Abstract
Background
GPs have high consultation rates for symptoms related to knee osteoarthritis (OA). Many risk factors for symptomatic knee OA progression remain unknown.
Aim
To define distinct knee pain trajectories in individuals with early symptomatic knee OA and determine the risk factors for these pain trajectories.
Design and setting
Data were obtained from the multicentre prospective Cohort Hip and Cohort Knee study in the Netherlands. Participants with knee OA, according to the clinical criteria of the American College of Rheumatology, and a completed 5-year follow-up were included.
Method
Baseline demographic, anamnestic, and physical examination characteristics were assessed. Outcome was annually assessed by the Numeric Rating Scale for pain. Pain trajectories were retrieved by latent class growth analysis. Multinomial logistic regression was used to calculate relative risk ratios.
Results
In total, 705 participants were included. Six distinct pain trajectories were identified with favourable and unfavourable courses. Statistically significant differences were found in baseline characteristics, including body mass index (BMI), symptom severity, and pain coping strategies between the different trajectories. Higher BMI, lower level of education, greater comorbidity, higher activity limitation scores, and joint space tenderness were more often associated with trajectories characterised by more pain at first presentation and pain progression — compared with the reference group with a mild pain trajectory. No association was found for baseline radiographic features.
Conclusion
These results can help differentiate those patients who require more specific monitoring in the management of early symptomatic knee OA from those for whom a ‘wait-and-see’ policy seems justifiable. Radiography provided no additional benefit over clinical diagnosis of early symptomatic knee OA in general practice.
Keywords: disease progression, knee osteoarthritis, knee pain, pain trajectories, primary health care
INTRODUCTION
Osteoarthritis of the knee (knee OA) is a common disease with a relatively high prevalence and incidence among older patients in the general population.1 Symptomatic knee OA varies greatly in affected individuals and many patients encounter the disabling effect of pain.1,2 Consequently, GPs have high consultation rates for OA-related symptoms and see a large variability in the evolution of the condition.3 As a result, they need to differentiate patients for whom a ‘wait-and-see’ policy seems justifiable from those for whom proactive management is necessary.
Many criteria have been developed to assess knee OA severity using clinical and radiographic features or magnetic resonance imaging (MRI) techniques to define disease progression.4–6 Numerous studies have determined risk factors for incident and radiographic progression of knee OA, but previous reviews have shown that only few studies have used symptomatic knee OA progression as an outcome measure.7,8
Discordance remains in the apparent correlation between stages of knee OA assessed by clinical and radiographic criteria and pain severity. This seems to imply that there are differences in risk factors for (radiographic) disease progression and pain progression in knee OA. Although the exact aetiology remains unclear, pain due to knee OA is known to fluctuate and multiple assessments of pain over time could give a better indication of pain than a single assessment.9,10 This course of pain, or pain trajectory, could be a more accurate or more relevant representation of clinical disease progression.
The ability to predict pain trajectories in an early stage of disease could help GPs and patients successfully to manage knee OA in a primary care setting. As such, this study aimed to:
define distinct knee pain trajectories in individuals with early symptomatic knee OA; and
determine patient or disease characteristics associated with these pain trajectories.
METHOD
Study design and population
The data for the current study were acquired from the Cohort Hip and Cohort Knee (CHECK) study.11 CHECK is a prospective, 10-year follow-up cohort of 1002 participants with early symptomatic OA of the knee and/or hip in the Netherlands. Its inclusion period ran from October 2002 until September 2005. Inclusion criteria for the CHECK study were:
pain and/or stiffness of the knee and/or hip;
aged 45–65 years; and
never consulted a physician for these symptoms, or had done so <6 months prior to recruitment to the study.
How this fits in
Symptom severity and progression of knee osteoarthritis (OA) are far more clinically relevant to the GP than radiographic severity or progression, but only few studies have assessed symptomatic knee OA progression in patients in general practice. This study identified distinct pain trajectories for symptomatic knee OA progression; the results provide evidence to support recommendations that could help GPs to manage early symptomatic knee OA.
Participants were excluded from the CHECK study if they had:
other pathological conditions that could explain the existing complaints (for example, other rheumatic disease, previous hip or knee joint replacement, congenital dysplasia, osteochondritis dissecans, intra-articular fractures, septic arthritis, Perthes disease, ligament or meniscus injury, plica syndrome, Baker’s cyst);
comorbidity that would not allow physical evaluation during 10 years’ follow-up;
malignancy in the past 5 years; and
inability to understand Dutch.
