Abstract
Chronic aortocaval fistula (ACP) is a rare complication of penetrating trauma to the abdomen. We report a case of traumatic ACP presenting with pulmonary hypertension and right heart failure symptoms 15 years after the initial penetrating injury. Although symptoms of pulmonary hypertension started 5 years ago, it was wrongly diagnosed and treated as chronic obstructive pulmonary disease. The presence of a continuous abdominal bruit and history of penetrating abdominal trauma gave rise to suspicion of a fistula, which was confirmed by computed tomography and angiography. Percutaneous closure of ACP was planned, but the patient died of severe pneumonia. The clinical presentation of chronic ACP can vary from being asymptomatic to symptoms related to pulmonary hypertension, right heart failure, and pulmonary embolism; thus, definitive diagnosis can be challenging.
Keywords: Chronic aortocaval fistula, computed tomography, penetrating abdominal trauma

INTRODUCTION
Aortocaval fistula (ACP) is a rare, life-threatening condition with complicated clinical symptoms and findings requiring urgent diagnosis. Of the ACP cases, 80% occur due to erosion or rupture of the abdominal aorta into inferior vena cava (IVC).[1] Otherwise, cases related to trauma or iatrogenic injury have also been reported.[1] Late onset complications of abdominal aorta or IVC injuries are rare. They can present as pseudoaneurysm, occlusion of the visceral aortic branches, or arteriovenous fistula.[2]
Hemodynamic effects of ACP when combined with patient age and other comorbidities impose a life-threatening condition for many patients.[3] Symptoms and signs of acute abdomen may present as venous hypertension and/or systemic hypoperfusion.[3] Open surgery is the conventional method of repair in these cases; however, surgical complication risk rates are very high.[3] Percutaneous endovascular treatment is the best treatment option in these patients.[1]
We are reporting a late diagnosed ACP case that presented with late onset right heart failure and pulmonary hypertension that had developed due to penetrating abdominal trauma.
CASE REPORT
A 50-year-old male presented to the Cardiology department due to edema of the legs, erythema, pain, abdominal swelling, and was hospitalized with pulmonary hypertension, right heart failure, atrial fibrillation, chronic obstructive pulmonary disease, and cellulitis. The patient had a history of appendectomy when he was 20 years old and had been stabbed in the abdomen when he was 35 years old. He had been stabbed twice, and 1 h after the stabbing, he had been successfully treated by general surgery. The patient had been discharged 1 week postoperatively. The patient also had a 4-year history of smoking during his adolescent period. Five years earlier, the patient (age 45 years) had presented to the Cardiology outpatient service and was diagnosed with New York Heart Association (NYHA) Class II shortness of breath. On echocardiography (echo), his right heart cavity dimensions were mildly increased and pulmonary artery systolic pressure was found to be 45 mmHg. After treatment, his complaints regressed completely. Three years earlier (age 47 years), he had been admitted to the Pulmonology department with abdominal and bilateral leg swelling, and shortness of breath (NYHA Class III) complaints. CT thorax was performed and he was diagnosed with right heart failure. But dilated IVC was missed or might have been accepted as secondary dilatation to pulmonary hypertension and right heart failure. Intravenous diuretics, steroids, teophylline, and inhaled bronchodilator treatment had been administered. Subsequently, his complaints regressed. His echo findings at that period of time were as follows: Left ventricular ejection fraction 65%, Grade I diastolic dysfunction, normal left heart cavities, significantly enlarged right heart cavities, pulmonary artery systolic pressure 70 mmHg, and Grade 2–3 tricuspid failure. He was being closely monitored by the pulmonologist for pulmonary hypertension. One year earlier (age 49 years), he had been admitted to the Pulmonology department due to newly developed high ventricular rate atrial fibrillation (168 bpm) resulting in significant abdominal ascites, diffuse edema of the lower extremities, and crackles in the middle and lower lung zones (NYHA functional Class IV). At that period of time, the echo had shown normal left