Abstract
Background
Rift Valley fever (RVF) is a fatal arthropod-borne zoonotic disease of livestock and humans. Since the identification of RVF in Kenya in the 1930s, repeated epizootics and epidemics coinciding with El Niño events have occurred in several locations in Africa and Saudi Arabia, causing mass deaths of livestock and humans. RVF is of great interest worldwide because of its negative effect on international livestock trade and its potential to spread globally. The latter is due to the increasing incidence of extreme climatic phenomena caused by global warming, as well as to the increase in global trade and international travel. How RVF is maintained and sustained between epidemics and epizootics is not clearly understood, but it has been speculated that wildlife reservoirs and trans-ovarian transmission in the vector may be important. Several studies have examined the role of wildlife and livestock in isolation or in a limited geographical location within the one country over a short time (usually less than a year). In this study, we examined the seroprevalence of anti-RVF antibodies in cattle and several wildlife species from several locations in Kenya over an inter-epidemic period spanning up to 7 years.
Methods
A serological survey of immunoglobulin G (IgG) antibodies to RVF using competitive ELISA was undertaken on 297 serum samples from different wildlife species at various locations in Kenya. The samples were collected between 2008 and 2015. Serum was also collected in 2014 from 177 cattle from Ol Pejeta Conservancy; 113 of the cattle were in close contact with wildlife and the other 64 were kept separate from buffalo and large game by an electric fence.
Results
The seroprevalence of RVF virus (RVFV) antibody was 11.6% in wildlife species during the study period. Cattle that could come in contact with wildlife and large game were all negative for RVFV. The seroprevalence was relatively high in elephants, rhinoceros, and buffalo, but there were no antibodies in zebras, baboons, vervet monkeys, or wildebeest.
Conclusions
Diverse species in conservation areas are exposed to RVFV. RVFV exposure in buffalo may indicate distribution of the virus over wide geographical areas beyond known RVFV foci in Kenya. This finding calls for thorough studies on the epizootology of RVFV in specific wildlife species and locations.
Keywords: inter-epidemic period, zoonosis, emerging disease, trans-boundary disease, cattle, one-health
Rift Valley fever (RVF) is a fatal arthropod-borne zoonotic disease, mainly of livestock and other ruminants, but it also affects humans. RVF virus (RVFV) belongs to the genus Phlebovirus of the family Bunyaviridae (1) and it is tran'smitted by several vectors, mainly mosquitoes of the genus Aedes (2). Since the identification of RVFV in Kenya in the 1930s, repeated epizootics and epidemics coinciding with El Niño events have occurred in several locations in Africa and Saudi Arabia, causing deaths in livestock and also mortality and morbidity in humans (3, 4).
RVFV is of great interest globally, because its range has been expanding outside mainland Africa, where it is known to be endemic. It is likely to spread further due to the increasing occurrence of extreme climatic phenomena driven by global warming and because of the high volume of global travel and trade. The presence of RVFV vectors outside of Africa, for example in North and South America, and improved habitat conditions for endemic establishment of the virus means that this disease has the potential to spread beyond the African continent. This potential has already been demonstrated by the recent outbreak of RVF disease in Saudi Arabia.
How RVFV is maintained and sustained between epidemics and epizootics is not clearly understood, although it is generally assumed that vertical transmission in vectors and wildlife may play a role. Evidence of vertical transmission (passage of the virus from adult mosquitoes to their offspring through trans-ovarian transmission) has been demonstrated in a few mosquito species (5, 6). Comparison of epidemic and inter-epidemic prevalence of RVFV in mosquitoes and humans has shown that there is a slight elevation during epidemics compared to the prevalence during inter-epidemic periods (7, 8). These studies, together with climatic changes associated with outbreaks of RVF disease, suggest that there may be a change in the predominance of mosquito species during epidemics and epizootics, which may be a key factor in causing outbreaks, or a switch of vectors to asymptomatic mammalian carriers. These asymptomatic mammalian carriers may then lead to an elevated infection rate in mosquitoes.
The role of wildlife in the transmission and maintenance of RVFV during the inter-epidemic period has recently been of great interest. In particular, it is not clear yet whether there is a particular wild host species that can be regarded as the reservoir for RVFV, or whether any wild species in the vicinity may be a suitable reservoir. Few serological surveys have examined the role of wildlife or livestock as hosts maintaining the virus during inter-epidemic periods. Such studies have detected antibodies to RVFV in a wide range of wildlife, including rodents, bats, ungulates, and rhinoceros (9, 10). In Kenya, neutralising antibodies to RVFV have been detected in diverse wildlife species born after the RVFV epidemic in Kenya in 2006–2007 (11). The most recent and elaborate serum survey in wildlife, which included samples obtained before (2000–2006), during (2007), and after (2008–2009) the RVFV epidemic in Kenya, showed that the seroprevalence increased during the epidemic and declined immediately after it (12). These studies found a great variation in seroprevalence among species from the same location, suggesting that some species may have an important role as host in maintenance of RVFV during inter-epidemic periods. Many of these studies concentrated on a few mammalian species over a short period of 1–3 years or covered a limited geographical area over a short time. In this study, we examined cattle and several wildlife species in several locations over a long inter-epidemic period spanning 1–7 years in Kenya.