For the analyses of the current study participants were included if, at baseline, they:
reported knee pain; and
were considered to have knee OA according to the clinical criteria of the American College of Rheumatology.4,11
If a participant had two affected knees, the knee with the worse score based on pain, Kellgren–Lawrence (KL) score, and physical examination findings was included. The physical examination assessed:
knee pain;
range of motion;
crepitus;
joint space tenderness;
palpable warmth; and
bony enlargement.
If all findings were identical in both knees, the right knee was included.
Baseline characteristics
The study included a baseline medical history, physical examination, and radiographs of the knee and hip to create variables that are available to the GP. The medical history was taken via questionnaires in which self-reported data were assessed. The following diseases were assessed as a relevant comorbidity:
asthma;
chronic sinusitis;
cardiovascular disease;
hypertension;
gastric ulcer;
gallstones;
liver disease;
renal disease;
diabetes;
thyroid gland disease;
epilepsy;
cancer (during follow-up);
severe skin disease; and
other chronic musculoskeletal diseases.
Furthermore, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscores were used to measure pain, stiffness, and physical functioning — a higher score (range 0–100) indicated worse health. To assess pain coping behaviour, the six-scale Pain Coping Inventory (PCI) was used, which represents active and passive pain coping dimensions.12 Active pain coping strategies are:
pain transformation (reinterpreting and transforming pain);
distraction (distracting oneself from pain); and
reducing demands (functioning but doing less than required).
Passive pain coping strategies are:
retreating (avoiding environmental stimuli);
worrying (catastrophising pain); and
All six items are scored according to a four-point Likert scale, ranging from 1 (hardly ever) to 4 (very often), in terms of frequency with which strategies are applied. Standardised radiographs of the tibiofemoral joint were made by a weight-bearing posteroanterior view, semi-flexed (7–10 degrees), according to protocols outlined by Buckland-Wright.14,15 For the hip, standardised weight-bearing anteroposterior radiographs of the pelvis were made. Radiographs were read with observers blinded to all patient characteristics. Scoring of radiographs was performed according to KL score.16 These two sets of radiographs were used as they are the radiographs that are available to GPs.
Outcome variable
Pain was assessed annually via questionnaires using the Numerical Rating Scale (NRS) for pain; scores range from 0–10, with a higher score indicating more pain. Participants were asked to score the pain they experienced in their most painful joint over the previous week. Using latent class growth analysis (LCGA), the annually assessed NRS for pain scores were plotted longitudinally and blinded to all other characteristics; this created various paiNRSn trajectory groups, which formed the outcome variable for this study. Pain scores of participants who underwent knee replacement surgery were scored as missing from the moment of surgery onwards. If participants missed more than two pain assessments they were excluded from analyses.
Statistical analysis
LCGA was used to identify the different pain trajectories. Previously described by Verkleij and colleagues,10 it is a technique that uncovers heterogeneity in a population and makes it possible to distinguish groups of people who are similar in their growth trajectories longitudinally. In short, it was tested whether the course of pain was best described by linear, quadratic, or cubic trajectories. The optimum model was determined on a combination of:
indices of fit (Bayesian information criterion [BIC], Vuong–Lo–Mendell– Rubin Likelihood Ratio Test [LRT] and the bootstrap LRT, and entropy indices);
clinical relevancy; and
interpretability of the model.
Baseline characteristics were calculated per pain trajectory group using descriptive statistics. After checking for collinearity, setting the cut-off value for Pearson’s correlation coefficient (r) at 0.70, multinomial logistic regression analyses were performed per variable to test whether differences were statistically different, obtaining a cut-off point of P<0.10. All variables with P<0.10 were later included in a final multivariable multinomial logistic regression model (P-removal, P<0.05) to obtain relative risk ratios (RRs) and 95% confidence intervals (CIs) for belonging in each trajectory. The final model was adjusted for age and sex to make the results more generalisable to the general population with symptoms related to knee OA.
The LCGA was performed using Mplus 6.1. All other analyses were performed using PASW 20.0.
RESULTS
Baseline characteristics
In total, 743 of the 1002 participants met the inclusion criteria at baseline. Of these, 38 (5%) participants missed more than two annual pain assessments or were lost to follow-up and, as such, were excluded from the analyses. The difference between the study population and the 38 excluded participants was not statistically significant with regard to the baseline values of body mass index (BMI), NRS for pain, age, sex, and KL score.
