 1) is comparable to the number of constitutive splice junctions in the coding fne region (144, 163, or 225 in females and 179, 246, or 294 in males) (Figure 1). This observation suggests that transcription initiating at the upstream 5′ UTR significantly contributes to the total fne RNA pool, and that the isoforms RD, RC, RE (Figure 1) represent the bulk of fne RNA.
Alternative splicing of fne in heads follows an unconventional pattern in that its sex-specific splicing is unchanged from CS females in tra or tra2 mutant XX pseudomales (Figure 3) (P ≥ 0.05) and significantly differs from splicing in CS males (Figure 3) (P ≤ 0.0006). This is unexpected and in striking contrast with changes of dsx, fru, and tango13 alternative transcripts showing quantitative differences in tra and tra2 XX pseudomales. Further, the very robust female mode splicing is unchanged not only in tra− and tra2− XX pseudomales but also in a Doa heteroallelic combination that causes sexual transformations (not shown) due to alteration of DOA, a kinase that phosphorylates TRA and TRA2 (Du et al. 1998). These data demonstrate that the sex-biased alternative splicing event in the upstream 5′ UTR of fne transcripts is not regulated by tra/tra2 in females.
Because we found that the sex-specific regulation of fne splicing does not require tra and tra2 function, we investigated alternative possibilities. There is some evidence for alternative sex determination/differentiation pathways dependent on Sxl but independent of tra and tra2 (Evans and Cline 2013) or independent of Sxl (Hartmann et al. 2011). We therefore examined the impact of Sxl mutations on fne alternative splicing. We found that in XX SxlM1,fΔ33/Sxlf7M1 pseudomales, fne splicing switches to a male mode (Figure 3). The enrichment of the fne-a vs. fne-b transcript in these XX pseudomales is similar to that of CS males (0.6-fold to 1.0-fold, P > 0.05), whereas it increases 8-fold to 12-fold compared to that in CS females (P < 0.0006). In contrast to the XX Sxl pseudomales, in both classes of our controls (y cm Sxlf7M1/Binscy: XX and SxlM1,fΔ33/Sxlf7M1, P(Sxl+w)9A females), the enrichment of fne-a vs. fne-b transcript is close to that in CS females (less than two-fold increase, P > 0.05) and is distinct from that in CS males (approximately eight-fold, P < 0.0006), indicating that this particular fne splicing regulation is intact in the controls (Figure 3). Importantly, the CS female-like splicing of fne in XX SxlM1,fΔ33/Sxlf7M1, P(Sxl+w)9A females confirms the specificity of the effect of Sxl on fne splicing in XX Sxl+ females.
FNE protein levels are independent of tra and tra2 in the two sexes but depend on Sxl in female heads
Due to posttranscriptional regulation, transcript levels only partially predict protein abundance, the latter being more directly relevant to function. Thus, to further evaluate the impact of Sxl on fne expression, we examined FNE protein levels in heads by immunoblot. We found that FNE levels are independent of sex in CS heads, with relative amounts in females of 0.9 vs. 1.0 in males (SEM = 0.119, based on four immunoblots). We found that in male and female CS, tra1/tra1 and tra2B/tra2B mutants all share similar FNE protein levels (Figure 4A), confirming independence of fne regulation and tra/tra2 function. However, FNE protein levels are increased, on average, 2.4-fold (four measures, SEM = 0.067) in XX SxlM1,fΔ33 / Sxlf7M1 pseudomales compared to CS females as well as all the other genotypes. In contrast, FNE levels remain unchanged in SxlM1,fΔ33 / Sxlf7M1, P(Sxl+w)9A females (1.1-fold variation, two measures, SEM = 0.15) where the Sxl mutation is rescued with a minigene (Figure 4A). Our data thus reveal a perfect correlation between changes in fne alternative splicing in XX Sxl- pseudomales and those in FNE protein levels beyond potential changes in levels of individual fne-a and fne-b transcripts. It is worth mentioning that the expression of ELAV, another pan neuronal protein paralogous to FNE, is unchanged in all the tested genotypes (not shown). This indicates that the increased expression of FNE in XX Sxl− pseudomales is not due to an overgrowth of neurons or the nervous system, that this effect is restricted to fne and is not found among all members of the family.
