Abstract
Background:
Prader-Willi syndrome (PWS) results from a deletion of the paternal genes in the region of chromosome 15q11-q13. PWS develops hyperphagia, which when left unmanaged, leads to an excessive ingestion of food. To date there is inadequate pharmacological treatment or supplementation for modification of the PWS hyperphagia and/or the associated behaviors. Therefore, the best practice is familial supervision and restriction of diet and environment.
Aim:
We aimed to determine if the natural supplement of Caralluma fimbriata extract (CFE) could attenuate hyperphagia or the associated appetite behaviors in children and adolescents with PWS over the 4-week pilot trial period.
Materials and Methods:
We conducted a placebo-controlled, double-blind, randomized crossover trial over a 10-week period to investigate the effects of CFE on hunger control, in a cohort of children and adolescents with confirmed PWS (n =15, mean age 9.27 ± 3.16 years, body weight 43.98 ± 23.99 kg). Participants from Australia and New Zealand ingested CFE or a placebo of maltodextrin/cabbage leaf over a 4-week period, with a 2-week washout before the crossover to the other treatment. Weekly comparisons in appetite behavior, severity, and drive were recorded by parents, as scaled time-point measures on a hyperphagia questionnaire validated for PWS.
Results:
CFE administration was found to induce a significant accumulative easing of hyperphagia (P = 0.05), with decreases evident in one-third of the participants. Furthermore due to CFE supplementation, a significant decrease (P ≤ 0.05) was recorded in the category of behavior and a decrease in hyperphagia (n = 8, P = 0.009) was observed at the highest dose 1,000 mg/day (recommended adult dose). There were no reported adverse effects at any dose.
Conclusion:
We demonstrate that an extract of the Indian cactus succulent Caralluma fimbriata eases hyperphagic appetite behavior within a cohort of children and adolescents (n = 15) with PWS without notable adverse effects. The outcomes of this study will have a potential positive impact on PWS management.
Keywords: Algorithm, emergency treatment, meta-analysis, reliability and validity, triage
Introduction
Prader-Willi syndrome (PWS) results from a deletion of the paternal genes in the region of 15q11-q13.[1] Though the condition is rare (prevalence of 1:10,000-1:30,000)[2,3,4,5] the fundamental issues of growth hormone deficiency, raised ghrelin levels, hyperphagia, and a well-defined impaired satiety[6,7] determine PWS to be the most prevalent genetic predictor of obesity. The appetite behaviors exhibited by individuals with PWS require a comprehensive intervention methodology though at this time there are inadequate strategies for long-term modification and pharmacological treatments are limited or nonexistent.
Due to complex syndrome characteristics, which include slowed metabolic rate, hypotonic muscle tone, and reduced energy expenditure, it has been acknowledged that all individuals with PWS will need to follow a diet of less calories than the general population (approximately 60%). In PWS, weight loss is difficult — once one becomes overweight — as any further caloric reductions may impede nutritional requirements.[5]
Eventually, individuals who possess any of the three distinct genotypes within the PWS “critical region” of chromosome 15q11-13 develop hyperphagia due to endocrine irregularities and discrete yet significant neurological differences.[7,8,9] The genotype delineations are: “deletion or noninherited” (approximately 70%), which is a loss of three to four megabases of paternal genetic material within the critical PWS region; “uniparental disomy” (UPD) (approximately 25%), which is a deletion of the paternal critical region with an inheritance of two silenced maternal alleles of these genes. The remaining individuals with PWS are delineated under the genotype “imprinting error” or “micro deletion,” which is an error during imprinting rendering the paternal contribution nonfunctional. The paternal contribution is of importance as the maternally derived genes are silenced due to structural modification or epigenetic modulation (DNA methylation).[10]
The literature does not really explore a capacity for full independence.[11,12] It has been proposed that early familial supervision of the proximity and choice of food may decrease the consistent preoccupation with food seen in individuals with PWS. Families are uniformly the axis of support and restriction.[11,13] For some individuals the regulation of the surroundings[1,14] includes locked food storage and/or restricted access in certain environments. This is mainly due to food seeking or foraging.[15] Continued opportunities for overeating may progress to morbid obesity often associated with cardiorespiratory disorders[16] or in some (especially where the individual has slimmed down) it may lead to gastric rupture or narcosis.[17]
The behavioral profile of individuals with PWS is complex; it includes issues with learning,[11] conceptual understanding,[18] attention-switching,[19] skin-picking, hoarding, redoing and extensive repetitive questioning,[18] anxiety, stubbornness, and/or temper tantrums.[14] Many of the PWS behaviors are components of an autism spectrum disorder (ASD)[1] and/or as obsessive compulsive disorder (OCD).
