Abstract
Backgrounds/Aims
Hepatocellular carcinoma (HCC) is uncommon in young adults and the prognosis of these patients is still unclear. In this retrospective study, we compared the clinicopathological characteristics and outcomes of young patients with HCC with those of older patients with HCC.
Methods
We retrospectively reviewed the clinicopathological characteristics of a total of 1,124 patients with HCC who underwent hepatectomy at our institution between 2006 and 2010. Patients ≤40 years of age at the time of HCC diagnosis were classified in the younger group.
Results
One hundred and three patients (9.2%) were classified in the younger group. whereas, 1021 patients were classified in the older group. The incidences of hepatitis B virus infection, alpha-fetoprotein (AFP) levels, and indocyanine green retention test were all higher in younger patients than in older patients (p<0.05). Disease-free survival and overall survival were longer in older patients than in younger patients, without statistical significance. In younger patients, increased levels of protein induced by vitamin K antagonist-II (PIVKA-II) and alkaline phosphatase, portal vein tumor thrombosis, and intrahepatic metastasis were all predisposing factors for tumor recurrence after hepatectomy.
Conclusions
Although the AFP levels were higher in younger patients with HCC than in older patients with HCC, disease-free survival and overall survival after liver resection were not significantly different between the two groups.
Keywords: Hepatocellular carcinoma, Hepatectomy, Young age, Prognosis, Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignancy worldwide. Although HCC is generally diagnosed in middle-aged and elderly individuals, the age of peak incidence differs substantially according to etiology and geographical region. In low-risk areas of western countries, HCC is rarely diagnosed in patients younger than 40 years of age. However, in high-risk areas such as East Asia, HCC is often diagnosed in individuals aged between 20 to 40 years.1 Therefore, HCC is a serious threat to public health in East Asia.
Patient age at the time of diagnosis has important prognostic value for certain cancers. While the prognosis for thyroid cancer is favorable if the patient is diagnosed at a young age,2 this is an unfavorable prognostic factor for breast cancer.3 The impact of diagnosis at a young age on the outcome of HCC remains controversial. This controversy could be due to geographic variability and differences in risk profiles between populations.4,5,6,7,8
Several reports described the factors associated with poor prognosis for young patients with HCC. Most of these studies included patients with advanced disease at presentation and high serum alpha-fetoprotein (AFP) levels.4,6,8 However, the difference in prognosis between young and old patients with HCC is not definitively established. There are several reports of worse outcomes for younger patients with HCC, as compared with older patients with HCC;5,7 whereas, others have reported no differences in survival between these two groups.4,6,8
In this study, we compared the clinicopathological characteristics and outcomes of younger patients with HCC with those of older patients with HCC treated at our institution.
MATERIALS AND METHODS
Patients
The study sample included 1,124 patients who underwent surgical resection of HCC between January 2005 and December 2010 at our institution. Of these patients, 103 (9.2%) were 40 years of age or younger (defined as the younger patient group), while 1021 patients (90.8%) were over 40 years of age (defined as the older patient group). The study was approved by the Samsung Medical Center Institutional Review Board and performed according to the guidelines of the Helsinki Declaration. The exclusion criteria were as follows: mixed HCC and cholangiocarcinoma on pathology; age <18 years; previous locoregional therapies such as hepatectomy, radiation, transarterial chemoembolization (TACE), radiofrequency ablation (RFA), and percutaneous ethanol injection (PEI); intraoperative RFA; fibrolamellar HCC, and loss to follow-up after hepatectomy. Demographic, preoperative laboratory, and pathologic data collected from the patients' electronic medical records were retrospectively reviewed.
None of the patients in either group received postoperative adjuvant therapy before recurrence. Patients with intrahepatic recurrences were treated with RFA, TACE, or radiation according to their functional liver reserve and the pattern of recurrence.
