Abstract
This review discusses the immunological roles of 5 major mushrooms in oncology: Agaricus blazei, Cordyceps sinensis, Grifola frondosa, Ganoderma lucidum, and Trametes versicolor. These mushrooms were selected based on the body of research performed on mushroom immunology in an oncology model. First, this article focuses on how mushrooms modify cytokines within specific cancer models and on how those cytokines affect the disease process. Second, this article examines the direct effect of mushrooms on cancer. Finally, this article presents an analysis of how mushrooms interact with chemotherapeutic agents, including their effects on its efficacy and on the myelosuppression that results from it. For these 5 mushrooms, an abundance of in vitro evidence exists that elucidates the anticancer immunological mechanisms. Preliminary research in humans is also available and is promising for treatment.
Medicinal mushrooms have been proposed as a novel therapy that may improve cancer treatment and patients’ survival. They have been used medicinally since at least 3000 bce. Mushrooms are reported to have antimicrobial, anti-inflammatory, cardiovascular-protective, antidiabetic, hepatoprotective, and anticancer properties. It is well-established that mushrooms are adept at immune modulation and affect hematopoietic stem cells, lymphocytes, macrophages, T cells, dendritic cells (DCs), and natural killer (NK) cells.1 Extensive research over the last 40 years has demonstrated that mushrooms have potent antineoplastic properties that slow growth of tumors, regulate tumor genes, decrease tumoral angioneogenesis, and increase malignant-cell phagocytosis. Additionally, evidence suggests that medicinal mushrooms may safely boost chemotherapeutic efficacy and simultaneously protect against bone marrow suppression.
Mushrooms represent a unique branch of botanical medicine and are classified in the kingdom of Fungi. They reproduce as spores. The fungal body can be a single cell or a structure called a hypha or mycelial threads. The fruiting body grows off the hyphae and produces spores for reproduction (Figure 1). The common and scientific names of the mushrooms discussed in this article are found in Table 1. The 5 mushrooms explored in this paper have many active constituents including, but not limited to, polysaccharides, polysaccharide peptides, proteins, terpenoids, and nucleotides (Table 1). Many of the compounds studied have yet to be named and are often referred to by gel chromatography fraction when they are studied. The most common medicinally active ingredient among mushrooms is β-glucan.
Figure 1.
Mushroom Anatomy
Table 1.
Scientific and Common Names of Mushrooms and Their Major Constituents
| Scientific Name | Common Name | Specific Constituent | Type of Constituent |
|---|---|---|---|
|
| |||
| Agaricus blazei | Agaricus | β-d-glucan | Polysaccharide |
|
| |||
| Ganoderma lucidum | Reishi, lingzhi | Ganoderic acid | Protein |
| Danoderiol | Protein | ||
| Danderenic acid | Protein | ||
| Lucidenic acid | Protein | ||
| GLPS | Polysaccharide | ||
|
| |||
| Cordyceps sinesis | Cordyceps, caterpillar mushroom | Adenosine | Nucleotide |
| Cordycepin | Nucleotide | ||
|
| |||
| Trametes versicolor (formerly Coriolus versicolor) | Turkey tail | PSP | Polysaccharide peptide |
| PSK | Polysaccharide peptide | ||
|
| |||
| Grifolia frondosa | Maitake | Grifolan | Polysaccharide |
| D-fraction | |||
| MD-fraction | Polysaccharide | ||
Abbreviations: GLPS = Ganoderma lucidum polysaccharide; PSP = polysaccharide peptide; PSK = polysaccharide K.
Cancer Immunology
One of the myriad effects of mushrooms occurs through their ability to stimulate cytokine production. Cytokines are small, soluble proteins that act as intracellular mediators in an immune response. In the effort to understand cytokine responses and the interrelationships between cytokines, one approach has been to characterize a certain set of cytokines for responses to different situations. The cytokines involved in different types of responses are defined as cytokine patterns. Patterns of importance in cancer research include TH1, TH2, TH3/T regulatory (Treg) cells, and the proinflammatory pathways. Each of these defined patterns can have a different physiological effect in a cancer patient (Table 2). Cytokines are cross-regulatory, and the expression of one pattern of cytokines can modulate other cytokine patterns. To evaluate the role of cytokines in disease, it is necessary to evaluate several cytokines from each pathway because the overall pattern may have a larger impact on the body than any individual cytokine.