In this study, we investigated an intervention to support food-related abstinence for children and adolescents with PWS to ease/decrease the hyperphagic behaviors exhibited during the habituated yet necessary familial restrictions. The intervention was a 4-week oral ingestion of a natural supplement, Caralluma fimbriata extract (CFE). This cacti-form succulent is a hardy roadside shrub well-known in Ayurvedic medicine for its attribute of hunger control. This traditional vegetable substitute has been ingested for centuries among tribal populations in India.[20] Though bitter to taste, CFE powder is easy to drink and its safety and toxicity profile have been rigourously studied.[21] CFE is commercially available in many countries, including Australia (Aust.) and New Zealand (N.Z.) where our participants resided.
Background
Our focus on CFE is due to anecdotal evidence, showing continuous decreased appetite behavior in a single case study for a child with PWS, recorded over an 8-year period with significant efficacy. In non-PWS controlled studies, ingestion of CFE has determined appetite suppression[20] and significant waist circumference reduction in overweight/obese individuals.[22] Animal studies have demonstrated dose responsive appetite suppression and significant nootropic and anxiolytic effects in rats.[23] With regard to toxicology, the powdered extract has been investigated at extremely high doses (5,000 mg/kg bw/day) and over a long treatment period with no significant toxicological effect.[21,23]
Materials and Methods
Clinical trial design
We conducted a placebo-controlled, double-blind, randomized crossover trial over a 10-week period investigating the effect of CFE on the typical hyperphagic appetite behavior in children and adolescents with confirmed PWS. Any change was observed and recorded by their parents on a questionnaire validated for PWS,[24] over each 4-week period, of both the ingestion of CFE and a placebo (PLAC) of maltodextrin/cabbage leaf. There was a 2-week washout period between the two treatment periods.
Participants, recruitment and inclusion
The clinical trial was registered through the Australian and New Zealand Clinical Trials Registry (ANZCTR); registration number: 00336712. Informed consent was obtained from all participants and their parents with verbal medical consent from their doctors. The research was approved by the PWS Associations and an enquiry with N.Z. Medsafe confirmed that approval was not obligatory for N.Z. as CFE is “listed” with the Therapeutic Goods Administration (TGA). Further to this, TGA was submitted a requested notification for CFE supplementation beyond its licensed use.
Due to cohort vulnerability, considerations of inclusion and exclusion followed strict criteria. All the recruited participants (n = 16) had diagnostically confirmed PWS and were between the age of 5 years and 17 years. The lower range limit was to allow for informed consent and the upper range was the maximum age for guardianship. Participants were expected to reside within their typical supervisory environment over the trial period. Accordingly, one participant's data was disqualified as the parental and environmental parameters had been transferred.
Exclusions included people who could possibly experience unexpected adverse side effects due to the severity of an established medical condition (e.g., respiratory disorders, kidney disease) or those on medication. Growth hormone treatment (GHT) was allowed if established more than 2 months earlier. The participating parents were required to be in communication with all those involved including school teachers, school aides, and respite workers.