Because it cannot be excluded that the increased FNE protein levels in the heads of XX Sxl− pseudomales are due to an increase in the amounts of fne stable coding transcripts, we examined those via qPCR in all the genotypes used in this study using the primers shown in Figure 1. Aside from high levels in CS males (with no impact on FNE protein levels), no significant difference in fne ORF-containing RNA is detected among other males, females, and pseudomales (P > 0.2) (Figure 4B). Further, we found that the enrichment of fne-a or fne-b relative to the fne ORF RNA follows XX-specific and XY-specific patterns, except in XX SxlM1,fΔ33/Sxlf7M1 pseudomales, where it departs from the CS female pattern (P ≤ 0.0006) and is male-like (P ≥ 0.05) (Figure 4C). Thus, we obtained further evidence that fne is regulated in a male model in the XX Sxl− pseudomales, but we see no evidence for increased fne transcript levels in these animals.
In summary, our data on Drosophila head RNA show that: (1) fne is differentially spliced in the two sexes; (2) tra and tra2 do not regulate fne; (3) Sxl does not impact coding transcript levels but regulates the splicing of fne, causing a switch from the default male splicing pattern to a female-specific pattern; and (4) Sxl downregulates the amount of FNE protein in XX female heads. The rescue of the effect of Sxl mutations by a Sxl minigene both at the level of fne-a and fne-b splicing and at the level of FNE protein demonstrates that the effects are specific to Sxl. Our data thus show that equal FNE protein abundance in male and females is the surprising outcome of complex regulation at multiple molecular levels.
Discussion
Transcript level fluctuations can occur independently of splicing regulation and with no impact on protein levels
Large-scale transcriptomic and proteomic analyses have contributed to the recognition of posttranscriptional regulation in the tuning of protein levels (Vogel and Marcotte 2012). In the case of fne, we measured distinctly higher transcript levels in CS males, although FNE protein levels are unchanged between CS males and females (and other examined genotypes aside from the Sxl− pseudomales). However, in both sexes and in all genotypes (but not in Sxl− pseudomales), we observed that the relative abundance of mutually exclusive alternative splice forms remains constant, in agreement with independent regulation of splicing and stable transcript levels. The transcript level fluctuations possibly reflect background differences. Thus, to evaluate potential splicing regulation in mutants, we focused on the ratios of alternative isoforms rather than on levels of individual transcripts.
Identification and regulation of genes expressed differentially in females and males
The Drosophila sex determination hierarchy is the classical model of developmentally regulated alternative splicing. To identify genes expressed differentially in males and females, we chose to work with head samples, thereby eliminating large numbers of events restricted to gonadal differentiation. Moreover, the neurons, enriched in heads, are the site of extensive regulation at the level of alternative splicing (Calarco et al. 2011; Zheng and Black 2013; Brown et al. 2014).
In addition to dsx and fru, canonical regulators of Drosophila sex determination, we identified and further characterized the expression of fne and tango13 as genes expressed in a sex-biased manner. We found that tango13 sex-specific expression responds to tra, tra2, and Sxl mutations in females as expected if under the control of the canonical sex determination pathway. An intriguing feature is the absence of reciprocity in the regulation of the mutually exclusive tango13-a and tango13-b splice forms, because tango13-a levels are reduced in tra, tra2, and Sxl mutants, but tango13-b levels are not. This observation suggests that tra and tra2 could possibly have an impact on the levels/stability of the tango13-a transcript rather than on the alternative splicing of tango13 RNA per se. The impact of tra/tra2 alleles on the expression of tango13-a is similar to that on the expression of dsx-F, consistent with regulation downstream of the sex determination pathway.
Sex-biased alternative splicing of fne and FNE protein levels are both dependent on Sxl but independent of tra/tra2
In contrast to fru, dsx, and tango13, the expression of fne is independent of TRA and TRA2. Crucially, fne splicing nevertheless depends on Sxl function in female heads: Sxl− pseudomales switch to a male mode of fne alternative splicing, consistent with a role for SXL in promoting, directly or indirectly, the formation of the fne-b isoform at the expense of fne-a in normal females. Further, although fne alternative splicing is male-like in XX Sxl pseudomales, FNE protein levels are also upregulated two-fold to three-fold compared to CS males and females. Both male-like splicing and increased FNE protein levels in the pseudomales are reverted by the introduction of a Sxl+ minigene, confirming the specificity of Sxl in the control of both the splicing and protein levels. Our data thus show that a Sxl-dependent, tra/tra2-independent mechanism regulates fne expression in females.
