Abstract
Background
Mild traumatic brain injury (mTBI) is a common problem in general practice settings, yet previous research does not take into account those who do not attend hospital after injury. This is important as there is evidence that effects may be far from mild.
Aim
To determine whether people sustain any persistent effects 1 year after mTBI, and to identify the predictors of health outcomes.
Design and setting
A community-based, longitudinal population study of an mTBI incidence cohort (n = 341) from a mixed urban and rural region (Hamilton and Waikato Districts) of the North Island of New Zealand (NZ).
Method
Adults (>16 years) completed assessments of cognitive functioning, global functioning, post-concussion symptoms, mood, and quality of life over the year after injury.
Results
Nearly half of participants (47.9%) reported experiencing four or more post-concussion symptoms 1 year post-injury. Additionally, 10.9% of participants revealed very low cognitive functioning. Levels of anxiety, depression, or reduced quality of life were comparable with the general population. Having at least one comorbidity, history of brain injury, living alone, non-white ethnic group, alcohol and medication use, and being female were significant predictors of poorer outcomes at 12 months.
Conclusion
Although some people make a spontaneous recovery after mTBI, nearly half continue to experience persistent symptoms linked to their injury. Monitoring of recovery from mTBI may be needed and interventions provided for those experiencing persistent difficulties. Demographic factors and medical history should be taken into account in treatment planning.
Keywords: epidemiology, head injury, mild traumatic brain injury, outcome, prediction
INTRODUCTION
Traumatic brain injury (TBI) occurs when there is an external force to the head resulting in an altered state of consciousness.1 Injuries are classified as mild in severity where any loss of consciousness is <30 minutes and disorientation lasts for <24 hours. Epidemiological studies have revealed that 90–95% of traumatic brain injuries are classified as being ‘mild’ in severity. Although labelled as ‘mild’, increasing evidence suggests that the burden of mild TBI (mTBI) may be ‘far from mild’.2 The Diagnostic and Statistical Manual of Mental Disorders (IV) recognises clusters of these symptoms as post-concussion syndrome.3 Diagnostic criteria include:
history of TBI causing ‘significant cerebral concussion’;
cognitive impairment in attention or memory; and
at least three of eight symptoms (fatigue, sleep disturbance, headache, dizziness, irritability, affective disturbance, personality change, apathy) appearing shortly after injury and persisting for at least 3 months.
Despite previous perceptions that the symptoms of mTBI resolve quickly, evidence is emerging that people experience an array of longer-term difficulties post mTBI.4–7 A study of patients attending an emergency department after minor head injury revealed that 63% of participants reported experiencing post-concussion symptoms 1 month post-injury.8 Longer-term studies suggest that symptoms may persist for up to 3 years after injury.2,9,10 Further longitudinal studies are required, however, to improve understanding of the extent of difficulties experienced after mTBI and the recovery trajectory.
There is wide heterogeneity in how people recover after mTBI,11 even when people experience similar injuries. There is a need to better understand this variability in outcome, and to identify the factors, in particular, modifiable factors that contribute to recovery to ensure that appropriate and timely treatment is provided. Existing prognostic models based on moderate to severe TBI perform poorly within the context of mTBI and it remains difficult to identify those at risk of developing persistent symptoms.12,13
In a systematic review of mTBI models, pre-injury mental health and post-injury cognitive functioning were found to be the most robust prognostic factors on persistent symptoms post-injury,12 although the authors note that many studies had suboptimal methodology including the exclusion of people who do not attend medical services after injury.
How this fits in
It was previously believed, and patients commonly expect, that most people recover spontaneously from mild traumatic brain injuries. This study highlights that nearly half of those who experience mild brain injury continue to experience persistent symptoms 1 year later. People who have a history of previous brain injuries, currently using psychotropic medication, or with additional comorbidities are at increased risk of ongoing difficulties. Persistent symptoms need to be identified and acknowledged by clinicians, with information and support provided to facilitate recovery and prevent re-injury.
One of the challenges in studying mTBI is that such an injury may not always be detected. People with mTBI may prefer to access community healthcare facilities such as GPs post-injury rather than attending hospital. People also may not be aware of the need to seek medical treatment or may not wish to declare an injury (due to domestic assault, for example), or do not recall that they have experienced an injury to the head. A person may be concerned that declaring an injury may affect their ability to return to work or sport.14 Outside of community settings, if there are multiple injuries then mTBI may also be overshadowed by more observable injuries requiring urgent medical treatment.