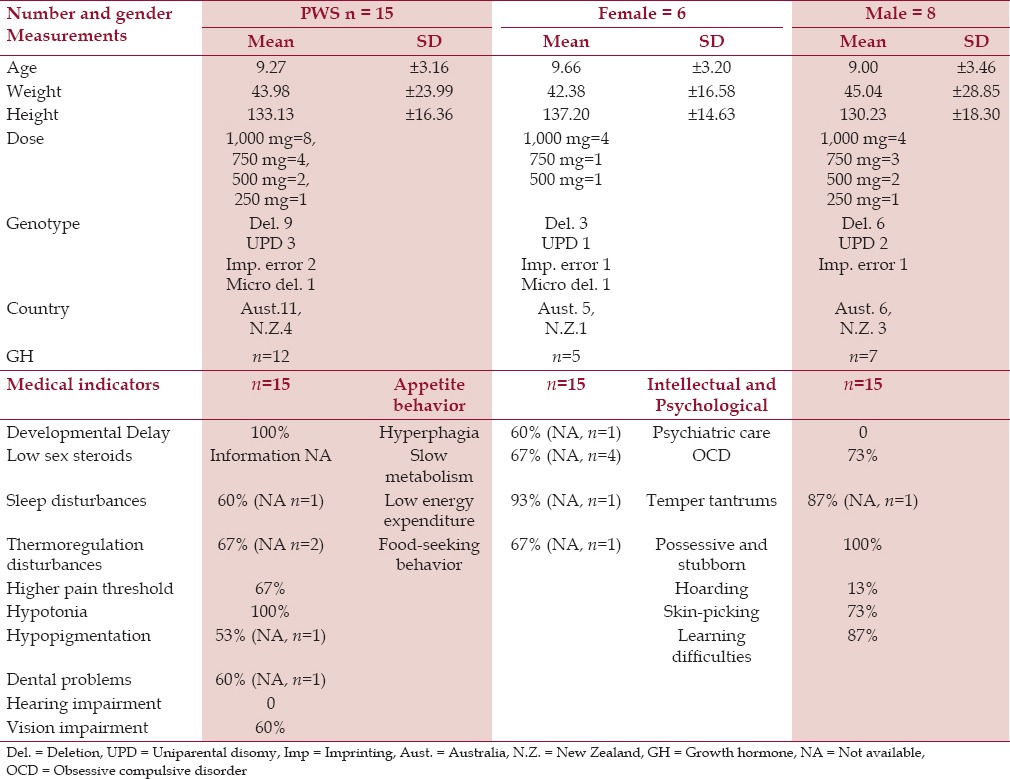
The final cohort had 15 participants (mean age 9.27 ± 3.16 years, body weight 44 ± 24 kg, height 133 ± 16 cm) with a mixed gender (males = 9 and females = 6). Anthropometric measurements and genotypes were also recorded, as shown in Table 1.
Table 1.
Anthropometric, behavioral, and clinical characteristics of children and adolescents with PWS
Caralluma fimbriata
Both CFE treatment and the placebo were supplied by Gencor Pacific International Ltd. (Hong Kong, China). The constituents of Caralluma fimbriata (the supplement) were extracted from the aerial parts of the plant with an alcohol solution, granulated, ground, and then dried. The powdered extract was then enclosed in 250 mg unlocked cylindrical capsules for storage at room temperature.
The placebo of maltodextrin (glucose polymers) and cabbage leaf — to match the CFE's color and the bitter taste — were also enclosed in coded 250 mg capsules (200 mg maltodextrin and 50 mg cabbage leaf powder). To prevent choking, each participant's coded full dose was dissolved by the parents in a tropical juice for breakfast while remaining blinded to the actual treatment.
All participants were sent their nonlabeled treatment and an adverse effects protocol by mail at baseline and midline. Compliance of the participants to the program was assessed weekly by phone or email.
Outcome measures
Anthropometric measures, dose, and blinding
The initial baseline anthropometric measurements were body height and weight in kilograms checked against medical questionnaires, to ensure correct dosage. We conservatively capped the intervention dose at the 1,000 mg/day recommended adult dose, taken from instructions on all commercial packaging and subsequent trials in humans[20] as well as dosages shown to reduce appetite in animals (50 mg/kg/day).[25] The agreement on dose was delineated for our cohort as 250 mg per 10 kg of body weight, up to and not exceeding the recommended adult dose (1,000 mg/day = 250 mg × 4).[20] The daily dose was ingested first thing in the morning.
