Abstract
Background:
Sparse published data are available regarding the prognostic importance of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients with acute ischemic stroke.
Materials and Methods:
We prospectively studied 74 consecutive patients presenting with acute ischemic stroke within 24 hours of onset. All of them underwent laboratory and imaging evaluation and were treated as per guidelines. In all subjects, plasma NT-proBNP levels were measured at initial admission and again on day 7.
Results:
Their mean age was 54 ± 13.5years; there were 49 males; 18 (24%) patients died during the hospital stay. A statistically significant negative correlation between log NT-proBNP and Glasgow coma scale (GCS) score (P < 0.001); and a significant positive correlation between log NT-proBNP and National Institutes of Health Stroke Scale (NIHSS) score (P < 0.001) were observed. Baseline log NT-proBNP levels were higher among non-survivors compared with survivors (6.7 ± 0.47 vs. 5.37 ± 0.62; P = 0.06); day 7 log NT-proBNP levels were significantly higher in non-survivors compared with survivors (7.3 ± 0.26 vs. 4.5 ± 0.4; P = 0.000). In survivors, there was a statistically significant decline in log NT-proBNP levels from baseline to day 7 (5.3710 ± 0.620 vs. 4.5320 ± 0.451; P < 0.001). In contrast, among non-survivors, log NT-proBNP levels showed a statistically significant increase from baseline to day 7 (4.5322 ± 0.451 vs. 7.2992 ± 0.263; P < 0.001). On receiver operator characteristic curve (ROC) analysis, at a cut-off value of ≥ 6.0661, log NT-proBNP had a sensitivity and specificity of 98.2 and 88.9, respectively, in predicting death.
Conclusions:
Plasma log NT-pro-BNP level appears to be a useful biological marker for predicting in-hospital mortality inpatients presenting with acute ischemic stroke.
Keywords: Acute ischemic stroke, N-terminal pro-BNP, outcome
Introduction
Acute ischemic stroke besides being a medical emergency is a significant social and economic problem as well.[1] Brain natriuretic peptide (BNP), which has diuretic, natriuretic, and vasodilatory effects, is a peptide-structured neurohormone released from cardiac ventricles in response to volume and pressure loads. Its role in diagnosis and treatment of cardiovascular diseases is well-reported, but less is known regarding its use in the diagnosis of ischemic stroke. In view of high mortality and morbidity associated with it, non-neurologic variables affecting stroke outcome are emerging to be important for treatment and prognosis in acute ischemic stroke.[2] Therefore, present study was done to assess pattern and prognostic values of plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients with acute ischemic stroke.
Materials and Methods
Consecutive adult patients admitted to neurology department between May 2011 to December 2012 with first episode of acute ischemic stroke presenting within 24 hours of onset were screened for eligibility for inclusion in the study. The diagnosis of acute ischemic stroke was based on clinical examination and confirmed with computed tomography (CT) and magnetic resonance imaging (MRI). Patients who were older than 80 years, patients with acute coronary syndromes, heart failure, valvular heart disease, atrial fibrillation, poor echocardiographic window, intracerebral bleed, cardioembolic stroke, previous stroke or transient ischemic attack (TIA), renal or liver failure, and patients developing fever after onset of stroke were excluded. Institutional Ethical Committee clearance was obtained for conducting the study. Written informed consent was obtained from all patients or from next responsible attendant in case the patient is unconscious for participation in study.
