Abstract
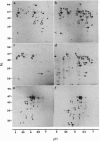
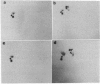
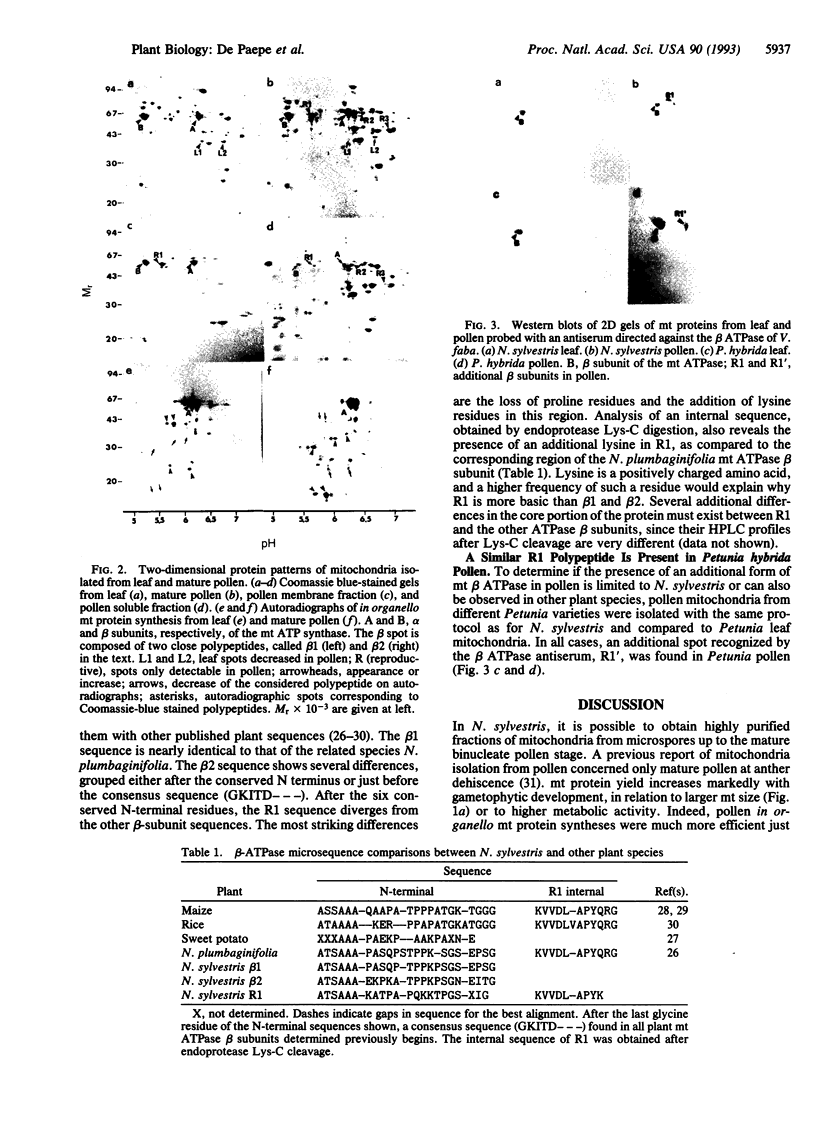
A protocol was designed to obtain a pure fraction of pollen mitochondria from the diploid species Nicotiana sylvestris, the female parent of the allotetraploid Nicotiana tabacum. Most organelles were morphologically intact and able to perform in organello mitochondrial (mt) protein synthesis. As revealed by two-dimensional protein electrophoresis, numerous quantitative differences exist between leaf and pollen mt proteins. Moreover, additional mt polypeptides, named R (for reproductive), encoded by either nuclear or mitochondrial genes, are found in pollen. The most abundant R polypeptide, R1 (M(r) 53,000, pI 5.6), is nuclearly encoded, is membrane bound, and cross-reacts with an antibody directed against the beta subunit of the mt ATP synthase (ATPase). N-terminal microsequence analysis showed that the two ATPase beta subunits present in leaves (beta 1 and beta 2) and the R1 pollen-specific subunit are encoded by distinct genes. A similar additional ATPase beta subunit was observed in pollen mitochondria from Petunia, suggesting that this polypeptide is of general importance for male gametophytic development in Solanaceaes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedinger P. The remarkable biology of pollen. Plant Cell. 1992 Aug;4(8):879–887. doi: 10.1105/tpc.4.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutry M., Briquet M., Goffeau A. The alpha subunit of a plant mitochondrial F1-ATPase is translated in mitochondria. J Biol Chem. 1983 Jul 25;258(14):8524–8526. [PubMed] [Google Scholar]
- Boutry M., Chua N. H. A nuclear gene encoding the beta subunit of the mitochondrial ATP synthase in Nicotiana plumbaginifolia. EMBO J. 1985 Sep;4(9):2159–2165. doi: 10.1002/j.1460-2075.1985.tb03910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe R., Chétrit P., Vitart V., Ambard-Bretteville F., Prat D., Vedel F. Several nuclear genes control both male sterility and mitochondrial protein synthesis in Nicotiana sylvestris protoclones. Mol Gen Genet. 1990 Jul;222(2-3):206–210. doi: 10.1007/BF00633819. [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H., Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5374–5378. doi: 10.1073/pnas.84.15.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenshaft M., Brambl R. Respiration and mitochondrial biogenesis in germinating embryos of maize. Plant Physiol. 1990 May;93(1):295–304. doi: 10.1104/pp.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Suzuki K. Production and separation of peptides from proteins stained with Coomassie brilliant blue R-250 after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1990 Dec;191(2):332–336. doi: 10.1016/0003-2697(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Lomax M. I., Grossman L. I. Tissue-specific genes for respiratory proteins. Trends Biochem Sci. 1989 Dec;14(12):501–503. doi: 10.1016/0968-0004(89)90185-0. [DOI] [PubMed] [Google Scholar]