Complex sex-specific fne regulation involving multiple molecular levels leads to an equal amount of FNE protein in males and females
CS female and male pools of fne RNA yield similar amounts of FNE protein in the two sexes. However, XX SxlM1,fΔ33 / Sxlf7M1 pseudomales have a male-like pool of fne RNA and two-fold to three-fold increased FNE protein levels compared to CS. Because fne is an X-linked gene, its expression is presumably influenced by the canonical dosage compensation pathway, which could be responsible for the upregulation of FNE levels in XX Sxl− pseudomales. However, according to the canonical model, higher fne transcript levels would be expected in pseudomales than in males and females, but that is not the case. Additional mechanisms must be at play.
First, increased FNE protein levels in XX Sxl− pseudomales compared to wild-type males do not result from increased transcript levels. Because males and pseudomales share similar spliced pools of fne RNA, their distinct FNE outputs necessarily result from a regulatory mechanism that operates independently of the effects of Sxl on alternative splicing. Formally, this mechanism appears to stimulate the translation of fne transcripts in XX individuals. Second, increased FNE protein levels, concomitant with changes in alternative splicing but not associated with changes in transcript levels, as in XX Sxl− pseudomales compared to wild-type females, are consistent with the existence of a Sxl-dependent mechanism that downregulates FNE protein levels in XX females. Only the XX-dependent upregulation would persist in Sxl− pseudomales, hence their increased FNE level. It is conceivable that fne regulation by Sxl occurs via direct binding of Sxl to fne transcripts (see File S4). An interesting alternative as a means to regulate its splicing is the possibility that the impact of Sxl on fne expression occurs indirectly (possibly via an hormonal axis), since the extensive impact of the germline on the expression of somatic genes has been documented (Parisi et al. 2010).
Regulation of fne by SXL
fne encodes an RNA-binding protein concentrated in the soma of neurons and present throughout development (Samson and Chalvet 2003). It is necessary for the normal development of the mushroom bodies of males and females, and it is involved in the regulation of male courtship (Zanini et al. 2012). It is intriguing that the expression of pan-neuronal fne is regulated in a sex-biased manner under the control of Sxl.
In addition to its role in the development of the germline, Sxl is involved in several regulatory pathways in the soma. It responds to a cell autonomous signal (number of X chromosomes) and is crucial both for the sexual development of somatic cells and for dosage compensation in males. SXL, but not TRA or TRA2, is also required independently of the somatic sex determination pathway for the development of a subset of sexually dimorphic neurons, with consequences on female ovulation (Evans and Cline 2013). Additional phenotypes independent of the canonical somatic sex determination pathway but dependent on Sxl are the control of the sexually dimorphic body size of flies (Cline and Meyer 1996) and the sex-specific bristle number on the A5 sternite (Penn and Schedl 2007). The latter occurs through general downregulation of the Notch pathway by SXL in multiple tissues (Penn and Schedl 2007). Thus, the Sxl-regulated expression of fne fits within the context of Sxl acting in parallel with the canonical Sxl-tra/tra2 cascade, constituting an example of its impact on tissues that do not show obvious sexual dimorphism.
What impact for the Sxl-dependent regulation of fne?
