Abstract
A 18-year-old male, screen printer by profession developed sensory motor polyneuropathy, change in his behavior, bilateral 6th and 7th cranial nerve palsies, down beat nystagmus and cerebellar dysarthria. He had bilaterally prolonged P100 latency; left: 137 ms; right: 144 ms. P 37 was not recordable on either side while N 20 was normal. The inter latency difference between Ipsilateral R2 and Contralateral R2 was 6.15 ms, on the left side and normal on the right side. In the follow-up, there was normalization of the blink reflex study, improvement in P100 latency [left: 114 ms; right: 120 ms.] but worsening of peripheral nerve conductions. The sequential clinical recovery was of the behavioral dysfunction, down beat nystagmus, 6th nerve, 7th nerve involvement and ataxia, in that order. Sural nerve biopsy showed loss of large diameter myelinated fibers.
Keywords: 6th and 7th nerve palsy, blink reflex, central nervous system involvement, down beat nystagmus, n- Hexane neuropathy
Introduction
Polyneuropathy related to occupational n-hexane toxicity has been attributed to its metabolite 2, 5-hexanedione,[1] Although documented in experimental animals,[1,2,3] there is paucity of reports suggesting a chronic neurotoxic effect of n-hexane on the central nervous system (CNS).[4,5,6,7]
There are reports of cerebral,[8] cerebellar[9,10] brain stem,[4] spinal cord, cranial nerve involvement[8,11,12] due to n-hexane. Involvement of 6th and 7th cranial nerves, blink reflex pathway and down beat nystagnus has not previously been reported.
We report here a case of n-hexane polyneuropathy with brain stem involvement manifesting with bilateral 6th, 7th cranial nerve involvement, down beat nystagmus, deranged blink reflex, cerebellar and cerebral dysfunction.
Case Report
A 18-years-old male, working in the screen-printing industry for the last 4 years, presented with complaints of numbness in both feet and impaired ground sensations in both feet for one and a half years. Within 9 months of the onset, the numbness progressed up to the knees. After another 3 months, he developed numbness in both hands up to the wrist. For about a month prior to presentation, he started experiencing dizziness, diplopia on looking towards both side, and oscillopsia. He also had a sense of lassitude, lack of interest in his work and excessive sleepiness. There was a change in his voice as if drunk. He was spending more time and even sleeping in his workshop for the last one month. His symptoms further worsened and he developed imbalance while walking and needed support to walk. For the past 1 week, he was bed bound. He denied fever, rash, joint pains, use of alcohol, drug abuse and glue-sniffing. Four of the seven people working with him had similar sensory complaints in their limbs but of variable magnitude.
Physical examination revealed desquamation of the skin over his palms. Higher mental functions, visual acuity, field of vision, color vision and both fundii were normal. He had cerebellar dysarthria, bilateral lateral rectus palsy, downbeat nystagmus and bilateral lower motor neuron facial palsy. All other cranial nerves were normal. All his limbs were hypotonic with normal power, and besides brisk knee jerks, all other reflexes were absent. Sensations of touch and pain were diminished in a glove-stocking distribution, while vibration was diminished in both lower limbs up to the level of the anterior superior iliac spine and in both upper limbs up to the level of radial styloid process. Joint position sensation was impaired in both lower limbs. Romberg's sign was positive.
His hemogram, and biochemical profile for blood sugar, LFT, blood urea, creatinine, serum electrolytes, acid base levels, thyroid profile, calcium, phosphorus were normal. Cerebro spinal fluid (CSF) examination was normal. C reactive protein (CRP) and rheumatoid arthritis (RA) factor, antinuclear antibody (ANA), double stranded DNA (ds-DNA), cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA), perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA), anti Ro, anti-La antibodies, HIV, Hepatitis B surface antigen (HBsAg), anti hepatitis C virus (HCV) were negative. Roentgenogram of Chest, electro cardiogram (ECG) and ultra sound examination of abdomen were normal. Urinary 2, 5-HD levels [qualitative] were high.