The total study population after 5 years consisted of 705 participants (n = 705 knees), whose mean age was 56.0 years (± 5.1 years); 81% were female. More details on participants’ characteristics are outlined in Tables 1 and 2. The baseline variables (‘NRS of the past week’ and ‘NRS at the moment of the questionnaire’) were strongly correlated (Pearson’s r = 0.83) — as were each of the WOMAC subscales (pain, joint stiffness, and physical function) — with ‘NRS of past week’ (Pearson’s r: 0.68, 0.51, and 0.63 respectively). The baseline ‘NRS at the moment of questionnaire’ was excluded from the final model due to strong collinearity.
Table 1.
Participants’ baseline characteristics
| Characteristic | Total population (n = 705) | Lost to follow-up (n = 38) | P-value |
|---|---|---|---|
| Mean age, years (SD) | 56.0 (5.1) | 56.0 (5.6) | 0.97 |
|
| |||
| Female, % | 81 | 89 | 0.19 |
|
| |||
| BMI (SD) | 26.5 (4.3) | 27.0 (3.3) | 0.43 |
|
| |||
| Baseline NRS in past week (SD) | 3.7 (2.1) | 3.6 (2.0) | 0.23 |
|
| |||
| WOMAC subscales score | |||
| Physical function (SD) | 24.8 (17.0) | 28.2 (20.8) | 0.25 |
|
| |||
| Kellgren–Lawrence grade | |||
| Distribution, % knees with grade 0/1 | 58/42 | 60/40 | 0.92 |
|
| |||
| TKA after 5 years’ follow-up | 14 | – | – |
BMI = body mass index. NRS = Numeric Rating Scale for pain. SD = standard deviation. TKA = total knee arthroplasty. WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index. P-values were calculated using ANOVA or Pearson’s χ2 test.
Table 2.
Characteristics of patients in the six pain trajectories
| Pain trajectory groups | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | A (constant mild pain) | C (moderate regression) | E (major regression) | B (severe progression) | D (moderate progression) | F (constant severe) |
|
| ||||||
| n = 186 | n = 207 | n = 23 | n = 35 | n = 180 | n = 74 | |
|
| ||||||
| Age, years (SD) | 56 (0.4) | 56 (0.4) | 57 (1.1) | 55 (0.9) | 56 (0.4) | 56 (0.5) |
|
| ||||||
| Female, % | 80 | 79 | 83 | 80 | 83 | 85 |
|
| ||||||
| BMI (SD)a,b | 25 (0.3) | 26 (0.3) | 25 (0.8) | 26 (0.8) | 27 (0.3) | 28 (0.6) |
|
| ||||||
| Highest achieved education levela,b | ||||||
| Primary or secondary school, % | 62 | 77 | 68 | 83 | 83 | 83 |
| University/college, % | 38 | 23 | 32 | 17 | 17 | 17 |
|
| ||||||
| White ethnicity, % | 99 | 98 | 100 | 97 | 97 | 92 |
|
| ||||||
| Participants with >1 comorbidity, %a,b | 32 | 48 | 59 | 57 | 49 | 69 |
|
| ||||||
| NRS in past week (IQR)a,b | 2.1 (1.0–3.0) | 3.4 (2.0–4.0) | 5.6 (5.0–7.0) | 2.3 (1.0–3.0) | 4.5 (4.0–6.0) | 6.2 (5.0–7.0) |
|
| ||||||
| NRS at moment of questionnaire (IQR)a,b | 1.7 (1.0–2.0) | 2.9 (2.0–4.0) | 4.5 (3.0–6.0) | 2.1 (1.0–3.0) | 4.0 (3.0–5.0) | 5.9 (5.0–7.0) |
|
| ||||||
| PCI subscales score | ||||||
| Pain transformation (IQR)a,b | 2.0 (1.5–2.5) | 2.1 (1.8–2.5) | 2.4 (1.9–3.0) | 2.2 (1.5–2.8) | 2.3 (1.8–2.8) | 2.4 (2.0–2.8) |