Table 2.
Basic Cytokine Patterns
| Pattern | Cytokines | Pattern Effect |
|---|---|---|
| TH1 | IFN-γ, IL-12, TNF-α | Stimulates immune response to cancer |
| TH2 | IL-4, IL-5, IL-13 | Decreases TH1 |
| TH3/Treg | TGF-β | Modulates TH1 |
| Proinflammatory | IL-1, IL-6, IL-8, TNF-α | Causes inflammation |
The cytokine pattern associated with a beneficial immune response to cancer is TH1. The dominant TH1 cytokine is IFN-γ, which is responsible for stimulating the cellular immune response. Cellular immunity is important in an antitumor response since NK and CD8+ T cells, as well as tumoricidal macrophages, can destroy tumor cells. In addition, a number of cellular functions, such as presentation of tumor-specific antigens and production of tumoricidal cytokines, are increased by IFN-γ. Thus, therapies, including use of mushrooms that increase IFN-γ and drive a TH1 response, are beneficial for cancer patients.2
In contrast to a TH1 response, a TH2 response is not typically associated with an immune response to cancer. TH2 responses are associated with allergies and asthma and involve the cytokines IL-4, IL-5, IL-13, and sometimes IL-10. Most important, IL-4 and IFN-γ cross-regulate each other. IFN-γ decreases production of IL-4, and IL-4 decreases production of IFN-γ. Thus, a TH2 response can be detrimental to cancer patients because it decreases IFN-γ and decreases the cellular immune response to cancer.
Regulation of the T-cell response is accomplished by Treg cells, also called TH3 or Treg cells. While many categories of Treg cells exist, most Tregs produce TGF-β (transforming growth factor β). This cytokine was discovered through its ability to increase the growth of tumor cells, mediated by decreasing the TH1 response. TGF-β can also decrease TH2 responses. Because it can decrease both TH1 and TH2, TGF-β is most commonly associated with tolerance and is found in high levels in the intestine and lungs, where large doses of innocuous antigens are frequently introduced. While it is beneficial to have a Treg response to self-antigens, Treg responses do not lead to cancer clearance.2
When associated with cancer, proinflammatory cytokines can contribute to inflammatory symptoms. These cytokines are released early in the immune response to infectious agents and are responsible for driving fever and stimulating the innate immune system. Many symptoms related to sickness—malaise, anxiety, and hostility, which are observed during infection are a result of these cytokines.3–10 For example, radiotherapy increases IL-1, IL-6, and TNF-α.11,12 A recent quantitative review of 1037 patients with cancer-related fatigue that partially resulted from radiotherapy demonstrated that IL-6 and IL-1RA were associated with fatigue; however, IL-1β and TNF-α were not linked to fatigue.13
In summary, when considering immunomodulatory effects of mushrooms, those that stimulate TH1 responses may be beneficial in cancer treatment, as are those that decrease TH2 and Treg responses. Mushrooms that decrease inflammation may have the added benefit of decreasing fatigue, anxiety, and other symptoms by decreasing inflammatory cytokines.
Immunomodulatory Effects of Mushrooms
Many studies have been conducted to elucidate the antitumor mechanisms of mushrooms. Rather than providing a summarization for each study in the text, this article provides Table 3, which summarizes cytokine modulation and the resulting pattern produced from Agaricus, maitake, reishi, Cordyceps, and turkey tail mushrooms. By the way they list each study, Table 3 and subsequent tables are organized such that human studies and in vivo studies are prioritized over in vitro and/or animal studies. Overall, the studies show a trend that indicates that each of these mushroom species increases TH1 cytokine production in both in vitro and in vivo models. At this stage of the immunological research, a notable lack of randomized, placebo-controlled trials is evident. Another important difficulty with the data lies in the delivery methods and types of mushroom extract used. Animal studies often, although not exclusively, use an intraperitoneal (IP) injection of the purified mushroom extract. The pharmacodynamics of IP injection versus oral ingestion of mushrooms is not well researched and, thus, it is difficult to translate dosage and form into human studies.