We wanted to determine the seroprevalence of antibodies to RVFV in both cattle and wildlife after the 2006–2007 outbreaks in Kenya, specifically in the years 2008–2015. We examined the influence of wildlife species, geographical location, and year of sampling on the variation in seroprevalence of RVFV antibody. The large number of buffalo examined in this study enabled us to examine the effect of age, sex, sampling period, and geographic location on the seroprevalence of antibodies to RVFV. We also compared the influence of recent circulation of the RVFV in cattle that were physically separated from buffalo and other wildlife and in cattle that were in close contact with buffalo and other wildlife.
Materials and methods
Ethical statement
Wildlife samples used in this study were obtained from the wildlife serum bank of the Kenya Wildlife Service (KWS). The samples had been collected previously during the implementation of various population management strategies, in particular translocations and routine disease surveillance. The samples of elephant serum were thus collected from the 2012 translocation of elephants within Narok-Maasai Mara to reduce conflicts between people and elephants in the area. Serum samples from other species such as warthogs (Phacochoerus aethiopicus delamerei), lesser kudu (Tragelaphus imberbis), reticulated giraffe (Giraffa camelopardalis reticulata), African buffalo (Syncerus caffer), olive baboons (Papio anubis), and vervet monkeys (Chlorocebus pygerythrus) were collected for surveillance of disease other than RVF.
Sampling
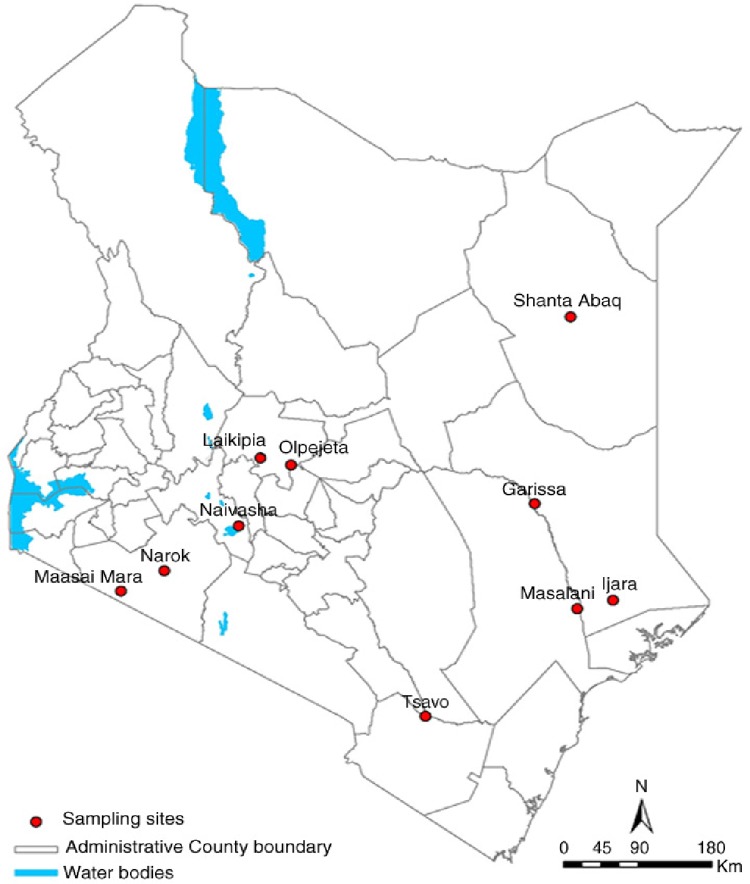
The serum samples were from animals located in several geographical locations in Kenya (Fig. 1), specifically Ijara (Garissa County), Ol Pejeta (Laikipia County), Narok-Maasai Mara (Narok County), Masalani (Tana River County), Shanta Abaq (Wajir County), and Tsavo (Taita Taveta County). However, to display the current extent of the spread of RVFV in Kenyan wildlife, we have included on the map the locations (counties) with RVFV exposure previously published by Evans et al. (2008) and Britch et al. (2013). The county locations with RVFV in wildlife are indicated by numbers on the map (Fig. 1).
Fig. 1.
Map of Kenya showing locations of sampled animals during the study.
Serum samples from cattle and wildlife
In 2014, we sampled a total of 177 cattle (Bos indicus) at Ol Pejeta wildlife conservancy. Of the cattle sampled, 113 were in contact with buffalo and other wildlife species, with a similar number of males (n=51) and females (n=62). We also sampled 64 cattle that were kept separate from buffalo and other large game by an electric fence. These cattle were mostly females (n=60), with a few males (n=4). The animals were restrained in a crush, and blood was drawn from the jugular vein by a veterinarian. It was drawn into plain vacutainer tubes. The samples were kept in a cool box and serum was harvested by centrifugation (3,000 g for 15 min). The samples were transferred to the KWS Veterinary Laboratory in Nairobi, where they were stored at −80°C until analysis.