Surgery and pathology
Liver function was evaluated using the Child-Pugh classification system. Patients were required to have Child-Pugh class A liver function. Patients were not considered for resection if they had a serum total bilirubin level ≥1.5 mg/dl, an indocyanine green retention test at 15 minutes (ICG-R15) ≥20%, or ascites. Patients with gross vascular invasion on imaging were only considered for resection if the main portal vein and the portal branch to the remaining liver lobe were patent. The platelet count and international normalization ratio (INR) were also recorded, in addition to the levels of serum albumin, total bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), creatinine, alpha-fetoprotein (AFP), and protein induced by vitamin K antagonist-II (PIVKA-II).
Standard operative techniques for hepatectomy are previously described.9,10 Major hepatectomy was defined as the resection of ≥3Couinaud segments and minor hepatectomy was defined as the resection of <3 segments. Anatomic resections involved resection of the tumor with the related portal vein branches and the corresponding hepatic territory. Both peripheral tumors and central tumors were treated by non-anatomic resection. Peripheral tumors and tumors with extrahepatic growths were treated by partial hepatectomy, because this method reportedly yields adequate surgical outcomes. Central tumors near the hepatic hilum and major vessels were treated by enucleation. In such cases, removing a large liver tissue only to obtain adequate margins was considered to be too difficult or risky.10
Postoperative histological parameters assessed included tumor diameter, number of tumors, encapsulation, portal vein tumor thrombosis, microvascular invasion, intrahepatic metastasis, multicentric occurrence, serosal involvement, and fibrosis grade. Intrahepatic metastasis and multicentric occurrence were defined based on guidelines from the Liver Cancer Study Group of Japan.11 The Edmonson-Steiner scale was used to grade HCC as well differentiated (grade I), moderately differentiated (grade II), or poorly differentiated (grade III, IV).12 Modified UICC staging was used to stage HCC.13 The Ludwig-Batts scoring system was used to assess hepatic fibrosis (stage) on the following scale of 0-4: F0=absent, F1=portal fibrosis, F2=periportal fibrosis, F3=bridging fibrosis; and F4=cirrhosis. Tumor recurrence and survival data were also recorded.
Surveillance after surgical resection
Patients were followed postoperatively every 2 to 3 months after surgery. Follow-up evaluations included physical examinations, AFP measurements, PIVKA-II measurements, liver function tests, and chest X-rays. Abdominal computed tomography was performed every 3 months or when recurrence was suspected. If no definitive evidence of recurrence was visible by CT, magnetic resonance imaging and/or positron emission tomography scanning were performed. Detailed information was recorded for each patient with recurrence. Patients with intrahepatic recurrences were treated with RFA, TACE, or sorafenib according to their functional liver reserve and recurrence pattern. Follow-up time was defined as the time from surgery to the time of the last follow-up or death.9,10
Statistical analysis
Categorical variables were expressed as percentages and compared using the χ2 test or Fisher' exact test. Continuous variables were expressed as means and standard deviations and compared using the Mann-Whitney U test. Patient survival and recurrence were calculated using the Kaplan-Meier method and compared using the log-rank test. The most suitable cut-off value for each continuous variable was determined by the receiver operating characteristics (ROC) curve. Clinical and pathologic variables with prognostic significance in univariate analysis were entered into a Cox multivariate proportional hazards model to determine which factors were independently predictive of HCC recurrence. p<0.05 was considered to indicate significance. Analyses were carried out using SPSS 21.0 (SPSS, Chicago, IL, USA).