Table 3.
Cytokine Modulation
| Mushroom | In Vivo/Vitro/Model | Cytokine | Pattern | Dose/Preparation | Reference |
|---|---|---|---|---|---|
| Agaricus | In vivo, mouse cancer |
 IFN-γ IFN-γ |
 TH1 TH1 |
350 mg PO QD; hot water extract | Takimoto et al, 200815 |
| Agaricus | In vivo, mouse leukemia |
 IFN-γ IFN-γ IL-6 IL-6 IL-1β IL-1β IL-4 IL-4 |
 TH1 TH1 PI PI |
3 or 6 mg/kg PO × 3 wk; hot water extract | Lin, Fan, and Tang, 201216 |
| Reishi | In vivo, advanced human lung cancer, prospective nonplacebo controlled trial with 36 participants |
 IL-2 56% IL-2 56% IL-6 56% IL-6 56% IFN-γ 56% IFN-γ 56% IL-1 56.7% IL-1 56.7% TNF-α 66.6% TNF-α 66.6% |
 TH1 TH1 PI PI |
5.4 g/d PO; Ganopoly × 12 wk; hot water extraction, then 75% ethanol extraction, then purified by gel filtration | Gao et al, 200517 |
| Reishi | In vivo, human late-stage cancer, prospective nonplacebo controlled trial with 34 participants |
 IL-2 IL-2 IL-6 IL-6 IFN-γ IFN-γ IL-1 IL-1 TNF-α TNF-α |
 TH1 TH1 PI PI |
1800 mg Ganopoly PO TID × 3 mo; hot water extraction, then 75% ethanol extraction, then purified by gel filtration | Gao et al, 200318 |
| Reishi | In vivo, mouse CT26 cancer |
 NF-κB NF-κB TNF-α TNF-α IL-1β IL-1β |
 TH1 TH1 PI PI |
50, 100, 200 mg/kg IP; standardized PSG-1 polysaccharide, compared to 5-fluorouracil or normal saline | Zhang et al, 201319 |
| Reishi | In vivo, mouse lung cancer |
 IL-2 IL-2 IFN-γ IFN-γ NF-κB NF-κB |
 TH1 TH1 |
28 mg/kg IP; ganoderic acid-Me purified from Ganoderma lucidum | Wang et al, 200720 |
| Reishi | In vivo, mouse sarcoma 180 |
 IFN-γ IFN-γ TNF-α TNF-α |
 TH1 TH1 PI PI |
50, 100, 200 mg/kg IP; Ganoderma polysaccharides | Wang et al, 201221 |
| Reishi | Ex vivo, S-180 sarcoma mouse model |
 IFN-γ IFN-γ IL-4 IL-4 IL-6 IL-6 |
 TH1 TH1 |
200 mg/kg IP/d; sporoderms and stipe broken extracts | Yue et al, 200822 |
| Reishi | In vitro, mouse cancer cell line |
 IL-6 IL-6 TNF-α TNF-α |
 PI PI |
50, 100, 200 mg/mL; broken spores dissolved in water, then extracted with ethanol | Guo et al, 200923 |
| Reishi | In vitro, precancerous uroepithelial cells (HUC-PC cell line) |
 IL-2 IL-2 IL-6 IL-6 NF-κB NF-κB IL-8 IL-8 |
 PI PI |
40, 80, 100 mg/mL; ethanol extraction only | Yuen, Gohel, and Ng, 201124 |
| Reishi | In vitro, inflammatory breast cancer cell line |
 IL-8 IL-8 |
 PI PI |
0.5, 1.0 mg/mL every 48 h for 96 h; extract of fruiting body and cracked spores | Martinez-Montemayor et al, 201125 |
| Maitake | Ex vivo, human breast cancer participants posttreatment |
 IFN-γ IFN-γ IL-10 IL-10 TNF-α TNF-α |
 TH1 TH1 |
