Abstract
Context:
Groove pancreatitis is a rare form of chronic pancreatitis affecting the “groove” of the pancreas among the pancreatic head, duodenum, and common bile duct. The exact cause is unknown, although there are associations with long-term alcohol abuse, smoking, peptic ulcer disease, heterotopic pancreas, gastric resection, biliary disease, and anatomical or functional obstruction of the minor papilla. The diagnosis can be challenging. Endoscopic ultrasound (EUS) and magnetic resonance cholangiopancreatography are the preferred imaging modalities. The treatment of choice is conservative although surgical intervention can sometimes be required.
Case Report:
A 57-year-old male with a history of human immunodeficiency virus and hepatitis B presented with 4 days of epigastric pain. Abdominal exam revealed absent bowel sounds and epigastric tenderness. He had a creatinine of 1.72 mg/dL, potassium of 2.9 mmol/L, and a normal lipase level of 86 U/L. Liver enzymes and total bilirubin were normal. Computed tomography abdomen showed high-grade obstruction of the second portion of the duodenum without any obvious mass. An esophagogastroduodenoscopy showed a mass at the duodenal bulb causing luminal narrowing, with biopsies negative for malignancy. Magnetic resonance imaging revealed a mass in the region of the pancreatic head and descending duodenum. EUS revealed a 3 cm mass in the region of pancreatic head with irregular borders and no vascular invasion. Fine needle aspiration (FNA) was nondiagnostic. The patient then underwent a Whipple's procedure. Pathology of these specimens was negative for malignancy but was consistent with para-duodenal or groove pancreatitis.
Conclusion:
The low incidence of groove pancreatitis is partly due to lack of familiarity with the disease. Groove pancreatitis should be considered in the differential for patients presenting with pancreatic head lesions and no cholestatic jaundice, especially when a duodenal obstruction is present, and neither duodenal biopsies nor pancreatic head FNA confirm adenocarcinoma.
Keywords: Chronic pancreatitis, groove pancreatitis, pancreticoduodenectomy, para-duodenal pancreatitis
Introduction
Groove pancreatitis is an uncommon form of chronic pancreatitis. Its presentation is very similar to that of pancreatic head adenocarcinoma, and its diagnosis is extremely challenging. Its low incidence is partly due to lack of familiarity. The term “pancreaticoduodenal groove” refers to the potential space between the head of the pancreas, the duodenum and the common bile duct (CBD). Patients present with symptoms of duodenal obstruction, very rarely with jaundice.[1,2,3] Endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) is generally inconclusive; therefore the diagnostic approach is challenging.[2]
Case Presentation
A 57-year-old male with a history of human immunodeficiency virus, chronic hepatitis B, and Fournier gangrene (with extensive debridement in the past requiring a colostomy) presented with a 4 day history of sharp epigastric pain with associated nausea and vomiting. He had not had a bowel movement for 4 days prior to admission. The patient denied tobacco use and used alcohol only occasionally. Family history was not significant.
On examination, he was mildly tachycardic but was in no acute distress. He had dry mucous membranes. Abdominal exam revealed absent bowel sounds and tenderness to palpation in the epigastric region without rebound or guarding. Physical exam was otherwise unremarkable. Initial laboratory evaluation revealed acute renal injury with a creatinine of 1.72 mg/dL, hypokalemia with a potassium of 2.9 mmol/L, and an unremarkable lipase level of 86 U/L. Liver enzymes and total bilirubin were normal. Computed tomography (CT) imaging of his abdomen and pelvis without contrast revealed a high-grade obstruction of the second portion of the duodenum without any obvious mass [Figure 1].
Figure 1.
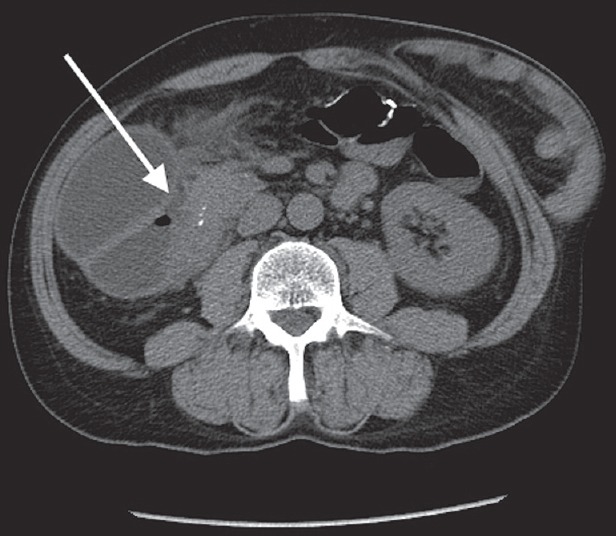
Computed tomography scan showing high-grade obstruction of the second portion of the duodenum (white arrow)
Esophagogastroduodenoscopy was performed due to duodenal obstruction and found a circumferential mass starting at the duodenal bulb causing luminal narrowing. Biopsies of this lesion were negative for malignancy; however, there was still a concern for cancer due to the location of the mass. Magnetic resonance imaging (MRI) of the abdomen was then obtained which revealed an ill-defined soft tissue mass in the region of the pancreatic head and descending duodenum with tethering of the duodenum causing obstruction [Figure 2]. A positron emission tomography scan demonstrated hypermetabolic activity of the mass without lymph node involvement. Tumor markers including carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) were both normal. The patient underwent EUS revealing a 3 cm hypoechoic mass in the region of pancreatic head with irregular borders and no vascular invasion. FNA was performed but was nondiagnostic with no lesional tissue identified. Given the mass seen on MRI and EUS as well as the presence of a high-grade duodenal obstruction, the patient went to surgery for a Whipple's procedure (pancreaticoduodenectomy).