Longitudinal studies of TBI have been based predominantly on participants who sought medical treatment after injury and have not accounted for the proportion of mTBI cases that may have gone undetected, potentially overestimating the longer-term effects identified. The incidence study that formed the basis of the current longitudinal cohort study15 revealed that 36% of TBI cases did not attend hospital, and would therefore have been missed if the study had ascertained cases solely from hospital or death records. The objectives of the current study are to determine the nature and frequency of difficulties experienced, and to identify the predictors of adverse outcomes over the year after injury based on a population-based incidence sample.
METHOD
This was a longitudinal study of a population- based mTBI incidence cohort identified as part of Brain Injury Incidence and Outcomes In the New Zealand Community (BIONIC). Full details of the methodology and incidence findings have been published separately.15,16 The incidence component of the study identified all cases (including all ages and severities) of TBI that occurred during a 1-year period (1 March 2010 through 28 February 2011) in a mixed urban and rural region (Hamilton and Waikato Districts) of the North Island of New Zealand (NZ). Multiple sources of case ascertainment were employed including searches of school and sports club accident records, GPs, allied health professional and self-referrals, in addition to searches of hospital admission and discharge records and national healthcare databases. To prevent missed TBIs skewing the results, all people involved in an accident where they sustained an injury to the upper half of their body were screened to see if a TBI had occurred. TBI was defined using the World Health Organization criteria,17 as an acute brain injury resulting from mechanical energy to the head from external physical forces. Information on all potential TBI cases based on self-report and information obtained from medical records was reviewed by a diagnostic adjudication group to determine if they met the inclusion criteria for TBI. Mild TBI severity was defined using the Glasgow Coma Scale (GCS; 13–15) and/or post-traumatic amnesia (<24 hours). All cases meeting the TBI inclusion criteria that did not have a recorded GCS score were classified as mild in severity.
All confirmed TBI cases were invited to participate in follow-up assessments at baseline (within 2 weeks of the injury), 1, 6, and at 12 months post-injury (±4 weeks). Assessments were completed in person at the participant’s place of residence or at another mutually convenient location such as a private room at a GP practice. Also, data were collected on other factors that could affect the outcome measures, for example, high alcohol use was classified as ≥16 standard drinks per week for males and ≥12 standard drinks per week for females.18 Data for all adult cases (≥16 years) who experienced mTBI in the 1-year period and who consented to follow-up were extracted from the BIONIC dataset for this analysis.
Outcome measures
The Rivermead Post Concussion Symptoms Questionnaire (RPQ)19 was specifically developed to assess the severity of symptoms experienced after a brain injury. Higher scores indicate greater severity of symptoms. A score of ≥2 (indicative that the symptom is problematic in daily life) on four or more items across both subscales was used to determine if post-concussion symptoms met the definition for caseness.
The CNS Vital Signs (CNS-VS)20 is a brief (30-minute) computerised test that measures cognitive functioning across different domains. Scores on each test were combined to yield an overall neurocognition index score. Scoring was automated, eliminating variability and rater bias, and age-adjusted standardised scores were used. A score of ≤70 on the CNS-VS neurocognition index was used to indicate very low performance.
The Hospital Anxiety and Depression Scale (HADS)21 contains two subscales assessing depression and anxiety, and has been found to be sensitive to changes in anxiety and depression during the course of disease. High scores (range 0–21) indicate poorer mood status. In this study a score of ≥8 on either subscale was applied to indicate clinical caseness.
The 36-item medical outcomes Short Form Survey (SF-36)22 assesses health-related quality of life and is commonly used in TBI research.23 The measure contains two component scales (mental health and physical health), which have sound psychometric properties.23,24 Higher scores (range 0–100) indicate better quality of life.
The Glasgow Outcome Scale (GOS)25 is a well-validated measure of global functioning as a combination of neurological functioning and dependence on others.26 The participants’ level of functioning is rated by the researcher conducting the assessment based on a 5-point scale (1 = complete recovery, to 5 = death). A score of ≥2 on the GOS was used to indicate a need for support to complete everyday activities.