Hyperphagia questionnaires
The life-threatening and often idiosyncratic appetite behaviors in PWS are determined by common expected markers, which may range from repeatedly asking for food, to obtaining food when not allowed. These are well-recognized by the parents due to a history of constant attention and supervision. We, therefore, tested our hypothesis by utilizing a 13-question hyperphagia questionnaire (HQ)-validated for PWS.[24] Evidence of any change in satiety or behavior was marked within a multiple choice range with a five-step gradient from 1 (low — not a problem) to 5 (high frequently — a problem). Measurements were taken at the baseline (week 1 — HQA), during the second and third weeks (HQB and C), and the day after the end of treatment in week 4 (HQD). After a 2-week washout, this process was repeated during the crossover treatment/placebo (HQE baseline — HQH posttreatment). Data were collected from the 13 questions, with 11 questions specifically designed to identify changes of appetite over the trial, grouped into severity, drive, and behavior. The other two variables for analysis were: the age the hyperphagia was detected (HQ12) and the variability over the trial (HQ13).[24]
Adverse events
The adverse effects protocol and weekly communications advised parents to be vigilant in noticing any side effects or gastrointestinal stomach upsets as individuals with PWS may have a reduced sense of pain.[2] The parents were also advised to monitor their child's fluid intake. Those with a strong recorded history of reduced vomiting were excluded. Other trials of CFE in non-PWS adults reported occasional moderate upsets, which subsided after 1 week.[20]
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS version 20.0 for Windows and Microsoft Office Excel 2010). Statistical significance was defined at 95% confidence intervals. Changes to appetite behavior were evaluated using independent paired, t-tests and analysis of variance (ANOVA) for repeated measures. Eleven of the questions were loaded for parametric delineation. HQ12 and HQ13 were not within the factor analyses. Time-point comparisons of variability from the baseline to week 4 of each treatment were analyzed and the results are expressed as “accumulative” in mean ± standard deviation (SD), with ≤0.05 considered to be significant. The results are also defined in categories of behavior, drive, and severity as per Dykens 2007.[24] The scores were also analyzed to determine if there was any significant interaction between the parameters CFE/dose or the participant's genotype, body weight, or the timeline of his/her hyperphagic drive.
Results
This is the first trial of a natural supplement for the control of the hyperphagic drive and associated behavior in children and adolescents with PWS. At the baseline (n = 15), the scores for either treatment (from HQA and HQE) were not significantly different though after 4 weeks’ treatment with CFE (n = 15), there was a significant reduction in the cumulative hyperphagia score during CFE treatment [CFE: - 0.18 ± 0.66 as opposed to the placebo (PLAC) −0.03 ± 0.76] with a significant (P = 0.05) delta score as shown in Table 2. A significantly decreased delta score was recorded in the category of behavior (n = 15) due to CFE −0.20 ± 0.61, PLAC 0.01 ± 0.81 (P = < 0.05); however, no marked changes were observed in the drive (P = 0.30) and severity (P = 0.54) [Table 2]. In addition, the results for gender determined significant reduction in the cumulative hyperphagia score during CFE treatment for the female group (n = 6), (P = 0.05) as opposed to the males, which was not significant (n = 9), (P = 0.24) [Table 2].
Table 2.
