Abstract
Background
Smoking is an important cardiovascular disease risk factor, but the mechanisms linking smoking to blood pressure are poorly understood.
Methods and Results
Data on 141,317 participants (62,666 never, 40,669 former, 37,982 current smokers) from 23 population-based studies were included in observational and Mendelian randomisation (MR) meta-analyses of the associations of smoking status and smoking heaviness with systolic and diastolic blood pressure (SBP, DBP), hypertension, and resting heart rate. For the MR analyses, a genetic variant rs16969968/rs1051730 was used as a proxy for smoking heaviness in current smokers. In observational analyses, current as compared with never smoking was associated with lower SBP, DBP, and lower hypertension risk, but with higher resting heart rate. In observational analyses amongst current smokers, one cigarette/day higher level of smoking heaviness was associated with higher (0.21 beats/minute; 95% CI 0.19; 0.24) resting heart rate, and slightly higher DBP (0.05 mmHg; 95% CI 0.02; 0.08) and SBP (0.08 mmHg; 95% CI 0.03; 0.13). However, in MR analyses amongst current smokers, while each smoking increasing allele of rs16969968/rs1051730 was associated with higher resting heart rate (0.36 beats/minute/allele; 95% CI 0.18; 0.54), there was no strong association with DBP, SBP, or hypertension. This would suggest a 7 beats/minute higher heart rate in those who smoke 20 cigarettes/day.
Conclusions
This MR meta-analysis supports a causal association of smoking heaviness with higher level of resting heart rate, but not with blood pressure. These findings suggest that part of the cardiovascular risk of smoking may operate through increasing resting heart rate.
Keywords: blood pressure, hypertension, Mendelian randomization, heart rate, smoking
Introduction
Smoking is a major risk factor for cardiovascular disease (CVD), but it is not clear by which mechanism smoking exerts its detrimental effects on CVD. Generally, epidemiological studies have reported lower blood pressure levels among current smokers compared with nonsmokers.1-14 On the other hand, smoking cessation has been reported to be followed by a decrease in blood pressure.15,16 Whether the apparent association between smoking and lower blood pressure is causal or can be explained by confounding by lifestyle or socioeconomic factors related to both smoking and blood pressure remains an open question. Furthermore, smoking is causally associated with lower body mass index (BMI),17 and higher level of adiposity is strongly associated with elevated blood pressure and is also considered a major risk factor for hypertension.18,19 Hence, there is a strong possibility that the lower blood pressure observed in smokers could be explained by lower body weight caused by smoking. Data from observational epidemiological studies also suggest that smoking is associated with higher level of resting heart rate.20 However, as for blood pressure, various confounding factors could explain this association.
In Mendelian randomisation, causal relationships in human populations are examined by using genetic variants as proxies for exposures of interest. The principle of Mendelian randomisation relies on the basic laws of Mendelian genetics, segregation and independent assortment. When these principles hold at a population level, genetic variants will not be associated with the confounding factors that generally distort conventional observational studies.21 In addition, an outcome measure cannot alter the germline genotype that an individual is born with, so these analyses should not be affected by reverse causality. It can be applied in two ways – first, to establish whether an observational association is likely to be causal, and second, using formal instrumental variable methods, to more accurately estimate the magnitude of this causal effect. Here we focus on the former approach.
A genetic variant, single nucleotide polymorphism (SNP), rs16969968, in the CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster on chromosome 15 has demonstrated robust association with smoking heaviness within smokers.22-24 The rs16969968 variant is functional and leads to an amino acid change (D398N) in the nicotinic receptor alpha5 subunit protein.25 The minor allele of the rs16969968 SNP is associated with an average increase in smoking amount of one cigarette per day in smokers and is even more strongly associated with serum cotinine (a metabolite of nicotine) levels.24,26 Importantly, the rs16969968 has not been robustly associated with smoking status and may therefore primarily be used as a marker of smoking heaviness in smokers. It is in perfect linkage disequilibrium with another SNP, rs1051730, and the two SNPs therefore represent the same genetic signal and can be used interchangeably. A large meta-analysis found that the minor allele of rs1051730 that increases smoking in current smokers is associated with lower BMI in current smokers, supporting the hypothesis that smoking is causally related to lower BMI.17 As expected, the effect of rs1051730 on BMI differed with smoking status; the negative effect was observed in current smokers but not in never smokers indicating that the association operate via smoking.17
A study in 5,402 young Finnish adults examined the effects of rs1051730 on blood pressure.23 The smoking increasing allele of rs1051730 tended to associate with lower systolic and diastolic blood pressure (SBP and DBP) among current smokers, but there was no strong statistical evidence for these associations. Furthermore, there was no evidence of interaction between smoking status and rs1051730 genotype. Åsvold and colleagues looked at the association between rs1051730 and cardiovascular risk factors in the HUNT study.27 In the total population including never, former, and current smokers, they found that the smoking increasing allele of rs1051730 was associated with lower SBP, but there was no association with DBP. Unexpectedly, the association between the rs1051730 and SBP was mainly seen among never smokers and there was no interaction between genotype and smoking status in relation to SBP possibly due to lack of power to detect interaction effects. Thus, it is not yet fully established whether smoking-related genetic variants are associated with blood pressure.
In this study, we investigated the associations between smoking and both blood pressure and resting heart rate, using both an observational and a Mendelian randomisation approach. First, we meta-analysed a total of 23 population-based studies participating in the consortium for Causal Analysis Research in Tobacco and Alcohol (CARTA).28 Second, we examined the possible causal effects of smoking on blood pressure and resting heart rate by Mendelian randomization using rs16969968/rs1051730 as unconfounded and unbiased proxies of smoking heaviness in current smokers.
Methods
Study Populations
The study was carried out within the CARTA consortium (http://www.bris.ac.uk/expsych/research/brain/targ/research/collaborations/carta).28,29 We used data on individuals (aged ≥16 years) of self-reported European ancestry from 23 studies: the 1958 British birth cohort (1958 BC), the Avon Longitudinal Study of Parents and Children (ALSPAC, including both mothers and children),30,31 the British Regional Heart Study (BRHS), the Caerphilly Prospective Study (CaPS), Cohorte Lausannoise (CoLaus/PsyCoLaus), the Dan-monica10 study, the English Longitudinal Study of Ageing (ELSA), the National FINRISK Study (FINRISK), the Danish GEMINAKAR study (The importance of genes, familiar and common environment for the development of insulin resistance, abdominal adiposity and cardiovascular risk factors), Genetics of Overweight Young Adults (GOYA) male, Generation Scotland: the Scottish Family Health Study (GS:SFHS), Health2006, the Helsinki Birth Cohort Study (HBCS), the Nord-Trøndelag health study (HUNT), Inter99, MIDSPAN Family Study, the Northern Finland Birth Cohorts (NFBC1966 and NFBC1986), the National Health and Nutrition Examination Survey (NHANES), the MRC National Survey of Health and Development (NSHD), the Netherlands Twin Registry (NTR), the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER), and the Whitehall II study. All studies received ethics approval from local research ethics committees. Further details of these studies are provided in supplemental material. Descriptive statistics are presented in Supplemental Table S1.
