Sir,
Non-fermenting Gram-negative bacteria (NFGNB) are a threat to the health care community because these cause opportunistic infections in critically ill or immunocompromised patients. Following Acinetobacter species and Pseudomonas aeruginosa, Burkholderia cepacia complex (Bcc) and Stenotrophomonas maltophilia are the third and fourth common NFGNB among the positive blood cultures at a tertiary care institute in north India1,2. The treatment of infections caused by these organisms is challenging because of high intrinsic and acquired resistance to all commonly used antibiotics including the antipseudomonal drugs3. The increasing incidence of infections by these organisms along with the rising drug resistance warrants a close monitoring of the antimicrobial susceptibility of these organisms.
We present the analysis of the antimicrobial susceptibility profiles from March 2007 to December 2012 of 186 Bcc isolates (63 isolates from 2007 to 2009, 89 isolates from 2010 to 2011 and 34 isolates in 2012) and 125 S. maltophilia isolates (38 isolates from 2007 to 2009, 54 isolates from 2010 to 2011 and 33 isolates in 2012) obtained from various clinical specimens (blood, cerebrospinal fluid, sputum, endotracheal aspirate, bronchoalveolar lavage and pus) at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. All the NFGNB isolates were identified by conventional biochemical reactions. Gram-negative, motile, NFGNB were identified further by the use of oxidase test, triple sugar iron agar with lead acetate paper strip and decarboxylase tests4. Molecular identification and typing of Bcc were done by recA polymerase chain reaction-restriction fragment length polymorphism (recA PCR-RFLP)4. Drug susceptibility was tested by Kirby-Bauer disk diffusion test (DD)5 against co-trimoxazole (TMP-SMX, 1.25 µg/23.75 µg), ceftazidime (30 µg), tetracycline (30 µg)/minocycline (30 µg), levofloxacin (5 µg) for S. maltophilia and additionally against meropenem (10 µg) for Bcc following Clinical Laboratory Standards Institute (CLSI) guidelines6. The minimum inhibitory concentrations (MIC) of selected number of isolates were determined by agar dilution method as per CLSI guidelines6 against minocycline (sensitive, S≤4 and resistant R≥16 μg/ml), levofloxacin (S≤2 & R≥8 μg/ml), ceftazidime (S≤8 & R≥32 μg/ml), chloramphenicol (S≤8 & R≥32 μg/ml) for Bcc, and minocycline (S≤4 & R≥16 μg/ml), levofloxacin (S≤2 & R≥8 μg/ml), co-trimoxazole (S≤2/38 & R≥4/76 μg/ml), chloramphenicol (S≤8 & R≥32 μg/ml), ceftazidime (S≤8 & R≥32 μg/ml) for S. maltophilia. The proportional data were analyzed by Z-test for proportions.
In 2012, by DD 100 per cent of the Bcc isolates were susceptible to minocycline, 71 per cent to co-trimoxazole, 71 per cent to meropenem and 59 per cent to ceftazidime. A significant decrease in susceptibility of Bcc for ceftazidime has been observed in 2012 (59%) when compared with previous years (83 and 85%, P<0.001). There was no significant decrease in susceptibility to co-trimoxazole and meropenem over the years (Fig. 1).
Fig. 1.
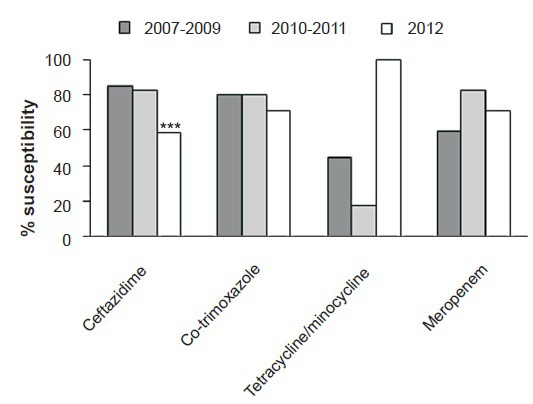
Percentage susceptibility of Bcc isolates by disc diffusion. ***P<0.001 compared with values in 2007-2009 and 2010-2011.
In 2012, by DD 100 per cent of the S. maltophilia isolates were susceptible to minocycline, 100 per cent to levofloxacin, 83 per cent to co-trimoxazole and 25 per cent to ceftazidime. The sensitivity of S. maltophilia to co-trimoxazole (70 to 91%) and levofloxacin (83 to 100%) showed significant variation in contrast to ceftazidime which remained low over the years (25 to 40%). The year-wise susceptibility of the S. maltophilia isolates is shown in Fig. 2.