Results for accumulative change baseline after treatment. Caralluma fimbriata extract against a placebo of maltodextrin/cabbage leaf in children and adolescent participants with Prader-Willi syndrome (PWS) (n = 15)
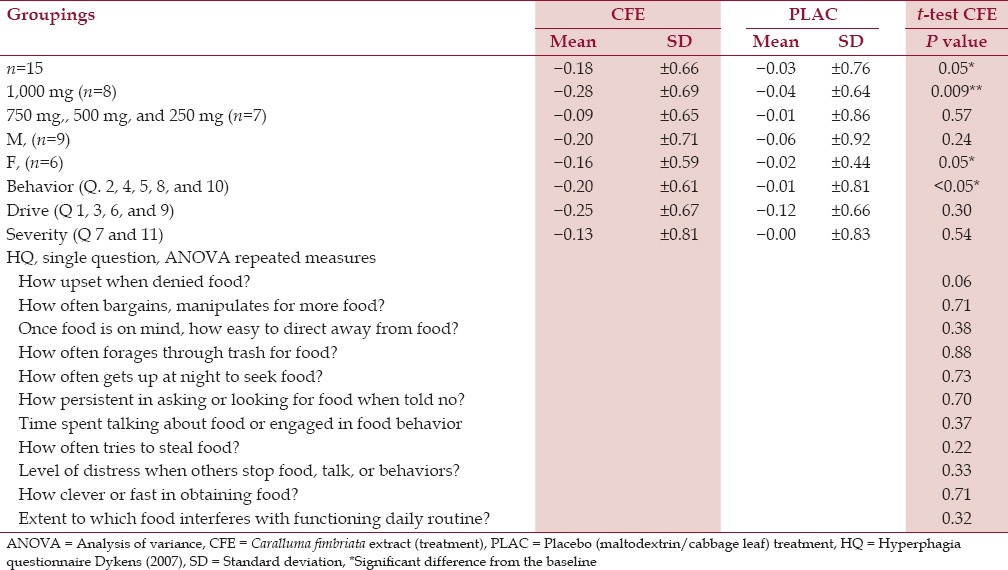
Caralluma ingestion demonstrated a significant accumulative hyperphagia decrease related to dose; with a mean decrease being observed at the highest dose of 1,000 mg/day (n = 8) of CFE 0.28 ± 0.69, PLAC −0.04 ± 0.64 (P = 0.009). Due to the small participant numbers, the lower doses (750 mg, 500 mg, and 250 mg) did not have sufficient statistical power to determine any dose-related effects.
There was a large variation between participant scores as some behaviors were less established within the cohort. Decreases ranged up to 4 points per question though due to the age of the cohort many scores on several behavioral measurements were already at the lowest rating, with no possibility of change. Therefore, the results created an overall trend; those with less apparent behaviors experienced less change. Individually, CFE administration induced an accumulative easing in appetite behavior for one-third of the participants.
Discussion
Individuals with PWS normally exhibit an appetite disorder. Accordingly, we focused our study on investigating an intervention for the hyperphagia and the associated problematic behaviors. This is the first trial demonstrating the efficacy of using a natural product to attenuate the hyperphagia in individuals with PWS. Importantly, this study also demonstrates the tolerability of CFE supplementation in children and adolescents with PWS with no adverse effects reported at any dose over the 4-week trial period.
In such a young cohort, these results present a novel approach in the management and treatment of this complex disorder. Clearly, changes were more likely to be present in individuals with the higher doses of CFE [Figure 1] and for those presenting with more severe PWS appetite characteristics at the baseline. There was also a significant result for the cumulative hyperphagia score for those in the highest dose subgroup (n = 8, 40-114 kg body weight) ingesting (1,000 mg/day). In general, the appetite reduction was more apparent in individuals with lower body weight.
Figure 1.
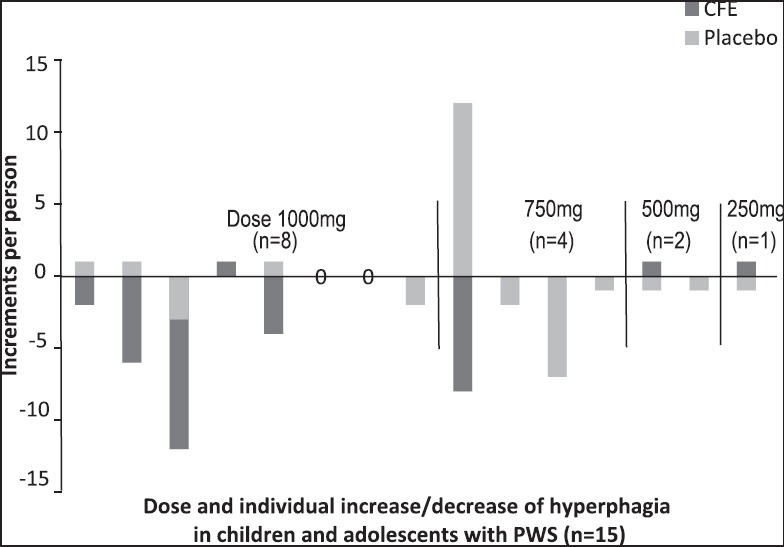
Placebo-controlled, double-blind, randomized crossover pilot trial over a 10-week period investigating the effect of Caralluma fimbriata extract (CFE) vs placebo maltodextrin/cabbage leaf, in children and adolescents with Prader-Willi syndrome (PWS) (n = 15). Delta scores were calculated over a 4-week period for both CFE and placebo groups
It is inevitable that our pilot study had several limitations. First, the small sample size and second, no blood samples were collected during the trial period due to the age and vulnerability of our cohort. In the future, it is important to address the relationship between PWS and blood serum levels including ghrelin, leptin, liver function, blood lipids, and glucose tolerance tests during CFE administration.