In all patients, a detailed history was obtained and a thorough physical examination including detailed neurological examination was carried out. Patients were categorized according to Glasgow Coma Scale (GCS) score, to have mild (GCS 13-15), moderate (GCS 9-12), and severe (GCS ≤8) degree of altered sensorium.[3] Neurological impairment was evaluated using National Institutes of Health Stroke Scale (NIHSS) score[4] done within 24 hours of admission and again on day 7. An NIHSS score of 0-6 carries a good prognosis, while a score of 7-15 carries an intermediate prognosis and a score of 16-42 carries a bad prognosis.[5]
All patients underwent following laboratory investigations: Complete hemogram, serum biochemistry, lipid profile, coagulation profile, serological testing for human immunodeficiency virus (HIV), vasculitis screening (where indicated), and urinalysis. Plasma NT-proBNP was measured using an automated electrochemiluminescence immunoassay (sandwich method) with an analytical range of 60-3000 pg/mL (7.09-354.73 pmol/L) within 24 hours of admission and on day 7 of admission; 1 mL of peripheral venous blood sample collected in ethylene diamine tetraaceticacid (EDTA) vial was kept at room temperature, and analyzed within 4 hours of sample collection as per the manufacturer's instructions (COBAS H 232 analyzer, Roche diagnostics GmbH, Mannheim, Germany).[6]
All patients underwent CT (Siemens Somatom Emotion Spiral CT Scanner, Muenchen, Germany), MRI (Siemens Symphony Maestro class MRI Scanner, Muenchen, Germany), and magnetic resonance angiography (MRA) of brain; the neuroradiologist and the neurologist together evaluated all images and opined on the same. The subjects were divided into three groups according to the size of infarction observed on MRI into: large (>10 cm3), medium size (4-10 cm3), and small infarction groups (<4 cm3).[7] Severe mass effect was defined as the presence of ventricular shift across the midline and/or enlargement of contralateral ventricle on MRI or CT.[8] Chest radiograph, electrocardiogram (ECG), two-dimensional echocardiogram (2D-ECHO) (IE 33 ECHO Philips medical systems, USA), and Doppler ultrasonography (Siemens Medical Solutions USA Inc., Mountain view, CA 94043, USA) of the carotid and vertebral arteries were done in all patients.
Further course of illness during hospital admission was assessed by progression of presenting complaints, neurological deficits, and their assessment by NIHSS throughout the period of admission, on day 7, and at the time of discharge from the hospital.
Statistical analysis
Data were recorded on a predesigned proforma and managed using Microsoft Excel 2010 (Microsoft Corp, Redmond, WA). All the entries were double-checked for any possible error. Descriptive statistics for the categorical variables were performed by computing the frequencies (percentages) in each category. For the quantitative variables, variables following normal distribution were summarized by mean and standard deviation. Correlation between plasma NT-proBNP, NIHSS, and other parameters was studied using Pearson's correlation coefficient.
As the NT-proBNP values were not normally distributed, log-transformation was undertaken. Log transformed NT-proBNP values at the time of initial presentation and on day 7 were used for analysis. Statistical analysis for intergroup differences was carried out using the independent sample t-test, paired t-test and one-way analysis of variance (ANOVA) for continuous variables and Chi-square for categorical variables. Receiver operating characteristic (ROC) method was used to define the optimal cut-off values of baseline log NT-proBNP and baseline NIHSS score for predicting mortality in patients presenting with acute stroke. A P - value less than 0.05 was considered to be significant. Statistical software IBM Statistical Package of Social Sciences (SPSS) Statistics 20, (IBM SPSS Statistics, Somers NY, USA); and MedCalc Version 11.3.0 for Windows 2000/XP/Vista/7 (MedCalc Software bvba, Belgium) were used for statistical analysis.
Results
During the study period, 237 patients with acute ischemic stroke were screened. Of these, 163 were excluded for various reasons and 74 patients were studied [Figure 1]. Their mean age was 54 ± 13.5 years; there were 49 men. Hypertension (65%) followed by tobacco smoking (46%), hyperlipidemia (29%), and diabetes mellitus (28%) were major risk factors. As per GCS score, severity of altered sensorium at presentation was mild, moderate, and severe in 35 (47%), 24 (33%), and 15 (20%), respectively.
Figure 1.
