Abstract
Introduction
In this study of preclinical Alzheimer’s disease (AD) we assessed the added diagnostic value of using cerebrospinal fluid (CSF) Aβ ratios rather than Aβ42 in isolation for detecting individuals who are positive on amyloid positron emission tomography (PET).
Methods
Thirty-eight community-recruited cognitively intact older adults (mean age 73, range 65–80 years) underwent 18F-flutemetamol PET and CSF measurement of Aβ1-42, Aβ1-40, Aβ1-38, and total tau (ttau). 18F-flutemetamol retention was quantified using standardized uptake value ratios in a composite cortical region (SUVRcomp) with reference to cerebellar grey matter. Based on a prior autopsy validation study, the SUVRcomp cut-off was 1.57. Sensitivities, specificities and cut-offs were defined based on receiver operating characteristic analysis with CSF analytes as variables of interest and 18F-flutemetamol positivity as the classifier. We also determined sensitivities and CSF cut-off values at fixed specificities of 90 % and 95 %.
Results
Seven out of 38 subjects (18 %) were positive on amyloid PET. Aβ42/ttau, Aβ42/Aβ40, Aβ42/Aβ38, and Aβ42 had the highest accuracy to identify amyloid-positive subjects (area under the curve (AUC) ≥ 0.908). Aβ40 and Aβ38 had significantly lower discriminative power (AUC = 0.571). When specificity was fixed at 90 % and 95 %, Aβ42/ttau had the highest sensitivity among the different CSF markers (85.71 % and 71.43 %, respectively). Sensitivity of Aβ42 alone was significantly lower under these conditions (57.14 % and 42.86 %, respectively).
Conclusion
For the CSF-based definition of preclinical AD, if a high specificity is required, our data support the use of Aβ42/ttau rather than using Aβ42 in isolation.
Introduction
Preclinical [1, 2], or asymptomatic [3], Alzheimer’s disease (AD) is characterized by the presence of AD-related pathophysiological processes in the absence of cognitive deficits. Evidence of brain amyloidosis is a requirement common to all three National Institute on Ageing–Alzheimer’s Association (NIA–AA) stages of preclinical AD [1] and is also a defining feature of the asymptomatic at risk for AD state according to the International Working Group IWG-2 criteria [3]. This can be detected directly in vivo by means of either amyloid-beta (Aβ) protein quantification in cerebrospinal fluid (CSF) or positron emission tomography (PET) amyloid imaging [1, 3–5].
Apart from Aβ1–42, other Aβ isoforms (e.g., Aβ1–40, Aβ1–38) have evoked interest from a clinical-diagnostic perspective, as either a separate biomarker tool or when combined (ratio) with Aβ1–42 [6–8]. Using ratios of Aβ isoforms (Aβ1–42/Aβ1–38, Aβ1–42/Aβ1–40) may have added value for the discrimination between AD and normal pressure hydrocephalus [9], cerebral amyloid angiopathy [10], frontotemporal dementia [11], and Lewy body dementia [12], and also between mild cognitive impairment (MCI) due to AD versus non-AD MCI [13]. In cognitively intact individuals, Aβ38 or Aβ40 does not correlate with amyloid PET positivity, in contrast with Aβ42 [5, 14].
In this study of preclinical AD, we assessed the added value of using ratios of Aβ42 to other C-terminal Aβ isoforms or to total tau (ttau) for discriminating amyloid-positive versus amyloid-negative cognitively intact healthy controls, with an autopsy-validated 18F-flutemetamol cutoff score [15] as standard of truth. The cutoff value was derived from the 18F-flutemetamol phase 3 study using a binarized measure of postmortem brain neuritic plaque density [16] (overall mean Bielschowsky score below or above 1.5 [15]). We also explored the diagnostic value of the Aβ38 and Aβ40 isoforms on their own.
For design of clinical trials in preclinical AD, the data presented may inform the decision on which CSF parameter to select for study eligibility based on its equivalence to an amyloid-PET-based definition. We not only provide the parameters providing optimal balance between sensitivity and specificity but also the parameters that provide an acceptable sensitivity for a fixed high specificity. Specificity may receive more weight in trials in preclinical AD because the definition of the target population often heavily relies on the biomarker value, healthy volunteers are exposed to potential adverse effects of study drugs for a long duration, and positive evidence for the presence of the study target increases the likelihood of success. Sensitivity will mainly determine the number needed to screen, and will therefore impact on the cost.
Methods
Participants
Thirty-eight cognitively intact older controls (mean age 73 years, standard deviation (SD) 5 years; Table 1) were recruited prospectively and consecutively, from 10 September 2012 until 4 April 2014, through advertisement in local newspapers and through websites for seniors, asking for healthy volunteers between 65 and 80 years of age for participation in a scientific study at the University Hospital Leuven, Belgium, involving brain imaging (sic). At screening, subjects underwent a detailed interview about medical history, a Mini Mental State Examination (MMSE), a Clinical Dementia Rating (CDR), blood sampling, and a conventional neuropsychological assessment. Inclusion criteria were age 65–80 years, MMSE ≥ 27, CDR = 0, and normal test scores on neuropsychological assessment according to the published norms adapted for age, gender, and education. Among the exclusion criteria were a neurological or psychiatric history and focal brain lesions on structural magnetic resonance imaging (MRI). Subjects who fulfilled all criteria underwent both 18F-flutemetamol PET and lumbar puncture. The target sample size of the PET-plus-CSF cohort was 40 but two subjects dropped out after the PET scan and prior to the lumbar puncture, giving a final sample size of 38.
Table 1.