Genotype
Within each study, individuals were genotyped for one of two SNPs in the CHRNA5-A3-B4 nicotinic receptor subunit gene cluster, rs16969968 or rs1051730. These SNPs are in perfect linkage disequilibrium with each other in Europeans (R2 = 1.00 in HapMap 3, http://hapmap.ncbi.nlm.nih.gov/) and therefore represent the same genetic signal. For studies with data available for both SNPs, the SNP that was genotyped in the largest number of participants was used. Details of genotyping methods within each study are provided in supplemental material.
Measurements of blood pressure and heart rate
Details on the methods used for measuring systolic (SBP) and diastolic (DBP) blood pressure (mmHg) and resting heart rate (beats per minute) in each study are provided in supplemental material. In cohorts where information about use of blood pressure lowering medication was available a constant was added to SBP (15 mmHg) and DBP (10 mmHg) in subjects on blood pressure lowering medication as recommended by Tobin and colleagues.32 If this information was not available SBP and DBP were analysed as they were. Availability of information on blood pressure lowering medication in each study is provided in Supplemental Table S1. The following dichotomous outcomes were defined: 1) ‘hypertension’ as SBP >140 mmHg or DBP >90 mmHg or taking blood pressure lowering medication, 2) ‘severe hypertension’ as SBP >160 mmHg or DBP >100 mmHg, or taking blood pressure lowering medication. Thus, participants taking blood pressure lowering medication were defined as having both hypertension and severe hypertension. BMI was calculated as weight/height2 (kg/m2). Age was calculated as years at the time of measurements of blood pressure and heart rate.
Smoking status
Individuals were classified as current, former, or never cigarette smokers. Where information on smoking frequency was available, current smokers were restricted to individuals smoking at least one cigarette per day. Smokers (occasional smokers) who reported smoking less frequently than this were excluded from analyses on smokers. Where information on pipe and cigar smoking was available, individuals reporting being current or former smokers of pipes or cigars but not cigarettes were excluded from all analyses.
For studies with adolescent populations (ALSPAC children and NFBC 86), analyses were restricted to current daily smokers who reported smoking at least one cigarette per day (current smokers) and individuals who had never tried smoking (never smokers).
Data on smoking heaviness in current smokers, measured as cigarettes smoked per day, were collected in most studies as a continuous variable and in a few studies as a categorical variable. For full details of the smoking measures collected within each study, see the supplemental material.
Statistical analysis
Analyses were conducted within each contributing study using Stata and R software following the same pre-specified analysis protocol. The analysis protocol is available on the CARTA website: http://www.bris.ac.uk/expsych/research/brain/targ/research/collaborations/carta/
Scripts for data analyses and output for Stata and R were developed and made available to ensure uniform analyses and minimize errors in data extraction. Thus, analyses were restricted to individuals with full data on blood pressure, heart rate, smoking status and rs16969968/rs1051730 genotype. Within each study, genotype frequencies were tested for deviation from Hardy Weinberg Equilibrium using a chi-squared test.
In observational analyses, sex- and age-adjusted associations of smoking status (never, former, current) and smoking heaviness with continuous measures of SBP and DBP, and heart rate, were assessed using linear regression. For the smoking status analysis, never smokers were used as the reference group. The smoking heaviness analysis was restricted to current daily smokers, and beta estimates represent change in the outcome measure per additional cigarette consumed per day. Sex- and age-adjusted associations expressed as odds ratios (ORs) of smoking status (never, former, current) and smoking heaviness with binary measures of hypertension (‘hypertension’ and ‘severe hypertension’) were assessed using logistic regression. In additional analyses, the above models were further adjusted for BMI (continuous variable) to observe changes (if any) in the estimates for the effects of smoking.
Mendelian randomisation analyses of the association between rs16969968/rs1051730 and continuous measures of blood pressure and heart rate were assessed using linear regression stratified by smoking status (never, former, current) and adjusted for age, and sex. Mendelian randomisation analyses of the association between rs16969968/rs1051730 and binary measures of hypertension (‘hypertension’ and ‘severe hypertension’) were assessed using logistic regression stratified by smoking status (never, former, current) adjusted for age, and sex. An additive genetic model was assumed, so risk estimates represent the difference in risk of the outcome per additional copy of the minor (risk) allele. In additional analyses, the above models were further adjusted for BMI (continuous variable) observing changes in the estimates for the effects of the risk allele.
Results from individual studies were meta-analysed in Stata (version 11) using the “metan” command. Results from observational analyses were combined by random effects model due to substantial heterogeneity (I2 > 50%). Results from Mendelian randomisation analyses were combined in a fixed effects model and the Cochran Q statistic was used to test for heterogeneity between genotype and smoking status in relation to the outcome measures.
Results
Descriptive statistics
In total, data on 141,317 iindividuals were available for analysis, including 62,666 never smokers, 40,669 former smokers, and 37,982 current smokers. Overall, 49% of the combined study population was male. The median age within the contributing studies ranged from 16 to 75 years. Descriptive statistics for each of the study populations are found in the Supplemental Table S1. Minor allele frequency for rs16969968/rs1051730 ranged between 0.29 and 0.36 (Supplemental Table S2). There was no strong evidence for deviation from Hardy Weinberg Equilibrium in any of the studies (p-values all ≥ 0.1) (Supplemental Table S2).