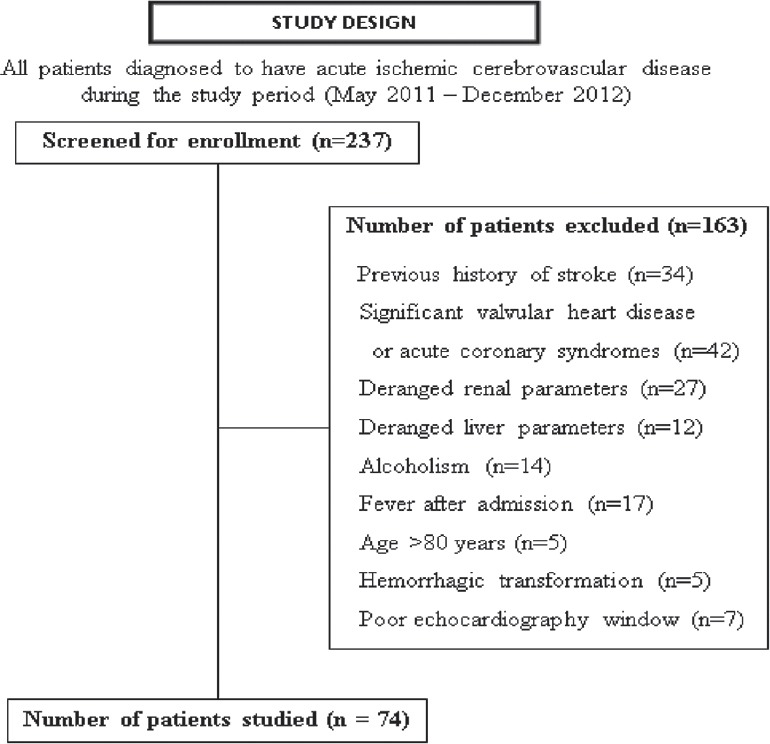
Study design and patient enrollment
A statistically significant negative correlation was observed between log NT-proBNP and baseline GCS score (P < 0.001); and a positive correlation was observed between log NT-proBNP and NIHSS score (P < 0.001) [Table 1]. Patients with severe degree of altered sensorium as per GCS score had a significantly higher levels of log NT-proBNP levels (P < 0.001) and NIHSS score in comparison with those with moderate and mild degree of altered sensorium (P < 0.001).
Table 1.
Correlation matrix showing correlation between NT-proBNP, NIHSS, and other parameters
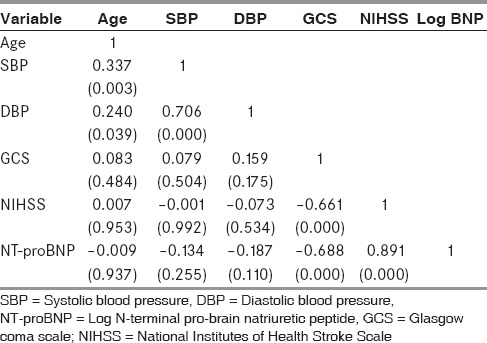
On imaging studies, majority of patients (n = 42; 57%) had large infarction followed by medium-sized (n = 13; 17%) and small (n = 19; 26%) infarction. One-way ANOVA revealed that patients with a NIHSS score of 17–42 had a statistically significant higher levels log NT-proBNP levels (P < 0.001). Patients with a large infarct had a statistically significant higher levels of log NT-proBNP as compared to those with medium and small infarcts (6.18 ± 0.55 vs. 5.61 ± 0.58 vs. 4.68 ± 0.45; F =13.163; P < 0.001).
Most of the patients (n = 59; 80%) received medical management and 14 (19%) required additional surgical measures like decompressive craniectomy. Eighteen patients (24.3%) died during the hospital stay. Of these, 5 died within 7 days and the remaining 13 patients died after 7 days of admission.
Comparison of demographic parameters, risk factors, stroke type, and clinical and laboratory parameters among alive (n = 56) and dead (n = 18) patients with acute ischemic stroke is shown in Tables 2 and 3. Hyperlipidemia was evident in a higher proportion of patients who died compared with those who were alive (12 vs. 17; P = 0.001). Patients who died had worsening course of illness (P = 0.001), lower mean GCS score (P = 0.002); larger infarct size (P = 0.004); and evidence of mass effect (P < 0.001). Even though the baseline log NT-proBNP level was higher among patients who died compared with those who were alive, this difference had borderline statistical significance (P = 0.06). However, the day 7 log NT-proBNP level was significantly higher in patients who died compared with those who were alive (P < 0.001). There was no statistically significant difference in the other parameters compared.
Table 2.
Comparison of demographic parameters among alive and dead patients with acute ischemic stroke
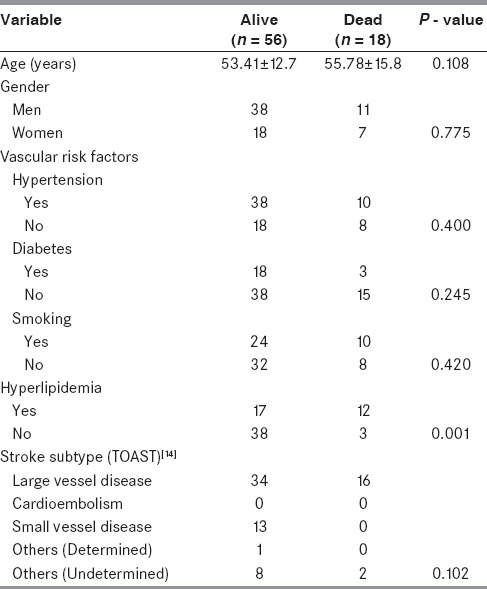
Table 3.