Demographics and CSF biomarker concentrations
| Gender (male/female) | 22/16 | LVF (number of words) | 36.0 (10.8, 17–64) |
|---|---|---|---|
| Age (years) | 73 (4.7, 65–80) | RPM (/60) | 36.1 (9.8, 15–53) |
| Education (years) | 13.4 (3.1, 8–20) | TMT B/A | 2.4 (0.5, 1.5–3.8) |
| APOE ε4 carriers (n) | 19 (50 %) | Aβ38 (pg/ml) | 2401 (654, 1057–3505) |
| BDNF met carriers (n) | 20 (53 %) | Aβ40 (pg/ml) | 8933 (2456, 3640–13273) |
| MMSE (/30) | 28.9 (1.0, 27–30) | Aβ42 (pg/ml) | 996 (430, 351–1859) |
| AVLT TL (/75) | 46.2 (8.4, 31–69) | ttau (pg/ml) | 360 (134, 126–660) |
| AVLT DR (/15) | 9.8 (2.5, 5–14) | Aβ42/Aβ38 | 0.412 (0.119, 0.136–0.596) |
| AVLT %DR | 83.7 (11.7, 55–108) | Aβ42/Aβ40 | 0.110 (0.030, 0.044–0.148) |
| BNT (/60) | 54.2 (4.2, 41–60) | Aβ42/ttau | 3.015 (1.246, 0.749–5.128) |
| AVF (number of words) | 24.0 (5.5, 14–40) | Amyloid+ (n) | 7 (18 %) |
Data presented as mean (standard deviation, range)
Aβ amyloid beta, APOE apolipoprotein E, AVF Animal Verbal Fluency Test, AVLT Rey Auditory Verbal Learning Test, BDNF brain-derived neurotrophic factor, BNT Boston Naming Test, CSF cerebrospinal fluid, DR delayed recall, LVF Letter Verbal Fluency Test, MMSE Mini Mental State Examination, RPM Raven’s Progressive Matrices, TL total learning, TMT Trail Making Test (part B divided by part A), ttau total tau
This PET-plus-CSF cohort belonged to a larger cohort of healthy older controls undergoing 18F-flutemetamol PET (target sample n = 180, recruited until time of writing n = 172) [17, 18]. The other subjects in this larger cohort did not undergo lumbar puncture per protocol. The primary aim of the full cohort was to investigate the interaction between brain-derived neurotrophic factor (BDNF) and apolipoprotein E (APOE) genetic polymorphisms on amyloid deposition and functional reorganization [17, 18]. The inclusion and exclusion criteria for the full cohort were identical to those of the PET-plus-CSF cohort apart from the age range (50–80 years for the full cohort). At inclusion, participants of the full cohort were stratified per age bin for two genetic factors: BDNF (met allele at codon 66 present or absent) and APOE (ε4 allele present or absent). The cells of this 2 × 2 factorial design were prospectively matched for number of cases, APOE and BDNF genetic status, age, sex, and education.
The PET-plus-CSF cohort (n = 38) did not differ from the remaining subjects (n = 134) with regards to sex, education, number of APOE ε4 carriers or BDNF met carriers, the presence of subjective memory complaints (29 % in each of the two groups), or neuropsychological test scores (P > 0.23). The CSF cohort was significantly older than the remaining subjects (mean age 73 years vs. mean age 67 years, P < 0.0001). The proportion of amyloid-positive cases did not differ significantly between the CSF-plus-PET cohort (18 %) and the remaining subjects (12 %) (P = 0.23).
The protocol (EudraCT: 2009-014475-45) was approved by the Ethics Committee University Hospitals Leuven, Belgium. Written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Amyloid PET
18F-flutemetamol PET was acquired on a 16-slice Siemens Biograph PET/CT scanner (Siemens, Erlangen, Germany). The tracer was injected as a bolus into an antecubital vein (mean activity 150 MBq, SD 5 MBq, range 134–162 MBq). Scan acquisition started 90 minutes after tracer injection and lasted for 30 minutes [17–20]. Prior to PET acquisition, a low-dose computed tomography scan of the head was performed for attenuation correction. Random and scatter correction were applied. The PET summed image was spatially normalized to Montreal Neurological Institute (MNI) space using a fully automated PET-only method [21]. On the basis of spatially normalized images (voxel size 2 × 2 × 2 mm3), standardized uptake value ratios (SUVR) were calculated with cerebellar gray matter as the reference region. The mean SUVR value was calculated in a composite cortical region (SUVRcomp) [15]. The composite cortical region and the cerebellar gray matter reference region were defined as a combination of narrow automated anatomic labeling-type regions [22] outlined on the ICBM-152 template masked with a gray matter probability mask [15]. Images were analyzed by an experienced medical imaging specialist blinded to all study information.
To estimate the SUVRcomp cutoff value for detecting amyloid positivity in vivo using the described method, receiver operating curve (ROC) analysis was performed by Thurfjell et al. [15] on an independent dataset of 68 SUVRcomp values (quantified based on the already described method) with the autopsy results as a standard of truth. The autopsy data were classified following Vemuri’s modification of the Consortium to Establish a Registry for AD criteria [16, 23]. Eight cortical regions (precuneus, midfrontal cortex, superior temporal cortex, middle temporal cortex, inferior parietal cortex, anterior cingulate gyrus, posterior cingulate gyrus, and primary visual cortex) were scored using an overall mean Bielschowsky score: 0 = no plaques, 1 = one to five plaques, 2 = six to 19 plaques, 3 = 20 or more plaques. If the mean Bielschowsky score was > 1.5 in at least one region, the brain was classified as amyloid-positive; if all regions scored ≤ 1.5, the brain was classified as amyloid-negative. The resulting SUVRcomp cutoff value was 1.57 [15].
Lumbar puncture and CSF analysis
Lumbar punctures were carried out at the L4/5 level in the morning (10 a.m.–2 p.m.) and collected in polypropylene tubes (total volume 15 ml, Greiner Bio-one Cellstar; VWR, Leuven, Belgium), discarding 1 ml to avoid traumatic blood contamination. Samples were centrifuged within 30 minutes after collection (2600 rpm, 10 minutes, 4 °C). After centrifugation, supernatants were transferred into polypropylene tubes and from there aliquoted in 1.5 ml polypropylene tubes (1 ml volume CSF/tube; Kartell, Noviglio, Italy). Samples were stored at –80 °C until batch analysis. Our primary analysis was based on the EUROIMMUN single analyte enzyme-linked immunosorbent assays (ELISA) (EUROIMMUN, Lübeck, Germany) of CSF Aβ1–42, Aβ1–40, Aβ1–38, and ttau. The assays were performed at ADx Ghent, Belgium by two experienced laboratory technicians blinded to all study information. The Aβ assays quantify the full length of the C-terminus-specific Aβ isoforms (Aβ1-specific assay format). The tau assay is designed with a capture antibody towards the central region and one monoclonal antibody with an epitope at the amino-terminus of the protein. The assay design includes lyophylized recombinant proteins as calibrators, run-validation control samples (calibrators added to a phosphate-buffered solution), as well as a qualification panel to evaluate the analytical performance(s) in the laboratory. These novel immunoassays are free from matrix interference and their intra-assay reproducibility has a coefficient of variation ≤ 5.0 % with an inter-assay reproducibility ≤ 8.3 % [24].