RESULTS
Preoperative patient characteristics
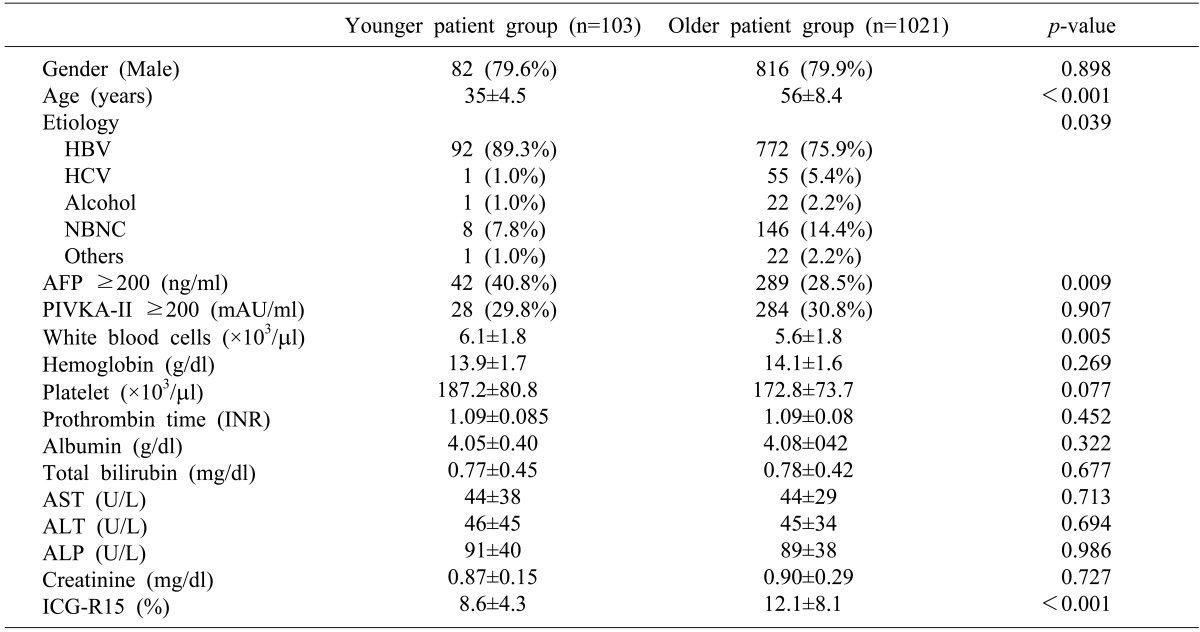
The demographic and preoperative characteristics of the patients were shown in Table 1. A total of 10.2% of the patients with HCC were classified in the young group. The ratio of males to females in the younger group was not significantly different from that of the older group. The younger group showed a higher incidence of HBV infection than the older group (89.3% vs. 79.9%); however, the incidences of non-B, non-C in the younger group were lower than in the older group (7.8% vs. 14.4%).
Table 1. Demographic characteristics.
*HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non-B non-C; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K antagonist-II; INR, internal normalization ratio; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; ICG-15, indocyanine green retention rate at 15 minutes
The mean AFP level in the younger group was higher than in the older group (5885.3±19750.5 ng/ml vs. 5644.2±69285.3 ng/ml; p<0.001); however, the mean PIVKA-II levels of the 2 groups were not significantly different (232.3±359.2 mAU/ml vs. 214.2±308.5 mAU/ml; p=0.496). Although the serum albumin and total bilirubin levels were not significantly different between the 2 groups, the younger group showed a lower mean ICG-R15 level than the older group (8.6% vs. 12.1%; p<0.001).