Comparison of clinical and laboratory parameters among alive and dead patients with acute ischemic stroke
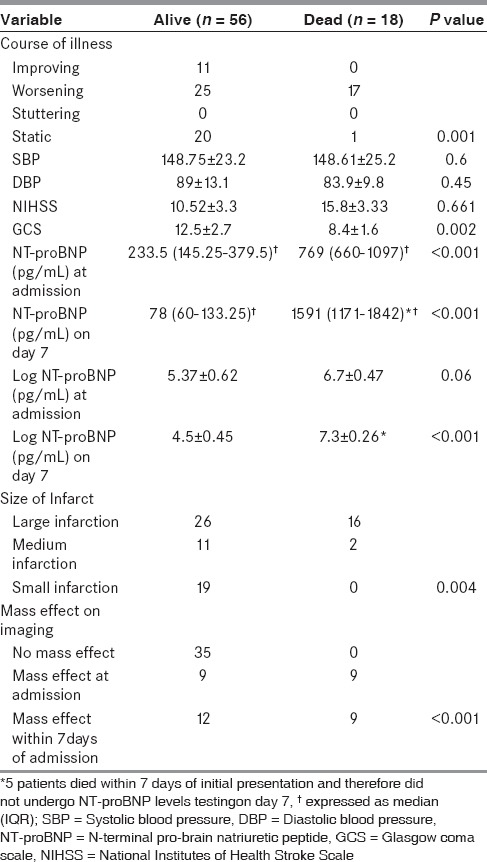
In the patients who were alive, there was a statistically significant decline in log NT-proBNP levels from baseline to day 7 (5.3710 ± 0.620 vs. 4.5320 ± 0.451; P < 0.001). In contrast, the opposite was observed among patients who died where the log NT-proBNP levels showed a statistically significant increase from baseline to day 7 (6.5322 ± 0.451 vs. 7.2992 ± 0.263; P < 0.001).
The ROC curve and interactive dot-diagram for calculating the optimal cut-off value of log NT-proBNP in predicting death is shown in Figures 2 and 3. At a cut-off value log NT-proBNP of greater than 6.0661, the sensitivity and specificity were 98.2 and 88.9, respectively.
Figure 2.
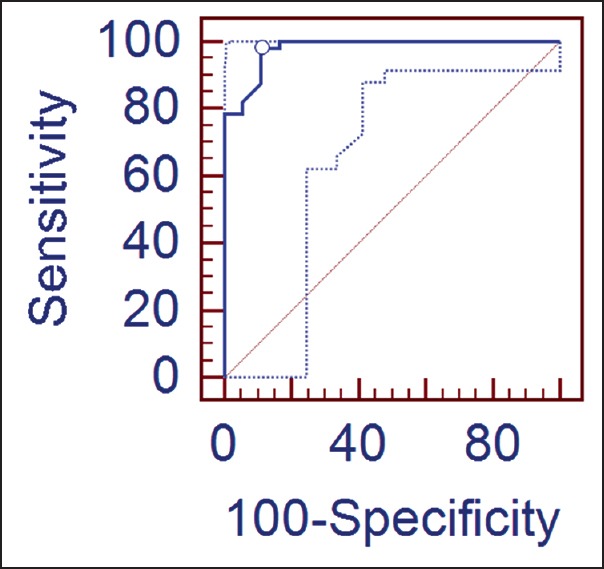
Receiver operator characteristic (ROC)-curve along with 95% confidence bounds for calculating the cut-off value of log N-terminal pro-brain natriuretic peptide (NT-proBNP) in predicting the outcome: Area under the ROC-curve = 0.979; standard error = 0.0153; 95% confidence intervals = 0.914 to 0.998; z-statistic = 31.346; significance level P (Area = 0.5) = 0.0001
Figure 3.