| Distraction (IQR)a,b | 2.1 (1.6–2.6) | 2.2 (1.8–2.6) | 2.2 (1.8–2.7) | 2.3 (1.6–3.0) | 2.3 (1.8–2.6) | 2.5 (2.2–2.8) |
| Reducing demands (IQR)a,b | 1.9 (1.7–2.3) | 2.0 (1.7–2.7) | 2.3 (2.0–2.8) | 1.9 (1.7–2.0) | 2.0 (1.7–2.7) | 2.3 (2.0–2.7) |
| Retreating (IQR)a,b | 1.5 (1.1–1.9) | 1.6 (1.1–1.9) | 1.7 (1.1–2.0) | 1.5 (1.1–1.9) | 1.5 (1.1–1.7) | 1.7 (1.3–2.0) |
| Worrying (IQR)a,b | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | 1.7 (1.4–1.9) | 1.6 (1.3–1.9) | 1.6 (1.3–1.9) | 1.8 (1.4–2.1) |
| Resting (IQR)a,b | 1.7 (1.4–2.0) | 1.8 (1.4–2.0) | 2.0 (1.6–2.3) | 1.7 (1.4–1.8) | 1.9 (1.6–2.2) | 2.1 (1.6–2.6) |
|
| ||||||
| WOMAC subscales score | ||||||
| Pain (IQR)a,b | 15 (5–20) | 24 (10–33) | 39 (25–55) | 19 (10–30) | 33 (25–40) | 46 (25–60) |
| Joint stiffness (IQR)a,b | 24 (13–38) | 34 (25–50) | 48 (38–63) | 24 (13–38) | 42 (25–50) | 53 (38–63) |
| Physical function (IQR)a,b | 14 (6–21) | 23 (11–31) | 37 (26–49) | 16 (6–21) | 31 (19–41) | 45 (33–58) |
|
| ||||||
| Uses pain medication, % | 36 | 38 | 26 | 35 | 39 | 44 |
|
| ||||||
| Drinks alcohol, %a | 78 | 78 | 91 | 74 | 76 | 63 |
|
| ||||||
| Smoker or previous smoker, % | 11 | 12 | 18 | 14 | 16 | 18 |
|
| ||||||
| Has additional supplements or vitamin intake, % | 54 | 52 | 46 | 51 | 52 | 57 |
|
| ||||||
| Pain in the ipsilateral hip, %a,b | 28 | 42 | 74 | 31 | 49 | 51 |
|
| ||||||
| Morning stiffness knees <30 minutes, %a,b | 64 | 67 | 61 | 59 | 76 | 80 |
|
| ||||||
| Palpable warmth knee, % | 6 | 6 | 9 | 0 | 6 | 4 |
|
| ||||||
| Joint space tenderness knee, %a,b | 36 | 51 | 52 | 61 | 70 | 68 |
|
| ||||||
| Bony enlargement knee, % | 4 | 4 | 0 | 9 | 6 | 4 |
|
| ||||||
| Crepitus during flexion knee, % | 53 | 56 | 43 | 51 | 50 | 57 |
|
| ||||||
| Positive re-fill test knee, % | 10 | 9 | 9 | 3 | 9 | 6 |
|
| ||||||
| ROM flexion knee, degrees (SD)a | 135 (1) | 134 (1) | 134 (2) | 132 (2) | 134 (1) | 131 (2) |
|
| ||||||
| ROM extension knee, degrees (SD) | 3 (0) | 3 (0) | 3 (0) | 3 (0) | 3 (0) | 3 (0) |
|
| ||||||
| Pain during active flexion knee, %a,b | 32 | 33 | 13 | 32 | 39 | 46 |
|
| ||||||
| Pain during active extension knee, %a | 12 | 21 | 18 | 12 | 24 | 29 |
|
| ||||||
| Pain during active internal rotation ipsilateral hip, %a,b | 20 | 28 | 55 | 25 | 39 | 46 |
|
| ||||||
| Bouchard swelling digit. 2–5 left or right, % | 18 | 19 | 17 | 20 | 19 | 24 |
|
| ||||||
| Heberden node digit. 2–5 left or right, % | 48 | 50 | 44 | 31 | 47 | 58 |
|
| ||||||
| Erythrocyte sedimentation rate, mm/hr (SD)a | 9.7 (0.6) | 9.4 (0.5) | 12.5 (2.3) | 9.9 (1.3) | 10.9 (0.6) | 12.3 (1.1) |
|
| ||||||
| Kellgren–Lawrence grade | ||||||
| Distribution, % knees with grade 0/1 | 57/43 | 63/37 | 70/30 | 56/44 | 55/45 | 49/51 |
|
| ||||||
| Kellgren–Lawrence score ipsilateral hip | ||||||
| Distribution, % hips with grade 0/1 | 81/19 | 75/25 | 70/30 | 85/15 | 80/20 | 79/21 |
|
| ||||||
| TKA after 5 years follow-up, total | 1 | 2 | 0 | 0 | 8 | 3 |
P-value <0.10 from the univariate multinomial regression analyses.