Another limitation was that the parametric testing of the hyperphagia was solely defined by nonparametric parental questionnaires. Though the daily scores of hunger on visual analogue scale (VAS) — before and after meals — may have generated further understanding of the cohort's hunger, the concentration on rating hunger may have confounded the data for certain questions on the HQ, i.e., time spent talking about food or engaged in food behavior (Q7). Further, appetite tests were beyond the scope of this trial due to geographic constraints.
To support the validity of the numerical markers, “best practice” for the management of the appetite behavior in PWS constitute supervision and routine,[2,8] The PWS HQ incorporates the typical close supervisory proximity and typically favors a constant routine because of the PWS behavioral profile. However, a parent's numerical rating of the behavior within his/her child's accumulative week's scores may be skewed by an unexpected change of routine or an incidental moment of food preoccupation. So too, expectations of future occasions (e.g., school holidays or birthday parties)[18,26,27] may heighten the level of food concentration and on occasion alter the routine. It is, therefore, recommended that to improve the trial stability in the future it may be useful to create an extra question regarding unusual occurrences and introduce an individualized appetite marker as “questions 13 and 14.”
Clearly, during CFE administration some parents reported a noticeable drop in their personalized predictors, e.g., less clock-watching and asking for breakfast, food, or snacks. This easing of problem behavior around food was not universal but it was clearly seen in those on a higher dose at the mid to lower range in weight, evident in the 1,000 mg group (n = 8) exhibiting a significant decrease in overall appetite behaviors (P = 0.009). Further, though 100% of the participants exhibited temper tantrums at the baseline, CFE was reported to ease these types of behaviors (P = <0.05). One could speculate that long-term CFE administration may contribute to a less habituated obsessive compulsive demeanor due to the nootropic and anxiolytic effect of CFE demonstrated in mice.[23]
Another limitation was that many of the younger children in our cohort had lower hyperphagic scores at baseline, which corresponded with the timeline of appetite behavior in the PWS phenotype. HQ question 12 pinpointed the onset of the initial hyperphagic behavior as mean 5.5 ± 2.5 years. Obviously, there is inadequate pharmacological treatment during the initial hyperphagic stage or for that matter into adulthood in PWS; so further research of CFE may need to determine if an earlier supplementation of CFE has any influence on the onset of the hyperphagia[28] and easing of the appetite behaviors into adulthood.
It is proposed that the active components within the hydroethanolic extract of Caralluma are the pregnane glycosides, which reportedly have selective serotonin reuptake inhibitor (SSRI)-like activity.[29] One proposed notion for the mechanism of hyperphagia in PWS, is namely, that reduced serotonin (5-hydroxytryptamine; 5-HT)-mediated signaling may cause decreased signaling of satiety in the brain.[10,19] Limited studies on SSRIs in PWS[30,31] have shown some clinical efficacy.
Conclusions
We demonstrated that an extract of the Indian cactus succulent Caralluma fimbriata eased hyperphagic appetite behavior within our initial cohort of children and adolescents (n = 15) with PWS. CFE administration was found to induce a significant overall easing of hyperphagia after 4 weeks of treatment compared to the placebo (P = 0.05). Due to CFE supplementation, there was a significant decrease in the category of behavior (P = <0.05) recorded by the parents and similarly, a significant decrease was observed after 4 weeks of CFE administration in response to the highest dose (1,000 mg/day CFE) (n = 8, P = 0.009), which was the recommended adult dose. The individual scores indicate that adjusting the dose according to body weight to a level higher than the cap (1,000 mg/day) used in this study should be investigated in a larger cohort of participants with PWS.