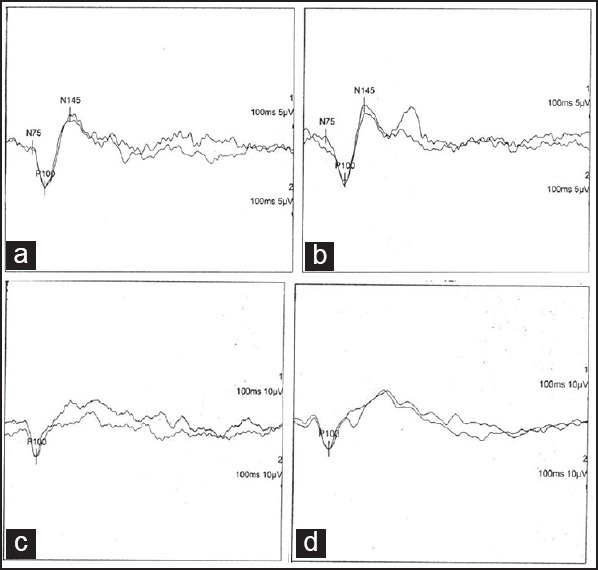
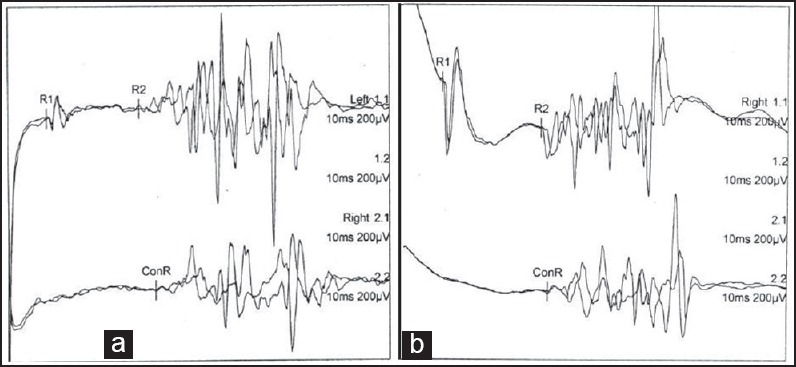
Nerve conduction studies [Table 1] revealed prolonged distal latency in both common peroneal (CP) and posterior tibial (PT) nerves, decreased CMAP amplitude of left CP, and both PT nerves, and partial conduction block [distal/proximal amplitudes and % drop; Right CP: 6.7/3.2 mV (52.2%); Left PT: 3.0/1.5 mV (50.0%)] in right common peroneal and left posterior tibial nerves. There was slow conduction in both common peroneal and posterior tibial nerves. The F wave was prolonged in both lower limb nerves along with chronodispersion of 6. 7 ms [normal ≤6 ms], in bilateral posterior tibial nerves. Temporal dispersion was not encountered in any of the studied nerves. Sensory nerve conduction studies revealed decreased Sensory nerve action potential (SNAP) amplitudes in both median and ulnar nerves while sural and superficial peroneal SNAPs, on both sides, were not recordable. However, dorsal ulnar cutaneous SNAPs were normally recorded. Needle Electromyography (EMG) revealed changes of chronic denervation in extensor digitorum brevis, and first dorsal interosseous, bilaterally. Grade one fibrillations were recorded from right Tibialis anterior and left Flexor digitorum sublimus. EMG of quadriceps, medial gastrocnemius, brachioradialis, pronator teres, biceps, deltoid, on both sides, was normal. The pattern evoked VEP revealed prolonged P100 latency (left: 137 ms; right: 144 ms.; normal, ≤104.5 ms) [Figure 1a and b] Brain stem evoked responses were within normal limits. Somatosensory evoked potential recording with stimulation of the median nerve revealed normal N 20 latency on both sides while P37 with stimulation of the posterior tibial nerve could not be recorded, from either side. Facial nerve study revealed normal distal latencies, while the CMAP amplitude was comparatively low on the left side (left, 2.7 mV; right, 3.9 mV, normal, ≥1.0 mV). Blink reflex study on stimulation of the supraorbital nerve revealed normal latency of R1 and R2, on both sides. However, the difference in latency