P-value <0.05 from the univariate multinomial regression analyses. BMI = body mass index. IQR = interquartile range. NRS = Numeric Rating Scale for pain. PCI = Pain Coping Inventory. ROM = range of motion. SD = standard deviation. TKA = total knee arthroplasty. WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Although Pearson’s r was <0.70 for all WOMAC subscales, only the WOMAC physical function subscale was included in the final model; this was to avoid overfitting the model and because this WOMAC subscale is most frequently used for assessing limitations due to knee OA.
Outcome variable
The most optimal and clinically relevant model retrieved by LCGA was a quadratic six-group model (low BIC [18 210], with best entropy indices [0.78] and a P-value of 0.53). The quadratic five-group model had a BIC of 18 237, entropy of 0.75, and LRT P-value of <0.05; the seven-group model had a BIC of 18 205, entropy of 0.76, and LRT P-value of >0.05. Although the P-value from the six-group LRT was >0.05, the model uncovered and distinguished sufficiently large groups of participants with distinct trajectories, which is highly informative and clinically relevant to both GPs and patients.
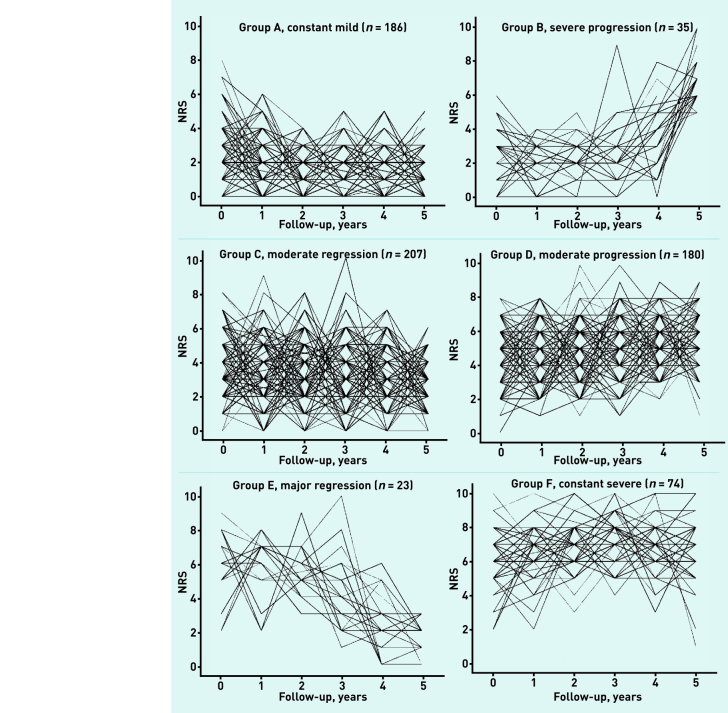
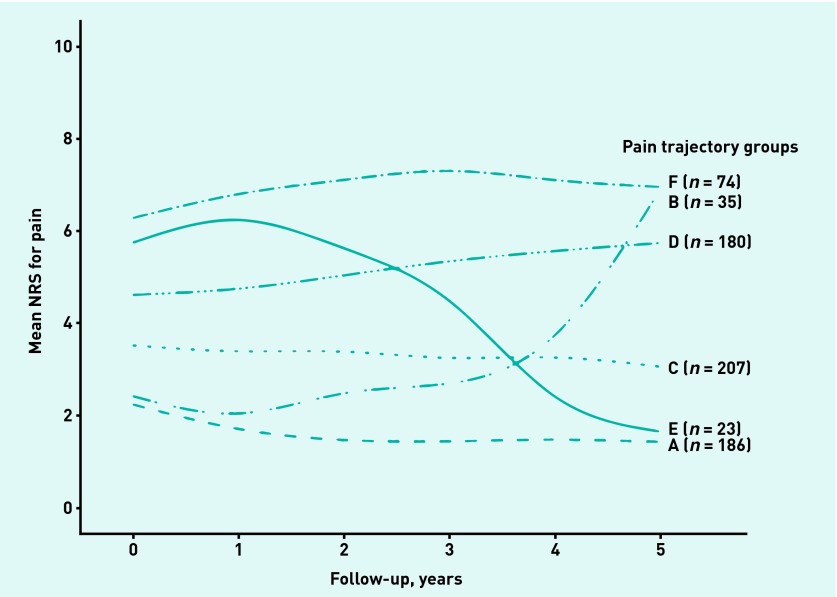
Figure 1 shows detailed depictions of the individual trajectories, with average fitted lines of these six pain trajectories shown in Figure 2. The figures show groups with different types of pain trajectories:
constant mild pain (group A, n = 186);
moderate pain regression (group C, n = 207);
major pain regression (group E, n = 23);
severe pain progression (group B, n = 35);
moderate pain progression (group D, n = 180); and
constant severe pain (group F, n = 74).