fne is a member of a fairly new multigene family restricted to dipterans (Samson 2008). The birth of this family predates the role of SXL in sex determination, which is restricted to the drosophilids (Meise et al. 1998). Based on our RNA-Seq data and the Flybase models (St Pierre et al. 2014), sex-specific alternative splicing has not been reported for either of the other two paralogues in this family, elav (embryonic lethal abnormal visual system, X linked) or rbp9 (RNA binding protein 9, second chromosome). elav is the result of a retrotransposition and is likely to have acquired new cis-regulatory elements in the process (Samson 2008). It autoregulates via a posttranscriptional mechanism involving its 3′ UTR (Samson 1998). It is unclear whether the Sxl-dependent regulation of fne is an ancestral property that has been lost for rbp9 or was recently acquired. Nevertheless, sex-specific alternative splicing provides fne with the ability to be differentially regulated in females, which may have an important impact on sex-specific nervous system function or development, for which there are numerous instances of a role for Sxl. Within the context of the canonical sex determination pathway, Sxl regulates the expression of fru and dsx, two transcription factors crucial for behavior and nervous system function. SXL also controls, via an independent pathway, specific aspects of female behavior (Evans and Cline 2013). Still outside of the context of the canonical sex determination pathway, Sxl regulates the neurogenic locus Notch (Penn and Schedl 2007). Further, in Drosophila virilis, SXL protein accumulates in the male developing nervous system, consistent with a role there (Bopp et al. 1996). Thus, the control exerted by Sxl on pan-neuronal fne outside of the context of the canonical sex determination pathway may be part of the heritage of an SXL ancestral function more focused on the nervous system than on sexual differentiation.
Supplementary Material
Acknowledgments
We thank Tom Cline for constructive criticisms of a prior version of the manuscript and for kindly providing the Sxl mutant stocks. More stocks were graciously provided by William Mattox, Bruce Baker, Trudy Mackay, the Indiana University Drosophila stock center, and the Drosophila Genetic Resource Center, Kyoto, Japan. We thank Karine Tuphile for cloning and sequencing of fne cDNAs and Bernadette Wiszniowski for assistance with stocks. We particularly thank S. Bate for providing the InVivoStat software. Financial support was provided to M.-L.S. and L.R. by the Indo-French Center for the Promotion of Advanced Research (IFCPAR, award #4903A), the Université Paris Sud, and the Centre National de la Recherche Scientifique (CNRS). This work was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases at NIH (NIDDK, to B.O.). X.S. was supported by a graduate fellowship from the China Scholarship Council. This work was submitted as a partial requirement for completion of a doctoral thesis (by X.S.).
Footnotes
Supporting information is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.023721/-/DC1
Sequence data reported in this manuscript have been assigned Genbank/EMBO accession numbers: KJ815141, KJ815142.
Communicating editor: J. A. Birchler
Literature Cited
- Amrein H., Axel R., 1997. Genes expressed in neurons of adult male Drosophila. Cell 88: 459–469. [DOI] [PubMed] [Google Scholar]
- Arbeitman M. N., Fleming A. A., Siegal M. L., Null B. H., Baker B. S., 2004. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development 131: 2007–2021. [DOI] [PubMed] [Google Scholar]
- Bashaw G. J., Baker B. S., 1995. The msl-2 dosage compensation gene of Drosophila encodes a putative DNA- binding protein whose expression is sex specifically regulated by Sex-lethal. Development 121: 3245–3258. [DOI] [PubMed] [Google Scholar]
- Birchler J., Sun L., Fernandez H., Donohue R., Xie W., et al. , 2011. Re-evaluation of the function of the male specific lethal complex in Drosophila. J. Genet. Genomics 38: 327–332. [DOI] [PubMed] [Google Scholar]
- Bopp D., Calhoun G., Horabin J. I., Samuels M., Schedl P., 1996. Sex-specific control of Sex-lethal is a conserved mechanism for sex determination in the genus Drosophila. Development 122: 971–982. [DOI] [PubMed] [Google Scholar]
- Brown J. B., Boley N., Eisman R., May G. E., Stoiber M. H., et al. , 2014. Diversity and dynamics of the Drosophila transcriptome. Nature 512: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco J. A., Zhen M., Blencowe B. J., 2011. Networking in a global world: establishing functional connections between neural splicing regulators and their target transcripts. RNA 17: 775–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. L., Dunham J. P., Nuzhdin S. V., Arbeitman M. N., 2011. Somatic sex-specific transcriptome differences in Drosophila revealed by whole transcriptome sequencing. BMC Genomics 12: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertemps T., Duportets L., Labeur C., Ueda R., Takahashi K., et al. , 2007. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 104: 4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Shoaib M., Hewitt K. N., Stanford S. C., Bate S. T., 2012. A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J. Psychopharmacol. 26: 1136–1142. [DOI] [PubMed] [Google Scholar]