Fig. 2.
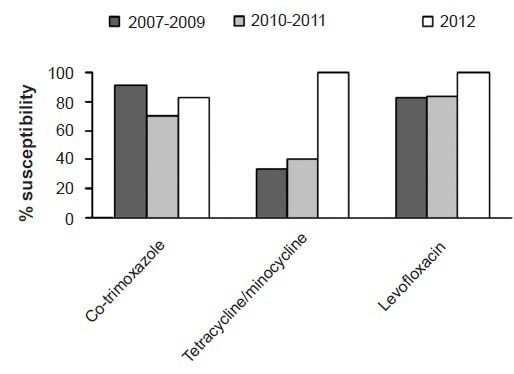
Percentage susceptibility of Stenotrophomonas maltophilia isolates by disc diffusion.
All Bcc and S. maltophilia isolates were sensitive to minocycline though the susceptibility to tetracycline tested in the previous years was less indicating minocycline as a better drug. The MIC values were calculated for 30 S. maltophilia and 60 Bcc isolates. In case of S. maltophilia the percentage of isolates which had MICs within the susceptible range was as follows: 100 per cent for minocycline and levofloxacin, 97 per cent for co-trimoxazole, 64 per cent for chloramphenicol and 50 per cent for ceftazidime. In case of Bcc the percentage of isolates which had MICs within the susceptible range was as follows: 75 per cent for minocycline, 27 per cent for levofloxacin and ceftazidime, 13 per cent for chloramphenicol.
Co-trimoxazole had most consistent antimicrobial activity against both Bcc and S. maltophilia. In a study carried out on NFGNB isolates collected globally7,8,9, co-trimoxazole was found to be the most active antibiotic tested against both of these organisms. Both the organisms were highly susceptible to minocycline (majority tested had MIC ≤ 4 µg/ml). S. maltophilia isolates showed lower MIC than the Bcc isolates. Similar variations in the antimicrobial susceptibility between species or between different genera among NFGNB has been documented in a previous study7. Based on the findings of the present and the previous studies7, levofloxacin can be considered as a good alternative in treating S. maltophilia infections. These findings emphasize the importance of correct identification of these organisms and their antimicrobial susceptibility as Bcc is known to be intrinsically resistant to polymyxin and S. maltophilia to carbapenems and both are resistant to aminoglycosides. Routine monitoring of antimicrobial susceptibility pattern of these organisms is mandatory for future policy in the management of such infections.
References
- 1.Arora S, Gautam V, Ray P. Changing susceptibility patterns of nonfermenting Gram-negative bacilli. Indian J Med Microbiol. 2012;30:485–6. doi: 10.4103/0255-0857.103785. [DOI] [PubMed] [Google Scholar]
- 2.Samanta P, Gautam V, Thapar R, Ray P. Emerging resistance of non-fermenting gram negative bacilli in a tertiary care centre. Indian J Pathol Microbiol. 2011;54:666–7. doi: 10.4103/0377-4929.85150. [DOI] [PubMed] [Google Scholar]
- 3.McGowan JE Jr. Resistance in non fermenting gram-negative bacteria: multidrug resistance to the maximum. Am J Med. 2006;119(6 Suppl 1):S29. doi: 10.1016/j.amjmed.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Gautam V, Ray P, Vandamme P, Chatterjee SS, Das A, Sharma K, et al. Identification of lysine positive non-fermenting gram negative bacilli (Stenotrophomonas maltophilia and Burkholderia cepacia complex) Indian J Med Microbiol. 2009;27:128–33. doi: 10.4103/0255-0857.49425. [DOI] [PubMed] [Google Scholar]
- 5.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 6.Wayne, USA: CLSI; 2012. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 22nd Informational Supplement M100-S22. [Google Scholar]
- 7.Sader HS, Jones RN. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. Int J Antimicrob Agents. 2005;25:95–109. doi: 10.1016/j.ijantimicag.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Messiaen AS, Verbrugghen T, Declerck C, Ortmann R, Schlitzer M, Nelis H, et al. Resistance of the Burkholderia cepacia complex to fosmidomycin and fosmidomycin derivatives. Int J Antimicrob Agents. 2011;38:261–4. doi: 10.1016/j.ijantimicag.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Mushtaq S, Warner M, Woodford N. Comparative in vitro activity of sulfametrole/trimethoprim and sulfamethoxazole/trimethoprim and other agents against multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69:1050–6. doi: 10.1093/jac/dkt455. [DOI] [PubMed] [Google Scholar]