Statistical analysis
Summary statistics and the proportion of participants meeting the specified criteria for caseness (poor recovery) were calculated at baseline, 1, 6, and 12 months post-injury. To explore predictors of recovery for the continuous outcomes, multiple linear regression was used. Stepwise selection was used to develop the predictive model for outcomes at 12 months post-injury. Predictors were retained in each model if the P-value was ≤0.05. All variables in Table 1 were considered as covariates in these analyses, in addition to additional comorbidities, current living situation, education level, prior and recurrent TBI, alcohol use, and use of psychotropic medication. As the data on the GOS were highly skewed to the less severe end of the scale, data were dichotomised and logistic regression was used to identify predictors of good or poor recovery for global functioning. As the value for missing data was low (13– 17%) and this appeared to occur at random, it was decided not to impute missing data to avoid biasing the rates of problems experienced at 12 months.
Table 1.
Comparison of participant and non-participant characteristics
| Participant mTBI sample (N = 341), mean (SD) | Non-consenting mTBI cases (N = 529), mean (SD) | Significance of test of difference, P-value | |
|---|---|---|---|
| Age, years | 37.5 (17.5) | 37.3 (20.4) | 0.91 |
|
| |||
| Frequency n (%) | Frequency n (%) | ||
|
|
|
||
| Sex | |||
| Male | 201 (58.9) | 331 (62.6) | 0.28 |
| Female | 140 (41.1) | 198 (37.4) | |
|
| |||
| Ethnic group | |||
| White | 226 (66.3) | 318 (60.1) | 0.07 |
| Maori | 96 (28.2) | 158 (29.9) | |
| Pacific | 9 (2.6) | 21 (4.0) | |
| Asian | 9 (2.6) | 23 (4.3) | |
| Other | 1 (0.3) | 9 (1.7) | |
|
| |||
| Consult within 24 hours | |||
| Yes | 276 (80.9) | 408 (77.1) | 0.54 |
| No | 65 (19.1) | 121 (22.9) | |
|
| |||
| Evidence of brain lesion on CT or skull fracture | |||
| Yes | 11 (3.2) | 19 (3.6) | 0.77 |
| No | 330 (96.8) | 510 (96.4) | |
|
| |||
| Additional injuries | |||
| Yes | 244 (71.6) | 368 (76.0) | 0.81 |
| No/not recorded | 97 (28.4) | 161 (24.0) | |
|
| |||
| Mechanism of injury | |||
| Falls | 114 (33.4) | 160 (30.2) | 0.69 |
| Motor vehicle accident | 80 (23.5) | 112 (21.2) | |
| Exposure to mechanical force | 66 (19.4) | 112 (21.2) | |
| Assault | 72 (21.1) | 118 (22.3) | |
| Other | 9 (2.6) | 27 (5.1) | |
|
| |||
| Area of residence | |||
| Urban | 259 (76.0) | 387 (73.2) | 0.36 |
| Rural | 82 (24.0) | 142 (26.8) | |
RESULTS
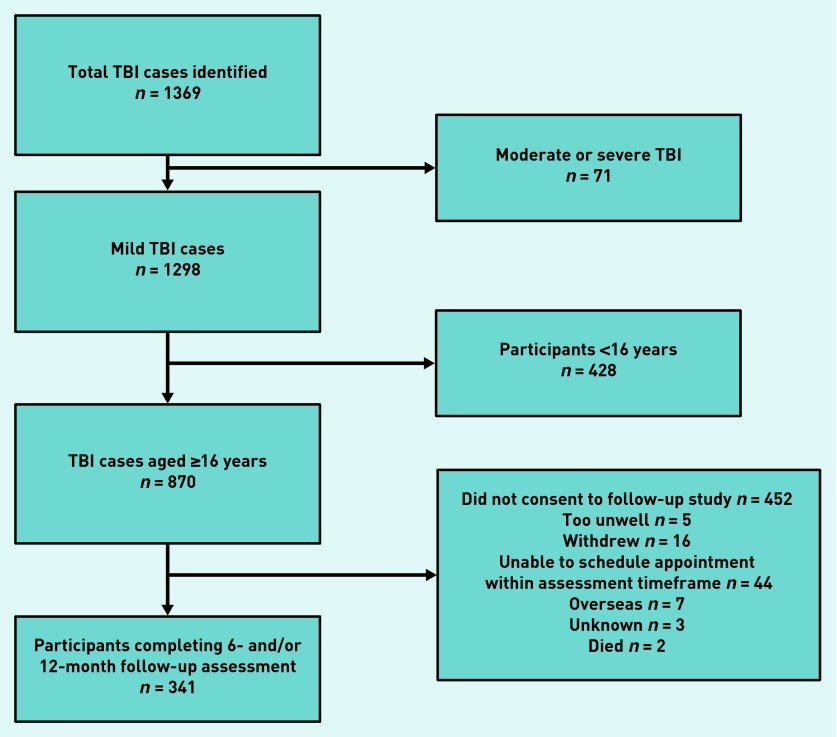
From the original incidence cohort of 870 adults who experienced a mild TBI, 341 (39.2%) completed a follow-up assessment post-injury (Figure 1).