As a secondary analysis, we verified our results using the INNOTEST ELISA for Aβ1–42, ttau, and 181phospho-tau (ptau) (Fujirebio Europe, Ghent, Belgium). The assays were performed at the Laboratory Medicine Department of UZ Leuven, Belgium, in a ISO-15189 and Joint Commission International accredited environment by an expert technician blinded to all study information. The assay design included ready-to-use recombinant proteins as calibrators, run-validation control samples, and internal quality controls samples (for which target value and acceptance criteria were established in the routine setting of AD biomarker quantification).
Statistical analysis
In the primary analysis, which was based on the EUROIMMUN assays, we compared the diagnostic accuracy of different CSF Aβ isoforms, their ratios, ttau, and Aβ42/ttau to detect amyloid-positive older individuals. We used a ROC analysis with CSF analytes as variables of interest and 18F-flutemetamol positivity defined based on the autopsy-derived SUVRcomp cutoff value as a classifier. We also evaluated whether case classification changed when we varied the cutoff value by ±1.5 %, corresponding to the test–retest variability estimated for SUVRcomp [20]. The highest Youden index (sensitivity + specificity – 1) was used to estimate the optimal ROC cutoff values. Statistical differences between ROCs were evaluated according to the method of DeLong et al. [25] for pairwise ROC comparisons. Correction for multiple comparisons (n = 21) was performed with the Bonferroni method. The Bonferroni corrected threshold for significance was P < 0.002, corresponding to Pcorrected < 0.05.
Depending on the study, a high specificity may be desirable even if this implies a loss of sensitivity. We therefore also evaluated sensitivities and cutoff values at a fixed prespecified specificity of 90 % and 95 %, respectively. We evaluated whether this changed case classification significantly (McNemar test).
As a secondary analysis, we performed ROC analyses based on the INNOTEST assay of Aβ42, ttau, and ptau and statistically compared the areas under the curves (AUCs) between the two types of assays. We also compared the AUCs between the different INNOTEST measures and determined the sensitivity and percentage of correct classifications at a fixed specificity of 90 % and 95 %.
As a further secondary analysis, we evaluated the continuous relationship between the different CSF analytes and 18F-flutemetamol SUVRcomp values. We tested whether a linear, polynomial (quadratic), exponential, or hyperbolic relation fitted best to these data. The model assumptions were assessed by evaluating normality and homoscedasticity of residuals with q–q plots and plots of residuals versus fitted values. The best fitting model was selected based on the Akaike information criterion (AIC), which is a measure of model fit. A lower AIC indicates a better fit. CSF analytes were used as dependent variables and 18F-flutemetamol SUVRcomp as an independent variable.
Statistical analyses were performed in R version 3.1.1 (https://www.r-project.org) and MedCalc version 14.8.1 (https://www.medcalc.org).
Results
Based on the autopsy-confirmed 18F-flutemetamol SUVRcomp cutoff value, seven out of 38 subjects (18 %) were assigned to the amyloid-positive category (Fig. 1a). Case assignment did not change when we varied the cutoff value according to the known test–retest replicability.
Fig. 1.
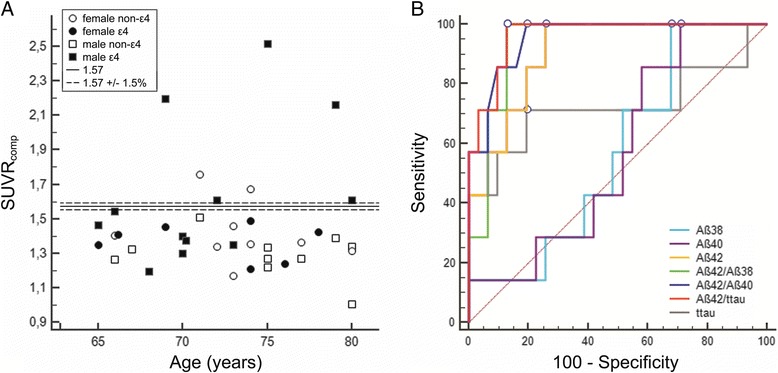
Distribution of 18F-flutemetamol SUVRcomp values and ROCs for different CSF analytes. a Distribution of 18F-flutemetamol SUVRcomp values according to age, sex, and APOE genotype. Solid line 1.57 SUVRcomp cutoff value; dashed line 1.57 SUVRcomp cutoff value ±1.5 % corresponding to a test–retest variability for SUVRcomp [20] (1.594 and 1.547). b ROCs for different CSF analytes, with 18F-flutemetamol positivity as classifier. Dots optimal cutoff values for each analyte, corresponding to the highest Youden index. Aβ Amyloid beta, SUVR comp standardized uptake value ratios in composite cortical region, ttau total tau
APOE ε4 carriers had significantly lower values of Aβ42, Aβ42/ttau, Aβ42/Aβ40, and Aβ42/Aβ38 than ε4 noncarriers (P < 0.003). CSF analyte concentrations did not differ between BDNF met carriers and noncarriers (P > 0.23).
Aβ42/ttau, Aβ42/Aβ40, Aβ42/Aβ38, and Aβ42 discriminated between 18F-flutemetamol-positive and 18F-flutemetamol-negative subjects with high accuracy (AUC ≥ 0.908; Table 2, Fig. 1b). Aβ38, Aβ40, and ttau showed a lower discriminative power with AUC ≤ 0.724 (Table 2). Aβ42/ttau, Aβ42/Aβ40, and Aβ42 had significantly higher AUCs than Aβ38 or Aβ40 alone (Table 2, P < 0.003). Aβ42/Aβ38 had significantly higher AUCs than Aβ40 (P = 0.002). There was no significant difference between the ratios Aβ42/ttau, Aβ42/Aβ40, and Aβ42/Aβ38, on the one hand, and Aβ42 alone, on the other (Table 2, P > 0.32). The AUCs of the three ratios were not statistically different from each other (Table 2, P > 0.30).