It is important to recognize that the participants within this trial had well-documented chronic behaviors of differing severity, which had not been ameliorated or modified by any previous process. The attenuation in appetite behaviors in PWS through CFE supplementation is therefore noteworthy. Importantly, we have established the tolerability of CFE as a natural and noninvasive approach for short-term treatment and management within children and adolescents with PWS. The outcomes of this study will have a potential positive impact on PWS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest for any of the authors in this trial.
Acknowledgments
We thank the participants and parents within the trial for their enthusiasm and commitment. National PWS Association, New Zealand PWS Association, Victorian PWS Association for their support and Neil Diamond, Mark Scarr, and Pat McLaughlin for their statistical advice. We are grateful to AZPA International and Gencor Pacific Ltd. for its generosity in supplying Victoria University with the supplements.
References
- 1.Cassidy SB, Driscoll DJ. Prader-Willi syndrome. Eur J Hum Genet. 2009;17:3–13. doi: 10.1038/ejhg.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-willi syndrome. Genet Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 3.Tuysuz B, Kartal N, Erener-Ercan T, Guclu-Geyik F, Vural M, Perk Y, et al. Prevalence of prader–willi syndrome among infants with hypotonia. J Pediatr. 2014;164:1064–7. doi: 10.1016/j.jpeds.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 4.Pignatti R, Mori I, Bertella L, Grugni G, Giardino D, Molinari E. Exploring patterns of unwanted behaviours in adults with prader–willi syndrome. J Appl Res Intellect Disabil. 2013;26:568–77. doi: 10.1111/jar.12047. [DOI] [PubMed] [Google Scholar]
- 5.Whittington J, Holland A. Neurobehavioral phenotype in Prader–Willi syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:438–47. doi: 10.1002/ajmg.c.30283. [DOI] [PubMed] [Google Scholar]
- 6.Holland AJ. The paradox of prader willi syndrome: A genetic model of starvation. J Intellect Disabil Res. 2008;52:811. [Google Scholar]
- 7.Miller JL, James GA, Goldstone AP, Couch JA, He G, Driscoll DJ, et al. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader-Willi syndrome. J Neurol Neurosurg Psychiatry. 2007;78:615–9. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Matsuzawa H, Uchiyama M, Kwee IL, Nakada T. Brain developmental abnormalities in Prader-Willi syndrome detected by diffusion tensor imaging. Pediatrics. 2006;118:e442–8. doi: 10.1542/peds.2006-0637. [DOI] [PubMed] [Google Scholar]
- 9.Hinton EC, Holland AJ, Owen AM. Functional neuroimaging in Prader-Willi syndrome. NPG. 2007;31:390–1. [Google Scholar]
- 10.Dimitropoulos A, Feurer ID, Roof E, Stone W, Butler MG, Sutcliffe J, et al. Appetitive behavior, compulsivity, and neurochemistry in Prader-Willi syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:125–30. doi: 10.1002/1098-2779(2000)6:2<125::AID-MRDD6>3.0.CO;2-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183–97. doi: 10.1210/jc.2008-0649. [DOI] [PubMed] [Google Scholar]
- 12.Dykens EM, Leckman J, Cassidy SB. Obsessions and compulsions in Prader-Willi Syndrome. J Child Psychol Psychiatry. 1996;37:995–1002. doi: 10.1111/j.1469-7610.1996.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 13.Russell H, Oliver C. The assessment of food-related problems in individuals with Prader-Willi syndrome. Br J Clin Psychol. 2003;42:379–92. doi: 10.1348/014466503322528928. [DOI] [PubMed] [Google Scholar]