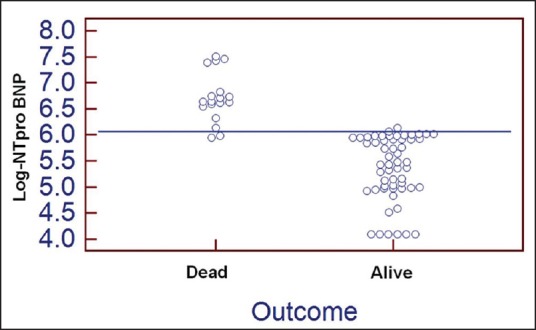
Interactive dot diagram for log N-terminal pro-brain natriuretic peptide (NT-proBNP) values observed among alive and dead patients with acute ischemic stroke. The horizontal line depicts the cut-off value
Discussion
As no currently validated serum biomarkers are available for prognostication in acute ischemic stroke, we evaluated the widely available, rapidly measured biomarker NT-proBNP. As cardiac abnormalities are known to result in increased NT-proBNP production by the heart,[9] care was taken in the present study to exclude ischemic stroke patients with cardiac abnormalities to ensure that these factors did not influence the NT-proBNP levels. The present study is the first of its kind to carefully exclude all patients with cardiac abnormalities while examining the association between serum levels of NT-proBNP and stroke outcome.
The mean age at presentation and gender distribution observed in the present study were similar to other reports.[10,11,12] While cardioembolic stroke constituted a major risk factor in some studies[11,13] none of the patients in the present study had cardioembolic stroke as this was an exclusion criterion. According to Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification of etiologyof stroke subtypes,[14] majority of patients (67%) had large vessel disease. However, in other studies from Europe[13,15] and Japan,[16] the prevalence of large vessel stroke was lower ranging from 8% to 30%. This may probably reflect the prevalence of atherosclerosis in the present study subjects.
We observed a statistically significant negative correlation between GCS and log NT-proBNP (P < 0.001). Furthermore, patients with severe degree of altered sensorium had a statistically significantly higher levels of log NT-proBNP levels (P < 0.001) and NIHSS scores (P < 0.001) in comparison with those with moderate and mild degree of altered sensorium. The probable reason might be due to the massive size of infarct with significant mass effect which usually leads to poor GCS with severely altered sensorium.
In the present study, patients with a large infarct had a statistically significant higher levels of log NT-proBNP as compared to those with medium-sized and small infarcts (P < 0.001). Log NT-pro-BNP level in the context of acute stroke may reflect volume expansion or pressure overload in which the resulting stress in the vessel wall initiates the synthesis of natriuretic peptides in the ventricular myocardium.[17] So, these observations reflect the fact that larger the infarct size, more the pressure overload and wall-stress leading to increased synthesis of natriuretic peptides in ventricular myocardium. Patients with a large infarct also had a significantly higher NIHSS score as compared to those with medium-sized and small infarcts (P < 0.001). This is due to the fact that larger the size of infarct, more neurological deficits will occur which leads to higher NIHSS score. Similar results were reported in another study[12,18] where a significant correlation of infarct size with NT-proBNP levels and NIHSS was reported.
Regarding outcome, in-hospital mortality was slightly high (24.3%) compared with previous studies.[10,11,12,13,17] Ours being a tertiary care hospital, most of the patients will be referred from other hospitals. In the present study, hyperlipidemia emerged as a significant predictor of death in patients with stroke; other variables were comparable. The size of the infarct also predicted death (P = 0.004). As reported in a study from Okayama, Japan,[19] a higher proportion of patients with mass effect had died (P < 0.001) in the present study. Although in other studies age at presentation[17,20] and blood pressure values at initial presentation[12,21] were found to be predictors of death, this was not evident in the present study. Selection bias because of the present study being a hospital-based study could be possible reason for this discordance.
In survivors, there was decline in log NT-proBNP levels from baseline to day 7 while among non-survivors an increase was observed in log NT-proBNP levels from baseline to day 7. This probably reflects the increase in the size of infarct or mass effect which led to an increase in NT-proBNP levels by day 7. Our observations suggest that by watching for an increasing trend in the NT-proBNP levels from the baseline, outcome can be predicted and this can facilitate institution of appropriate care and timely intervention.
Though baseline log NT-proBNP levels were higher among non-survivors, compared with survivors, this difference did not attain statistical significance (P = 0.06). However, log NT-proBNP levels done on day 7 were significantly higher among non-survivors, compared with survivors (P < 0.001). In the present study, ROC-curve analysis revealed that log NT-proBNP value of 6.0661 or more had a sensitivity of 98.2 and specificity of 88.9 in predicting death in patients presenting with acute stroke.