Observational analysis
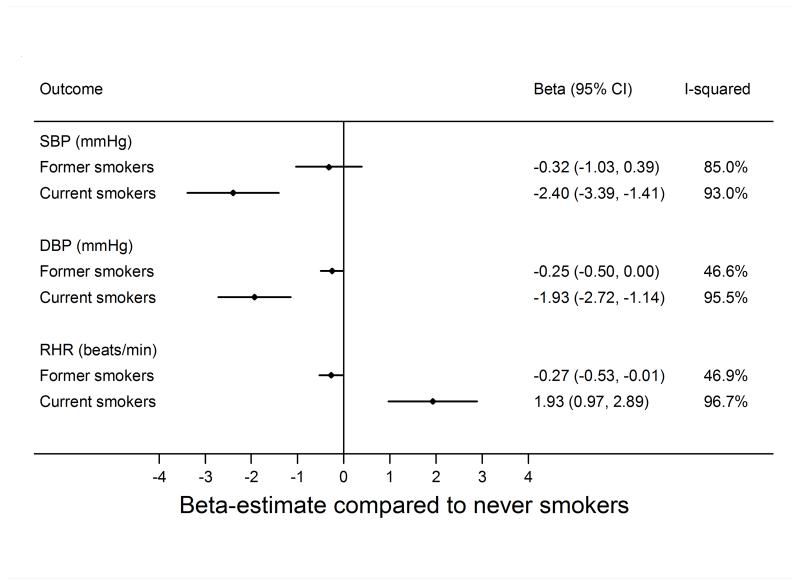
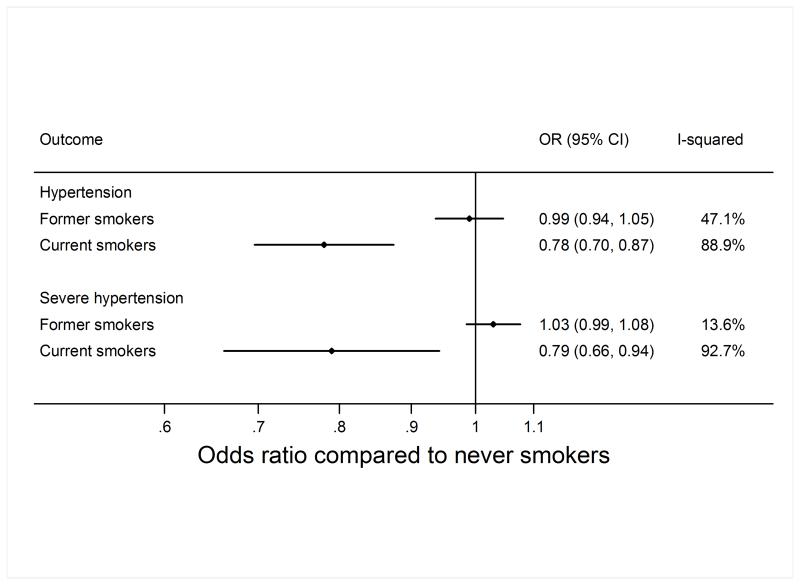
The meta-analysed estimates of the age- and sex-adjusted associations of smoking status (never, former, current smoking) with SBP, DBP, and resting heart rate are shown in Figure 1. Study-specific estimates are shown in supplemental material. Current as compared with never smoking was associated with lower SBP (−2.40 mmHg; 95% CI −3.39; −1.41) and DBP (−1.93 mmHg; 95% CI −2.72; −1.15). Accordingly, current as compared with never smoking was associated with a lower risk of hypertension (OR 0.78; 95% CI: 0.70; 0.88) and severe hypertension (OR 0.79; 95% CI: 0.66; 0.94) (Figure 2).
Figure 1.
Association of smoking status with systolic blood pressure (SBP) and diastolic blood pressure (DBP), and resting heart rate (RHR). Former and current smoking status are compared to never smoking status; the difference was estimated by linear regression adjusted for sex and age.
Figure 2.
Association of smoking status with hypertension and severe hypertension. Former and current smoking status are compared to never smoking status; the difference was estimated by logistic regression adjusted for sex and age. OR, Odds ratio.
The above associations were attenuated, but remained, when further adjusted for BMI (Supplemental Figures S1 and S2). For example, after additional adjustment for BMI, current as compared with never smoking was associated with a lower risk of hypertension (OR 0.84; 95% CI: 0.73; 0.94) and severe hypertension (OR 0.85; 95% CI: 0.72; 1.01) (Supplemental Figure S2).
Current as compared with never smoking was associated with 1.93 beats per minute (95% CI: 0.97; 2.89) higher resting heart rate (Figure 1) and this association was not attenuated by adjustment for BMI (Supplemental Figure S1).
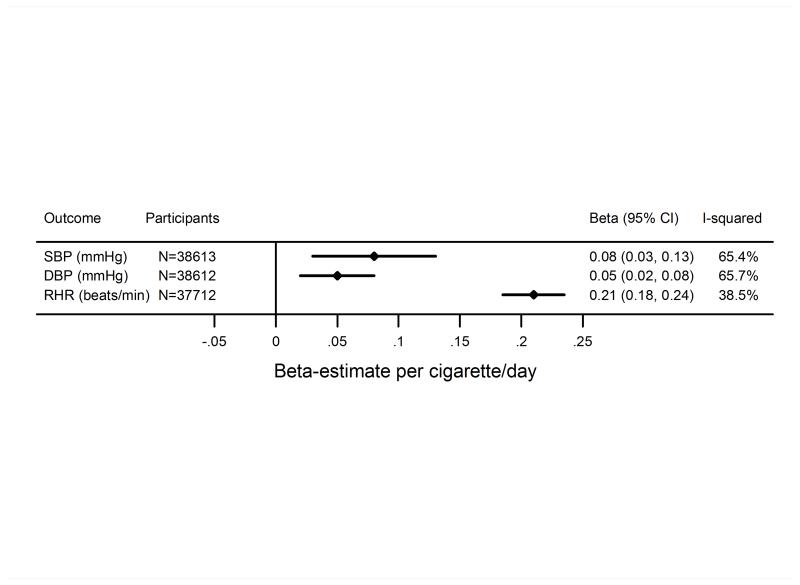
The analysis of smoking heaviness (performed only in current smokers) showed that higher level of smoking heaviness was associated with slightly higher levels of SBP and DBP (Figure 3). In age- and sex-adjusted analyses, one cigarette per day higher level of smoking heaviness was associated with a 0.08 (95% CI: 0.03; 0.13) and 0.05 (95% CI: 0.02; 0.08) mmHg higher SBP and DBP, respectively. These associations attenuated after further adjustment for BMI (Figure S3). One cigarette per day higher level of smoking heaviness was associated with a 0.21 (95% CI: 0.19; 0.24) beats per minute higher level of resting heart rate (Figure 3), and this association was not attenuated by adjustment for BMI (Supplemental Figure S3).
Figure 3.
Association of smoking heaviness in current smokers with systolic blood pressure (SBP) and diastolic blood pressure (DBP) and resting heart rate (RHR). The difference per one cigarette per day increase in smoking heaviness was estimated by linear regression adjusted for sex and age.
Mendelian randomization analysis
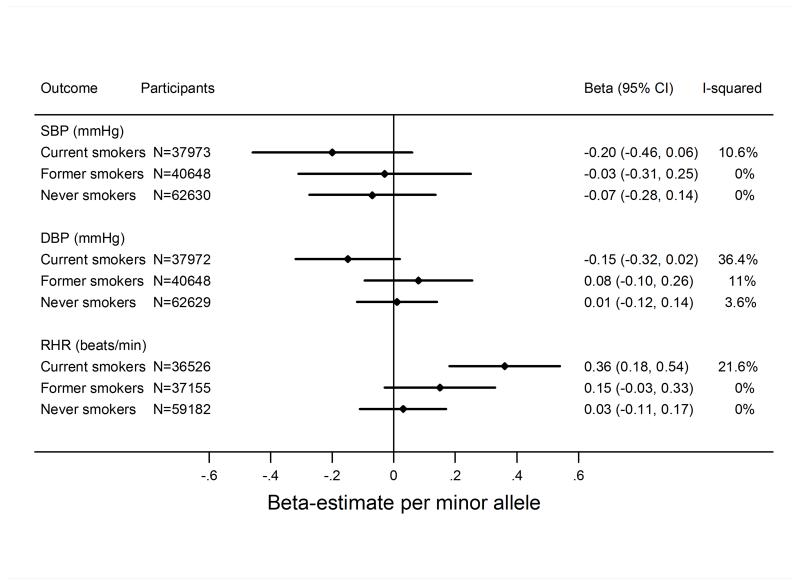
In meta-analyses stratified by smoking status, there was no strong evidence that rs16969968/rs1051730 was associated with either continuous measures of blood pressure (Table 1)(Figure 4) or with binary measures of hypertension (hypertension and severe hypertension) (Table 1)(Supplemental Figure S4). Associations were closer to the null after adjustment for BMI (Supplemental Figures S5 and S6). There was no evidence that the effect of the CHRNA5-A3-B4 variant differed between never, former and current smokers (p-values for heterogeneity from Cochran Q test all >= 0.1).