A total of 297 serum samples from ten mammalian species, collected from nine locations in Kenya, were retrieved from the KWS sample bank for analysis. The samples were from baboons (Papio anubis), black rhino (Diceros bicornis), African buffalo (Syncerus caffer), African elephant (Loxodonta africana), reticulated giraffe (Giraffa camelopardalis reticulata), lesser kudu (Tragelaphus imberbis), vervet monkeys (Chlorocebus pygerythrus), warthogs (Phachocoerus aethiopicus delamerie), blue wildebeest (Connochaetes taurinus), and plains zebra (Equus quagga). The samples had been collected over the period 2008–2015 from immobilised animals during population management activities such as translocations, surveillance of other diseases, and clinical interventions. For buffalo, we had data on location, age, sex, and date of sampling, whereas for the majority of wildlife samples we only had data on the species and the date and location of sampling.
Competitive IgG ELISA
Four hundred and seventy-four sera sampled from cattle and the different species of wildlife listed above were screened for the presence of IgG antibodies to RVFV using ID Screen® RVF competition multi-species ELISA kits (ID-Vet; Innovative Diagnostics, Montpellier, France). The competitive ELISA was carried out according to the manufacturer's protocol. Briefly, 50 µl of the dilution buffer was dispensed into each well of a 96-well ELISA plate. Then 50 µl of the internal positive control was added to wells A1 and B1, whereas the internal negative control was added to wells C1 and D1. To the remaining 94 wells, 50 µl of each sample was added. The samples and controls were mixed appropriately with the dilution buffer. One hundred microlitres of the mixture was transferred to a labelled ELISA plate pre-coated with recombinant RVF nucleoprotein and incubated at 37°C for 1 hour. The ELISA plate was washed three times with washing buffer. Next 100 µl of anti-nucleoprotein peroxidase (HRP) conjugate was added to the microwells and the plate was incubated at room temperature for 30 min. It was washed three times to remove excess conjugate. To the microwells, 100 µl of substrate solution was added and the plate was incubated at room temperature for 15 min in the dark. Then 100 µl of stop solution was added to terminate the reaction. The presence of antibodies to RVFV was shown by lack of a colour change, whereas absence of antibodies to RVFV was shown by a change in substrate colour to blue. (After the addition of stop solution, the colour turned from blue to yellow.) The ELISA plate was read at a wavelength of 450 nm using an iMark microplate absorbance reader (BioRad, Hercules, CA, USA).
Interpretation of ELISA results
Each ELISA test had duplicate internal controls incorporated in each run on a 96-well plate. The optical densities (ODs) of the control were read after each assay at a wavelength of 450 nm using the ELISA reader. To verify the reliability and validity of the results obtained from each ELISA test, the average of the ODs of the two negative controls (NCs) was >0.7 while the average of the two positive controls divided by the average OD of the NCs was <0.3. For each sample, the competition percentage was calculated by dividing the OD of the sample by the average OD of the NCs multiplied by 100 ([ODsample/ODNC]×100). A sample was considered positive if the value obtained from the formula was ≤40%. Any sample with a value of >50% was considered to be negative, whereas values ranging from 40 to 50% were considered to be inconclusive.
Statistical analysis
In order to identify whether certain wildlife species, year of sample collection, and the location of sampling had any influence on the seroprevalence of antibodies to RVFV, we used a generalised linear model (GLM) framework. More specifically, we employed a GLM with a binomial family and a logit link function. The RVFV antibody result was used as a dependent binary variable. Bivariate GLM rather than multivariate GLM was used to determine the influence of several independent variables listed above on the seroprevalence of antibodies to RVFV, because our data were not balanced, due to the opportunistic nature of the sample collection. Where assumptions were met, we repeated these bivariate tests with chi-square statistics to confirm the results from the GLM. We used multivariate GLM on data on buffalo only and tested for the effect on prevalence of location, age, sex, and year of sample collection. We tested all bivariate and multivariate permutations of all independent variables and selected the best model using Akaike information criteria (AIC). For buffalo, using chi-square analysis we also investigated associations between the prevalence of RVFV IgG antibodies and exposure to RVFV during the epidemic and during the inter-epidemic. All analyses were carried out using XLSTAT software for Excel, version 2013.3.03 (Addinsoft, New York, NY, USA) and R software for statistical computing version 3.1.3 (R Core Team, 2015).
Results
The seroprevalence of antibody to RVFV for the 10 wildlife species combined, over all the years of sampling, was 11.6%. The seroprevalence varied among wildlife species, and this difference between species was statistically significant (Table 1). The seroprevalence of IgG antibodies to RVFV was highest (~29%) in the black rhinoceros, followed by the African elephant (~22%) and the buffalo (~18%) (Table 1). A single sample from lesser kudu was positive for IgG antibodies to RVFV. Such antibodies were not detected in several animals, including the giraffe, zebra, baboon, and vervet monkey.
Table 1.