Our study demonstrates the utility of NT-proBNP to predict mortality in patients presenting with acute stroke. Similar observations were reported in other studies.[10,11,12,13,17,21,22,23] Prognostic value of natriuretic peptides have previously been described in patients with heart failure, acute coronary syndromes, and general population, and is being used as a cardiac biomarker in predicting adverse cardiac outcomes. This study suggests a further application of NT-proBNP in risk stratification of stroke patients.
Data regarding levels of BNP after acute ischemic stroke are lacking. Increased sympathetic activity after stroke leads to activation of RAAS and endothelial signal pathways, abnormal accumulation of calcium, release of inflammatory cytokines, oxidative stress, and mechanical stress leading to increased catecholamine release. Increased catecholamines cause toxic effects on myocardium leading to contractile dysfunction, necrosis in cardiac myocytes, and apoptosis. All these factors ultimately lead to myocardial stress. The resulting myocardial wall stress initiates synthesis of natriuretic peptide in the ventricular myocardium and also in brain tissue.[10,24] The more damage sustained as indicated by severity, the poorer the outcome reflected by increased release of NT-proBNP. Natriuretic peptide system is suggested to have a role in mechanism of atherosclerotic plaque formation in coronary arteries. The severity of coronary artery disease is reported to correlate with natriuretic peptide levels. These findings imply that the severity of atherosclerosis underlying the high natriuretic peptide levels might also account for the poor outcome of stroke patients.[25]
Diagnostic applications of NT-proBNP are emerging in recent years. While normative data have been reported in an earlier study,[26] there is a need to derive and define normative data for NT-proBNP for various ethnic regions in the world for its wider applications. High NT-proBNP levels indicate more severe ischemic stroke, and this high NT-proBNP may be an index of occult cardiac impairment contributing to higher mortality. Therefore, estimation of that plasma NT-proBNP level at admission can be useful in predicting the outcome of patients with acute ischemic stroke.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Stroke-1989, Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–31. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 2.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: A systematic review. Cerebrovasc Dis. 2009;28:220–6. doi: 10.1159/000226773. [DOI] [PubMed] [Google Scholar]
- 3.Durant E, Sporer KA. Characteristics of patients with an abnormal glasgow coma scale score in the prehospital setting. West J Emerg Med. 2011;12:30–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Saritas A, Cakir Z, Emet M, Uzkeser M, Akoz A, Acemoglu H. Factors affecting the B-type natriuretic peptide levels in stroke patients. Ann Acad Med Singapore. 2010;39:385–9. [PubMed] [Google Scholar]
- 5.Bulut M, Aydın SA. Stroke. In: Satar S, Karcioglu O, editors. Cardiac emergencies. 1st ed. Adana: Nobel Bookshop; 2008. pp. 457–80. [Google Scholar]
- 6.Cobas h 232 system. Handling. [Last accessed on 2013 1 Apr]. Available from URL: http://www.concern-energomash.am/products/labeq/poc/pdf/cobas_h_232.pdf .