Figure 1.
Participant flowchart.
Comparisons were explored of key demographic and injury characteristics between adult cases included in the analyses and mTBI cases who did not consent to participate in the follow-up assessment. There were no significant differences between participants who completed the follow-up assessment and those who did not consent (Table 1). Further demographic details were available from the TBI sample who completed the follow-up assessment. Of these participants, 87.8% (n = 300) were living with others, 79.8% (n = 272) had at least one comorbid condition, 11.7% (n = 40) reported high levels of alcohol use, 37.0% (n = 127) had experienced more than one TBI, 49.6% (n = 169) had undertaken tertiary education, (that is, university or polytechnic level), and 15.0% (n = 51) were currently using psychotropic medication. Only a few participants (6%) reported using illegal substances, and therefore this variable was not included in the model because of insufficient observations.
The numbers of people experiencing clinically significant difficulties based on the cut-off scores for each of the outcomes are shown in Table 2. It was revealed that nearly half (47.9%) of participants reported experiencing four or more mild to severe post-concussion symptoms at 1 year post-injury.
Table 2.
Proportion of participants experiencing significant impairment over the year after mild TBI
| Impairment, n participants (%) | ||||
|---|---|---|---|---|
|
| ||||
| At baseline | At 1 month | At 6 months | At 12 months | |
| Post-concussion symptomsa | 107 (70.9) | 130 (57.8) | 122 (49.8) | 139 (47.9) |
| Health-related quality of life — mental component (SF-36)b | 93 (62.8) | 120 (53.6) | 117 (48.3) | 140 (48.1) |
| Health-related quality of life — physical component (SF-36)b | 112 (75.7) | 138 (61.1) | 117 (48.3) | 138 (47.4) |
| Cognitive functioning (CNS-VS Neurocognitive Index)b | 15 (12.5) | 19 (10.0) | 21 (11.4) | 26 (10.5) |
| Depression (HADS)a | 30 (18.6) | 38 (16.5) | 38 (15.0) | 36 (12.1) |
| Anxiety (HADS)a | 68 (42.2) | 69 (30.0) | 84 (33.2) | 87 (29.3) |
| Global functioning (GOS)b | 67 (20.0) | 35 (15.1) | 31 (12.2) | 31 (10.4) |
Higher scores indicate poorer outcome.
Higher scores indicate better outcome. GOS = Glasgow Outcome Scale. HADS = Hospital Anxiety and Depression Scale. SF-36 = 36-item medical outcomes Short Form Survey.
To identify predictors of outcome, demographic factors (age, sex, ethnic group, comorbidities, residential area, and whether they live alone), injury characteristics (medical consultation, evidence of skull fracture/lesion, additional injuries sustained, mechanism of injury), and risk factors (alcohol use, multiple TBIs) were entered into linear regression models of outcomes 1 year after injury (Table 3).
Table 3.