Perioperative and pathological characteristics
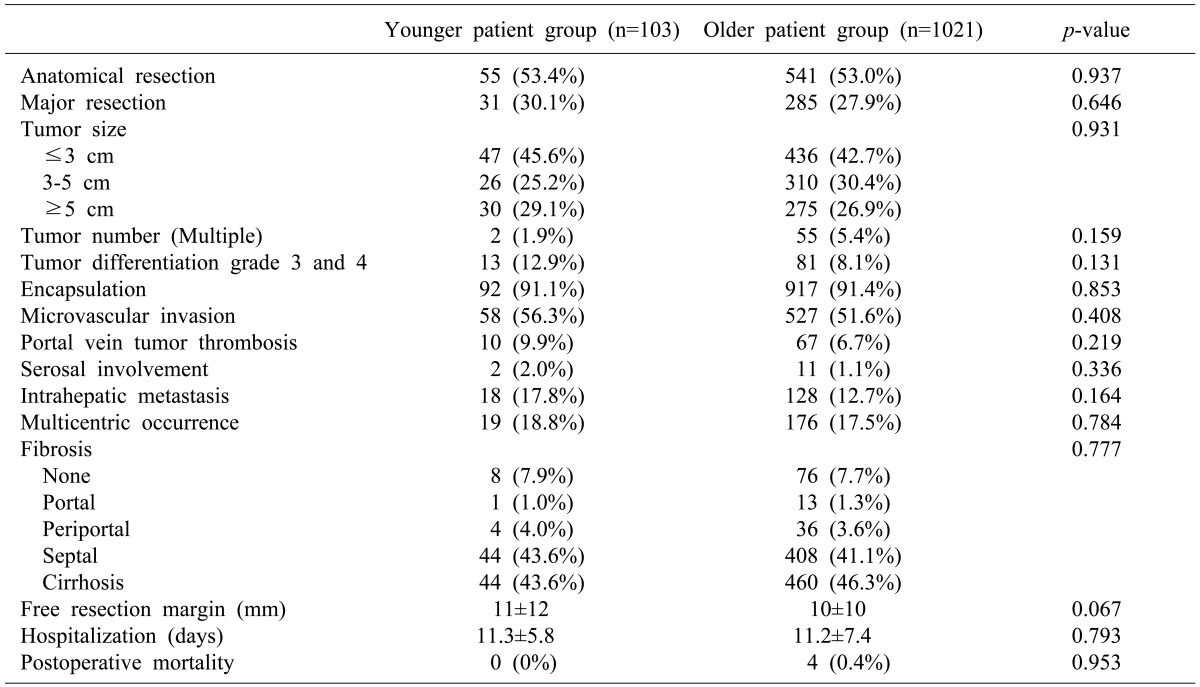
The rates of anatomical resection and major resections in the younger group were not significantly different from those of the older group. The mean tumor sizes in the younger and older groups were 4.8±4.0 cm and 4.4±3.1 cm, respectively. No significant differences in tumor size, tumor number, grade, encapsulation, microvascular invasion, portal vein tumor thrombosis, serosal involvement, intrahepatic metastasis, multicentric occurrence, fibrosis, or hospitalization were observed between the 2 groups. There was no mortality after treatment among patients in the younger group (Table 2).
Table 2. Perioperative and pathologic characteristics.
Disease-free and overall survival
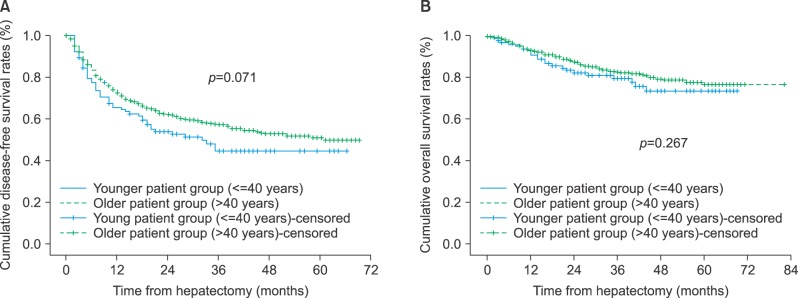
The mean follow-up duration in the younger and older groups were 35±18 months and 32.3±16.7 months, respectively (p=0.156). The 1-year, 2-year and 3-year disease-free survival rates were 65.5%, 53.9%, and 44.6% in the younger group, and 72.5%, 62.1%, and 57.3% in the older group, respectively. Disease-free survival in the younger group was lower than in the older group, without statistical significance (p=0.071) (Fig. 1). The 1-year, 2-year, and 3-year overall survival rates were 93.1%, 82.5%, and 79.7% in the younger group, and 93.6%, 87.8%, and 83.1% in the older group, respectively (p=0.267).