- 7.Pullicino P, Nelson RF, Kendall BE, Marshall J. Small deep infarcts diagnosed on computed tomography. Neurology. 1980;30:1090–6. doi: 10.1212/wnl.30.10.1090. [DOI] [PubMed] [Google Scholar]
- 8.Shibazaki K, Kimura K, Iguchi Y, Aoki J, Sakai K, Kobayashi K. Plasma brain natriuretic peptide predicts death during hospitalization in acute ischaemic stroke and transient ischaemic attack patients with atrial fibrillation. Eur J Neurol. 2011;18:165–9. doi: 10.1111/j.1468-1331.2010.03101.x. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Hamada M, Yamamoto K, Kazatani Y, Hiwada K. Brain natriuretic peptide as a risk marker for incident hypertensive cardiovascular events. Hypertens Res. 2002;25:669–76. doi: 10.1291/hypres.25.669. [DOI] [PubMed] [Google Scholar]
- 10.Iltumur K, Yavavli A, Apak I, Ariturk Z, Toprak N. Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am Heart J. 2006;151:1115–22. doi: 10.1016/j.ahj.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Yip HK, Sun CK, Chang LT, Chen MC, Liou CW. Time course and prognostic value of plasma levels of N-terminal pro-brain natriuretic peptide in patients after ischemic stroke. Circ J. 2006;70:447–52. doi: 10.1253/circj.70.447. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhan X, Chen M, Lei H, Wang Y, Wei D, et al. The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern Med. 2012;51:2887–92. doi: 10.2169/internalmedicine.51.8027. [DOI] [PubMed] [Google Scholar]
- 13.Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005;36:270–5. doi: 10.1161/01.STR.0000151364.19066.a1. [DOI] [PubMed] [Google Scholar]
- 14.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Hajdinjak E, Klemen P, Grmec S. Prognostic value of a single prehospital measurement of N-terminal pro-brain natriuretic peptide and troponin T after acute ischaemic stroke. J Int Med Res. 2012;40:768–76. doi: 10.1177/147323001204000243. [DOI] [PubMed] [Google Scholar]
- 16.Shibazaki K, Kimura K, Okada Y, Iguchi Y, Uemura J, Terasawa Y, et al. Plasma brain natriuretic peptide as an independent predictor of in-hospital mortality after acute ischemic stroke. Intern Med. 2009;48:1601–6. doi: 10.2169/internalmedicine.48.2166. [DOI] [PubMed] [Google Scholar]
- 17.Jensen JK, Atar D, Kristensen SR, Mickley H, Januzzi JL., Jr Usefulness of natriuretic peptide testing for long-term risk assessment following acute ischemic stroke. Am J Cardiol. 2009;104:287–91. doi: 10.1016/j.amjcard.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 18.McLean AS, Huang SJ, Nalos M, Tang B, Stewart DE. The confounding effects of age, gender, serum creatinine, and electrolyte concentrations on plasma B-type natriuretic peptide concentrations in critically ill patients. Crit Care Med. 2003;31:2611–8. doi: 10.1097/01.CCM.0000094225.18237.20. [DOI] [PubMed] [Google Scholar]
- 19.Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, et al. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43:441–5. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomita H, Metoki N, Saitoh G, Ashitate T, Echizen T, Katoh C, et al. Elevated plasma brain natriuretic peptide levels independent of heart disease in acute ischemic stroke: Correlation with stroke severity. Hypertens Res. 2008;31:1695–702. doi: 10.1291/hypres.31.1695. [DOI] [PubMed] [Google Scholar]
- 21.Mäkikallio AM, Mäkikallio TH, Korpelainen JT, Vuolteenaho O, Tapanainen JM, Ylitalo K, et al. Natriuretic peptides and mortality after stroke. Stroke. 2005;36:1016–20. doi: 10.1161/01.STR.0000162751.54349.ae. [DOI] [PubMed] [Google Scholar]
- 22.Sharma JC, Ananda K, Ross I, Hill R, Vassallo M. N-terminal proBrain natriuretic peptide levels predict short-term post stroke survival. J Stroke Cerebrovasc Dis. 2006;15:121–7. doi: 10.1016/j.jstrokecerebrovasdis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Idris I, Hill R, Ross I, Sharma JC. N-terminal probrain natriuretic peptide predicts 1-year mortality following acute stroke: Possible evidence of occult cardiac dysfunction among patients with acute stroke. Age Ageing. 2010;39:752–5. doi: 10.1093/ageing/afq098. [DOI] [PubMed] [Google Scholar]
- 24.Tomida M, Muraki M, Uemura K, Yamasaki K. Plasma concentrations of brain natriuretic peptide in patients with subarachnoid hemorrhage. Stroke. 1998;29:1584–7. doi: 10.1161/01.str.29.8.1584. [DOI] [PubMed] [Google Scholar]
- 25.Duschek N, Skrinjar E, Waldhör T, Vutuc C, Daniel G, Hübl W, et al. N-terminal pro B-type natriuretic peptide (NT pro-BNP) is a predictor oflong-term survival in male patients of 75 years and older with high-grade asymptomatic internal carotid artery stenosis. J Vasc Surg. 2011;53:1242–50. doi: 10.1016/j.jvs.2010.10.123. [DOI] [PubMed] [Google Scholar]
- 26.Daniels LB, Allison MA, Clopton P, Redwine L, Siecke N, Taylor K, et al. Use of natriuretic peptides in pre-participation screening of college athletes. Int J Cardiol. 2008;124:411–4. doi: 10.1016/j.ijcard.2006.12.076. [DOI] [PubMed] [Google Scholar]