Figure 2.
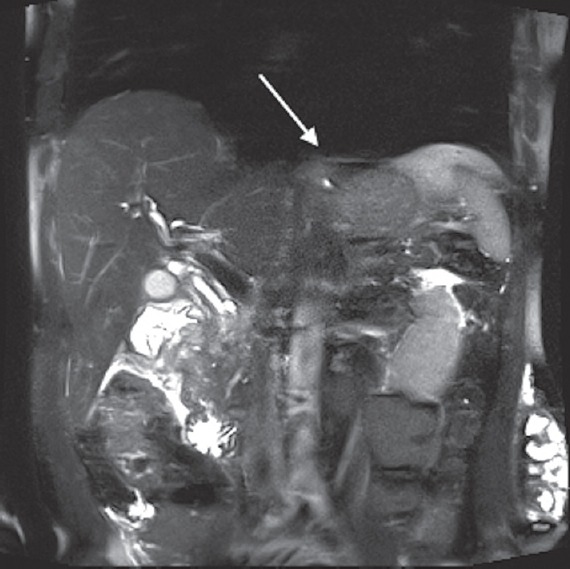
Magnetic resonance cholangiopancreatography showing ill-defined soft tissue mass in the region of the pancreatic head (white arrow)
Pathology of these specimens was negative for malignancy and consistent with para-duodenal or “groove” pancreatitis. The duodenal submucosa had Brunner's gland hyperplasia with fibrosis and inflammatory infiltrate into the pancreas [Figures 3 and 4]. The pancreatic tissue had some focal fibrosis [Figure 5]. Staining for acid-fast bacilli and fungus were negative. There was scattered immunoglobulin G4 positivity throughout the specimen, but this was nonspecific. The patient was discharged after his pain was controlled, and he was tolerating an oral diet.
Figure 3.
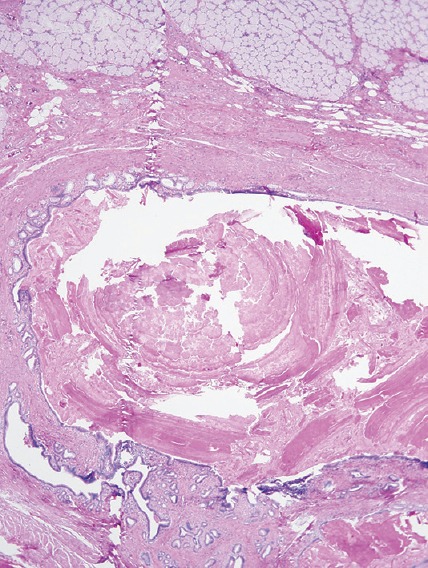
Low power view showing prominent Brunner's gland hyperplasia and submucosal fibrosis (Duodenum, ×4)
Figure 4.
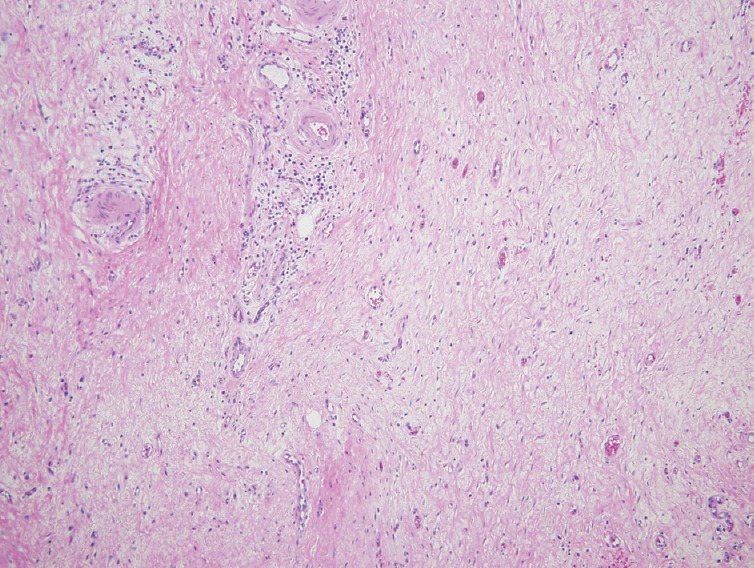
Hyalinized fibrosis of the duodenum submucosa. A sparse chronic inflammatory infiltrate is present but there is no storiform fibrosis, phlebitis or prominent collections of plasma cells (Duodenal fibrosis, ×10)
Figure 5.