Variation in seroprevalence of antibodies to RVFV in different wildlife species
| Category | n | Seroprevalence (%) | β estimate | Chi-square | Probability |
|---|---|---|---|---|---|
| Baboon | 34 | 0 | 0.000 | ||
| Black rhino | 7 | 28.6 | 5.932 | 12.745 | <0.001 |
| Buffalo | 95 | 17.9 | 5.431 | 12.487 | <0.001 |
| Elephant | 45 | 22.2 | 5.690 | 13.527 | <0.001 |
| Giraffe | 9 | 0 | 2.515 | 1.103 | 0.294 |
| Vervet monkey | 25 | 0 | 1.441 | 0.301 | 0.584 |
| Warthog | 57 | 8.8 | 4.632 | 8.750 | 0.003 |
| Wildebeest | 21 | 4.8 | 4.039 | 5.904 | 0.015 |
| Zebra | 3 | 0 | 3.582 | 2.059 | 0.151 |
Logistic regression coefficients were compared to baboons as the reference group. Kudu was removed from the analysis because there was only a single sample. RVFV, Rift Valley fever virus.
The cattle that were in contact with buffalo and other wildlife species ranged in age from 0.8 years to 2.6 years with a mean (± SD) age of 1.15±0.49 years. All the cattle sampled were negative for IgG antibodies to RVFV irrespective of whether they were in contact with buffalo and other large game or whether they were kept separate.
There was an apparent variation in seroprevalence of antibodies to RVFV between years (χ 2=13.64, P=0.018) (Table 2), with the highest seroprevalence recorded in 2014. Seroprevalence varied with location, with Narok-Maasai Mara having the highest prevalence of anti-RVFV antibodies while Naivasha had significantly lower prevalence than Ijara (Table 3).
Table 2.
Annual variation in seroprevalence of IgG to RVFV in wildlife in the years 2008–2015 in Kenya
| Year | Negative | Positive | Seroprevalence (%) |
|---|---|---|---|
| 2008 | 23 | 4 | 17.4 |
| 2009 | 52 | 5 | 9.6 |
| 2010 | 25 | 0 | 0.0 |
| 2012 | 62 | 11 | 17.7 |
| 2014 | 52 | 13 | 25.0 |
| 2015 | 34 | 0 | 0.0 |
| Total | 248 | 33 | 11.6 |
Table 3.
Spatial variation in seroprevalence of IgG to RVFV in Kenyan wildlife by using seroprevalence in Ijara as a reference for assessment of variation between sampling sites
| Source | n | Seroprevalence (%) | β estimate | Chi-square | Probability |
|---|---|---|---|---|---|
| Ijara | 42 | 7.1 | 0 | ||
| Laikipia | 16 | 12.5 | 0.618 | 1.023 | 0.312 |
| Narok-Maasai Mara | 135 | 17.8 | 1.027 | 6.955 | 0.008 |
| Tsavo | 27 | 14.8 | 0.807 | 2.476 | 0.116 |
| Shanta Abaq | 21 | 9.5 | 0.331 | 0.322 | 0.570 |
| Garissa | 5 | 0.0 | −1.333 | 0.546 | 0.460 |
| Naivasha | 29 | 0.0 | −4.654 | 8.841 | 0.003 |
| Masalani | 20 | 5.0 | −0.253 | 0.144 | 0.704 |
The seroprevalence of antibodies to RVFV in African buffalo varied according to the year and sampling period, but using chi-square analysis this variation was not significantly different from what would be expected at random (χ 2=2.59, P=0.274). The seroprevalence was higher in female buffalo (26.2%, n=11/42) than in male buffalo (13.0%, n=6/46) buffalo, but there was no statistical association between sex and seroprevalence of antibodies to RVFV (χ 2=2.44, P=0.12). However, in a bivariate GLM analysis there was a positive correlation between the seroprevalence of antibodies to RVFV in buffalo and age (β=0.14, P=0.037) (Table 4). Multivariable analysis revealed that the best model for seroprevalence of anti-RVFV antibodies based on AIC was one containing age and sex (Table 5). The prevalence of RVFV antibodies was higher (21.1%, n=12/57) in animals born before or during the epidemic period in 2006–2007 than in animals born after the epidemic (16.1%, n=5/31), but this difference did not reach statistical significance (χ 2=0.312, P=0.312).
Table 4.
Generalised linear model results showing coefficients of different bivariate models for prediction of seroprevalence of IgG to RVFV in buffalo
| Models/predictor variables | β estimate | Standard error | Z-statistic | Probability |
|---|---|---|---|---|
| (Intercept) | −2.29507 | 0.53453 | −4.294 | <0.001 |
| Age | 0.14041 | 0.06726 | 2.088 | 0.037 |
| (Intercept) | −1.0361 | 0.351 | −2.952 | 0.003 |
| Males (compared to females) | −0.861 | 0.5611 | −1.535 | 0.125 |
| (Intercept) | −195.164 | 219.9378 | −0.887 | 0.375 |
| Year | 0.09628 | 0.10929 | 0.881 | 0.378 |
| (Intercept) | −1.3269 | 0.312 | −4.253 | <0.001 |
| Tsavo (compared to Narok-Maasai Mara) | −0.3779 | 0.6267 | −0.603 | 0.547 |
Table 5.