Predictors of outcomes at 1 year
| Outcome variable | Parameter | Estimate | P -value | CIs | R2 |
|---|---|---|---|---|---|
| Post-concussion symptoms (RPQ)a | Female sex | 8.86 | <0.0001 | 5.95 to 11.79 | |
| Living alone | 8.93 | <0.0001 | 5.04 to 12.82 | ||
| Multiple TBI | 5.76 | <0.0001 | 3.22 to 8.29 | ||
| White ethnic group | −3.77 | <0.01 | −6.46 to −1.09 | ||
| Medication use | 5.19 | <0.01 | 1.60 to 8.78 | ||
| Overall model | 0.22 | ||||
|
| |||||
| Health-related quality of life — physical component (SF-36)b | Age (1 year increase) | −0.20 | <0.0001 | −0.027 to −0.014 | |
| White ethnic group | 3.64 | <0.01 | 1.25 to 6.03 | ||
| ≥1 comorbidity | −4.12 | <0.01 | −6.95 to −1.28 | ||
| Living alone | −4.61 | <0.02 | −6.77 to −0.45 | ||
| Medication use | −3.61 | <0.03 | −6.78 to −0.45 | ||
| Overall model | 0.24 | ||||
|
| |||||
| Health-related quality of life — mental component (SF-36)b | Age (1 year increase) | 0.12 | <0.01 | 0.04 to 0.20 | |
| Female sex | −4.56 | <0.001 | −7.23 to −1.89 | ||
| White ethnic group | 3.12 | <0.03 | 0.35 to 5.88 | ||
| ≥1 comorbidity | −5.47 | <0.002 | −8.78 to −2.16 | ||
| Living alone | −9.79 | <0.01 | −13.93 to −5.60 | ||
| Medication use | 5.12 | <0.01 | 1.41 to 8.84 | ||
| Overall model | 0.20 | ||||
|
| |||||
| Cognitive functioning (CNS-VS Neurocognitive Index)b | White ethnic group | 10.44 | <0.001 | 4.99 to 15.90 | |
| Overall model | 0.05 | ||||
|
| |||||
| Anxiety (HADS)a | Age | −0.03 | <0.02 | 3.47 to 6.12 | |
| Female sex | 1.20 | 0.02 | 0.24 to 2.15 | ||
| ≥1 comorbidity | 1.49 | <0.02 | 0.31 to 2.68 | ||
| Living alone | 1.89 | <0.02 | 0.41 to 3.38 | ||
| Medication use | 1.51 | <0.03 | 0.18 to 2.83 | ||
| Overall model | 0.10 | ||||
|
| |||||
| Depression (HADS)a | ≥1 comorbidity | 1.27 | <0.02 | 0.30 to 2.24 | |
| Living alone | 1.54 | <0.02 | 0.34 to 2.75 | ||
| Medication use | 1.34 | <0.02 | 0.24 to 2.42 | ||
| Overall model | 0.08 | ||||
|
| |||||
| Poor functioning (GOS) | Age (1 year increase) | 0.04 | 0.0002 | 1.02 to 1.07 | |
| Female sex | 0.43 | 0.4150 | 1.03 to 5.49 | ||
| Multiple TBIs | 0.83 | 0.0016 | 1.88 to 14.83 | ||
| Living alone | 0.50 | 0.0403 | 1.05 to 7.16 | ||
| Overall model | 0.81 | ||||
| C statistic | |||||
Higher scores indicate poorer outcome.
Higher scores indicate better outcome. GOS = Glasgow Outcome Scale. HADS = Hospital Anxiety and Depression Scale. RPQ = Rivermead Post Concussion Symptoms Questionnaire. SF-36 = 36-item medical outcomes Short Form Survey.
Being female, living alone, having more than one comorbidity, multiple TBIs, use of psychotropic medication, and being of non-white ethnic group were predictive of poorer outcomes at 12 months. Age had a variable impact on outcome, with older adults having poorer physical but improved mental health outcomes.
To explore the most problematic symptoms encountered after mTBI, the number of people experiencing individual symptoms over time are outlined. As shown in Table 4, headaches, fatigue, forgetfulness, poor concentration, and taking longer to think (slower processing of information and remembering things) were the most common symptoms experienced at all follow-up time-points.
Table 4.