Table 1.
Mendelian randomization meta-analysis: results of meta-analysed estimates of the association of the smoking increasing allele of rs16969968/rs1051730 with continuous measures of blood pressure, binary measures of hypertension (hypertension and severe hypertension), and the continuous measure of resting heart rate. Results were stratified by smoking status.
| Never smokers | Former smokers | Current smokers | |
|---|---|---|---|
|
Systolic blood pressure
beta-coefficient* (95% CI); mmHg/allele |
−0.07 (−0.28; 0.13) P = 0.479 |
−0.03 (−0.31; 0.25) P = 0.824 |
−0.20 (−0.46; 0.06) P = 0.136 |
|
Diastolic blood pressure
beta-coefficient* (95% CI); mmHg/allele |
0.01 (−0.12; 0.14) P = 0.879 |
0.08 (−0.09; 0.26) P = 0.331 |
−0.15 (−0.32; 0.02) P = 0.079 |
|
Hypertension
Odds ratio* (95% CI) per allele |
1.00 (0.97; 1.03) P = 0.923 |
1.00 (0.96; 1.03) P = 0.811 |
0.98 (0.95; 1.02) P = 0.277 |
|
Severe hypertension
Odds ratio* (95% CI) per allele |
1.02 (0.98; 1.05) P = 0.362 |
1.01 (0.97; 1.05) P = 0.543 |
0.96 (0.92; 1.00) P = 0.061 |
|
Resting heart rate
beta-coefficient* (95% CI); beats/minute/allele |
0.03 (−0.11; 0.17) P = 0.686 |
0.15 (−0.03; 0.33) P = 0.109 |
0.36 (0.18; 0.54) P < 0.001 † |
associations have been adjusted for sex and age by linear (continuous outcomes) or logistic (binary outcomes) regression
Bonferroni-adjusted (15 tests) P-value was 0.001
CI confidence interval
Figure 4.
Mendelian randomisation analysis of the association of the smoking increasing allele (minor allele) of rs1051730/rs16969968 with systolic blood pressure (SBP) and diastolic blood pressure (DBP) and resting heart rate (RHR). Analyses were stratified by smoking status (current, former, and never smoking). The difference per one allele was estimated by linear regression adjusted for sex and age. Overall test for heterogeneity by smoking status: SBP: P=0.656; DBP: P=0.138; RHR: P=0.015;
In contrast, in current smokers, the smoking increasing allele of rs16969968/rs1051730 was associated with a 0.36 (95% CI 0.18; 0.54) beats per minute higher heart rate per allele (Table 1)(Figure 4). This estimate was 0.39 (95% CI 0.21; 0.57)) after additional adjustment for BMI (Supplemental Figure S5). In addition, there was evidence of interaction (p-value for heterogeneity from Cochran Q test 0.003) between smoking status and the CHRNA5-A3-B4 variant in age, sex and BMI-adjusted analyses lending further support to a causal effect of smoking on resting heart rate.
The results of observational and Mendelian Randomization analyses stratified by two smoking status categories (never and ever smokers) were in line with the analyses stratified by three smoking categories (never, former, and current smokers) (data not shown).
Additionally, sensitivity analyses were performed excluding each of the following studies: HUNT, ALSPAC children, and Prosper. The reasons for this were the large number of participants contributed by one study (HUNT), relatedness between participants (mothers and children in ALSPAC), and results being outliers (Prosper). These analyses yielded generally results similar to results based on all studies. However, when excluding the HUNT study, the association of the smoking increasing allele of rs16969968/rs1051730 with resting heart rate in current smokers attenuated and became statistically insignificant.
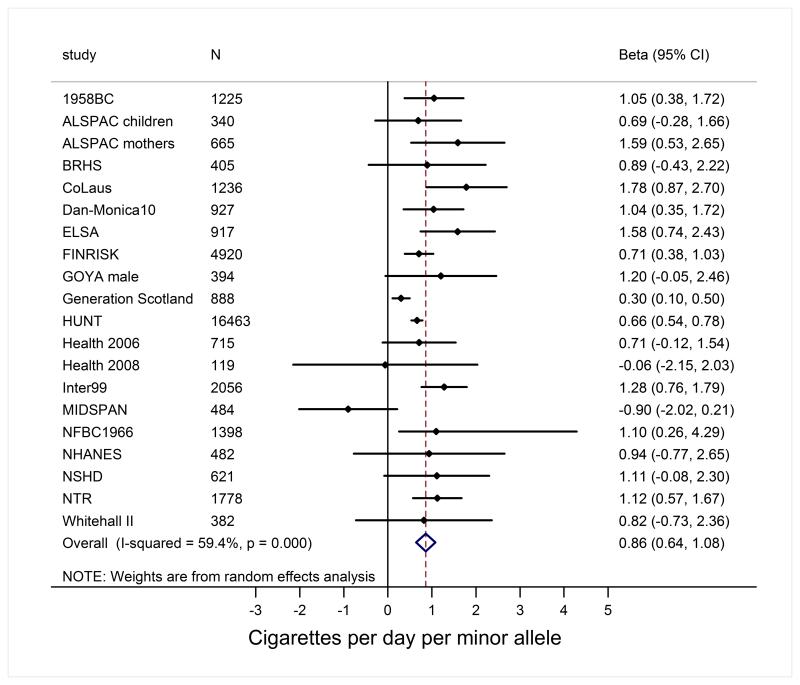
Finally, we investigated the association of the smoking increasing allele of rs16969968/rs1051730 with smoking status and smoking heaviness. In current smokers, the smoking increasing allele of rs16969968/rs1051730 was associated with 0.9 cigarettes/day/allele increase in smoking heaviness (Figure 5). Our results confirmed previous findings in that the smoking increasing allele is not associated with smoking initiation (the allele is not associated with ever versus never smoking), but may be associated with smoking cessation (the allele is significantly associated with current versus former smoking) (Supplemental Figure S7).
Figure 5.