Figure 1.
Pain trajectories per individual as obtained by Latent Class Growth Analysis (LCGA). NRS = numeric rating scale.
Figure 2.
Average fitted lines of pain trajectories retrieved by Latent Class Growth Analysis (LCGA). NRS = Numeric Rating Scale.
Multinomial logistic regression analyses
The mean of all characteristics per pain trajectory group are presented in Table 2. Statistically significant differences were found for various demographic and anamnestic features, including baseline pain and use of pain coping strategies. There were no significant differences in baseline radiographic severity scores.
The results from the final multivariable model are shown in Table 3 (Nagelkerke R2=0.42). The mildest trajectory group (group A) was set as the reference group. Compared with this group, participants with a higher BMI, lower level of education, greater comorbidity, higher WOMAC physical function score, and knee joint space tenderness had increased risks for pain trajectories characterised by greater pain.
Table 3.
Trajectory relative risk ratiosa
| Baseline characteristic/factor RR (95% CI) | Pain trajectory groups | ||||
|---|---|---|---|---|---|
|
| |||||
| C (moderate regression) | E (major regression) | B (severe progression) | D (moderate progression) | F (constant severe) | |
|
| |||||
| n = 207 | n = 23 | n = 35 | n = 180 | n = 74 | |
|
| |||||
| BMI | 1.06 (1.00 to 1.13) | 0.96 (0.85 to 1.10) | 1.07 (0.96 to 1.19) | 1.09 (1.02 to 1.16)b | 1.10 (1.01 to 1.20)b |
|
| |||||
| Highest achieved education level | |||||
| Primary or secondary school | ref | ref | ref | ref | ref |
| University/college | 0.53 (0.32 to 0.87)b | 0.83 (0.27 to 2.51) | 0.33 (0.16 to 0.95)b | 0.44 (0.24 to 0.80)b | 0.55 (0.23 to1.31) |
|
| |||||
| >1 comorbidity | 1.75 (1.10 to 2.77)b | 1.37 (0.48 to 3.85) | 2.87 (1.23 to 6.67)b | 1.23 (0.74 to 2.08) | 2.65 (1.25 to 6.99)b |
| WOMAC physical function subscalec | 1.04 (1.02 to 1.06)b | 1.13 (1.09 to 1.17)b | 1.01 (0.98 to 1.05) | 1.09 (1.07 to 1.12)b | 1.14 (1.11 to 1.18)b |
|
| |||||
| Knee joint space tenderness | 1.70 (1.07 to 2.69)b | 2.13 (0.76 to 6.02) | 2.84 (1.21 to 6.67)b | 3.86 (2.28 to 6.62)b | 2.03 (0.96 to 4.33) |
|
| |||||
| Painful flexion knee | 0.91 (0.56 to 1.49) | 0.14 (0.03 to 0.69)b | 0.90 (0.37 to 2.19) | 0.77 (0.44 to 1.33) | 1.08 (0.51 to 2.29) |
Relative to reference trajectory (constant mild, group A), n = 186.
P<0.05.
RR per unit increase. A higher WOMAC score indicates more limitations due to physical health. BMI = body mass index. RR = relative risk ratio (obtained by multinomial logistic regression, adjusted for age and sex. Nagelkerke R2 = 0.42 for the model). WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
DISCUSSION
Summary
In this study six pain trajectories were uncovered over 5 years’ follow-up in individuals with early symptomatic knee OA in a primary care setting. A substantial group (group A) of 186 participants (26% of study population) that had a mild pain trajectory was identified. The largest group (group C) comprised 207 participants (29% of the study population) and showed a similar trajectory, but the pain they experienced was moderate. Nonetheless, 56% of the study population showed a constant mild or moderate pain trajectory during 5 years.
The results from the multivariate analyses indicate that, when compared with the mild trajectory group, participants in groups B, D, and F had a higher BMI, suffered more comorbidity, had lower levels of education, and had joint space tenderness of the knee more often (which was borderline significant in group F).
Noteworthy are the results from group E. Participants from this group reported severe knee pain at baseline; however, joint space tenderness was not statistically significant in multivariate analysis. This group also had the highest percentage of pain in the ipsilateral hip.