heart cavity dimensions, left ventricular ejection fraction 50%, paradoxical interventricular septal motion, significantly enlarged right heart cavities, pulmonary artery systolic pressure 95 mmHg, and Grade 3 tricuspid insufficiency. The patient was started on digoxin 1 × 1 tb, diltiazem 2 × 120 mg, and warfarin by the cardiologist. In 1 week, his symptoms regressed to NYHA Class III and he was discharged from the hospital. Fifteen days later, the patient was admitted to Emergency department with symptoms of worsening right heart failure and shortness of breath; therefore, he was moved to the Cardiology department and started on intravenous furosemide, spironolactone 25 mg, clexane 2 × 0.6 cc (because of subtherapeutic international normalized ratio), and continued on diltiazem and digoxin. According to the dermatologist's recommendation, he was also started on ampicillin/sulbactam 4 × 1 g IV. Laboratory findings were as follows: Blood urea nitrogen 60 mg/dl (normal range 7–22 mg/dl), creatinine 1.2 mg/dl (normal range 0.8–1.3 mg/dl), aspartate aminotransferase 40 U/l (normal range 0–35 U/l), alanine aminotransferase 45 U/l (normal range 0 –35 U/l), hemoglobin 12.8 g/dl (14.0–17.4 g/dl), white blood cells 14,500/µl (normal range 3,500–10,500 µl). On the second day of admission, the patient first developed respiratory arrest and then cardiac arrest. Cardiopulmonary resuscitation (CPR) was initiated, the patient was intubated, and on the 5th minute of CPR, he gained basal rhythm of atrial fibrillation and a blood pressure of 100/60 mmHg. The patient was then transferred to the intensive care unit and placed on a mechanic ventilator. With an initial diagnosis of pulmonary embolism, the patient had undergone pulmonary CT angiography; however, no thrombi were detected. Deep vein thrombosis (DVT) protocol CT was not performed. Only thorax CT angiography and lower extremity venous Doppler ultrasonography were performed. But no thrombotic formation was detected at Doppler examination. The diagnosis was missed by physicians. But simple abdominal auscultation revealed the diagnosis. The IVC diameter was found to be 19 cm. On cardiologic evaluation, at the tricuspid focus, on the left and right corners, a harsh pansystolic murmur at 4–5/6 intensity was heard. The liver was palpable 7–8 cm under the costal arch, and diffuse ascites was detected in the abdomen. Edema of the legs was 3+. On the umbilical region of the abdomen, a continuous thrill and a machine-like murmur were detected [Figure 1]. On echo, right cardiac cavities were severely enlarged, and 3–4 degree of tricuspid failure, pulmonary artery pressure of 75–80 mmHg, and normal left heart cavities were detected. IVC was measured and found to be 18 cm. On the region of abdominal murmur, the echo probe showed fistula between the abdominal aorta and the IVC on transverse plane [Figure 2a and b]. Transesophageal echocardiography showed intact interventricular septum and patent foramen ovale of the interatrial septum. Right to left shunt of the patent foramen ovale was seen. CT angiography was performed in order to confirm the ACP and to determine its dimensions. Right at the lower level of renal artery and bifurcation of the aorta, between IVC and abdominal aorta, a fistula was detected. At the localization of fistula on the lower level of the renal artery [Figure 3a], the diameter of the aorta was 22 mm, IVC diameter was 99 mm, and the diameter of the fistula tract was 11 mm. At the localization of fistula on the abdominal aortic bifurcation [Figure 3b], the aortic diameter was 25 mm, IVC diameter was 169 mm, and fistula tract diameter was 17 mm. Hemodynamic work-up and shunt measurements were planned. On abdominal aortography, findings consistent with CT angiography (two fistulas from the abdominal aorta to the IVC) were found [Figure 4]. Oxymetrically calculated left-to-right shunt was 2.8. On coronary angiography, the coronary arteries were normal and systolic pulmonary artery pressure was 75 mmHg [Figure 5]. The patient's condition was hemodynamically stabile; however, considering the possible complications, the ACP was decided to be closed percutaneously. The procedure was to be performed after the pneumonia secondary to intubation had resolved. Despite the antibiotic therapy, the pneumonia did not resolve. The patient developed sepsis and died of septic shock in the intensive care unit.
Figure 1.