- Cline T., Meyer B., 1996. Vive la difference: males vs. females in flies vs. worms. Annu. Rev. Genet. 30: 637–702. [DOI] [PubMed] [Google Scholar]
- Conrad T., Akhtar A., 2012. Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription. Nat. Rev. Genet. 13: 123–134. [DOI] [PubMed] [Google Scholar]
- Coschigano K. T., Wensink P. C., 1993. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 7: 42–54. [DOI] [PubMed] [Google Scholar]
- Du C., McGuffin M. E., Dauwalder B., Rabinow L., Mattox W., 1998. Protein phosphorylation plays an essential role in the regulation of alternative splicing and sex determination in Drosophila. Mol. Cell 2: 741–750. [DOI] [PubMed] [Google Scholar]
- Edwards A. C., Rollmann S. M., Morgan T. J., Mackay T. F., 2006. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2: e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. W., Quintero J. J., 2007. Indirect effects of ploidy suggest X chromosome dose, not the X:A ratio, signals sex in Drosophila. PLoS Biol. 5: e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. S., Cline T. W., 2013. Drosophila switch gene Sex-lethal can bypass its switch-gene target transformer to regulate aspects of female behavior. Proc. Natl. Acad. Sci. USA 110: E4474–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay, D. S., and K. Gerow, 2013 A biologist’s guide to statistical thinking and analysis. WormBook 1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissac S., Sammeth M., 2007. Astalavista: dynamic and flexible analysis of alternative splicing events in custom gene datasets. Nucleic Acids Res. 35: W297–W299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Amrein H., 2002. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 21: 5353–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Toyama A., Amrein H., 2008. A male-specific fatty acid ω-hydroxylase, SXE1, is necessary for efficient male mating in Drosophila melanogaster. Genetics 180: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Merendino L., Hentze M., Valcárcel J., 1998. The Drosophila splicing regulator Sex-lethal directly inhibits translation of Male-specific-lethal 2 mRNA. RNA 4: 142–150. [PMC free article] [PubMed] [Google Scholar]
- Goldman T. D., Arbeitman M. N., 2007. Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 3: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A. N., Lu H., Erickson J. W., 2008. A shared enhancer controls a temporal switch between promoters during Drosophila primary sex determination. Proc. Natl. Acad. Sci. USA 105: 18436–18441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graindorge A., Carré C., Gebauer F., 2013. Sex-lethal promotes nuclear retention of msl2 mRNA via interactions with the STAR protein HOW. Genes Dev. 27: 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Castelo R., Minana B., Peden E., Blanchette M., et al. , 2011. Distinct regulatory programs establish widespread sex-specific alternative splicing in Drosophila melanogaster. RNA 17: 453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers V., Perry M. W., Hendrix D., Stark A., Levine M., et al. , 2011. Neural-specific elongation of 3′ UTRs during Drosophila development. Proc. Natl. Acad. Sci. USA 108: 15864–15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungreis I., Lin M. F., Spokony R., Chan C. S., Negre N., et al. , 2011. Evidence of abundant stop codon readthrough in Drosophila and other metazoa. Genome Res. 21: 2096–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Y., Wang E., Silterra J., Schwartz S., Wong B., et al. , 2015. Quantitative visualization of alternative exon expression from RNA-seq data. Bioinformatics 31: 2400–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R., Solovyeva I., Lyman L., Richman R., Solovyev V., et al. , 1995. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81: 867–877. [DOI] [PubMed] [Google Scholar]
- Kelley R., Wang J., Bell L., Kuroda M., 1997. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387: 195–199. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L., 2012. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Bahn J. H., Park J. H., 2006. Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila Proc Natl Acad Sci U S A 103: 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., B. Handsaker, A. Wysoker, T. Fennell, J. Ruan et al, 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattox W., Baker B. S., 1991. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes Dev. 5: 786–796. [DOI] [PubMed] [Google Scholar]