- Monéger F., Mandaron P., Niogret M. F., Freyssinet G., Mache R. Expression of Chloroplast and Mitochondrial Genes during Microsporogenesis in Maize. Plant Physiol. 1992 Jun;99(2):396–400. doi: 10.1104/pp.99.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K. J., Walbot V. Maize mitochondria synthesize organ-specific polypeptides. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6879–6883. doi: 10.1073/pnas.82.20.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Shimada H., Fujimura T. Nucleotide sequence of a cDNA encoding a beta subunit of the mitochondrial ATPase from rice (Oryza sativa). Plant Mol Biol. 1992 Oct;20(1):171–174. doi: 10.1007/BF00029164. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Deno H., Kato A., Sugiura M. Overlap and cotranscription of the genes for the beta and epsilon subunits of tobacco chloroplast ATPase. Gene. 1983 Oct;24(2-3):147–155. doi: 10.1016/0378-1119(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Sommarin M., Petit P. X., Møller I. M. Endogenous protein phosphorylation in purified plant mitochondria. Biochim Biophys Acta. 1990 Apr 9;1052(1):195–203. doi: 10.1016/0167-4889(90)90076-p. [DOI] [PubMed] [Google Scholar]
- Stinson J. R., Eisenberg A. J., Willing R. P., Pe M. E., Hanson D. D., Mascarenhas J. P. Genes expressed in the male gametophyte of flowering plants and their isolation. Plant Physiol. 1987 Feb;83(2):442–447. doi: 10.1104/pp.83.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff A., Myers A. M. Genetics of mitochondrial biogenesis. Annu Rev Biochem. 1986;55:249–285. doi: 10.1146/annurev.bi.55.070186.001341. [DOI] [PubMed] [Google Scholar]
- Winning B. M., Bathgate B., Purdue P. E., Leaver C. J. Nucleotide sequence of a cDNA encoding the beta subunit of the mitochondrial ATP synthase from Zea mays. Nucleic Acids Res. 1990 Oct 11;18(19):5885–5885. doi: 10.1093/nar/18.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. G., Hanson M. R. A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell. 1987 Jul 3;50(1):41–49. doi: 10.1016/0092-8674(87)90660-x. [DOI] [PubMed] [Google Scholar]
- des Francs-Small C. C., Ambard-Bretteville F., Darpas A., Sallantin M., Huet J. C., Pernollet J. C., Rémy R. Variation of the polypeptide composition of mitochondria isolated from different potato tissues. Plant Physiol. 1992 Jan;98(1):273–278. doi: 10.1104/pp.98.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]