Mendelian randomisation analysis of the association of the smoking increasing allele (minor allele) of rs1051730/rs16969968 with smoking heaviness in current smokers. The difference (increase in smoking quantity) per one allele was estimated by linear regression adjusted for sex and age.
Discussion
We performed a meta-analysis of 23 population-based studies including a total of 141,317 individuals, using both observational and Mendelian randomisation analyses. In observational analyses, we found that current smoking is associated with lower blood pressure and lower prevalence of hypertension. However, observational and Mendelian randomisation analyses did not support a causal association between smoking heaviness in current smokers and blood pressure. In contrast, both observational and Mendelian randomisation analyses consistently suggested that smoking heaviness is causally related to increasing resting heart rate amongst smokers.
In our meta-analyses, the risk estimate for the effect of rs16969968/rs1051730 on blood pressure in current smokers was close to the null, with the confidence interval overlapping the null. Thus, the Mendelian randomisation analysis did not support a causal effect of smoking heaviness on blood pressure. It should be stressed that the unadjusted analysis captures the overall effect of smoking on blood pressure. Given evidence from previous Mendelian randomisation studies that smoking reduces BMI, and BMI causes higher blood pressure, we might have expected to see a negative association between the smoking increasing allele and blood pressure in current smokers, unless smoking increases blood pressure through other pathways. There was some indication that adjustment for BMI in our Mendelian randomisation analyses attenuated the estimates for the effects of the smoking increasing allele among current smokers even more towards the null, an observation that may suggest that any potential effect of smoking on blood pressure would be mediated through the BMI-decreasing effect of smoking.17 It should be stressed, that if BMI acts as a mediator, adjustment for BMI would be inappropriate and caution should be exercised when interpreting the BMI adjusted results. Future studies could employ a double Mendelian randomization approach using genetic makers of both smoking and BMI.
Amongst current smokers, results of our Mendelian randomisation analysis supported that smoking heaviness is causally associated with increasing resting heart rate. These results thus corroborate results of our observational analysis and are furthermore in line with studies showing that resting heart rate tends to decrease following smoking cessation.16,33,34 The mechanisms by which smoking increases resting heart rate are not clear. Human experimental studies show that administration of nicotine increases resting heart rate and that this is an acute effect.35,36 Thus, when nicotine is administered intravenously the heart rate increasing effect peaks within the first minute after administration.36 Interestingly, it has been observed that reductions in hospital admissions with acute myocardial infarction following smoking regulation and bans in public places appear to have a surprisingly rapid onset.37
The clinical implications of smoking-induced higher resting heart rate are not clear. However, considering the well-documented detrimental effects of smoking on risk of cardiovascular disease and our finding that this might not involve a strong direct effect on blood pressure, it may be speculated that more attention should be paid to resting heart rate as a marker of cardiovascular health and risk prediction.38 Several studies have shown that resting heart rate is a predictor of cardiovascular events even after controlling for other cardiovascular risk factors such as blood pressure.39-44 Of potential relevance is that heart-rate lowering drugs improve long-term survival of patients with myocardial infarction.45 Our results roughly translated estimate that someone smoking 20 cigarettes per day could increase their resting heart rate by around 7 beats per min, and vice versa that someone stopping smoking could have this meaningful reduction in HR.
The minor allele of the rs16969968/rs1051730 is known to be associated with smoking heaviness by approximately 1 cigarette per day per copy of the allele in current smokers,46 and we observed a 0.36 (95% CI 0.18; 0.54) beats per minute higher resting heart rate per one extra copy of the smoking increasing allele in current smokers (Figure 4). Using the CHRNA5-A3-B4 variant (rs16969968/rs1051730) as an instrument for tobacco exposure may be advantageous compared to using a self-reported measure of tobacco exposure. Firstly, self-reported tobacco use is likely subject to some degree of misclassification and reporting bias. Secondly, self-reported tobacco consumption does not take into account variation in smoking topography, such as the amount of a cigarette an individual smokes or the depth of inhalation.24 Thirdly, the CHRNA5-A3-B4 variant is an instrument for lifetime cumulated tobacco exposure, and this is not fully captured by cigarettes per day. In further support of the genetic variant being a better measure of smoking heaviness, it has been shown that rs16969968/rs1051730 explains more of the variance (4%) in serum cotinine, a biomarker of tobacco exposure, than in self-reported number of cigarettes per day (1%)24,26. Notably, the effect (0.21 beats per minute increase per cigarette per day) of smoking heaviness on resting heart rate observed in our observational analyses based on self-reported smoking habits was smaller than indicated by the Mendelian randomisation analyses, a finding that is consistent with the idea that the rs16969968/rs1051730 SNP is a more accurate marker of smoking heaviness than self-reports. Of note, it has previously been shown that the use of instrumental variable analysis in Mendelian Randomisation studies estimate the magnitude of any causal effect of cigarette smoking, where measured self-reported cigarette consumption is used as the exposure, is likely inappropriate and may lead to biased estimates.47
We would not expect to see an effect of rs16969968/rs1051730 on resting heart rate in never smokers, because the variant cannot be associated with smoking heaviness within these individuals without any exposure. Thus, this group can be used to test the assumption of no pleiotrophy (i.e. that the gene affects only one exposure) in Mendelian randomisation analyses. Thus, our observation that there was no clear statistical evidence for an association of the rs16969968/rs1051730 variant with heart rate in never smokers, and that we observed statistical evidence of heterogeneity of the effect of the variant between smoking categories, lends further support to the notion that smoking is causally related to higher resting heart rate.
The principle of Mendelian randomization is based of assumptions, e.g. a) the genetic marker is associated with the exposure, b) the genetic marker is independent of the outcome given the exposure and all confounders (pleitrophy, see above), and c) the genetic marker is independent of factors that confound the exposure-outcome relationship. It should be recognized that these assumptions may not all be easy to evaluate.
Stratification of Mendelian randomisation analyses may induce collider bias, if the instrument is predictive of the stratification variable.48 We do not think that this is a major concern as our instrument rs16969968/rs1051730 is primarily a genetic variant for smoking heaviness within smokers and there is no clear evidence that it is associated with smoking initiation (i.e. being an ever versus a never smoker). It does show some evidence for an association with smoking cessation,49 but analyses performed in ever smokers yielded results similar to analyses performed in current smokers. The rs16969968/rs1051730 variant has been used in a similar way (i.e. stratified on smoking status) in a number of other Mendelian Randomisation studies to demonstrate the expected causal associations of smoking with increased all-cause mortality,50 decreased lung function,51 and body mass index. The fact that stratification by smoking status has been used to show that smoking is, as expected, causally related these phenotypes, supports our view that this approach is appropriate.
Conclusions
This large Mendelian randomization meta-analysis suggests that smoking is causally related to higher level of resting heart rate, but not to alterations in blood pressure and risk of hypertension. These findings are consistent with the hypothesis that smoking exerts its detrimental effects on cardiovascular disease at least partly via increasing resting heart rate.