- Mattox W., Palmer M. J., Baker B. S., 1990. Alternative splicing of the sex determination gene transformer-2 is sex-specific in the germ line but not in the soma. Genes Dev. 4: 789–805. [DOI] [PubMed] [Google Scholar]
- McIntyre L. M., Lisa L. M., Bono M., Genissel A., Westerman R., et al. , 2006. Sex-specific expression of alternative transcripts in Drosophila. Genome Biol. 7: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meise M., Hilfiker-Kleiner D., Dubendorfer A., Brunner C., Nothiger R., et al. , 1998. Sex-lethal, the master sex-determining gene in Drosophila, is not sex-specifically regulated in Musca domestica. Development 125: 1487–1494. [DOI] [PubMed] [Google Scholar]
- Parisi M. J., Gupta V., Sturgill D., Warren J. T., Jallon J.-M., et al. , 2010. Germline-dependent gene expression in distant non-gonadal somatic tissues of Drosophila. BMC Genomics 11: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn J. K. M., Schedl P., 2007. The master switch gene Sex-lethal promotes female development by negatively regulating the N signaling pathway. Dev. Cell 12: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M.-L., 1998. Evidence for 3′ untranslated region-dependent autoregulation of the Drosophila gene encoding the neuronal nuclear RNA-binding protein ELAV. Genetics 150: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M.-L., 2008. Rapid functional diversification in the structurally conserved ELAV family of neuronal RNA binding proteins. BMC Genomics 9: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M. L., Chalvet F., 2003. found in neurons, a third member of the Drosophila elav gene family, encodes a neuronal protein and interacts with elav. Mech. Dev. 120: 373–383. [DOI] [PubMed] [Google Scholar]
- Samson M. L., Rabinow L., 2014. Transcriptomic analysis of sexual differentiation in somatic tissues of Drosophila melanogaster: successes and caveats. Sex Dev. 8: 113–126. [DOI] [PubMed] [Google Scholar]
- Shirangi T. R., Dufour H. D., Williams T. M., Carroll S. B., 2009. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 7: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Pierre S. E., Ponting L., Stefancsik R., Mc Quilton P., Consortium F., 2014. FlyBase 102—advanced approaches to interrogating FlyBase. Nucleic Acids Res. 42: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill D., Malone J. H., Sun X., Smith H. E., Rabinow L., et al. , 2013. Design of RNA splicing analysis null models for post hoc filtering of Drosophila head RNA-Seq data with the splicing analysis kit (Spanki). BMC Bioinformatics 14: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telonis-Scott M., Kopp A., Wayne M. L., Nuzhdin S. V., McIntyre L. M., 2009. Sex-specific splicing in Drosophila: widespread occurrence, tissue specificity and evolutionary conservation. Genetics 181: 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni E., Su S., Zraly C. B., Mattox W., 2003. Analysis of a null mutation in the Drosophila splicing regulator Tra2 suggests its function is restricted to sexual differentiation. Genesis 37: 76–83. [DOI] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P., and M. R. Green, 1993. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature 362: 171–175. [DOI] [PubMed] [Google Scholar]
- Vogel C., Marcotte E. M., 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. M., Selegue J. E., Werner T., Gompel N., Kopp A., et al. , 2008. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., Zwarts L., Callaerts P., Norga K., Mackay T. F., et al. , 2008. Neurogenetic networks for startle-induced locomotion in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 105: 12393–12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Belote J. M., 1995. Determination of the molecular lesions associated with loss-of-function transformer alleles of Drosophila melanogaster. Biochim. Biophys. Acta 1260: 369–370. [DOI] [PubMed] [Google Scholar]
- Zanini D., Jallon J. M., Rabinow L., Samson M. L., 2012. Deletion of the Drosophila neuronal gene found in neurons disrupts brain anatomy and male courtship. Genes Brain Behav. 11: 819–827. [DOI] [PubMed] [Google Scholar]
- Zheng S., Black D. L., 2013. Alternative pre-mRNA splicing in neurons: growing up and extending its reach. Trends Genet. 29: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figure S1 is a graphic representation of sex-biased alternative splicing for key splice events. File S1 is the complete Spanki analysis of the RNA-Seq data. File S2 and File S3 are detailed statistical analyses, respectively for qPCR results and for enrichments. File S4 is a supplemental discussion. Table S1 contains the Spanki analysis of the RNA-Seq data and qPCR validation for key splice events. Table S2 provides details on the primers. Accession numbers for sequence and gene expression data are specified in the Materials and Methods.