Table 2.
Diagnostic performance of different CSF analytes with 18F-flutemetamol PET as autopsy-validated standard of truth (EUROIMMUN assay)
| AUC | SE | 95 % CI | Cutoffa | Sensitivity (%) | Specificity (%) | Correctly classifiedb (%) | |
|---|---|---|---|---|---|---|---|
| Aβ38 | 0.571 | 0.111 | 0.401–0.730 | 2909 | 100 | 32.26 | 45 |
| Aβ40 | 0.571 | 0.112 | 0.401–0.730 | 10738 | 100 | 29.03 | 42 |
| Aβ42*† | 0.908 | 0.051 | 0.769–0.977 | 745 | 100 | 74.19 | 79 |
| ttau | 0.724 | 0.148 | 0.555–0.856 | 436 | 71.43 | 80.65 | 76 |
| Aβ42/Aβ38* | 0.935 | 0.039 | 0.806–0.989 | 0.332 | 100 | 87.10 | 89 |
| Aβ42/Aβ40*† | 0.954 | 0.033 | 0.832–0.995 | 0.096 | 100 | 80.65 | 84 |
| Aβ42/ttau*† | 0.963 | 0.028 | 0.846–0.998 | 2.006 | 100 | 87.10 | 89 |
Analyte concentrations are described as pg/ml or calculated as ratios between concentrations of two analytes
Statistically significant differences of AUCs between analytes: *P corrected < 0.05 compared with Aβ40; †P corrected < 0.05 compared with Aβ38. No other differences of AUCs were found
aCutoff value corresponding to the highest Youden index
bPercentage of positively classified cases based on the CSF cutoff compared with amyloid PET classification
Aβ amyloid beta, AUC area under the receiver operating characteristic curve, CI confidence interval, CSF cerebrospinal fluid, PET positron emission tomography, SE standard error, ttau total tau
When specificity was fixed at 90 %, Aβ42/ttau and Aβ42/Aβ40 had the highest sensitivity and Aβ42/Aβ38 the second highest sensitivity (Table 3). All three Aβ isoforms (Aβ42, Aβ40, Aβ38) used on their own detected significantly fewer amyloid PET-positive cases when specificity was fixed a priori at 90 % than when the cutoff value was based on the highest Youden index (Table 3), indicative of a significant loss in sensitivity. This was not the case for Aβ42/ttau, Aβ42/Aβ40, and Aβ42/Aβ38 ratios and ttau (Table 3).
Table 3.
Clinical accuracy: estimated sensitivities and cutoff values at a fixed specificity of 90 % or 95 % (EUROIMMUN assay)
| Sensitivity (%) | 95 % CI | Cutoff value | Differencea (%) | P valueb | Correctly classifiedc (%) | |
|---|---|---|---|---|---|---|
| Specificity of 90 % | ||||||
| Aβ38 | 14.29 | 0.00–71.43 | 1446 | 65.79 | <0.0001 | 79 |
| Aβ40 | 14.29 | 0.00–71.43 | 5602 | 65.79 | <0.0001 | 76 |
| Aβ42 | 57.14 | 0.00–100.00 | 546 | 21.05 | 0.008 | 84 |
| ttau | 57.14 | 14.29–100.00 | 471 | 10.53 | 0.125 | 82 |
| Aβ42/Aβ38 | 71.43 | 0.00–100.00 | 0.268 | 7.89 | 0.250 | 87 |
| Aβ42/Aβ40 | 85.71 | 14.29–100.00 | 0.074 | 10.53 | 0.125 | 89 |
| Aβ42/ttau | 85.71 | 14.29–100.00 | 1.852 | 5.26 | 0.500 | 89 |
| Specificity of 95 % | ||||||
| Aβ38 | 14.29 | 0.00–71.43 | 1342 | 68.42 | <0.0001 | 82 |
| Aβ40 | 14.29 | 0.00–71.43 | 5254 | 71.05 | <0.0001 | 82 |
| Aβ42 | 42.86 | 0.00–85.71 | 493 | 28.95 | 0.001 | 87 |
| ttau | 42.86 | 0.00–85.71 | 539 | 18.42 | 0.016 | 84 |
| Aβ42/Aβ38 | 28.57 | 0.00–71.43 | 0.251 | 21.05 | 0.008 | 84 |
| Aβ42/Aβ40 | 57.14 | 8.62–85.71 | 0.067 | 21.05 | 0.008 | 89 |
| Aβ42/ttau | 71.43 | 28.57–100.00 | 1.415 | 13.16 | 0.063 | 92 |
Analyte concentrations are described as pg/ml or calculated as ratios between concentrations of two analytes
aPercentage of subjects who were classified differently based on the cutoff values from fixed specificities compared with the cutoff values corresponding to the highest Youden index (Table 2)
bSignificance for the “Difference”cPercentage of positively classified cases based on the CSF cutoffs from fixed specificities compared with amyloid PET classification
Aβ amyloid beta, CI confidence interval, ttau total tau
When specificity was fixed at 95 %, Aβ42/ttau had the highest sensitivity (Table 3). All Aβ isoforms, ttau, and all ratios detected significantly less amyloid-positive cases when the specificity was fixed a priori at 95 % compared with the highest Youden index-based cutoff value, with one exception—namely the ratio Aβ42/ttau (Table 3). At a specificity of 95 %, the number of amyloid PET-positive cases detected based on the ratio Aβ42/ttau did not differ significantly from the number detected based on the highest Youden index-based cutoff value, although it was numerically lower.
As a secondary analysis, we compared the AUCs between two types of assays, EUROIMMUN and INNOTEST. The AUCs for Aβ42, ttau, and Aβ42/ttau did not differ between the EUROIMMUN and INNOTEST assays (Aβ42, P = 0.33; ttau, P = 0.91; and Aβ42/ttau, P = 0.25) (Tables 2 vs. 4). When we compared the AUCs between the different INNOTEST measures, the AUC for Aβ42/ttau differed significantly from the AUC for ttau (uncorrected P = 0.0172) or ptau (uncorrected P = 0.0096). When specificity was fixed at 90 %, Aβ42 and Aβ42/ttau had the highest sensitivity (Table 4). When specificity was fixed at 95 %, Aβ42/ttau had the highest sensitivity (Table 4).