The best model for seroprevalence of antibodies to RVFV in buffalo
| Models/predictor variables | β estimate | Standard error | Z-statistic | Probability |
|---|---|---|---|---|
| (Intercept) | −1.930 | 0.581 | −3.322 | 0.001 |
| Age | 0.146 | 0.070 | 2.090 | 0.037 |
| Male (compared to female) | −0.893 | 0.580 | −1.541 | 0.123 |
Discussion
We found that on average 11.6% of wildlife samples collected after the RVF outbreak in 2006–2007 in Kenya had IgG antibodies to RVFV. A host infected with the virus usually mounts a humoral immune response in which IgM antibodies peak between 2 and 4 weeks after infection but wane within 50 days (13, 14). Four weeks after infection with the virus, IgG antibodies become detectable (15, 16). However, it is not clear how long IgG antibodies remain in the host circulation. The persistence of IgG antibodies varies among different host species; for example, in cattle the IgG remains detectable 5 months after infection (17). This fact means that in the present study detection of IgG does not signify current infection at the time that samples were collected but indicates that the host was exposed to the virus earlier. Interestingly, we even detected IgG seropositivity in samples collected 7 years after the last major epidemic in Kenya. This finding could either suggest long-term persistence of anti-RVFV antibodies in hosts or a low level of relatively recent circulation of RVFV. Clearance of the virus from tissues has been associated with both cellular and humoral responses: mainly CD4+ cells and increased IgG titres (18).
The overall IgG seroprevalence of 11.6% that we observed during the inter-epidemic period was lower than the IgG seroprevalence recorded during the RVF outbreak in Kenya in 2007 (11, 12). The annual trend in anti-RVFV seroprevalence showed a decline after the RVF outbreak in 2007, with no IgG-seropositive hosts in 2010 but with a detectable peak in anti-RVFV seroprevalence in wildlife in 2014. The apparent variation in annual prevalence that we observed could have been an artefact of a lack of systematic sampling of all hosts in all years. If host species vary in their seroprevalence of IgG to RVFV, the annual variation in host species sampled could have caused this temporal variation.
We detected a high seroprevalence of IgG antibodies to RVFV in five species of wildlife (Table 3), where black rhinoceros and African elephants had a higher seroprevalence than buffalo, warthogs, and wildebeest. An earlier survey of anti-RVFV antibodies in black rhinoceros serum samples collected in the years 1987–1997 from populations in Tsavo, Nairobi, and Laikipia, Kenya, did not reveal any seropositivity (19). However, during the RVF outbreak in Kenya in 2006, the black rhinoceros was one of the hosts with the highest seroprevalence of neutralising antibodies (11). In fact, during the 2006 epizootic, Evans et al. (2008) recorded a higher proportion of animals with neutralising antibodies in the black rhinoceros (32.6%) than in the African buffalo (15.6%), African elephant (6%), or warthog (2.5%).
All the cattle tested were seronegative for anti-RVFV IgG antibodies. This result is contrary to the results of previous studies on the inter-epizootic seroprevalence of anti-RVFV antibodies in livestock in Tanzania born after the epidemic, which was found to be about 5.5% (20). The absence of anti-RVFV antibodies in young cattle (2 years old and younger) suggests that there was no recent circulation of RVF virus in the Laikipia region of central Kenya where we sampled cattle and that the cattle would be susceptible to RVFV infection.
The epizootology of RVFV in ruminant hosts of both wildlife and livestock species, especially in the African buffalo, has been well investigated (11, 21, 22). However, from our study, the prevalence of RVFV in non-ruminant hosts such as elephants, rhinos, and warthogs was high, which suggests that these animals may have an important role in the epizootology of RVFV. Inclusion of these species in epidemiological surveillance should be paramount. In Kenya, the black rhinoceros and the African elephant are frequently translocated – as a way of minimising inbreeding in meta-populations in the case of rhino and to minimise conflicts between humans and elephants. Translocation of viraemic animals could facilitate the spread of RVFV. Moving live animals has the inherent risk of spreading pathogens, and this risk is acknowledged more often in the livestock sector. Movement of wildlife or wildlife products (such as smuggled bush meat) is also a huge risk for the local or international spread of pathogens such as RVFV (23).
The species of wildlife that have consistently been found to be seropositive in the pre-epizootic, epizootic, and post-epizootic periods, either by ELISA or by virus neutralisation test, include the African buffalo, the warthog, and the waterbuck (11, 12). During the RVF outbreak in South Africa in 1999, abortions in waterbuck and buffalo were associated with virus infection, clearly demonstrating that these hosts can have clinical signs of RVF disease (9). We assume that hydrophilic traits – especially resting and wallowing in and near marshes and dambos by the African buffalo, waterbuck, and warthog (24, 25), sites that may have a high vector density – would most likely predispose them to RVFV infection even during periods of low virus circulation.