Dose escalation up to 5 mg/kg PO BID for 21 d; hot water extraction followed by alcohol precipitation, packaged by Gaia Herbs | Deng et al, 200926 |
| Maitake | Ex vivo, mouse colon cancer model |
 IFN-γ IFN-γ IL-12p70 IL-12p70 |
 TH1 TH1 |
7.8 mg/kg/d IP for 19 d; D-fraction of dried maitake | Kodama et al, 200227 |
| Maitake | In vivo, mouse cancer cisplatin treatment |
 IL-12p70 IL-12p70 IL-12p40 IL-12p40 IFN-γ IFN-γ G-CSF G-CSF M-CSF M-CSF |
 TH1 TH1 |
8 mg/kg/d IP; water extraction followed by alcohol precipitation, MD-fraction | Masuda et al, 200928 |
| Maitake | In vivo, mouse colon-cancer model |
 IL-12 IL-12 |
 TH1 TH1 |
8 mg/kg/d IP; water extraction followed by alcohol precipitation, MD-fraction | Masuda et al, 200829 |
| Maitake | In vivo, mouse carcinoma model |
 TNF-α TNF-α IFN-γ IFN-γ IL-12 IL-12 |
 TH1 TH1 |
5 mg/kg/d PO for 19 d; water extraction followed by alcohol precipitation, D-fraction | Kodama et al, 200230 |
| Maitake | In vivo, mouse carcinoma model |
 IL-4 IL-4 IFN-γ IFN-γ IL-12p70 IL-12p70 IL-18 IL-18 |
 TH1 TH1 TH2 TH2 |
5 mg/kg/d PO QD for 20 d; D-fraction | Inoue, Kodama, and Nanba, 200231 |
| Maitake | In vivo, mouse colon-cancer model |
 TNF-α TNF-α IFN-γ IFN-γ IL-12 IL-12 IL-1 IL-1 |
 TH1 TH1 |
7.5, 15.0 mg IP QD for 7 d; hot water extract with an ethanol precipitation, followed by complex gel column fractionations for MLP fraction | Kodama et al, 201032 |
| Maitake | In vivo, mouse colon-cancer model |
 IFN-γ IFN-γ IL-12p70 IL-12p70 |
 TH1 TH1 |
7.8 mg/kg/d IP; hot water extract with an the ethanol precipitation for D fraction | Harada, Kodama, and Nanba, 200333 |
| Maitake | In vitro, human mononuclear cells |
 IFN-γ IFN-γ TNF-α TNF-α |
 TH1 TH1 |
12.5, 11, and 200 mg/mL; intracellular fractions of fruiting body | Svagelj et al, 200834 |
| Cordyceps | In vitro, mouse lymphoma cell line |
 IL-1 IL-1 IL-2 IL-2 |
 PI PI |
200 mg/mL Cordyceps sinensis or 100 mg/mL 1,3-β-glucan | Kawanishi et al, 201035 |
| Turkey Tail | In vivo, TLR2 knockout Mice vs normal mice |
 IL-12 only in normal mice IL-12 only in normal mice |
 TH1 TH1 |
1–100 mg/mL × 96 h; purified PSK | Lu et al, 201136 |
| Turkey Tail | In vitro, breast cancer cell line |
 TNF-α TNF-α IFN-γ IFN-γ IL-12 IL-12 |
 TH1 TH1 |
10 mg/mL; purified PSK | Lu et al, 201137 |
| Turkey Tail | In vitro, TLR2 knockout mice vs normal mice |
 IFN-γ IFN-γ IL-12p70 IL-12p70 TNF-α TNF-α IL-12p40 IL-12p40 IL-2 all inhibited by TLR2 knockout IL-2 all inhibited by TLR2 knockout |
 TH1 TH1 |
1–100 mg/mL × 96 h; purified PSK | Lu et al, 201136 |
Abbreviations: PO=by mouth; QD=every day; TID=3×/d; IP=intraperitoneal; PSG-1=Ganoderma atrum polysaccharide; BID=2×/d; PSK=polysaccharide K.