Percentage of participants reporting a score of ≥2 on the individual post-concussion symptom items over time
| Symptom | Baseline (%) | 1 month (%) | 6 months (%) | 12 months (%) |
|---|---|---|---|---|
| Headaches | 96 (63.6) | 99 (44.0) | 88 (35.9) | 105 (36.1) |
| Feelings of dizziness | 78 (51.7) | 93 (41.3) | 76 (31.0) | 83 (28.5) |
| Nausea/vomiting | 34 (22.5) | 31 (13.8) | 35 (14.3) | 37 (12.7) |
| Noise sensitivity | 67 (44.4) | 71 (31.6) | 75 (30.6) | 80 (27.5) |
| Sleep disturbance | 65 (43.0) | 86 (38.2) | 87 (35.5) | 93 (32.0) |
| Fatigue/tiring more easily | 97 (64.2) | 123 (54.7) | 117 (47.8) | 118 (40.5) |
| Being irritable/easily angered | 73 (48.3) | 82 (36.4) | 84 (34.3) | 94 (32.3) |
| Feeling depressed or tearful | 46 (30.5) | 54 (24.0) | 62 (25.3) | 70 (24.1) |
| Feeling frustrated or impatient | 77 (51.0) | 96 (42.7) | 86 (35.1) | 98 (33.7) |
| Forgetfulness/poor memory | 85 (56.3) | 108 (48.0) | 110 (44.9) | 119 (40.9) |
| Poor concentration | 78 (51.7) | 104 (46.2) | 97 (39.6) | 99 (34.0) |
| Taking longer to think | 91 (60.3) | 123 (54.7) | 107 (43.7) | 118 (40.5) |
| Blurred vision | 50 (33.1) | 60 (26.7) | 51 (20.8) | 72 (24.7) |
| Light sensitivity | 53 (35.0) | 63 (28.0) | 62 (25.3) | 68 (23.4) |
| Double vision | 16 (10.6) | 31 (13.8) | 28 (11.4) | 34 (11.7) |
| Restlessness | 66 (43.7) | 78 (34.7) | 75 (30.6) | 75 (25.8) |
DISCUSSION
Summary
This study aimed to determine the extent to which adults experience ongoing difficulties 12 months after mTBI and identifying the predictors of outcome. Findings indicated that after mTBI nearly half of people continue to experience difficulties with post-concussion symptoms 1 year after injury. Impairments in cognitive functioning and global functioning were observed in 10–11% of the sample. Levels of depression, anxiety, or reduced quality of life post-TBI were comparable to the general population. Being female, living alone, having multiple TBIs, comorbidities, high alcohol use, use of psychotropic medication, and being of nonwhite ethnic group were associated with poorer outcomes at 12 months post-injury.
Strengths and limitations
A key strength of this study was the use of a population-based and proactive approach to case ascertainment that identified and included cases who did not attend hospital after injury or cases where a TBI had been missed. Although injuries that do not present to hospital are often assumed to be ‘milder’ in severity, this study highlights that people may experience ongoing difficulties after mTBI. Every attempt was made to capture all TBI cases within the study region, but some people still may have chosen not to make themselves known to the study team or may have been missed. A further strength of the study was consideration of both recurrent and subsequent brain injuries in the analysis as highlighted in a recent review.27 Whereas incident and subsequent TBIs were verified by clinicians, history of prior injuries was based on self-report, and results on prior TBI should be interpreted with caution.
Information on prior mood, psychiatric, and medical conditions was included in the comorbidities variable, but data on pre-injury functioning were not available, which may account for some of the additional variance in outcome in the models. Without information on pre-injury measures it remains unclear as to the extent the injury itself influenced people’s scores on the measures and the extent attributable to pre-injury life events. This issue is an inherent difficulty in the collection of data at a population level.
On contacting participants about the study it was emphasised that the research team was interested in recruiting people who had recovered well with no problems, as well as those experiencing ongoing difficulties. There were no differences in demographic or injury characteristics between those who completed the followup assessment and those who did not. However, it was not possible to determine whether those who did not participate experienced any differences in outcome (for example, recovering well and therefore having less interest in taking part). In terms of living status, comorbidities and multiple TBI may have affected the findings.
Comparison with existing literature
Rates of post-concussion symptoms identified in the current study are comparable with rates identified in previous studies based on patients attending hospital after injury at 1 year.2,10 Interestingly, the consult within 24 hours variable was not predictive of outcome, which suggests that those who do not immediately seek health care may still be at risk of ongoing difficulties. As New Zealand has a no-fault compensation injury scheme, compensation, which has previously been identified as a factor linked to outcome after mTBI, was not investigated in this study.
It should be considered that post- concussion symptoms are not unique to TBI and can occur as a result of other medical conditions or acute illness. For example, in a recent New Zealand survey, 35.4% of the general population reported experiencing problems with headaches, 35.5% fatigue, 17.4% irritability, and 12.1% memory difficulties.28 The occurrence of headaches in the NZ general population was found to be relatively comparable with the present TBI sample, suggesting that the experience of headaches reported in the present sample may not be a result of TBI specifically. In contrast, the occurrences of memory difficulties, fatigue, and irritability symptoms were far higher in the present sample, suggesting an increased likelihood of symptoms being associated as a long-term sequelae of mTBI. The study shows that 10.9% of participants were performing within the ‘very low’ percentile of overall cognitive performance compared with general population norms.29 The nature of the association remains to be determined as these symptoms could be a direct result of the TBI or could be a consequence of other symptoms such as sleep disturbance, side effects of medications taken, or loss of, or difficulties in, employment. It is of note that levels of depression, anxiety, and quality of life were found to be equivalent to mean scores on these measures within general population samples.30,31
In comparison with the models found to be predictive of disability in a hospital-based sample,13 age and comorbidities were also found to be predictive of outcome in the current study. In addition, the current models also identified female sex, experiencing multiple TBIs, living alone, and use of psychotropic medication as predictors of poor outcome across a range of domains.