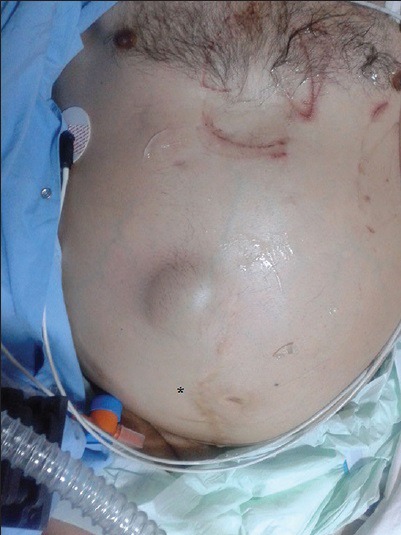
50-year-old male presented with edema of legs, erythema, pain, and abdominal swelling and was later diagnosed with chronic aortocaval fistula. Abdominal photograph of patient shows a continuous thrill (star), and a machine-like murmur was detected on the umbilical region of the abdomen.
Figure 2.
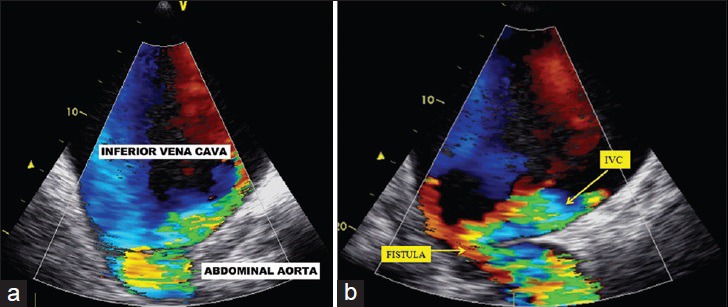
50-year-old male presented with edema of legs, erythema, pain, and abdominal swelling and was later diagnosed with chronic aortocaval fistula. Transesophageal echocardiography (a) on transverse plane, (b) on the region of abdominal murmur, show fistula between the abdominal aorta and the IVC.
Figure 3.
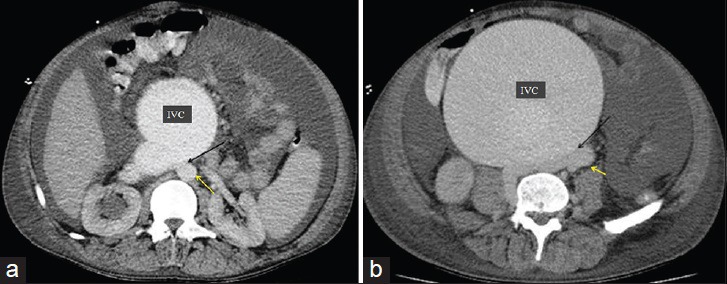
50-year-old male presented with edema of legs, erythema, pain, and abdominal swelling and was later diagnosed with chronic aortocaval fistula. Axial contrast-enhanced CT angiography, (120/350 cc contrast medium, injection rate 4 cc/sec, scan time 30. sec) shows fistula (black arrow) at the lower level of renal artery and the bifurcation of the aorta between the abdominal aorta (yellow arrow) and the IVC. (b) Axial contrast-enhanced CT angiography, showed fistula (black arrow) at the at the abdominal aortic bifurcation, between the abdominal aorta (yellow arrow) and the IVC.
Figure 4.
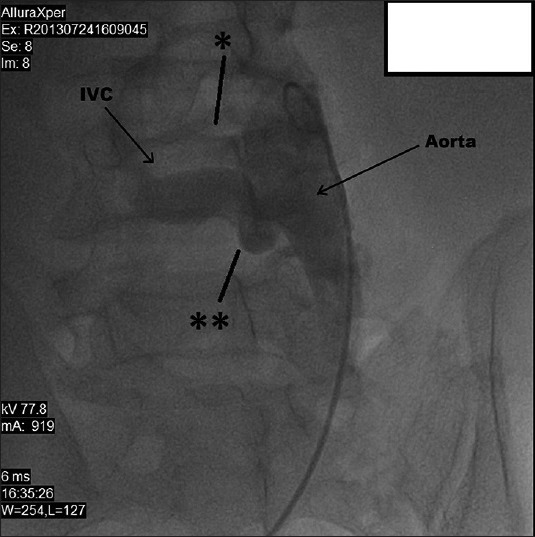
50-year-old male presented with edema of legs, erythema, pain, and abdominal swelling and was later diagnosed with chronic aortocaval fistula. Abdominal aortography shows two fistulas (stars) between the abdominal aorta and the IVC.
Figure 5.