Table 4.
Diagnostic performance of different CSF analytes measured with the INNOTEST assay for Aβ42, ttau, and ptau at an optimal specificity and at a specificity fixed at 90 % or 95 %
| AUC | SE | 95 % CI | Cutoffa | Sensitivity (%) | Specificity (%) | Correctly classifiedb (%) | |
|---|---|---|---|---|---|---|---|
| Aβ42 | 0.935 | 0.0394 | 0.806–0.989 | 853 | 100 | 83.87 | 87 |
| ttau | 0.733 | 0.132 | 0.565–0.863 | 352 | 71.43 | 77.42 | 76 |
| ptau | 0.675 | 0.139 | 0.504–0.818 | 86 | 42.86 | 93.55 | 84 |
| Aβ42/ttau | 0.880 | 0.0878 | 0.734–0.963 | 2.258 | 85.71 | 90.32 | 89 |
| Specificity of 90 % | Sensitivity (%) | 95 % CI | Cutoffa | Differencec (%) | P valued | Correctly classifiedb (%) | |
| Aβ42 | 85.71 | 11.54–100.00 | 798 | 7.90 | 0.25 | 89 | |
| ttau | 57.14 | 14.29–100.00 | 465 | 10.53 | 0.125 | 82 | |
| ptau | 42.96 | 0.00–85.71 | 87 | 5.26 | 0.5 | 79 | |
| Aβ42/ttau | 85.71 | 28.57–100.00 | 2.263 | 0 | 1 | 89 | |
| Specificity of 95 % | Sensitivity (%) | 95 % CI | Cutoffa | Differencec (%) | P valued | Correctly classifiedb (%) | |
| Aβ42 | 42.86 | 4.05–100.00 | 672 | 21.05 | 0.008 | 87 | |
| ttau | 14.29 | 0.00–85.71 | 566 | 23.69 | 0.004 | 82 | |
| ptau | 28.57 | 0.00–71.43 | 94 | 2.63 | 1 | 82 | |
| Aβ42/ttau | 71.43 | 8.71–100.00 | 2.093 | 7.90 | 0.25 | 92 | |
Analyte concentrations are described as pg/ml or calculated as ratios between concentrations of two analytes
aCutoff value corresponding to the highest Youden index
bPercentage of positively classified cases based on the CSF cutoff value compared with amyloid PET classification
cPercentage of subjects who were classified differently based on the cutoff values from fixed specificities compared with the cutoff values corresponding to the highest Youden index
dSignificance for the “Difference”
Aβ amyloid beta, AUC area under the receiver operating characteristic curve, CI confidence interval, CSF cerebrospinal fluid, PET positron emission tomography, ptau 181phospho-tau, SE standard error, ttau total tau
Four CSF analytes—Aβ42/ttau, Aβ42/Aβ40, Aβ42/Aβ38, and Aβ42—showed a significant correlation with the 18F-flutemetamol SUVRcomp values (Fig. 2). The linear model was rejected because it did not satisfy assumptions of the model. The hyperbolic model fitted best to the relationship between Aβ42 and 18F-flutemetamol SUVRcomp. The relationships between 18F-flutemetamol SUVRcomp and Aβ42/ttau, Aβ42/Aβ40, and Aβ42/Aβ38 were best described by the exponential model. However, differences between the models were small. There was no correlation between 18F-flutemetamol SUVRcomp values and Aβ38, Aβ40, and ttau (Fig. 2).
Fig. 2.
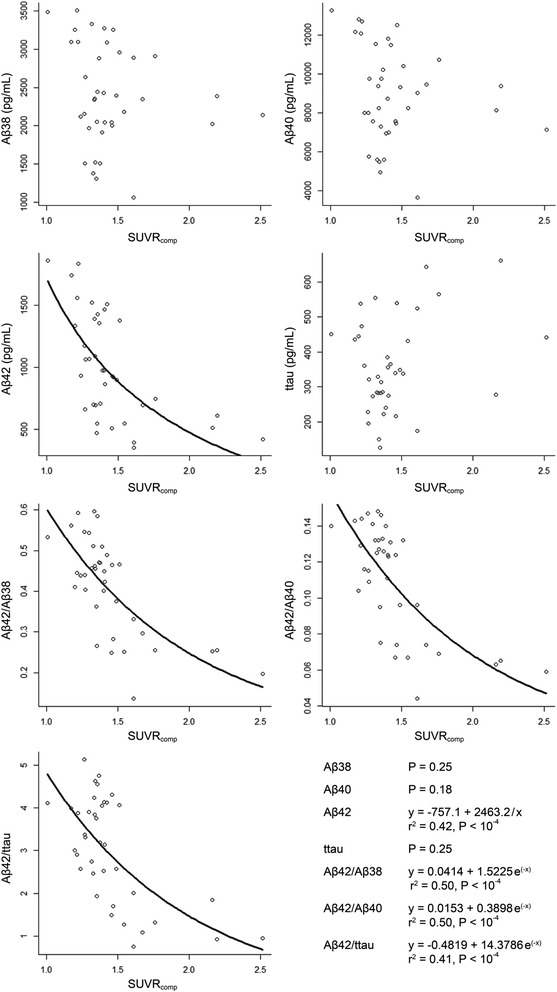
Associations between the different CSF analytes and 18F-flutemetamol SUVRcomp. Black lines fitting of the model, shown only for the significant correlations. Aβ Amyloid beta, SUVR comp standardized uptake value ratios in composite cortical region, ttau total tau
Discussion
Overall, when sensitivity and specificity were combined, the ability to discriminate amyloid-positive from amyloid-negative cognitively healthy older adults was comparable between Aβ42 on its own and the ratio of Aβ42 over the isoforms examined or over ttau. However, when a high specificity of 90–95 % was imposed as a criterion, the sensitivity of Aβ42 alone diminished to 43–57 %. The sensitivity of the ratio over Aβ40 was acceptable at a specificity of 90 % (86 %), but at a specificity of 95 % the sensitivity decreased to 57 %. Under these requirements, the ratio over ttau was the only measure which retained an acceptable sensitivity (71–86 %). A high specificity would for instance be desirable if the potential benefit of a study drug depends on the amyloid positivity of cognitively normal subjects and the study drug has potentially noxious effects or a high cost. A favorable tradeoff in terms of sensitivity, as was the case only for Aβ42 over ttau, would decrease the number of subjects needed to scan to reach a prespecified number of positive cases.