Older buffalo were more likely to be anti-RVFV seropositive than younger ones, which is consistent with results on South African buffalo obtained by Beechler et al. (2013). A similar correlation between seropositivity and age has been noted in livestock (26) and humans (8, 27–29). One theory suggests that the positive correlation between age and seroprevalence is due to accumulation of antibodies in the host population, since neutralising antibodies to RVFV are long-lasting (22). Thus the older animals with a higher prevalence of antibodies are likely to be those that were present during the epidemic. However, when we examined the prevalence in animals born after the epidemic, we still detected the presence of IgG antibodies to RVFV – suggesting exposure to RVFV after the 2006–2007 epidemic. These results support those from several other studies in which the virus has been found to be circulating in domestic ungulates in Tanzania (30).
We also tested the effect of sex on seroprevalence, and the results indicated lack of an association between sex and RVFV antibodies. Our findings are consistent with those of Beechler et al. (2013), which indicated that male and females have an equal chance of being infected with RVFV.
Although giraffes were seronegative in the present analysis, they are among the susceptible wild host species that have previously been shown to have anti-RVFV antibodies (11, 12). The absence of detectable antibodies in zebra is consistent with the findings of Evans et al. (2008) (11). We also did not detect any anti-RVFV antibodies in the baboon and vervet monkey, although these species are known to be able to develop viraemia after infection (9, 31–33).
In the present study, seropositivity for anti-RVFV antibodies was detected in wildlife species in the Ijara, Masalani, and Shanta Abaq regions of north-eastern Kenya, which are situated within the traditional foci of recurrent RVF epizootics. Shanta Abaq was an epicentre of the outbreak in humans and livestock in 2006–2007, where 16 human cases with three deaths were recorded, and warthogs were found to be seropositive (11). In fact, in the last epizootic of 2006–2007, the Ijara region was one of the locations in which African buffalo were found to have a high seroprevalence, with 131 human cases and 27 deaths (11). We detected anti-RVFV antibodies in warthogs and lesser kudu in samples from 2009–2010, which either indicates persistence of the antibodies or post-epizootic activity of the virus. We therefore suggest that the Ijara region should be one of the important locations for RVFV serum surveillance in wildlife, specifically targeting lesser kudu and warthogs, which are the most abundant species in the area.
Although Naivasha is a hotspot for RVF in Kenya (Fig. 1), being the location where RVFV was originally isolated from livestock (34), and although it has been an important and continuing source of outbreaks, there were no seropositive animals detected there during this study. However, during the RVF epizootics of 2007, Britch et al. (2013) found that several wildlife species were seropositive in both the Naivasha and Maasai Mara regions. Interestingly, Narok-Maasai Mara had the highest seroprevalence in the present study. The seroprevalence in wildlife in Narok-Maasai Mara has already been reported (11, 12), which would suggest the possibility of spread of RVFV from Naivasha through movement of viraemic livestock. The Narok-Maasai Mara region is inhabited by the pastoralist community, whose extensive use of grazing may facilitate spread of the virus in the region. Movement of livestock during the viraemic phase has been associated with the spread of RVFV to new areas (35, 36).
Tsavo was the location that had the second-highest seroprevalence. The first record of seroprevalence in Tsavo was in 2008 and involved African buffalo (Britch et al., 2013). The RVF outbreaks in Kenya in 2006–2007 were the most extensive, involving many locations for the first time (36). The seropositivity in Tsavo in 2008 and in the current analysis possibly indicates current virus circulation or long-term retention of anti-RVFV antibodies in hosts.
A basic finding of the present study was that seropositivity in wildlife species was widespread and occurred beyond the known foci of RVF epizootics in Kenya. This finding could be due to a wildlife-vector cycle of RVFV operating at a very low level in such areas or to migration of either wildlife or livestock. For instance, the occurrence of seropositive animals in Lake Nakuru National Park, a completely ring-fence-protected area, may indicate a possible wildlife-vector cycle that does not involve livestock. Furthermore, translocation of wildlife is likely to be a risk factor for the spread of RVFV to new areas. Wildlife translocation is carried out frequently in Kenya (37) as a strategy for wildlife population management, to reduce human–wildlife conflicts and especially to promote meta-population growth of rhinoceros.
We had hypothesised that wildlife populations in certain locations would be more likely to be seropositive than populations in other regions. Our results show that, compared to Ijara, the seroprevalence was higher in Narok-Maasai Mara and lowest in Naivasha, whereas the rest of the locations had seroprevalence for antibodies against RVFV that were not significantly different from those of Ijara.
Anti-RVF antibodies in wildlife populations in Laikipa, Narok-Maasai Mara, and Tsavo show long-term persistence, which makes these locations important foci for serum surveillance of wildlife and livestock during inter-epidemic periods.
Conclusions
Several animal species in diverse conservation areas in Kenya are exposed to RVFV. We have shown that buffalo are also exposed to RVFV during the inter-epidemic periods, suggesting that there is continuous circulation of the virus in the environment. Furthermore, RVFV antibody-positive wildlife species were widespread and occurred beyond the known foci of RVF epizootics in Kenya.
Acknowledgements
We thank the KWS Veterinary Services Department for incredible work in the field during sample collection. Special thanks to Edward Kingori for his immense assistance in cattle sampling and data entry. We would like to thank Ms. Lina Okondo and Mr. Simon Kamande Muiruri for their technical support. This research was co-funded by Kenya Wildlife Service and the Swedish International Development Agency.