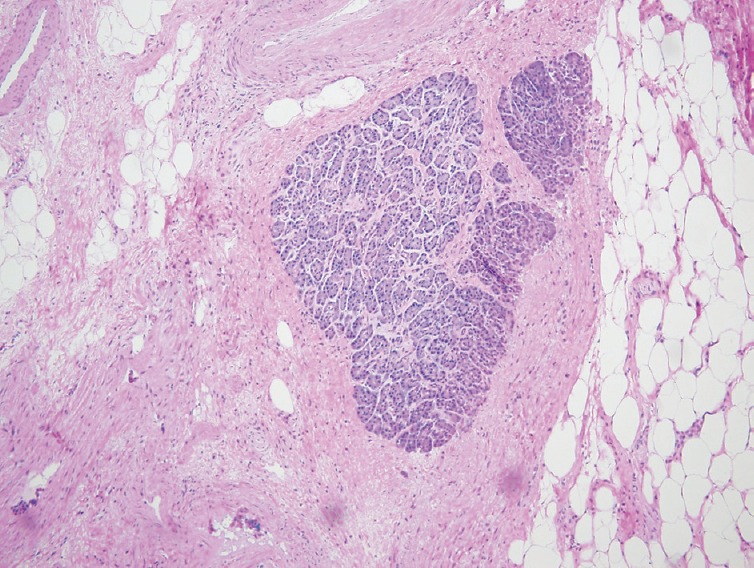
Isolated benign pancreatic tissue completely surrounded by dense fibrosis (Pancreatic fibrosis, ×10)
Discussion
Groove Pancreatitis was first reported by Becker in 1973.[4] Stolte et al. in 1982 then defined it as fibrous scars of the anatomic space between the dorsocranial part of the head of the pancreas, the duodenum, and the CBD.[5] Becker and Mischke in the early 1990s recognized two forms: The “segmental” form, which involves both the pancreatic head and the groove; and the “pure” form, which affects the groove only, sparing the pancreatic head.[6] In 2004, Adsay and Zamboni proposed the umbrella term “Para-duodenal Pancreatitis” which termed a distinct form of chronic pancreatitis and occurred predominantly in and around the duodenum along the minor papilla. This term included cystic dystrophy of the heterotopic pancreas (CDHP), pancreatic hamartoma of duodenum, para-duodenal wall cyst, myoadenomatosis, and groove pancreatitis.[7] Para-duodenal pancreatitis was described as either cystic lesions (cystic variant) or a solid-mass occurring predominantly in and around the minor papilla (solid variant) by Casetti et al. in 2009.[8] The pathogenesis of groove pancreatitis is uncertain, however, alcohol abuse is thought to have the strongest association by increasing the viscosity of pancreatic secretions.[9,10]
Other suggested etiologies include peptic ulcer disease, heterotopic pancreas, gastric resection, biliary disease, and anatomical or functional obstruction of the minor papilla.[5,11,12,13] Interestingly, a recent study by Wagner et al. which looked at multi-detector CT signs of CDHP, showed that minor papilla abnormalities do not seem to be a predisposing factor for Child Health and Disability Prevention.[14]
The clinical manifestations include upper abdominal pain, weight loss, postprandial nausea, and vomiting due to duodenal stenosis, but jaundice is rare[1,2,3] as was seen in our case. However, bilirubin levels can be elevated if the CBD is obstructed, and alkaline phosphatase levels can also be elevated even in the absence of ductal obstruction.[10] Pancreatic enzymes are often normal or only minimally elevated, and tumor markers (like CEA and CA 19-9) are usually negative.[3]
While groove pancreatitis is a very uncommon form of chronic pancreatitis, similarities in a presentation to pancreatic head carcinoma represent a diagnostic challenge. EUS and magnetic resonance cholangiopancreatography are preferred imaging modalities and may help to differentiate between both entities.[8,15] Kalb et al. described characteristic MRI features of groove pancreatitis including thickening and increased enhancement of the second part of the duodenum, and cystic changes near the pancreatic accessory duct.[16] FNA findings can be misleading and inconclusive due to sampling error or due to over calling a specimen and labeling it as cancer.[17]
The treatment of choice is similar to chronic pancreatitis and is conservative in the acute phase of groove pancreatitis and includes bowel rest, analgesia, and intravenous fluids.[2] Groove pancreatitis can be resistant to medical treatment and require surgical intervention. The surgical treatment of choice is a Whipple procedure or a pylorus-preserving pancreaticoduodenectomy.[3]
Conclusion