Added value of Aβ isoforms Aβ38 and Aβ40
The shorter isoforms Aβ38 and Aβ40 on their own had no diagnostic value to discriminate preclinical AD, in line with previous studies in cognitively intact healthy controls [14], and also in clinical AD patients [26]. In the context of preclinical AD, the added value of the Aβ isoforms mainly occurred when used for calculating ratios. The ratio over Aβ40 performed better than Aβ42 alone if a high specificity was required (Table 3).
The impact of using Aβ isoforms on the clinical accuracy is linked in part to the context of use. In some studies comparing clinical AD with healthy controls, the ratio of Aβ42 over Aβ38 or Aβ40 improved overall diagnostic accuracy [27, 28], but in others it did not [26, 29]. For the discrimination between clinically probable AD and non-AD dementias, the discriminative value of Aβ42/Aβ40 was similar to that of the ratio over ttau and better than Aβ42 alone [30, 31]. In the MCI stage of the disease, the predictive value for progression to dementia over a 4-year interval was higher for Aβ42/Aβ40 (AUC = 0.866) than for Aβ42 alone (AUC = 0.768) [13]. In our study, Aβ42/Aβ40 still allowed acceptable sensitivity for a specificity of 90 %, and more so than Aβ42 in isolation.
The reason why ratios perform better than Aβ42 in isolation may be methodological: the normalization procedure may remove a portion of the preanalytical and analytical variability in the measurement of the protein levels that is in itself unrelated to AD. In that case, as better standards become available for Aβ42 measurement, the benefit of using ratios will diminish. Alternatively, the ratio may perform better than Aβ42 for biological reasons. Many autosomal dominant forms of AD are associated with an increase in the ratio of Aβ42 over Aβ40 [32, 33]. Others, such as the Dutch and the Arctic APP mutation, are associated with the inverse effect [32]. If the driving force in the initial phases of sporadic AD is related to disequilibrium between different isoforms rather than the absolute amount of Aβ42 on its own, this could theoretically explain why the ratio would be better.
Ratio of Aβ42 over ttau
For a fixed specificity of 95 %, the highest sensitivity (71 %) was obtained for Aβ42 over ttau. Generally, ttau is thought to reflect neuronal loss. Adding the separate measurement of a biomarker that increases with the intensity of the neurodegenerative process may enhance specificity because AD is a multidimensional disease [34, 35] so that adding a second dimension (neuronal loss) improves accuracy of classification. The added value of combining Aβ42 with ttau for the definition of preclinical AD is in line with the International Working Group IWG-2 criteria for preclinical AD which advocate for the combined use of both Aβ42 and ttau or ptau [3].
CSF cutoff value for positive classification
The optimal Aβ42 cutoff value for the INNOTEST assay was higher than what is commonly applied in clinical practice. Previous studies have also suggested that cutoff values derived from studies in patients with more or less advanced stages of AD versus controls may not be entirely appropriate for distinguishing amyloid-positive from amyloid-negative healthy cognitively intact older adults [14, 36]. This has implications for clinical trials aiming to sensitively select cognitively intact subjects with increased Aβ aggregation [36].
Potential study limitations
Our study has some limitations. The sample size was relatively low and the number of amyloid-positive cases was relatively small. Larger studies of preclinical AD will be needed to confirm the estimates of sensitivity and specificity. The low sample size is related to the strict inclusion and exclusion criteria. All subjects were recruited from the community and volunteered for the lumbar puncture purely for research purposes and were informed beforehand that they would not receive any feedback about their proper CSF results. We also applied strict criteria regarding the normality of the neuropsychological test scores. Given the small sample size we were careful to base our conclusions on the most robust findings: we applied strict correction for multiple comparisons and ascertained that our findings were replicable across different assay types and did not depend on small variations of the PET cutoff value within the range of the known test–retest variability of 18F-flutemetamol PET. For all these reasons we consider our results reliable despite the relatively small sample size, in particular the comparisons between AUC analyses. The repercussions of fixing specificity at 90–95 % on sensitivity have to be interpreted more cautiously: given the relatively low number of true positives, a change in classification of an individual case from positive to negative may lead to a disproportionately large decrease in sensitivity.
A community-recruited cohort is not equivalent to a population-based cohort and could be prone to a selection bias, targeting subjects concerned about their cognition, subjects who were more educated or more mobile, etc. We were careful not to mention memory, cognition, or related terms in our advertisement. The research question at hand, namely the comparison between CSF and PET for the research definition of preclinical AD, is most pertinent for a community-recruited setting: clinical trials targeting preclinical AD will generally not be based on population-based nor on memory clinic-based cohorts but on community-recruited cohorts. There was no evidence for a positive selection bias compared with other community-recruited cohorts. If anything, also taking into account the prior stratification for APOE ε4 in our study, our percentage of amyloid-positive cases was lower than in most other community-recruited studies [37]. In a population-based cross-sectional study of cognitively intact adults 50–89 years old, the frequency of amyloid-positive individuals was similar to that in our study [38]. The proportion of subjects who confirmed subjective memory complaints was also not particularly elevated compared with community-based [39, 40] or population-based studies [41].
Our standard of truth was 18F-flutemetamol positivity based on an autopsy-validated cutoff value. We have previously demonstrated a high concordance between 18F-flutemetamol and 11C-Pittsburgh Compound B for the definition of preclinical AD [42]. The autopsy study covered the different Thal stages 1–5 [43]. However, it remains possible, theoretically, that if measured in a population restricted to cognitively intact older adults, the cutoff value for distinguishing moderate to high neuritic amyloid density from sparse to low density may be lower than what is found in a mixed group including patients with advanced dementia along with dementia-free individuals [43]. According to the current study logic, a case who has low Aβ42 values but a normal 18F-flutemetamol value would be considered a false-positive. We cannot, however, exclude that this case is in a preclinical state preceding amyloid deposition detectable by PET [14]. In the selection of subjects who have increased risk of amyloid deposition but who have not yet reached the amyloid positivity threshold, there could still be a role for Aβ isoforms beyond Aβ42, although this remains to be demonstrated. The specificity required to define preclinical AD based on biomarkers will depend on the type of clinical trial. Different therapeutic strategies may target different preclinical stages of the disease. Our findings are mainly relevant for those trials that target a phase where amyloid aggregation has already occurred and where a marker must be selected, CSF versus amyloid PET.