Conflict of interest and funding
The authors declare that there are no conflicts of interests.
References
- 1.Garcia S, Crance JM, Billecocq A, Peinnequin A, Jouan A, Bouloy M, et al. Quantitative real-time PCR detection of Rift Valley fever virus and its application to evaluation of antiviral compounds. J Clin Microbiol. 2001;39:4456–61. doi: 10.1128/JCM.39.12.4456-4461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balenghien T, Cardinale E, Chevalier V, Elissa N, Failloux A-B, Jean Jose Nipomichene T, et al. Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet Res. 2013;44:2–10. doi: 10.1186/1297-9716-44-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linthicum KJ, Anyamba A, Tucker CJ, Kelley PW, Myers MF, Peters CJ. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science. 1999;285:397–400. doi: 10.1126/science.285.5426.397. [DOI] [PubMed] [Google Scholar]
- 4.Indeje M, Ward MN, Ogallo LJ, Davies G, Dilley M, Anyamba A. Predictability of the normalized difference vegetation index in Kenya and potential applications as an indicator of Rift Valley fever outbreaks in the Greater Horn of Africa. J Clim. 2006;19:1673–87. [Google Scholar]
- 5.Romoser WS, Oviedo MN, Lerdthusnee K, Patrican LA, Turell MJ, Dohm DJ, et al. Rift Valley fever virus-infected mosquito ova and associated pathology: possible implications for endemic maintenance. Res Rep Trop Med. 2011;2:121–7. doi: 10.2147/RRTM.S13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed RAEH, Abdelgadir DM, Bashab HM. Transovarian transmission of Rift Valley fever virus by two species of mosquitoes in Khartoum State (Sudan): Aedes vexans (Meigen) and Culex quinquefasciatus (Say) Sudanese J Public Health. 2013;8:164–170. [Google Scholar]
- 7.Sang R, Kioko E, Lutomiah J, Warigia M, Ochieng C, O'Guinn M, et al. Rift Valley fever virus epidemic in Kenya, 2006/2007: the entomologic investigations. Am J Trop Med Hyg. 2010;83(2 Suppl):28–37. doi: 10.4269/ajtmh.2010.09-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaBeaud AD, Muchiri EM, Ndzovu M, Mwanje MT, Muiruri S, Peters CJ, et al. Interepidemic Rift Valley fever virus seropositivity, northeastern Kenya. Emerg Infect Dis. 2008;14:1240. doi: 10.3201/eid1408.080082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olive M-M, Goodman SM, Reynes J-M. The role of wild mammals in the maintenance of Rift Valley fever virus. J Wildl Dis. 2012;48:241–66. doi: 10.7589/0090-3558-48.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Herve G. Enzootic activity of Rift Valley fever virus in Senegal. Am J Trop Med Hyg. 1997;56:265–72. doi: 10.4269/ajtmh.1997.56.265. [DOI] [PubMed] [Google Scholar]
- 11.Evans A, Gakuya F, Paweska J, Rostal M, Akoolo L, Van Vuren P, et al. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–9. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britch SC, Binepal YS, Ruder MG, Kariithi HM, Linthicum KJ, Anyamba A, et al. Rift Valley fever risk map model and seroprevalence in selected wild ungulates and camels from Kenya. PLoS One. 2013;8:e66626. doi: 10.1371/journal.pone.0066626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paweska JT, Burt FJ, Anthony F, Smith SJ, Grobbelaar AA, Croft JE, et al. IgG-sandwich and IgM-capture enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in domestic ruminants. J Virol Meth. 2003;113:103–12. doi: 10.1016/s0166-0934(03)00228-3. [DOI] [PubMed] [Google Scholar]
- 14.Niklasson B, Peters C, Grandien M, Wood O. Detection of human immunoglobulins G and M antibodies to Rift Valley fever virus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;19:225–9. doi: 10.1128/jcm.19.2.225-229.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paweska JT, Smith S, Wright I, Williams R. Indirect enzyme-linked immunosorbent assay for the detection of antibody against Rift Valley fever virus in domestic and wild ruminant sera. Onderstepoort J Vet Res. 2003;70:49. [PubMed] [Google Scholar]
- 16.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morvan J, Rollin P, Laventure S, Roux J. Duration of immunoglobulin M antibodies against Rift Valley fever virus in cattle after natural infection. Trans R Soc Trop Med Hyg. 1992;86:675. doi: 10.1016/0035-9203(92)90187-h. [DOI] [PubMed] [Google Scholar]
- 18.Dodd KA, McElroy AK, Jones ME, Nichol ST, Spiropoulou CF. Rift Valley fever virus clearance and protection from neurologic disease are dependent on CD4+ T cell and virus-specific antibody responses. J Virol. 2013;87:6161–71. doi: 10.1128/JVI.00337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer-Tenhagen C, Hamblin C, Quandt S, Frölich K. Serosurvey for selected infectious disease agents in free-ranging black and white rhinoceros in Africa. J Wildl Dis. 2000;36:316–23. doi: 10.7589/0090-3558-36.2.316. [DOI] [PubMed] [Google Scholar]