between Ipsilateral R2 and Contralateral R2 was 6.15 ms [normal, <5.0 ms], on the left side [Figure 2a] and 2.4 ms, on the right side. R/D ratio [R1/direct facial nerve latency] was 3.70 [normal, 2.6-4.6]. magnetic resonance imaging (MRI) brain was normal. Biopsy of the right sural nerve showed loss of large diameter myelinated fibers with no evidence of inflammation or fibrosis [Figure 3]. The patient was managed symptomatically. By the seventh day of his admission, his downbeat nystagmus had resolved completely and by the 10th day, both the 6th and 7th nerve palsies disappeared. Ataxia also showed marked improvement in the first week, however, tandem walking remained impaired even at the end of two and a half months. The deep tendon jerks remained the same. On 70 days of follow up, his electrophysiological evaluation revealed normalization of the blink reflex study [Figure 2b]. The R/D ratio was 3.21. There was improvement in P100 latency [left: 114 ms; right: 120 ms Figure 1c and d], non recordable sensory potentials in both sural and superficial peroneal nerves and decreased SNAP amplitude in both median and ulnar nerves. The distal latencies of both lower limb nerves [Rt. CP 9.6 ms; Lt. CP 10.6 ms; Rt. PT 9.75 ms; Lt. PT 10.50 ms] had further prolonged with F wave showing chronodispersion in right common peroneal [6.30 ms] and left posterior tibial nerves [9.70 ms] along with prolonged minimal latency, in all the motor nerves studied of both upper and lower limbs.
Table 1.
Motor and sensory nerve conduction studies
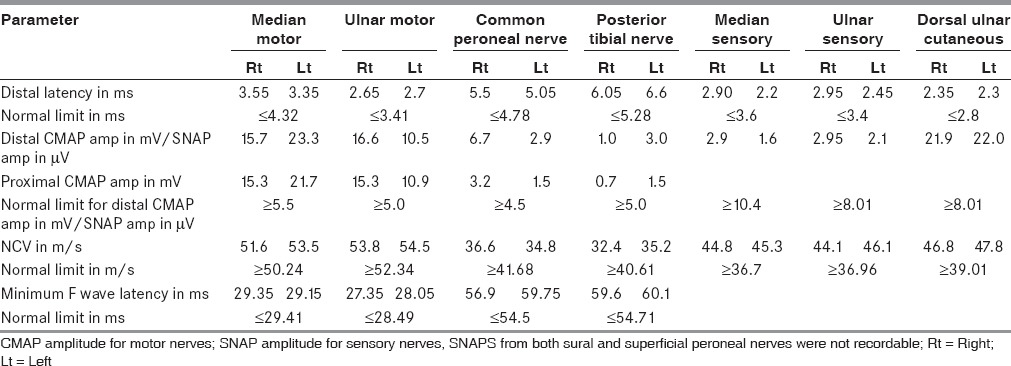
Figure 1.
P 100 latency of pattern evoked visual responses: At presentation; (a) Left, 137 ms, (b) Right 144 ms, After 70 days; (c) Left, 114 ms, (d) Right, 120 ms
Figure 2.
Blink reflex after stimulation of left supra orbital nerve: (a) At presentation R1 was 10.55 ms, R2, 34.3 ms, Contra lateral R 2, 40.45 ms; on direct facial nerve stimulation, latency was 2.85 ms, (b) After 70 days; R1 was 11.25 ms, R2, 39.6 ms, Contra lateral R 2, 41.7 ms; on direct facial nerve stimulation, latency was 3.5 ms
Figure 3.