Fig. 1. (A) Disease-free survival and (B) overall survival in patients with hepatocellular carcinoma who underwent hepatic resection.
Tumor recurrence
Tumor recurrence was observed in 52 (50.5%) younger patients and 402 (39.7%) older patients. Intrahepatic recurrence occurred in 31 (59.6%) younger patients and 239 (59.5%) older patients. The differences in systemic recurrence (n=5, 9.6% vs. n=49, 12.2%) and concurrent intrahepatic and systemic recurrence (n=16, 30.8% vs. n=114, 28.4%) between the 2 groups were not significant.
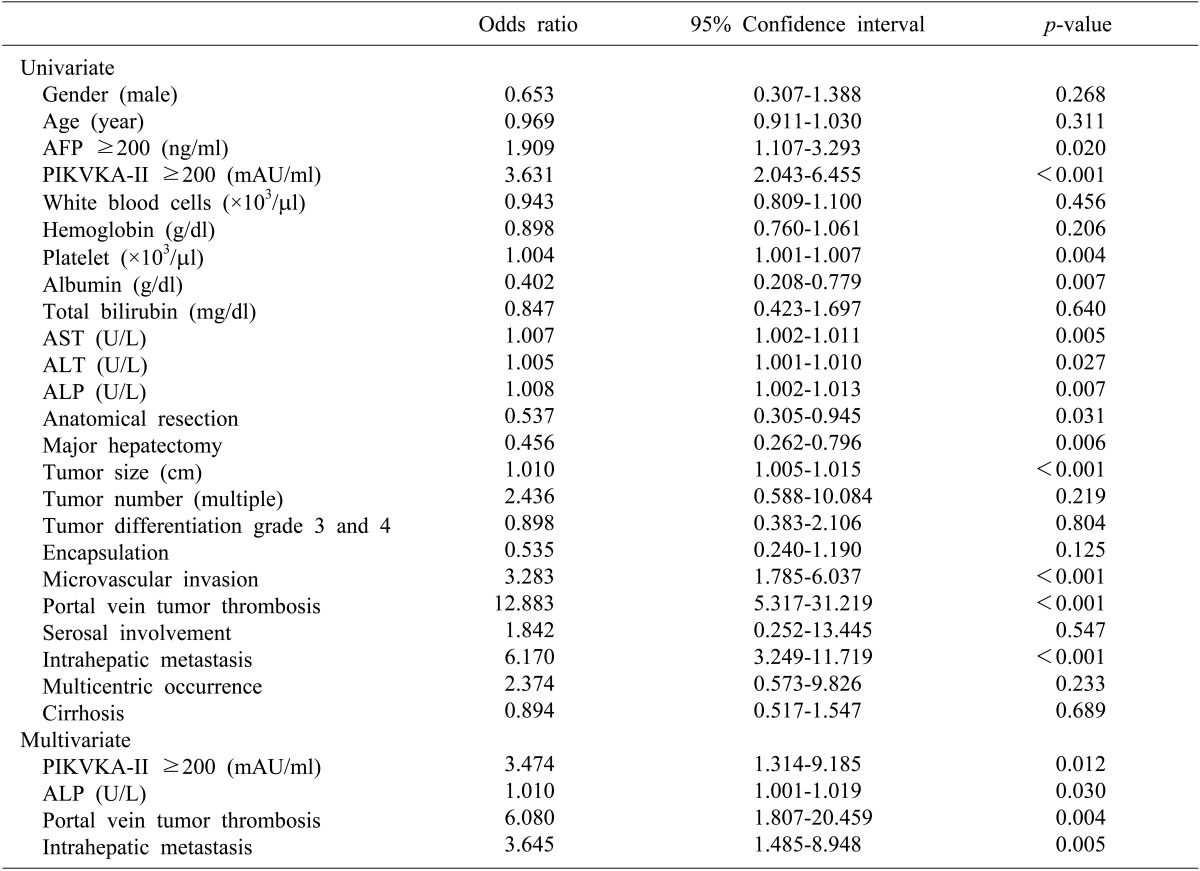
The risk factors for HCC recurrence after hepatectomy in the younger group were summarized in Table 3. Multivariate analysis revealed that increased PIVKA-II levels, increased ALP levels, portal vein tumor thrombosis, and intrahepatic metastasis were independent predisposing factors for tumor recurrence.