- 20.Sumaye RD, Geubbels E, Mbeyela E, Berkvens D. Inter-epidemic transmission of Rift Valley fever in livestock in the Kilombero River Valley, Tanzania: a cross-sectional survey. PLoS Negl Trop Dis. 2013;7:e2356. doi: 10.1371/journal.pntd.0002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bird BH, Githinji JW, Macharia JM, Kasiiti JL, Muriithi RM, Gacheru SG, et al. Multiple virus lineages sharing recent common ancestry were associated with a large Rift Valley fever outbreak among livestock in Kenya during 2006–2007. J Virol. 2008;82:11152–66. doi: 10.1128/JVI.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beechler BR, Bengis R, Swanepoel R, Paweska JT, Kemp A, Vuren PJ, et al. Rift Valley Fever in Kruger national park: do buffalo play a role in the inter-epidemic circulation of virus? Transbound Emerg Dis. 2013;62:24–32. doi: 10.1111/tbed.12197. [DOI] [PubMed] [Google Scholar]
- 23.Smith KM, Anthony SJ, Switzer WM, Epstein JH, Seimon T, Jia H, et al. Zoonotic viruses associated with illegally imported wildlife products. PLoS One. 2012;7:e29505. doi: 10.1371/journal.pone.0029505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obanda VO, Lekolool I, Munyao M, Chege SM, Manyibe T, Gakuya F. New distribution records for the desert Warthog (Phacochoerus aethiopicus delamerei–Pallas 1766) Afr J Ecol. 2011;49:373. [Google Scholar]
- 25.Nyirenda VR, Siamudaala VM, Kaula M. Management effectiveness and potential for tourism of Peri-Urban Lusaka national park, Zambia: a preliminary assessment. Environ Nat Resour Res. 2014;4:117. [Google Scholar]
- 26.Di Nardo A, Rossi D, Saleh SM, Lejlifa SM, Hamdi SJ, Di Gennaro A, et al. Evidence of Rift Valley fever seroprevalence in the Sahrawi semi-nomadic pastoralist system, Western Sahara. BMC Vet Res. 2014;10:92. doi: 10.1186/1746-6148-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson ML. Rift Valley fever virus ecology and the epidemiology of disease emergence. Ann N Y Acad Sci. 1994;740:169–80. doi: 10.1111/j.1749-6632.1994.tb19867.x. [DOI] [PubMed] [Google Scholar]
- 28.Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, et al. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37:1084–92. doi: 10.1086/378747. [DOI] [PubMed] [Google Scholar]
- 29.LaBeaud AD. New understanding of the epidemiology of Rift Valley fever virus in Kenya. Cleveland, OH: Case Western Reserve University; 2009. [Google Scholar]
- 30.Kifaro EG, Nkangaga J, Joshua G, Sallu R, Yongolo M, Dautu G, et al. Epidemiological study of Rift Valley fever virus in Kigoma, Tanzania. Onderstepoort J Vet Res. 2014;81:E1–5. doi: 10.4102/ojvr.v81i2.717. [DOI] [PubMed] [Google Scholar]
- 31.Davies F, Onyango E. Rift Valley Fever: the role of the vervet monkey as a reservoir or maintenance host for this virus. Trans R Soc Trop Med Hyg. 1978;72:213–14. doi: 10.1016/0035-9203(78)90074-3. [DOI] [PubMed] [Google Scholar]
- 32.Johnson B, Gitau L, Gichogo A, Tukei P, Else J, Suleman M, et al. Marburg, Ebola and Rift Valley fever virus antibodies in East African primates. Trans R Soc Trop Med Hyg. 1982;76:307–10. doi: 10.1016/0035-9203(82)90175-4. [DOI] [PubMed] [Google Scholar]
- 33.Hartman AL, Powell DS, Bethel LM, Caroline AL, Schmid RJ, Oury T, et al. Aerosolized Rift Valley Fever virus causes fatal encephalitis in African green monkeys and common marmosets. J Virol. 2014;88:2235–45. doi: 10.1128/JVI.02341-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daubney R, Hudson J, Garnham P. Enzootic hepatitis or Rift Valley fever. An undescribed virus disease of sheep cattle and man from East Africa. J Pathol Bacteriol. 1931;34:545–79. [Google Scholar]
- 35.Anyangu AS, Gould LH, Sharif SK, Nguku PM, Omolo JO, Mutonga D, et al. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 2010;83(2 Suppl):14–21. doi: 10.4269/ajtmh.2010.09-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munyua P, Murithi RM, Wainwright S, Githinji J, Hightower A, Mutonga D, et al. Rift Valley fever outbreak in livestock in Kenya, 2006–2007. Am J Trop Med Hyg. 2010;83(2 Suppl):58–64. doi: 10.4269/ajtmh.2010.09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lekolool I, editor. Mega-translocations: The Kenya Wildlife Service at its best. George Wright Forum. 2012;29:93–9. [Google Scholar]