Supplementary Material
Clinical Perspective.
Smoking is a major risk factor for cardiovascular disease, but it is not clear by which mechanism smoking exerts its detrimental effects on cardiovascular disease. Previous observational epidemiological studies show that smokers as compared to non-smokers have lower blood pressure and risk of hypertension. However, these studies may have been hampered by confounding and biases. Mendelian randomization is an approach where genetic markers of exposures are used for testing causal associations. We performed a meta-analysis of 23 population-based studies including a total of 141,317 individuals, using both observational and Mendelian randomization analyses. Our results suggested that smoking is causally related to higher level of resting heart rate, but not to significant alterations in blood pressure and risk of hypertension. These findings are consistent with the hypothesis that smoking exerts its detrimental effects on cardiovascular disease at least partly via increasing resting heart rate. Our results roughly translated estimate that someone smoking 20 cigarettes per day could increase their resting heart rate by around 7 beats per min, and vice versa that someone stopping smoking could have this meaningful reduction in heart rate. Considering the well-documented detrimental effects of smoking on risk of cardiovascular disease and our finding that this might not involve a strong direct effect on blood pressure, it may be speculated that more attention should be paid to resting heart rate as a marker of cardiovascular health and risk prediction.
Acknowledgments
Funding Sources: 1958BC Statistical analyses were funded by the Academy of Finland (Project 24300796 and SALVE/PREVMEDSYN). DNA collection was funded by MRC grant G0000934 and cell-line creation by Wellcome Trust grant 068545/Z/02. This research used resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development, and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. This study makes use of data generated by the Wellcome Trust Case-Control Consortium. A full list of investigators who contributed to generation of the data is available from the Wellcome Trust Case-Control Consortium website. Funding for the project was provided by the Wellcome Trust under the award 076113. Great Ormond Street Hospital/University College London, Institute of Child Health receives a proportion of funding from the Department of Health's National Institute for Health Research (NIHR) ('Biomedical Research Centres' funding). ALSPAC The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. The genetic data for the ALSPAC mothers were funded by a grant from the Wellcome Trust (WT088806) and the blood pressure and pulse data by a grant from the British Heart Foundation (SP/07/008/24066). This work was supported by the Wellcome Trust (grant number 086684) and the Medical Research Council (grant numbers MR/J01351X/1, G0800612, G0802736, MC_UU_12013/1, MC_UU_12013/5, MC_UU_12013/6). The British Regional Heart Study (BRHS) BRHS is a British Heart Foundation (BHF) Research Group. The Caerphilly Prospective Study (CaPS) The CaPS was conducted by the former MRC Epidemiology Unit (South Wales). The Caerphilly archive is now maintained by the School of Social and Community Medicine in Bristol University. CoLaus/PsyCoLaus The CoLaus/PsyCoLaus study was supported by four grants of the Swiss National Science Foundation (#105993, 118308, 139468 and 122661), two unrestricted grants from GlaxoSmithKline as well as by the Faculty of Biology and Medicine of the University of Lausanne. The Dan-MONICA10 The Dan-MONICA10 was sponsored by The Danish Heart Foundation; the Danish Medical Research Council; The Danish Hospital Foundation of Medical Research, region of Copenhagen, the Faroe Islands and Greenland; The Danish Health Insurance Foundation; The Foundation of E. & M. Wedel-Wedellsborg; Landsforeningen til Bekampelse af Kredslobssygdomme; The Augustinus Foundation; The Becket Foundation; and The Foundation of senior registrar J. & L. Boserup. The English Longitudinal Study of Ageing (ELSA) ELSA is funded by the National Institute on Aging in the US (R01 AG017644;R01AG1764406S1) and by a consortium of UK Government departments (including: Department for Communities and Local Government, Department for Transport, Department for Work and Pensions, Department of Health, HM Revenue and Customs and Office for National Statistics). The National FINRISK Study The FINRISK study was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (grant numbers 213506, 129680), the Academy of Finland (grant numbers 139635, 129494, 136895, 263836 and 141054), the Sigrid Juselius Foundation , and ENGAGE – European Network for Genetic and Genomic Epidemiology, FP7-HEALTH-F4-2007, grant agreement number 201413. Finnish Foundation for Cardiovascular Research. The Danish GEMINAKAR study The GEMINAKAR study was supported by grants from the Medical Research Fund, the Danish Diabetes Association, the NOVO Foundation, and the Danish Heart Foundation. The Generation Scotland study Generation Scotland has received core funding from the Chief Scientist Office of the Scottish Government Health Directorates CZD/16/6 and the Scottish Funding Council HR03006. Genotyping of the GS:SFHS samples was carried out by the Genetics Core Laboratory at the Wellcome Trust Clinical Research Facility, Edinburgh, Scotland and was funded by the UK’s Medical Research Council. Genomics of Overweight Young Adults (GOYA) male The GOYA study was conducted as part of the activities of the Danish Obesity Research Centre (DanORC, www.danorc.dk) and The MRC centre for Causal Analyses in Translational Epidemiology (MRC CAiTE). The genotyping for GOYA was funded by the Wellcome Trust (WT 084762). GOYA is a nested study within The Danish National Birth Cohort which was established with major funding from the Danish National Research Foundation. Additional support for this cohort has been obtained from the Pharmacy Foundation, the Egmont Foundation, The March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation. TSA was supported by the Gene Diet Interactions in Obesity (GENDINOB, www.gendinob.dk ) postdoctoral fellowship grant. LP is funded by an MRC Population Health Scientist Fellowship (MR/J012165/1). The Health2006 study The Health2006 study was financially supported by grants from the Velux Foundation; the Danish Medical Research Council, Danish Agency for Science, Technology and Innovation; the Aase and Ejner Danielsens Foundation; ALK-Abello’ A/S (Horsholm, Denmark), Timber Merchant Vilhelm Bangs Foundation, MEKOS Laboratories (Denmark) and Research Centre for Prevention and Health, the Capital Region of Denmark. The Helsinki Birth Cohort Study (HBCS) The HBCS has been supported by grants from the Academy of Finland, the Finnish Diabetes Research Society, Samfundet Folkhalsan, Novo Nordisk Foundation, Finska Lakaresallskapet, Signe and Ane Gyllenberg Foundation, University of Helsinki, Ministry of Education, Ahokas Foundation, Emil Aaltonen Foundation. Nord-Trondelag Health Study (The HUNT Study) HUNT is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trondelag