Table 3. Risk factors related to tumor recurrence in patients with large HCC after hepatic resection.
*AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K antagonist-II; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; ICG-15, indocyanine green retention rate at 15 minutes
DISCUSSION
The prognosis of young adults with HCC is controversial. In the present study, younger patients with HCC who underwent hepatectomy were more likely than older patients to be HBV-positive, to have high AFP levels at diagnosis, and better liver function status. However, tumor size, tumor number, serum PIVKA-II levels at presentation and extent of liver resection were not significantly different in younger patients, as compared to older patients. Therefore, the survival outcomes of young adults with HCC are not measurably different from those of older patients after liver resection.
The outcome of young patients with HCC is highly debated. Among the tumor factors, AFP levels are reportedly higher in younger patients than older patients. Specifically, more than 50% of all patients with HCC are shown to have an AFP level equal to or exceeding 400 ng/ml.5,6,14 In addition, younger patients tend to have larger tumors than older patients, with the maximum tumor diameter in younger patients exceeding 6 cm.6,8,14,15 Thus, younger patients with HCC should be offered aggressive hepatectomy to preserve liver function. In the present study, the younger group had higher AFP levels and showed better liver function than the older group; these findings are consistent with previous reports.6,14,15 However, tumor size did not differ significantly between the 2 groups. In addition, the presence of initial metastases was similar between the 2 groups. Specifically, younger patients with HCC had worse disease-free and overall survival rates, presumably because they presented with higher AFP levels. However, the survival rates of younger and older patients were not significantly different. Therefore, the natural histories of younger patients with HCC may be similar to those of older patients with HCC after hepatectomy. Thus, early detection may be the best way to improve the prognosis of young patients.
Previous studies have reported that young patients with HCC (aged <40 years) tend to have poor prognoses, as compared to older patients. This is most likely because young patients are often diagnosed at more advanced stages of disease, as indicated by their high serum AFP levels and advanced TNM stages.4,6,8 The results of our study are consistent with previous reports on the importance of higher serum AFP levels; however, we did not find any significant differences in disease-free or overall survival between younger and older patients with HCC. In addition, high serum AFP levels were not associated with tumor recurrence in younger patients.
We defined younger patients with HCC as patients less than 40 years of age at the time of HCC diagnosis. In the present study, 10.2% of all patients with HCC were classified as younger patients. The rates of HCC in this age category (40 years and younger) are reported to range from 0.6 to 10.9% in Asia.7,8,14,15 Although most studies were carried out in Asia, the frequency of HCC is shown to vary according to region. Moreover, many young Asian patients with HCC are also HBV-positive. HBV is an underlying cause of HCC in young patients, and many carriers of HBV live in Asia.16 Moreover, HCC can also develop in non-cirrhotic livers. According to our data, about 57% of all patients with HCC develop cancer in the absence of cirrhosis.
The American Association for the Study of Liver Diseases (AASLD) recommends HCC screening for all patients with cirrhotic HBV infection, Asian male patients older than 40 years with HBV infection, and Asian female patients older than 50 years with HBV infection.17 Based on a strict application of these guidelines, young patients (under the age of 40 years) should be excluded from cancer screening programs. However, our study results suggest that patients less than 40 years of age with HBV infection should receive cancer screening, because early diagnosis of HCC in young patients may improve survival.