Implications for practice
As many mild injuries present at primary care, these findings emphasise the role of the GP in monitoring longer-term effects and ensuring that patients experiencing difficulties receive support to facilitate recovery in line with recommended guidelines.32 The finding that being of female sex and being of non-white ethnic group were predictive of poorer outcome highlights sex and ethnic disparities in outcome from mTBI, and suggests that some people may require additional support or different types of support to meet their needs after mTBI. Additionally, the finding that experiencing multiple TBIs was also predictive of outcome supports previous evidence proposing that the brain remains vulnerable after initial injury, which can negatively affect recovery,33 and that, for mild TBI, wider contextual factors play a greater role in influencing outcome.13 The models highlight that demographic, medical history, and injury characteristics are important in the prediction of outcome after mild TBI.
Current guidelines for mTBI32 highlight that a TBI injury history and assessment of effects should be conducted on presentation after mTBI. Advice on what to expect after injury, signs to look out for (for example, indications that secondary inflammation or bleeding are occurring), and when to seek follow-up should be provided. As this study has highlighted that a number of contextual and injury factors can influence recovery, clinicians should consider these factors in their management plans. Patients should be monitored every 2–4 weeks until symptom resolution or until a referral is made to a brain injury specialist/service.34 Acknowledgement of the injury and its effects as well as lifestyle advice may facilitate recovery, for example, gradual return to activities after resolution of symptoms, avoidance of activities that can trigger symptoms (such as computer use or driving), and taking regular rest breaks. There remains a lack of evidence on the use of pharmacological and non-pharmacological treatments after mTBI, although early information and advice has been found to have beneficial effects on recovery.35,36
Overall, this study has highlighted that, although some people recover well after TBI, nearly half continue to experience significant persistent symptoms 1 year after mTBI. The impact of these symptoms on people’s ability to function in everyday life suggests that early intervention is needed and can improve longer-term outcomes to facilitate the resolution of symptoms.
Acknowledgments
The authors thank the research team for their dedication and performance; the staff at the Coroner’s office in Hamilton; the staff of the NZ Accident Compensation Corporation and Health Information Service; Waikato District Health Board; Waikato University Staff; the many doctors, nurses, and rehabilitation professionals and service providers such as ABI Management and administrative staff within and outside Hamilton; and the BIONIC participants and their families and friends. Thanks also to Helen McDonald for her administrative support for the study and CNS Vital Signs for their support with the neuropsychological assessment.
Funding
This work was funded by the Health Research Council of New Zealand (09/063A, 11/192), although the research was conducted independently from the sponsor. Alice Theadom was co-funded by ABI Management Rehabilitation and Kathryn McPherson holds the Laura Fergusson Trust Chair.
Ethical approval
Ethical approval was obtained from the Northern Y Health and Disability ethics committee of New Zealand (NTY/09/09/095) and the Auckland University of Technology ethics committee (09/265).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Menon DK, Schwab K, Wright DW, et al. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2.McMahon P, Hricik A, Yue JK, et al. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI Study. J Neurotrauma. 2014;31(1):26–33. doi: 10.1089/neu.2013.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edition. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 4.Dischinger P, Read K, Kerns T, et al. Causes and outcomes of mild traumatic brain injury: an analysis of CIREN data. Annu Proc Assoc Adv Automot Med. 2003;47:577–589. [PMC free article] [PubMed] [Google Scholar]
- 5.Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. New Engl J Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 6.O’Keeffe FM, Dockree PM, Robertson IH. Poor insight in traumatic brain injury mediated by impaired error processing? Evidence from electrodermal activity. Brain Res: Cog Brain Res. 2004;22(1):101–112. doi: 10.1016/j.cogbrainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):904–911. doi: 10.1016/j.apmr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham J, Brison RJ, Pickett W. Concussive symptoms in emergency department patients diagnosed with minor head injury. J Emerg Med. 2011;40(3):262–266. doi: 10.1016/j.jemermed.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Ahman S, Saveman BI, Styrke J, et al. Long-term follow-up of patients with mild traumatic brain injury: a mixed-method study. J Rehabil Med. 2013;45(8):758–764. doi: 10.2340/16501977-1182. [DOI] [PubMed] [Google Scholar]