County Council and the Norwegian Institute of Public Health. The Inter99 study Data collection in the Inter99 study was supported economically by The Danish Medical Research Council, The Danish Centre for Evaluation and Health Technology Assessment, Novo Nordisk, Copenhagen County, The Danish Heart Foundation, The Danish Pharmaceutical Association, Augustinus foundation, Ib Henriksen foundation and Becket foundation. LLNH was supported by the Health Insurance Foundation (grant No. 2010 B 131). The MIDSPAN family study The MIDSPAN Family Study was funded as part of the NHS Research and Development Cardiovascular Research Programme. The Northern Finland Birth Cohort 1966 and 1986 (NFBC1966 and NFBC1986) The NFBC1966 and NFBC1986 received financial support from the Academy of Finland (project grants 104781, 120315, 129269, 1114194, 24300796, 141042 Center of Excellence in Complex Disease Genetics and SALVE), University Hospital Oulu, Biocenter, University of Oulu, Finland (75617), NHLBI grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01), NIH/NIMH (5R01MH63706:02), the European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000-01643), ENGAGE project and grant agreement HEALTH-F4-2007-201413, EU FP7 EurHEALTHAgeing - 277849, the Medical Research Council, UK (G0500539, G0600705, G1002319, PrevMetSyn/SALVE) and the MRC, Centenary Early Career Award. The DNA extractions, sample quality controls, biobank up-keeping and aliquotting was performed in the National Public Health Institute, Biomedicum Helsinki, Finland and supported financially by the Academy of Finland and Biocentrum Helsinki. The National Health and Nutrition Examination Survey (NHANES) The NHANES (http://www.cdc.gov/nchs/nhanes.htm) is a program of health surveys run by the National Center for Health Statistics, part of the Centers for Disease Control and Prevention in the United States. The Medical Research Council National Survey of Health and Development (NSHD) The NSHD was funded by the Medical Research Council [MC_UU_12019/1]. The Netherlands Twin Register (NTR) This study was supported by the European Research Council (ERC Starting Grant 284167 PI Vink), Netherlands Organization for Scientific Research (NWO: MagW/ZonMW grants 904-61-090, 985-10-002, 904-61-193, 480-04-004, 400-05-717, Addiction-31160008 Middelgroot-911-09-032, Spinozapremie 56-464-14192), BBRMI-NL (Biobanking and Biomolecular Resources Research Infrastructure), VU University’s Institutes for Health and Care Research and Neuroscience Campus Amsterdam. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) trial The PROSPER was supported by an investigator initiated grant from Bristol-Myers Squibb, USA. The study was conducted, analysed, and reported independently of the company. The GWAS project PHASE has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement HEALTH-F2-2009-223004. A part of the genotyping was funded by The Netherlands Consortium for Healthy Ageing (NGI: 05060810). JWJ is an established clinical investigator of The Netherlands Heart Foundation (2001 D 032). Whitehall II The Whitehall II study has been supported by grants from the Medical Research Council (K013351); British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (NHLBI: HL36310) and National Institute on Aging (AG13196), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. MeKu is partially supported by the Economic and Social Research Council International Centre for Life Course Studies in Society and Health (RES-596-28-0001). MK is partially supported by the Medical Research Council and the Economic and Social Research Council.
Footnotes
Conflict of Interest Disclosures: Tellervo Korhonen consulted for Pfizer in 2011-2014 on nicotine dependence. Jaakko Kaprio consulted for Pfizer on nicotine dependence in 2013 & 2014. Diana Kuh has received funding from the Medical Research Council to pay for her salary and the NSHD data collection. Peter H. Whincup has received a research grant from the British Heart Foundation. Gerard Waeber has received a research grant from the Swiss National Science Foundation for recruitment for the population-based study COLAUS. Blair H Smith has received a research grant from the Scottish Government, Chief Scientist Office, for collection of data (Generation Scotland).
References
- 1.Goldbourt U, Medalie JH. Characteristics of smokers, non-smokers and ex-smokers among 10,000 adult males inIsrael. II. Physiologic, biochemical and genetic characteristics. Am J Epidemiol. 1977;105:75–86. doi: 10.1093/oxfordjournals.aje.a112358. [DOI] [PubMed] [Google Scholar]
- 2.Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J. 1986;111:932–940. doi: 10.1016/0002-8703(86)90645-9. [DOI] [PubMed] [Google Scholar]
- 3.Savdie E, Grosslight GM, Adena MA. Relation of alcohol and cigarette consumption to blood pressure and serum creatinine levels. J Chronic Dis. 1984;37:617–623. doi: 10.1016/0021-9681(84)90111-5. [DOI] [PubMed] [Google Scholar]
- 4.Berglund G, Wilhelmsen L. Factors related to blood pressure in a general population sample of Swedish men. Acta Med Scand. 1975;198:291–298. doi: 10.1111/j.0954-6820.1975.tb19543.x. [DOI] [PubMed] [Google Scholar]
- 5.Reid DD, Holland WW, Rose GA. An Anglo-American cardiovascular comparison. Lancet. 1967;2:1375–1378. doi: 10.1016/s0140-6736(67)93019-x. [DOI] [PubMed] [Google Scholar]
- 6.Seltzer CC. Effect of smoking on blood pressure. Am Heart J. 1974;87:558–564. doi: 10.1016/0002-8703(74)90492-x. [DOI] [PubMed] [Google Scholar]
- 7.Higgins MW, Kjelsberg M. Characteristics of smokers and nonsmokers in Tecumseh, Michigan. II. The distribution of selected physical measurements and physiologic variables and the prevalence of certain diseases in smokers and nonsmokers. Am J Epidemiol. 1967;86:60–77. doi: 10.1093/oxfordjournals.aje.a120734. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins CD, Rosenman RH, Zyzanski SJ. Cigarette smoking. Its relationship to coronary heart disease and related risk factors in the Western Collaborative Group Study. Circulation. 1968;38:1140–1155. doi: 10.1161/01.cir.38.6.1140. [DOI] [PubMed] [Google Scholar]
- 9.Kesteloot H, Van HO. The epidemiology of arterial blood pressure. Brux Med. 1973;Spec No:7–38. [PubMed] [Google Scholar]
- 10.Klatsky AL, Friedman GD, Siegelaub AB, Gerard MJ. Alcohol consumption and blood pressure Kaiser-Permanente Multiphasic Health Examination data. N Engl J Med. 1977;296:1194–1200. doi: 10.1056/NEJM197705262962103. [DOI] [PubMed] [Google Scholar]
- 11.Ueshima H, Shimamoto T, Iida M, Konishi M, Tanigaki M, Doi M, et al. Alcohol intake and hypertension among urban and rural Japanese populations. J Chronic Dis. 1984;37:585–592. doi: 10.1016/0021-9681(84)90008-0. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen KL, Wiinberg N, Hoegholm A, Christensen HR, Bang LE, Nielsen PE, et al. Smoking related to 24-h ambulatory blood pressure and heart rate: a study in 352 normotensive Danish subjects. Am J Hypertens. 1997;10:483–491. doi: 10.1016/s0895-7061(96)00487-6. [DOI] [PubMed] [Google Scholar]