The present study had some limitations. First, the signs and symptoms of the patients with HCC were not included in the clinical data because it was impossible to identify these warning signs or symptoms for early detection in young patients. Second, the detailed treatment records taken at the time when young patients experienced HCC recurrence did not include sufficient data for analysis. It was difficult to collect treatment data for patients with recurrent HCC because many of these patients were treated at other centers. Third, since the observational data were analyzed at a single institution, there was possible selection bias; specifically, younger patients may have been more likely to be referred to our institution for evaluation and treatment, while older patients may have received less aggressive treatments and had lower referral rates. In support of this idea, older patients are often managed at community centers. Fourth, patients with unresectable HCC were not included in our analysis. Our study focused on patients who underwent hepatectomy, and its results may therefore have been biased.
In conclusion, younger patients with HCC have different clinical characteristics than older patients with HCC. Specifically, younger patients with HCC are more likely to have hepatitis B infection, high AFP levels, and better liver function. However, younger and older patients with HCC after surgical resection did not exhibit any significant differences regarding disease-free survival and overall survival.
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 Suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973-1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Peres J. Advanced breast cancer in young women. J Natl Cancer Inst. 2013;105:1257–1258. doi: 10.1093/jnci/djt245. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki Y, Kakizaki S, Sohara N, Sato K, Takagi H, Arai H, et al. Hepatocellular carcinoma in young adults: the clinical characteristics, prognosis, and findings of a patient survival analysis. Dig Dis Sci. 2007;52:1103–1107. doi: 10.1007/s10620-006-9578-2. [DOI] [PubMed] [Google Scholar]
- 5.Cho SJ, Yoon JH, Hwang SS, Lee HS. Do young hepatocellular carcinoma patients with relatively good liver function have poorer outcomes than elderly patients. J Gastroenterol Hepatol. 2007;22:1226–1231. doi: 10.1111/j.1440-1746.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Choi MS, Lee H, Kim do Y, Lee JH, Koh KC, et al. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2006;21:588–594. doi: 10.1111/j.1440-1746.2005.04127.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen CH, Chang TT, Cheng KS, Su WW, Yang SS, Lin HH, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26:766–773. doi: 10.1111/j.1478-3231.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 8.Lam CM, Chan AO, Ho P, Ng IO, Lo CM, Liu CL, et al. Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients - implications for screening. Aliment Pharmacol Ther. 2004;19:771–777. doi: 10.1111/j.1365-2036.2004.01912.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 2014;110:976–981. doi: 10.1002/jso.23772. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 2014;21:458–465. doi: 10.1245/s10434-013-3302-1. [DOI] [PubMed] [Google Scholar]
- 11.Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer. 2nd ed. Tokyo: Kaneharap; 2003. [Google Scholar]
- 12.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Korean Liver Cancer Study Group and National Cancer Center, Korea. Practice guidelines for management of hepatocellular carcinoma 2009. Korean J Hepatol. 2009;15:391–423. doi: 10.3350/kjhep.2009.15.3.391. [DOI] [PubMed] [Google Scholar]
- 14.Chang PE, Ong WC, Lui HF, Tan CK. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients? A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol. 2008;43:881–888. doi: 10.1007/s00535-008-2238-x. [DOI] [PubMed] [Google Scholar]
- 15.Takeishi K, Shirabe K, Muto J, Toshima T, Taketomi A, Maehara Y. Clinicopathological features and outcomes of young patients with hepatocellular carcinoma after hepatectomy. World J Surg. 2011;35:1063–1071. doi: 10.1007/s00268-011-1017-7. [DOI] [PubMed] [Google Scholar]
- 16.Dan YY, Aung MO, Lim SG. The economics of treating chronic hepatitis B in Asia. Hepatol Int. 2008;2:284–295. doi: 10.1007/s12072-008-9049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]