Strengths and limitations
The fact that patients in group E had the highest percentage of pain in the ipsilateral hip may suggest that they do not actually have clinical knee OA; instead, they may have referred pain in the knee due to hip OA. The results from group E should therefore be interpreted with caution.
The trajectory groups B and E are both relatively small and, as a result, although the findings are informative and noteworthy, they should be interpreted carefully.
A limitation of this study is that, although participants were asked where pain was located, the NRS for pain and WOMAC scales were assessed on the joint with the most severe pain — hence, an individual with both hip and knee symptoms could consequently have a high NRS relating to pain in the hip. It is possible that the NRS, therefore, does not fully correspond with the pain the individual experienced in the knee. Another limitation is that the NRS assessment was only undertaken once annually, whereas an NRS assessment that was undertaken more frequently would lead to an even more precise estimation of the pain trajectories.
A large number of variables that could have led to bias were tested in the analyses. To deal with this, however, data reduction methods were used by testing for collinearity and by entering variables based on univariate P-values. Moreover, most included variables in the analyses are part of clinical examination and are assumed to relate to disease severity or overall health.
Comparison with existing literature
Overweight has often been recognised as a potent risk factor for incident knee OA.17 In a recent systematic review, strong evidence for the associations between BMI and clinical progression of knee OA was reported by the authors,7 which is consistent with the findings presented here. The authors also found strong evidence for the association with comorbidity count,7 and moderate evidence was found for the association between education level and symptomatic knee OA.7 In this study a strong association for joint space tenderness, for which the evidence was limited in the systematic review, was found.7 The findings related to joint space tenderness are consistent with earlier findings described by Altman and colleagues, and underline the importance of physical examination.4,18 What is not included in the criteria described by Altman and colleagues is painful knee flexion. Lastly, there were no significant differences in the distribution of baseline radiographic knee OA severity, which underlines current OA guideline recommendations to refrain from radiography in the early stages of disease.18–20
Using LCGA to define pain trajectories in knee OA is a relatively new technique and has only been applied by a few authors to date.21–23 Holla and colleagues applied this technique on the same study population (CHECK), but used WOMAC physical function as the outcome variable;22 they identified a three-group model and found similar associations to the study findings presented here. Collins and colleagues applied LCGA on a study population from the Osteoarthritis Initiative (OAI) and used WOMAC pain as the outcome measure; they identified five trajectories21 and suggest knee OA is characterised by persistent, rather than severe, inevitable progression — this is in contrast with the findings presented here. However, in a previous study comparing CHECK with OAI, it was concluded that CHECK expectedly represents participants in an earlier stage of OA compared with OAI.11
Nicholls and colleagues applied LCGA on a study population from the Knee Clinical Assessment Study and matched their model with a population drawn from the OAI.23 They also used WOMAC pain as an outcome variable and identified five trajectories. They concluded that different types of symptom progression in knee OA exist, varying from severe progression to regression, which is in accordance with the findings presented here.
Implications for research and practice
The six distinct pain trajectories presented here can help GPs to differentiate those patients for whom, in accordance with current guideline recommendations,18–20 a ‘wait-and-see’ policy seems justifiable (that is, groups A, C, and E) from those participants who require more specific monitoring in the management of early symptomatic knee OA (that is, groups B, D, and F).
For patients with moderate, severe, or progressing pain, it seems justifiable to maintain a proactive management plan and offer re-assessments of pain and function limitations after at least 1 year. In that way, GPs can better assess which pain trajectory the patient is most likely to follow and can act accordingly — by promoting weight loss, prescribing pain medication, or referring patients for specialist treatment.
The results also show that proper physical examination of the knee is essential in the management of symptomatic knee OA. Those individuals with knee pain who have a higher BMI, are less educated, experience more comorbidity, have a higher WOMAC physical function score, and show joint space tenderness should be proactively monitored during the first year of management, as opposed to a ‘wait-and-see’ approach.
Baseline radiographic severity was not associated with the pain trajectories. As a result of these findings, the authors would recommend that GPs who are consulted by patients with early symptomatic knee OA:
assess pain severity, limitations in daily activities, and presence of comorbidity;
properly examine the knee (focusing on joint space tenderness); and
refrain from radiographic examination.
Future research should be aimed at measuring symptomatic progression of knee OA with even more frequent symptom assessment to further identify those patients in whom an active monitoring policy from general practice is required.