- 13.Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60:1393–1399. doi: 10.1161/HYPERTENSIONAHA.112.201780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brummett BH, Babyak MA, Siegler IC, Shanahan M, Harris KM, Elder GH, et al. Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US young adult sample. Hypertension. 2011;58:161–166. doi: 10.1161/HYPERTENSIONAHA.111.171272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward KD, Bliss RE, Vokonas PS, Garvey AJ. Effects of smoking cessation on blood pressure. Am J Cardiol. 1993;72:979–981. doi: 10.1016/0002-9149(93)91121-w. [DOI] [PubMed] [Google Scholar]
- 16.Hatsukami DK, Kotlyar M, Allen S, Jensen J, Li S, Le C, et al. Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest. 2005;128:2528–2537. doi: 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- 17.Freathy RM, Kazeem GR, Morris RW, Johnson PC, Paternoster L, Ebrahim S, et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int J Epidemiol. 2011;40:1617–1628. doi: 10.1093/ije/dyr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timpson NJ, Harbord R, Davey SG, Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension. 2009;54:84–90. doi: 10.1161/HYPERTENSIONAHA.109.130005. [DOI] [PubMed] [Google Scholar]
- 19.Seven E, Husemoen LL, Wachtell K, Ibsen H, Linneberg A, Jeppesen JL. Five-year weight changes associate with blood pressure alterations independent of changes in serum insulin. J Hypertens. 2014;32:2231–2237. doi: 10.1097/HJH.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 20.Gillum RF. Epidemiology of resting pulse rate of persons ages 25-74--data from NHANES 1971-74. Public Health Rep. 1992;107:193–201. [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 22.Ware JJ, van den Bree M, Munafo MR. From men to mice: CHRNA5/CHRNA3, smoking behavior and disease. Nicotine Tob Res. 2012;14:1291–1299. doi: 10.1093/ntr/nts106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaakinen M, Ducci F, Sillanpaa MJ, Laara E, Jarvelin MR. Associations between variation in CHRNA5-CHRNA3-CHRNB4, body mass index and blood pressure in the Northern Finland Birth Cohort 1966. PLoS One. 2012;7:e46557. doi: 10.1371/journal.pone.0046557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munafo MR, Timofeeva MN, Morris RW, Prieto-Merino D, Sattar N, Brennan P, et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst. 2012;104:740–748. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keskitalo K, Broms U, Heliovaara M, Ripatti S, Surakka I, Perola M, et al. Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet. 2009;18:4007–4012. doi: 10.1093/hmg/ddp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asvold BO, Bjorngaard JH, Carslake D, Gabrielsen ME, Skorpen F, Smith GD, et al. Causal associations of tobacco smoking with cardiovascular risk factors: a Mendelian randomization analysis of the HUNT Study in Norway. Int J Epidemiol. 2014;43:1458–1470. doi: 10.1093/ije/dyu113. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AE, Fluharty ME, Bjorngaard JH, Gabrielsen ME, Skorpen F, Marioni RE, et al. Investigating the possible causal association of smoking with depression and anxiety using Mendelian randomisation meta-analysis: the CARTA consortium. BMJ Open. 2014;4:e006141. doi: 10.1136/bmjopen-2014-006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor AE, Morris RW, Fluharty ME, Bjorngaard JH, Asvold BO, Gabrielsen ME, et al. Stratification by Smoking Status Reveals an Association of CHRNA5-A3-B4 Genotype with Body Mass Index in Never Smokers. PLoS Genet. 2014;10:e1004799. doi: 10.1371/journal.pgen.1004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the 'children of the 90s'--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey SG, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 33.Ward KD, Garvey AJ, Bliss RE. Evidence of transient heart rate change after smoking cessation. Psychopharmacology (Berl) 1992;106:337–340. doi: 10.1007/BF02245414. [DOI] [PubMed] [Google Scholar]
- 34.Korhonen T, Goodwin A, Miesmaa P, Dupuis EA, Kinnunen T. Smoking cessation program with exercise improves cardiovascular disease biomarkers in sedentary women. J Womens Health (Larchmt ) 2011;20:1051–1064. doi: 10.1089/jwh.2010.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolma CD, Samson RA, Klewer SE, Donnerstein RL, Goldberg SJ. Acute cardiac effects of nicotine in healthy young adults. Echocardiography. 2002;19:443–448. doi: 10.1046/j.1540-8175.2002.00443.x. [DOI] [PubMed] [Google Scholar]
- 36.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–2183. doi: 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooney MT, Vartiainen E, Laatikainen T, Joulevi A, Dudina A, Graham I. Simplifying cardiovascular risk estimation using resting heart rate. Eur Heart J. 2010;31:2141–2147. doi: 10.1093/eurheartj/ehq164. [DOI] [PubMed] [Google Scholar]
- 39.Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: a cardiovascular risk factor? Eur Heart J. 2006;27:2387–2393. doi: 10.1093/eurheartj/ehl259. [DOI] [PubMed] [Google Scholar]
- 40.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 42.Jensen MT, Marott JL, Allin KH, Nordestgaard BG, Jensen GB. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: the Copenhagen City Heart Study. Eur J Prev Cardiol. 2012;19:102–108. doi: 10.1177/1741826710394274. [DOI] [PubMed] [Google Scholar]
- 43.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 44.Legeai C, Jouven X, Tafflet M, Dartigues JF, Helmer C, Ritchie K, et al. Resting heart rate, mortality and future coronary heart disease in the elderly: the 3C Study. Eur J Cardiovasc Prev Rehabil. 2011;18:488–497. doi: 10.1177/1741826710389365. [DOI] [PubMed] [Google Scholar]
- 45.Reil JC, Custodis F, Swedberg K, Komajda M, Borer JS, Ford I, et al. Heart rate reduction in cardiovascular disease and therapy. Clin Res Cardiol. 2011;100:11–19. doi: 10.1007/s00392-010-0207-x. [DOI] [PubMed] [Google Scholar]
- 46.Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor AE, Davies NM, Ware JJ, VanderWeele T, Smith GD, Munafo MR. Mendelian randomization in health research: using appropriate genetic variants and avoiding biased estimates. Econ Hum Biol. 2014;13:99–106. doi: 10.1016/j.ehb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glymour MM, Tchetgen Tchetgen EJ, Robins JM. Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol. 2012;175:332–339. doi: 10.1093/aje/kwr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor AE, Munafo MR. Commentary: Does mortality from smoking have implications for future Mendelian randomization studies? Int J Epidemiol. 2014;43:1483–1486. doi: 10.1093/ije/dyu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rode L, Bojesen SE, Weischer M, Nordestgaard BG. High tobacco consumption is causally associated with increased all-cause mortality in a general population sample of 55,568 individuals, but not with short telomeres: a Mendelian randomization study. Int J Epidemiol. 2014;43:1473–1483. doi: 10.1093/ije/dyu119. [DOI] [PubMed] [Google Scholar]
- 51.Kaur-Knudsen D, Nordestgaard BG, Bojesen SE. CHRNA3 genotype, nicotine dependence, lung function and disease in the general population. Eur Respir J. 2012;40:1538–1544. doi: 10.1183/09031936.00176811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.