- 14.McCandless SE. Committee on Genetics. Clinical report-health supervision for children with prader-willi syndrome. Pediatrics. 2011;127:195–204. doi: 10.1542/peds.2010-2820. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Visootsak J, Dills S, John MG., Jr Prader-Willi syndrome: An update and review for the primary pediatrician. Clin Pediatr (Phila) 2007;46:580–91. doi: 10.1177/0009922807299314. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, von Deneen KM, Kobeissy FH, Gold MS. Food addiction and obesity: Evidence from bench to bedside. J Psychoactive Drugs. 2010;42:133–45. doi: 10.1080/02791072.2010.10400686. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DA, Heinemann J, Angulo M, Butler MG, Loker J, Rupe N, et al. Gastric rupture and necrosis in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2007;45:272. doi: 10.1097/MPG.0b013e31805b82b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dykens E, Shah B. Psychiatric disorders in prader-willi syndrome: Epidemiology and management. CNS Drugs. 2003;17:167–78. doi: 10.2165/00023210-200317030-00003. [DOI] [PubMed] [Google Scholar]
- 19.Zipf WB, O’Dorisio TM, Bernston GG. Short-term infusion of pancreatic polypeptide: Effect on children with Prader-Willi syndrome. Am J Clin Nutr. 1990;51:162–6. doi: 10.1093/ajcn/51.2.162. [DOI] [PubMed] [Google Scholar]
- 20.Kuriyan R, Raj T, Srinivas SK, Vaz M, Rajendran R, Kurpad AV. Effect of caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite. 2007;48:338–44. doi: 10.1016/j.appet.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Odendaal AY, Deshmukh NS, Marx TK, Schauss AG, Endres JR, Clewell AE. Safety assessment of a hydroethanolic extract of caralluma fimbriata. Int J Toxicol. 2013;32:385–94. doi: 10.1177/1091581813492827. [DOI] [PubMed] [Google Scholar]
- 22.Astell KJ, Mathai ML, McAinch AJ, Stathis CG, Su XQ. A pilot study investigating the effect of Caralluma fimbriata extract on the risk factors of metabolic syndrome in overweight and obese subjects: A randomised controlled clinical trial. Complement Ther Med. 2013;21:180–9. doi: 10.1016/j.ctim.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran R, Ambikar D, Khandare R, Sannapuri V, Clayton P, Vyawahare N. Nootropic activity of Caralluma fimbriata extract in mice. FNS. 2014;5:147–52. [Google Scholar]
- 24.Dykens EM. Assessment of hyperphagia in prader-willi syndrome. Obesity. 2007;15:1816–26. doi: 10.1038/oby.2007.216. [DOI] [PubMed] [Google Scholar]
- 25.Kamalakkannan S, Rajendran R, Venkatesh RV, Clayton P, Akbarsha MA. Antiobesogenic and antiatherosclerotic properties of caralluma fimbriata extract. J Nutr Metab 2010. 2010 doi: 10.1155/2010/285301. 285301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke DJ, Boer H, Whittington J, Holland A, Butler J, Webb T. Prader-Willi syndrome, compulsive and ritualistic behaviours: The first population-based survey. Br J Psychiatry. 2002;180:358–62. doi: 10.1192/bjp.180.4.358. [DOI] [PubMed] [Google Scholar]
- 27.Bertella L, Mori I, Grugni G, Pignatti R, Ceriani F, Molinari E, et al. Quality of life and psychological well-being in GH-treated, adult PWS patients: A longitudinal study. J Intellect Disabil Res. 2007;51:302–11. doi: 10.1111/j.1365-2788.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- 28.Goldstone AP, Holland AJ, Butler JV, Whittington JE. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes (Lond) 2012;36:1564–70. doi: 10.1038/ijo.2011.274. [DOI] [PubMed] [Google Scholar]
- 29.Kunert O, Rao VG, Babu GS, Sujatha P, Sivagamy M, Anuradha S, et al. Pregnane glycosides from Caralluma adscendens var. fimbriata. Chem Biodivers. 2008;5:239–50. doi: 10.1002/cbdv.200890021. [DOI] [PubMed] [Google Scholar]
- 30.Selikowitz M, Sunman J, Pendergast A, Wright S. Fenfluramine in Prader-Willi syndrome: A double blind, placebo controlled trial. Arch Dis Child. 1990;65:112–4. doi: 10.1136/adc.65.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin E, Buot-Smith T. Naltrexone and fluoxetine in Prader-Willi syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:870–3. doi: 10.1097/00004583-199307000-00025. [DOI] [PubMed] [Google Scholar]