Acknowledgments
The authors would like to thank all participants of the Cohort Hip and Cohort Knee study and all the collaborators of the different sites for their efforts.
Funding
This study was partly funded by a programme grant of the Dutch Arthritis Foundation for its centre of excellence, Osteoarthritis in Primary Care.
Ethical approval
Medical ethics committees of all participating centres of the Cohort Hip and Cohort Knee (CHECK) study approved the study, and all participants in CHECK gave written informed consent. The participating centres were: Erasmus Medical Center Rotterdam; Kennemer Gasthuis Haarlem; Leiden University Medical Centre; Maastricht University Medical Centre; Martini Hospital Groningen/Allied Health Care Centre for Rheumatology and Rehabilitation Groningen; Medical Spectrum Twente Enschede/Ziekenhuisgroep Twente Almelo; Reade/VU Medical Centre Amsterdam; St Maartens-kliniek Nijmegen; University Medical Center Utrecht; and Wilhelmina Hospital Assen.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RR, Cahalan C, Mensing G, et al. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 3.Croft P, Porcheret M, Peat G. Managing osteoarthritis in primary care: the GP as public health physician and surgical gatekeeper. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X588231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, Arden N, Conaghan PG, et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19(8):963–969. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiphof D, Boers M, Bierma-Zeinstra SMA. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67(7):1034–1036. doi: 10.1136/ard.2007.079020. [DOI] [PubMed] [Google Scholar]
- 7.Bastick A, Runhaar J, Belo J, Bierma-Zeinstra S. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17(1):152. doi: 10.1186/s13075-015-0670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belo JN, Berger MY, Reijman M, et al. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57(1):13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 9.Gooberman-Hill R, Woolhead G, Mackichan F, et al. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rheum. 2007;57(4):666–671. doi: 10.1002/art.22681. [DOI] [PubMed] [Google Scholar]
- 10.Verkleij SPJ, Hoekstra T, Rozendaal RM, et al. Defining discriminative pain trajectories in hip osteoarthritis over a 2-year time period. Ann Rheum Dis. 2012;71(9):1517–1523. doi: 10.1136/annrheumdis-2011-200687. [DOI] [PubMed] [Google Scholar]
- 11.Wesseling J, Dekker J, van den Berg WB, et al. CHECK (Cohort Hip and Cohort Knee): similarities and differences with the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68(9):1413–1419. doi: 10.1136/ard.2008.096164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraaimaat FW, Bakker A, Evers AWM. Pijncoping-strategieën bij chronische pijnpatiënten: De ontwikkeling van de Pijn-Coping-Inventarisatielijst (PCI). [Pain coping strategies in chronic pain patients: the development of the Pain Coping Inventory (PCI)] Gedragstherapie. 1997;30(3):185–201. [Google Scholar]
- 13.Kraaimaat FW, Evers AWM. Pain-coping strategies in chronic pain patients: psychometric characteristics of the pain-coping inventory (PCI) Int J Behav Med. 2003;10(4):343–363. doi: 10.1207/s15327558ijbm1004_5. [DOI] [PubMed] [Google Scholar]
- 14.Buckland-Wright C. Protocols for precise radio-anatomical positioning of the tibiofemoral and patellofemoral compartments of the knee. Osteoarthritis Cartilage. 1995;3(Suppl A):71–80. [PubMed] [Google Scholar]
- 15.Kinds MB, Vincken KL, Vignon EP, et al. Radiographic features of knee and hip osteoarthritis represent characteristics of an individual, in addition to severity of osteoarthritis. Scand J Rheumatol. 2012;41(2):141–149. doi: 10.3109/03009742.2011.617311. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.[no author listed] Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43(9):1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Nederlands Huisartsen Genootschap. [The Dutch College of General Practitioners] NHG-Standaard Niet traumatische knieproblemen bij volwassenen [NHG-guideline non-traumatic knee complaints in adults]. [Guideline in Dutch] Huisarts en Wetenschap. 2008;5:229–240. [Google Scholar]
- 19.National Institute for Health and Care Excellence . Osteoarthritis: care and management in adults. CG177. London: NICE; 2014. [PubMed] [Google Scholar]
- 20.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2014;22(5):622–630. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holla JFM, van der Leeden M, Heymans MW, et al. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: a 5-year follow-up study. Ann Rheum Dis. 2014;73(7):1369–1375. doi: 10.1136/annrheumdis-2012-202984. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls E, Thomas E, van der Windt DA, et al. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22(12):2041–2050. doi: 10.1016/j.joca.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]