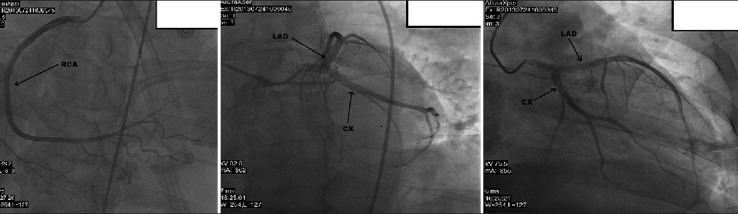
50-year-old male presented with edema of legs, erythema, pain, and abdominal swelling later diagnosed with chronic aortocaval fistula. Coronary arteries are seen on coronary angiography.
DISCUSSION
ACP was first described by Syme in 1831.[1] The first succesful open surgery was performed in 1954 by Cooley et al.[3] Eighty to ninety percent of the ACPs occur spontaneously. They may occur as a result of infrarenal abdominal aortic aneurysm erosion or rupturing of the aneurysm into the vena cava inferior. Very rarely, the aneurysm may fistulize to the left renal vein. In these cases, the left renal vein is generally retroaortic. Ten to twenty percent of the cases are iatrogenic.[4] The most common cause of ACPs are penetrating firearm (piece, fragment) or stabbing (knife, skewer) injuries. Iatrogenic injuries during arterial and cardiac catheterizations or during lumbal disc surgeries, and blunt traumas are rarer etiologic factors.[2]
Clinical manifestations of traumatic ACP occur in two ways. In acute cases, most of the patients die at the place of injury or are rushed into the emergency department in serious hypotensive shock accompanied by other injuries. The mortality rate in this group of patients is 40–50%.[2] In chronic conditions, however, the presence of ACP is detected weeks, months, or years after the injury. The fistula may grow or spontaneously close in time.[2] Accordingly, chronic ACP may be asymptomatic or at the side of injury, it may show signs of a consistent thrill, pain, peripheral edema, arterial insufficiency, and congestive heart failure.[2] Factors determining the severity of compensatory changes are the dimension of the fistula, the diameter of the involved artery or vein, patient's age, and proximity to the heart.[2] Chronic traumatic ACPs are more often seen in young and healthy males. These patients adapt to the hemodynamic changes caused by ACPs easier as they rarely have cardiac problems.[2]
Clinical symptoms vary according to the diameter of the fistula, anatomical position (proximal or distal), and time of occurence (acute or chronic). Large arteriovenous fistulas have common symptoms and signs such as high-output heart failure, abdominal thrill, hypotensive shock, oliguria, regional venous hypertension, and stasis (leg edema, hematuria, rectal bleed).[5]
Most common symptoms and findings in chronic ACPs are high-output cardiac failure (dyspnea, elevated jugular venous pressure, pulmonary edema, wide pulse pressure), abdominal thrill, palpable abdominal aneurysm (if related to aneurysm), oliguria, and regional hypertension findings (edema of the legs, cyanosis, pulsatile varicose veins, hematuria, rectal bleed). Shock, abdominal pain, chest pain, lower back pain, scrotal edema, tenesmus, priapism, and weak peripheral pulse are the other symptoms and signs encountered.[6] Furthermore, recurrent pulmonary embolisms may be seen.[6]
ACP may be closed with conventional open surgery. However, mortality rate in this procedure is 67%, which is very high.[7] Massive intraoperative hemorrhages related to enlarged retroperitoneal veins are inevitable, particularly in chronic fistulas due to arterialization of the veins.[6] Increased hemorrhage risk may be seen during large arteriovenous fistula repairs due to arterialization of the veins and perivascular inflammation. Furthermore, reccurent paradoxal pulmonary embolisms and cardiac decompensation are the other complications encountered.[8]
In 1999, Parodini et al., performed the first endovascular repair of chronic ACP.[9] Not requiring laparotomy, lower loss of blood, use of local anesthetics instead of general anesthesia, lower related post-operative complications, and reduced costs have made endovascular stent graft procedure more attractive than open surgery. Coated stents, abdominal aortic endografts, coil embolization, and patent ductus arteriosus device are the endovascular techniques invented until now.[10]
The use of aortic stent grafting has benefits such as faster cardiopulmonary recovery and reduced blood loss.[9]
Considering the cardiopulmonary condition of the patient presented in the report, the ACP was decided to be closed using thoracic graft stent due to the high surgical risks involved. However, given the risk of possibly developing infective endocarditis from the graft, priority had been given to his recovery from pneumonia. Despite intensive antibiotic therapy, the pneumonia did not resolve, and the patient developed ARDS and sepsis, and succumbed due to multiorgan failure. It is clear that if the ACP had been detected 5 years earlier during early symptoms, the benefits of endovascular or surgical procedure would have been very high. It should also be considered that in early phase where IVC is not significantly enlarged, and pulmonary hypertension and right heart failure have not developed, interventions would carry much lesser amount of complication risk.