- 10.Wäljas M, Iverson GL, Lange RT, et al. A prospective biopsychosocial study of the persistent post-concussion symptoms following mild traumatic brain injury. J Neurotrauma. 2015;32(8):534–547. doi: 10.1089/neu.2014.3339. [DOI] [PubMed] [Google Scholar]
- 11.Lingsma HF, Roozenbeek B, Steyerberg EW, et al. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9(5):543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg ND, Gardner AJ, Brubacher JR, et al. Systematic review of multivariable prognostic models for mild traumatic brain injury. J Neurotrauma. 2015;32(8):517–526. doi: 10.1089/neu.2014.3600. [DOI] [PubMed] [Google Scholar]
- 13.Lingsma HF, Roozenbeek B, Steyerberg EW, et al. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9(5):543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 15.Feigin VF, Theadom A, Barker-Collo SL, et al. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol. 2013;12(1):53–64. doi: 10.1016/S1474-4422(12)70262-4. [DOI] [PubMed] [Google Scholar]
- 16.Theadom A, Barker-Collo S, Feigin V, et al. The spectrum captured: a methodological approach to studying incidence and outcomes of traumatic brain injury on a population level. Neuroepidemiology. 2012;38(1):18–29. doi: 10.1159/000334746. [DOI] [PubMed] [Google Scholar]
- 17.Carroll LJ, Cassidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;(43 Suppl):113–125. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Craig M, Wilkinson DA, Davila R. Empirically based guidelines for moderate drinking: one year results from three studies with problem drinkers. Am J Public Health. 1995;85(6):823–828. doi: 10.2105/ajph.85.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King NS, Crawford S, Wenden FJ, et al. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- 20.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski M, Keller SD. SF-36 physical and mental health summary scales: a user’s manual. Boston, MA: Health Institute, New England Medical Centre; 1994. [Google Scholar]
- 23.Emanuelson I, Andersson Elgmark E, Bjorklund R, Stalhammar D. Quality of life and post-concussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand. 2003;108(5):332–338. doi: 10.1034/j.1600-0404.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 24.Findler M, Cantor J, Haddad L, et al. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Inj. 2001;15(8):715–723. doi: 10.1080/02699050010013941. [DOI] [PubMed] [Google Scholar]
- 25.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 26.Pettigrew LEL, Wilson JTL, Teasdale GM. Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J Head Trauma Rehabil. 2003;18(3):252–258. doi: 10.1097/00001199-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(Suppl):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 28.Petrie K, Faase K, Crichton F, Grey A. How common are symptoms? Evidence from a New Zealand national telephone survey. BMJ Open. 2014;4:e005374. doi: 10.1136/bmjopen-2014-005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gualtieri CT, Johnson LG. A computerized test battery sensitive to mild and severe brain injury. Medscape J Med. 2008;10(4):90. [PMC free article] [PubMed] [Google Scholar]
- 30.MaGPIe Research Group The nature and prevalence of psychological problems in New Zealand primary healthcare: a report on Mental Health and General Practice Investigation (MaGPIe) N Z Med J. 2003;116(1171):U379. [PubMed] [Google Scholar]
- 31.Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40(Pt 4):429–434. doi: 10.1348/014466501163904. [DOI] [PubMed] [Google Scholar]
- 32.Ontario Neurotrauma Foundation . Guidelines for concussion/mild traumatic brain injury and persistent symptoms. 2nd edn. Toronto, ON: ONF; 2013. [Google Scholar]
- 33.Iverson GL, Echemendia RJ, Lamarre AK, et al. Possible lingering effects of multiple past concussions. Rehabil Res Pract. 2012;2012:316575. doi: 10.1155/2012/316575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall S, Bayley M, McCullagh S, et al. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58(3):257–267. [PMC free article] [PubMed] [Google Scholar]
- 35.Barker-Collo S, Starkey N, Theadom A. Treatment for depression following mild traumatic brain injury in adults: a meta-analysis. Brain Inj. 2013;27(10):1124–1133. doi: 10.3109/02699052.2013.801513. [DOI] [PubMed] [Google Scholar]
- 36.Ponsford J. Rehabilitation interventions after mild head injury. Curr Opin Neurol. 2005;18(6):692–697. doi: 10.1097/01.wco.0000186840.61431.44. [DOI] [PubMed] [Google Scholar]