Conclusion
For selection of subjects with increased PET amyloid load, if a high specificity is required, our data support the use of Aβ42 over ttau rather than using Aβ42 alone or the ratios to other Aβ isoforms.
Acknowledgements
The authors would like to thank the staff of Nuclear Medicine, Neurology, and Radiology at the University Hospitals Leuven. Special thanks to Carine Schildermans, Dorien Timmers, Kwinten Porters, Mieke Steukers, and Veerle Neyens for help with the study, and to Kimberley Mauroo, Leentje Demeyer, Erik Stoops, and Erik Van Herck for biomarker analysis in CSF. Financial support was provided by the Foundation for Alzheimer Research SAO-FRMA (09013, 11020, 13007); Research Foundation Flanders FWO (G.0660.09); KU Leuven (OT/08/056, OT/12/097); IWT VIND; IWT TGO BioAdapt AD; Belspo IAP (P7/11); Research Foundation Flanders FWO senior clinical investigator grant to RV and KVL; Research Foundation Flanders FWO doctoral fellowship to KA; and for KH by the Research Fund KU Leuven (OT/11/087 and CREA/14/023). 18F-flutemetamol was provided by GE Healthcare free of charge for this academic investigator-driven trial.
Abbreviations
- Aβ
Amyloid beta
- AD
Alzheimer’s disease
- AIC
Akaike information criterion
- APOE
Apolipoprotein E
- AUC
Area under the receiver operating characteristic curve
- BDNF
Brain-derived neurotrophic factor
- CDR
Clinical Dementia Rating
- comp
Composite cortical volume of interest
- CSF
Cerebrospinal fluid
- ELISA
Enzyme-linked immunosorbent assays
- MCI
Mild cognitive impairment
- MNI
Montreal Neurological Institute
- MMSE
Mini Mental State Examination
- MRI
Magnetic resonance imaging
- NIA–AA
National Institute on Ageing–Alzheimer’s Association
- PET
Positron emission tomography
- ptau
181Phospho-tau
- ROC
Receiver operating characteristic curve
- SD
Standard deviation
- SUVR
Standardized uptake value ratios
- ttau
Total tau
Footnotes
Competing interests
RV has received research grants from Research Foundation Flanders FWO and KU Leuven, has had a clinical trial agreement for phase 1 and 2 study between University Hospitals Leuven and GEHC, has received nonfinancial support from GEHC (provision of 18F-flutemetamol for conduct of investigator-driven trial free of cost), and has a clinical trial agreement (local principal investigator) between University Hospitals Leuven and Merck, Forum, and Roche. HMJV is an employee of ADx NeuroSciences. JL was an employee of GE Healthcare. The remaining authors declare that they have no competing interests.
Authors’ contributions
KA contributed to the study concept and design, acquired the data, performed genotyping, performed statistical analyses, interpreted the data, and drafted the manuscript. JS acquired the data, performed genotyping, and revised the manuscript. HMJV analyzed CSF samples and revised the manuscript. JL analyzed neuroimaging data and helped to revise the manuscript. NN interpreted the data and revised the manuscript. KVL contributed to the study concept and design, and revised the manuscript. PD contributed to the study concept and design, and revised the manuscript. KH performed genotyping and helped to revise the manuscript. KP analyzed CSF samples and revised the manuscript. RV contributed to the study concept and design, interpreted the data and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Katarzyna Adamczuk, Email: kate.adamczuk@med.kuleuven.be.
Jolien Schaeverbeke, Email: jolien.schaeverbeke@med.kuleuven.be.
Hugo M. J. Vanderstichele, Email: hugo.vanderstichele@adxneurosciences.com
Johan Lilja, Email: johan.lilja@radiol.uu.se.
Natalie Nelissen, Email: natalie.nelissen@psych.ox.ac.uk.
Koen Van Laere, Email: koen.vanlaere@uzleuven.be.
Patrick Dupont, Email: patrick.dupont@med.kuleuven.be.
Kelly Hilven, Email: kelly.hilven@med.kuleuven.be.
Koen Poesen, Email: koen.poesen@uzleuven.be.
Rik Vandenberghe, Phone: ++ 32 (0)16 344280, Email: rik.vandenberghe@uz.kuleuven.ac.be.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 4.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid β-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 2014;71:1282–1289. doi: 10.1001/jamaneurol.2014.1358. [DOI] [PubMed] [Google Scholar]
- 5.Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, et al. Independent information from cerebrospinal fluid amyloid-β and florbetapir imaging in Alzheimer’s disease. Brain. 2015;138:772–783. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiltfang J, Esselmann H, Bibl M, Hüll M, Hampel H, Kessler H, et al. Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-Tau in patients with low- and high-CSF A beta 40 load. J Neurochem. 2007;101:1053–1059. doi: 10.1111/j.1471-4159.2006.04404.x. [DOI] [PubMed] [Google Scholar]
- 7.Roher AE, Cribbs DH, Kim RC, Maarouf CL, Whiteside CM, Kokjohn TA, et al. Bapineuzumab alters aβ composition: implications for the amyloid cascade hypothesis and anti-amyloid immunotherapy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roher AE, Maarouf CL, Kokjohn TA, Whiteside CM, Kalback WM, Serrano G, et al. Neuropathological and biochemical assessments of an Alzheimer’s disease patient treated with the γ-secretase inhibitor semagacestat. Am J Neurodegener Dis. 2014;3:115–133. [PMC free article] [PubMed] [Google Scholar]
- 9.Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80:1385–1392. doi: 10.1212/WNL.0b013e31828c2fda. [DOI] [PubMed] [Google Scholar]
- 10.Verbeek MM, Kremer BPH, Rikkert MO, Van Domburg PHMF, Skehan ME, Greenberg SM. Cerebrospinal fluid amyloid beta(40) is decreased in cerebral amyloid angiopathy. Ann Neurol. 2009;66:245–249. doi: 10.1002/ana.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibl M, Gallus M, Welge V, Esselmann H, Wolf S, Rüther E, et al. Cerebrospinal fluid amyloid-β 2–42 is decreased in Alzheimer’s, but not in frontotemporal dementia. J Neural Transm. 2012;119:805–813. doi: 10.1007/s00702-012-0801-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulugeta E, Londos E, Ballard C, Alves G, Zetterberg H, Blennow K, et al. CSF amyloid β38 as a novel diagnostic marker for dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2011;82:160–164. doi: 10.1136/jnnp.2009.199398. [DOI] [PubMed] [Google Scholar]
- 13.Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, et al. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 14.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer’s disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, et al. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55:1623–1628. doi: 10.2967/jnumed.114.142109. [DOI] [PubMed] [Google Scholar]
- 16.Vemuri P, Whitwell JL, Kantarci K, Josephs KA, Parisi JE, Shiung MS, et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008;42:559–567. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamczuk K, De Weer AS, Nelissen N, Chen K, Sleegers K, Bettens K, et al. Polymorphism of brain derived neurotrophic factor influences β amyloid load in cognitively intact apolipoprotein E ε4 carriers. Neuroimage Clin. 2013;2:512–520. doi: 10.1016/j.nicl.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamczuk K, De Weer AS, Nelissen N, Dupont P, Sunaert S, Bettens K, et al. Functional changes in the language network in response to increased amyloid deposition in cognitively intact older adults. Cereb Cortex. 2014. doi:10.1093/cercor/bhu286.