20× Immunohistochemistry for Myelin Basic Protein of sural nerve showing near total loss of large diameter myelinated fibers with few preserved small diameter myelinated fibers
The chemical analysis of the liquid used in his place of work revealed n-hexane. Suitable instructions were given, regarding the handling of n-hexane, to the patient and his co-workers.
Discussion
In experimental animals,[1,2,3] the pathological hall mark of hexacarbon intoxication is the occurrence of giant axonal swellings in the peripheral nerves along with endoneurial edema. Nerve fiber degeneration is most prominent in the distal portions of the tibial nerve and its branches, and rarely encountered in the proximal regions of the sciatic nerve or in the spinal roots. The lumbosacral anterior horn cells and dorsal root ganglion cells remain uninvolved. The CNS reveals the involvement of rostral regions of long, ascending tracts (dorso-spino-cerebellar, gracile and cuneate) and the caudal end of long, descending tracts (lateral column, ventrolateral and ventromedial tracts) in the medulla and the spinal cord. In cats intoxicated by low-level but prolonged administration of 2, 5-hexanedione, early axonal swellings in the mammillary bodies, the lateral geniculate body and distal optic tract, and the superior colliculi have been reported.[13]
However, in humans a sequential involvement of the CNS, in a case of polyneuropathy, has not been documented. The only autopsy report of a patient with chronic exposure revealed diffuse neuronal loss in cerebral and cerebellar cortex with giant axonal central neuronopathy affecting the long ascending and descending tracts. The corpus callosum was atrophic secondarily to neuron loss in the neocortex. Giant axonal degeneration was also observed in the white matter of the spinal cord, mainly in the posterior and lateral columns.[14]
Perhaps metabolic disturbances induced by hexo carbons may be the underlying mechanism for the development of various pathological changes.
Acute exposure to high air concentration of n-hexane can induce nausea, headache, giddiness, euphoria, hallucination, and mild narcosis. However, acute as well as chronic encephalopathy is much more frequent among glue sniffers.
The clinical profile of our patient was of chronic, sensory motor peripheral neuropathy, more severe in the lower limbs, similar to that described for n- hexane neuropathy. However, he also had almost reversible non-specific features of encephalopathy [lassitude, lack of interest in his work, excessive sleepiness] and brain stem involvement [bilateral 6th, 7th, down beat nystagmus, cerebellar dysfunction]. The bilateral presence of knee jerks could perhaps be due to pyramidal tract involvement. Electrophysiological evaluation revealed asymmetrical, mixed but predominant demyelinating pattern of neuropathy involving both lower limbs. The sensory conduction was severely affected in lower limbs as compared to the upper limbs. The normal dorsal ulnar cutaneous sensory nerve studies could be due to sparing of proximal originating nerve in the dying back phenomenon. Futhermore, the chronic denervation in distal muscles was also evidence of existent dying back phenomenon. The bilateral prolonged pattern VEPs could be due to the demyelinating but subclinical involvement of optic nerves. The non recordable P37, on both sides, may be the resultant of distal axonal involvement of central and peripheral projections. The prolonged inter latency between Ipsilateral R2 and Contra lateral R2 on left side [Figure 1] can be explained with involvement of the pathway between the nucleus of spinal tract of 5th cranial nerve in the ipsilateral pons and medulla and inter neurons forming connections to the contra lateral facial nuclei.