Thrombotic complications may also be seen as a result of endovascular treatment, causing life-threatening situations and clinical emergencies. Acute IVC occlusion (highly decreased blood flow in highly enlarged IVC due to stasis), persistent severe lowere extremity edema, and continuous worsening of the clinical condition are some of these complications. In such cases, further evaluation by venography and recanalization to the IVC should be performed. Compression of the IVC by the aortic aneurysm sack may result in intraluminal thrombus formation. Paradoxic pulmonary embolism is one of the reported complications that may occur before, during, or after the treatment.[7,8] Compared to open surgery, endovascular treatment has lower operative and 30-day mortality rates and overall low morbidity rates.
CONCLUSION
Chronic ACP is a rare clinical condition that might have simple to serious manifestations. Early diagnosis and intervention before cardiopulmonary deterioration is important and life saving in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2015/5/1/62/170731
REFERENCES
- 1.Akwei S, Altaf N, Tennant W, MacSweeney S, Braithwaite B. Emergency endovascular repair of aortocaval fistula-a single center experience. Vasc Endovascular Surg. 2011;45:442–6. doi: 10.1177/1538574411407087. [DOI] [PubMed] [Google Scholar]
- 2.Spencer TA, Smyth SH, Wittich G, Hunter GC. Delayed presentation of traumatic aortocaval fistula: A report of two cases and a review of the associated compensatory hemodynamic and structural changes. J Vasc Surg. 2006;43:836–40. doi: 10.1016/j.jvs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Takkar C, Choi L, Mastouri N, Kadambi PV. Aortocaval fistula: A rare cause of venous hypertension and acute renal failure. Case Rep Surg 2012. 2012:487079. doi: 10.1155/2012/487079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidovic LB, Kostic DM, Cvetkovic SD, Jakovljevic NS, Stojanov PL, Kacar AS, et al. Aorto-caval fistulas. Cardiovasc Surg. 2002;10:555–60. doi: 10.1016/s0967-2109(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 5.Leigh-Smith S, Smith RC. Aorto caval fistula-the “bursting heart syndrome”. J Accid Emerg Med. 2000;17:223–5. doi: 10.1136/emj.17.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightwell RE, Pegna V, Boyne N. Aortocaval fistula: Current management strategies. ANZ J Surg. 2013;83:31–5. doi: 10.1111/j.1445-2197.2012.06294.x. [DOI] [PubMed] [Google Scholar]
- 7.Laporte F, Olivier A, Groben L, Admant P, Aliot E. Aortocaval fistula: An uncommon cause of paradoxical embolism. Cardiovasc Med (Hagerstown) 2012;13:68–71. doi: 10.2459/JCM.0b013e32834039d7. [DOI] [PubMed] [Google Scholar]
- 8.DeRango P, Parlani G, Cieri E, Verzini F, Isernia G, Silvestri V, et al. Paradoxical pulmonary embolism with spontaneous aortocaval fistula. Ann Vasc Surg. 2012;26:739–46. doi: 10.1016/j.avsg.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou GA, Koutsias S, Karathanos C, Sfyroeras GS, Vretzakis G, Giannoukas AD. Endovascular stent-graft repair of major abdominal arteriovenous fistula: A systematic review. J Endovasc Ther. 2009;16:514–23. doi: 10.1583/09-2725.1. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell ME, McDaniel HB, Rushton FW., Jr Endovascular repair of a chronic aortocaval fistula using a thoracic aortic endoprosthesis. Ann Vasc Surg. 2009;23:150–2. doi: 10.1016/j.avsg.2007.10.007. [DOI] [PubMed] [Google Scholar]