- 19.Nelissen N, Van Laere K, Thurfjell L, Owenius R, Vandenbulcke M, Koole M, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–1259. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 21.Lundqvist R, Lilja J, Thomas BA, Lötjönen J, Villemagne VL, Rowe CC, et al. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med. 2013;54:1472–1478. doi: 10.2967/jnumed.112.115006. [DOI] [PubMed] [Google Scholar]
- 22.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 23.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/WNL.41.4.479. [DOI] [PubMed] [Google Scholar]
- 24.Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72:1029-42 [DOI] [PMC free article] [PubMed]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 26.Schoonenboom NS, Mulder C, Van Kamp GJ, Mehta SP, Scheltens P, Blankenstein MA, et al. Amyloid beta 38, 40, and 42 species in cerebrospinal fluid more of the same? Ann Neurol. 2005;58:139–142. doi: 10.1002/ana.20508. [DOI] [PubMed] [Google Scholar]
- 27.Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, De Deyn PP, et al. Cerebrospinal fluid Aβ1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J Alzheimer’s Dis. 2013;36:759–767. doi: 10.3233/JAD-130107. [DOI] [PubMed] [Google Scholar]
- 28.Lewczuk P, Lelental N, Spitzer P, Maler JM, Kornhuber J. Amyloid-β 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. J Alzheimers Dis. 2015;43:183–191. doi: 10.3233/JAD-140771. [DOI] [PubMed] [Google Scholar]
- 29.Mehta PD, Pirttila T. Increased cerebrospinal fluid A beta38/A beta42 ratio in Alzheimer disease. Neurodegener Dis. 2005;2:242–245. doi: 10.1159/000090363. [DOI] [PubMed] [Google Scholar]
- 30.Welge V, Fiege O, Lewczuk P, Mollenhauer B, Esselmann H, Klafki HW, et al. Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer’s disease. J Neural Transm. 2009;116:203–212. doi: 10.1007/s00702-008-0177-6. [DOI] [PubMed] [Google Scholar]
- 31.Spies PE, Slats D, Sjögren JMC, Kremer BPH, Verhey FRJ, Rikkert MGMO, et al. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer’s disease from non-Alzheimer’s dementia. Curr Alzheimer Res. 2010;7:470–476. doi: 10.2174/156720510791383796. [DOI] [PubMed] [Google Scholar]
- 32.Theuns J, Marjaux E, Vandenbulcke M, Van Laere K, Kumar-Singh S, Bormans G, et al. Alzheimer dementia caused by a novel mutation located in the APP C-terminal intracytosolic fragment. Hum Mutat. 2006;27:888–896. doi: 10.1002/humu.20402. [DOI] [PubMed] [Google Scholar]
- 33.Zhou L, Brouwers N, Benilova I, Vandersteen A, Mercken M, Van Laere K, et al. Amyloid precursor protein mutation E682K at the alternative β-secretase cleavage β′-site increases Aβ generation. EMBO Mol Med. 2011;3:291–302. doi: 10.1002/emmm.201100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenberghe R, Adamczuk K, Dupont P, Van Laere K, Chételat G. Amyloid PET in clinical practice: its place in the multidimensional space of Alzheimer’s disease. Neuroimage Clin. 2013;2:497–511. doi: 10.1016/j.nicl.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberghe R. The relationship between amyloid deposition, neurodegeneration, and cognitive decline in dementia. Curr Neurol Neurosci Rep. 2014;14:498. doi: 10.1007/s11910-014-0498-9. [DOI] [PubMed] [Google Scholar]
- 36.Mattsson N, Insel PS, Donohue M, Jagust W, Sperling R, Aisen P, et al. Predicting reduction of cerebrospinal fluid β-amyloid 42 in cognitively healthy controls. JAMA Neurol. 2015;72:554–560. doi: 10.1001/jamaneurol.2014.4530. [DOI] [PubMed] [Google Scholar]
- 37.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia. A review of clinical and population-based studies. Int J Geriat Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::AID-GPS238>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montejo P, Montenegro M, Fernandez MA, Maestu F. Subjective memory complaints in the elderly: prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging Ment Health. 2011;15:85–96. doi: 10.1080/13607863.2010.501062. [DOI] [PubMed] [Google Scholar]
- 42.Adamczuk K, Schaeverbeke J, Nelissen N, Neyens V, Vandenbulcke M, Goffin K, et al. Amyloid imaging in cognitively normal older adults: Comparison between 18F-flutemetamol and 11C-Pittsburgh Compound B. Eur J Nucl Med Mol Imaging. 2015. doi:10.1007/s00259-015-3156-9. [DOI] [PubMed]
- 43.Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 2015;72:287–294. doi: 10.1001/jamaneurol.2014.4144. [DOI] [PubMed] [Google Scholar]