Groove pancreatitis is deceptively similar in presentation to pancreatic adenocarcinoma and can be difficult to differentiate using clinical features, imaging, and laboratory markers. Therefore, several cases eventually require surgery. Groove pancreatitis should always be considered in the differential for patients presenting with pancreatic head lesions with no cholestatic jaundice especially when a duodenal obstruction is present, and neither duodenal biopsies nor pancreatic head FNA confirm adenocarcinoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Adsay NV, Zamboni G. Paraduodenal pancreatitis: A clinico-pathologically distinct entity unifying “cystic dystrophy of the heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Shudo R, Yazaki Y, Sakurai S, Uenishi H, Yamada H, Sugawara K, et al. Groove pancreatitis: Report of a case and review of the clinical and radiologic features of groove pancreatitis reported in Japan. Intern Med. 2002;41:537–42. doi: 10.2169/internalmedicine.41.537. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan V, Chatni S, Radhakrishnan L, Narayanan VA, Nair P. Groove pancreatitis: A case report and review of literature. JOP. 2007;8:592–7. [PubMed] [Google Scholar]
- 4.Becker V. Proceedings: Fundamental morphological aspects of acute and chronic pancreatitis (author's transl) Langenbecks Arch Chir. 1973;334:317–22. doi: 10.1007/BF01286576. [DOI] [PubMed] [Google Scholar]
- 5.Stolte M, Weiss W, Volkholz H, Rösch W. A special form of segmental pancreatitis: “groove pancreatitis”. Hepatogastroenterology. 1982;29:198–208. [PubMed] [Google Scholar]
- 6.Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol. 1991;10:173–82. doi: 10.1007/BF02924155. [DOI] [PubMed] [Google Scholar]
- 7.Adsay NV, Zamboni G. Paraduodenal pancreatitis: A clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;2:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Casetti L, Bassi C, Salvia R, Butturini G, Graziani R, Falconi M, et al. “Paraduodenal” pancreatitis: Results of surgery on 58 consecutives patients from a single institution. World J Surg. 2009;33:2664–9. doi: 10.1007/s00268-009-0238-5. [DOI] [PubMed] [Google Scholar]
- 9.Blasbalg R, Baroni RH, Costa DN, Machado MC. MRI features of groove pancreatitis. AJR Am J Roentgenol. 2007;189:73–80. doi: 10.2214/AJR.06.1244. [DOI] [PubMed] [Google Scholar]
- 10.Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P. Groove pancreatitis: A diagnostic challenge. Eur Radiol. 2009;19:1736–43. doi: 10.1007/s00330-009-1332-7. [DOI] [PubMed] [Google Scholar]
- 11.Irie H, Honda H, Kuroiwa T, Hanada K, Yoshimitsu K, Tajima T, et al. MRI of groove pancreatitis. J Comput Assist Tomogr. 1998;22:651–5. doi: 10.1097/00004728-199807000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Itoh S, Yamakawa K, Shimamoto K, Endo T, Ishigaki T. CT findings in groove pancreatitis: Correlation with histopathological findings. J Comput Assist Tomogr. 1994;18:911–5. doi: 10.1097/00004728-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Zamboni G, Capelli P, Scarpa A, Bogina G, Pesci A, Brunello E, et al. Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:439–53. doi: 10.5858/133.3.439. [DOI] [PubMed] [Google Scholar]
- 14.Wagner M, Vullierme MP, Rebours V, Ronot M, Ruszniewski P, Vilgrain V. Cystic form of paraduodenal pancreatitis (cystic dystrophy in heterotopic pancreas (CDHP)): A potential link with minor papilla abnormalities? A study in a large series. Eur Radiol. 2015;1:1–7. doi: 10.1007/s00330-015-3799-8. [DOI] [PubMed] [Google Scholar]
- 15.Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging. 2008;33:342–8. doi: 10.1007/s00261-007-9245-x. [DOI] [PubMed] [Google Scholar]
- 16.Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, et al. Paraduodenal pancreatitis: Clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475–81. doi: 10.1148/radiology.13112056. [DOI] [PubMed] [Google Scholar]
- 17.Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: An efficient approach. Gastrointest Endosc. 2001;54:485–90. doi: 10.1067/mge.2001.118445. [DOI] [PubMed] [Google Scholar]