Our patient was spending most of his time in his workplace and this chronic exposure may explain the development of his encephalopathy. The recovery substantiates the reversibility of this process at this early stage of the disease. The sequential recovery was of the cerebral dysfunction, down beat nystagmus, 6th nerve, 7th nerve involvement and ataxia, in that order. Although he had clinical recovery, there was electrophysiological worsening of his motor conduction studies and improvement in blink reflex and VEP. This could be due to coasting effect of n-hexane only on peripheral nerves and sparing of the optic nerve and the CNS. Sural nerve biopsy with features of large fiber axonal loss with evidence of demyelination with and without axonal swelling has been reported in n-hexane neuropathy,[15] as in our case. Perhaps the co-occurrence of similar pathological changes in different parts of neuroaxis was expressing with various clinical as well as electrophysiological changes, in the present case. Further, in the early stage of exposure the neurotoxic effects of n-hexane are reversible
Thus, this case is unique to manifest with n-hexane peripheral neuropathy and reversible CNS neurotoxicity. Safety guidelines in handling n-hexane need to be strictly followed to prevent such occurrences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Spencer PS, Schaumburg HH. Experimental neuropathy produced by 2,5-hexanedione — a major metabolite of the neurotoxic industrial solvent methyl n-butyl ketone. J Neurol Neurosurg Psychiatry. 1975;38:771–5. doi: 10.1136/jnnp.38.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process, IV. Differential vulnerability of PNS and CNS fibres in experimental central-peripheral distal axonopathies. J Neuropathol Exp Neurol. 1977;36:300–20. doi: 10.1097/00005072-197703000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Schaumburg HH, Spencer PS. Degeneration in central and peripheral nervous systems produced by pure n-hexane: An experimental study. Brain. 1976;99:183–92. doi: 10.1093/brain/99.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Chang YC. Neurotoxic effects of n-hexane on the human central nervous system: Evoked potential abnormalities in n-hexane polyneuropathy. J Neurol Neurosurg Psychiatry. 1987;50:269–74. doi: 10.1136/jnnp.50.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korobkin R, Asbury AK, Summer AJ, Nielsen SL. Glue-sniffing neuropathy. Arch Neurol. 1975;32:158–62. doi: 10.1001/archneur.1975.00490450038004. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura M, Inoue N, Ohnishi A, Santo T, Goto I. Polyneuropathy due to glue-sniffing --2 cases involving identical twins. Rinsho Shinkeigaku. 1972;12:290–6. [PubMed] [Google Scholar]
- 7.Sobue I, Yamamura Y, Ando K, Iida M, Takayanagi T. n-Hexane polyneuropathy-outbreak among vinyl sandal manufacturers. Clin Neurol (Jpn) 1968;8:393–403. [Google Scholar]
- 8.Ehyai A, Freemon FR. Progressive optic neuropathy and sensorineural hearing loss due to chronic glue sniffing. J Neurol Neurosurg Psychiatry. 1983;46:349–51. doi: 10.1136/jnnp.46.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabski DA. Toluene sniffing producing cerebellar degeneration. Am J Psychiatry. 1961;118:461–2. doi: 10.1176/ajp.118.5.461. [DOI] [PubMed] [Google Scholar]
- 10.Kelly TW. Prolonged cerebellar dysfunction associated with paint-sniffing. Pediatrics. 1975;56:605–6. [PubMed] [Google Scholar]
- 11.Chang CM, Yu CW, Fong KY, Leung SY, Tsin TW, Yu YL, et al. N-hexane neuropathy in offset printers. J Neurol Neurosurg Psychiatry. 1993;56:538–42. doi: 10.1136/jnnp.56.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seppalainen AM, Raitta C, Huuskonen MS. n-Hexane induced changes in visual evoked potentials and electroretinograms of industrial workers. Electroencephalogr Clin Neurophysiol. 1979;47:492–8. doi: 10.1016/0013-4694(79)90165-2. [DOI] [PubMed] [Google Scholar]
- 13.Schaumburg HH, Spencer PS. Environmental hydrocarbons produce degeneration in cat hypothalamus and optic tract. Science. 1978;199:199–200. doi: 10.1126/science.413192. [DOI] [PubMed] [Google Scholar]
- 14.Escobar A, Aruffo C. Chronic thinner intoxication: Clinico-pathologic report of a human case. J Neuro Neurosurg Psychiatry. 1980;43:986–94. doi: 10.1136/jnnp.43.11.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puri V, Chaudhry N, Tatke M. N-hexane neuropathy in screen printers. Electromyogr Clin Neurophysiol. 2007;47:145–52. [PubMed] [Google Scholar]