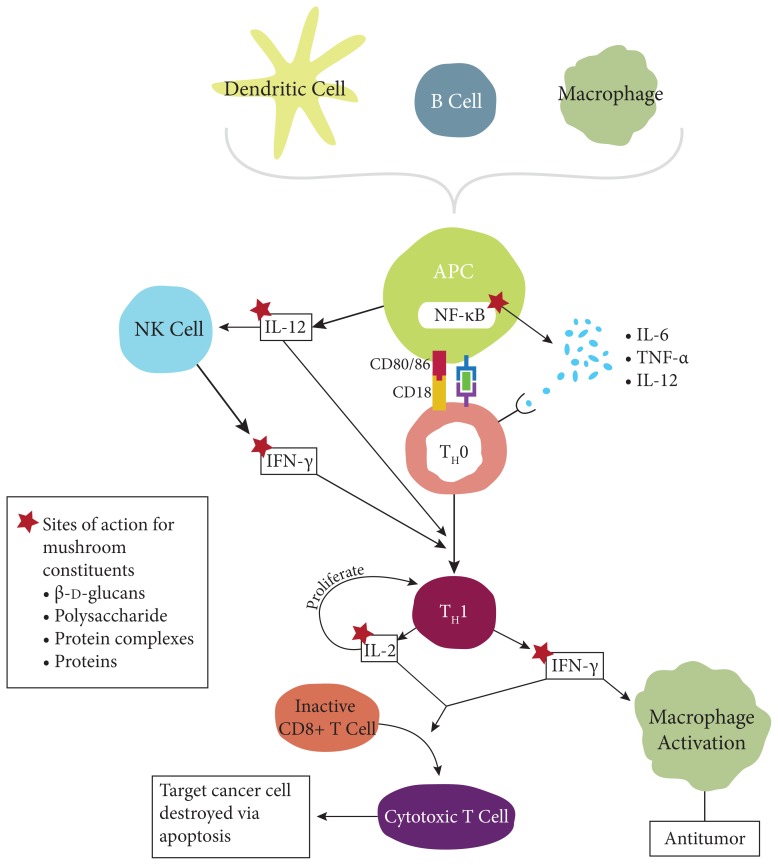
Modulation of non-TH1 cytokines is not as clear-cut. For example, TNF-α is often elevated within in vitro studies, but when it is measured in vivo, it decreases. This result is difficult to interpret and exemplifies the fact that researchers cannot simply study a substance’s immunological activity outside of the organism. The Agaricus, maitake, reishi, Cordyceps, and turkey tail mushrooms often downregulate TH2 cytokines, which again suggests a benefit in treating cancer. Figure 2 illustrates potential sites of action for constituents of mushrooms that impact immunological pathways in a cancer model.
Figure 2.
Potential Sites of Action
Cellular immunity stimulated through TH1 responses can be measured in a variety of ways. In addition to examining cytokine patterns, some mushroom studies have examined cellular immunity directly by assessing NK cell and macrophage activity. Increased NK cell killing and phagocytosis can lead to increased tumor destruction. An indirect method of evaluating cellular stimulation is to look at markers of cellular activation. For example, when NK cells are activated, they increase the amount of CD56 and CD69 on their surface. Therefore, increased CD56 and CD69 indicate a beneficial response to cancer. Increasing CD3 suggests an increase of T-cell activity, whereas increasing CD19 is indicative of increasing B cells. The MMP-9 marker is elevated in many cancers and is related to poor prognosis. Thus, mushrooms that downregulate MMP-9 expression would be expected to be beneficial to patients with cancer. Table 4 shows findings from studies using evaluations of cell surface biomarkers and cellular activity to determine how mushrooms activate different cell types.
Table 4.
Cellular Immune Response to Cancer
| Mushroom | Model | Cellular Response | Dose | Reference |
|---|---|---|---|---|
| Agaricus | In vivo, mouse cancer |
 CD69 and CD49 T cells CD69 and CD49 T cells |
350 mg QD; hot water, standardized to 7.2 μg/mL of β-glucan | Takimoto et al, 200815 |
| Agaricus | In vivo, mouse colon cancer |
 Phagocytosis of spleen cells Phagocytosis of spleen cells |
100–150 g/d PO; 10% ground, dried mushroom | Ishii et al, 201138 |
| Agaricus | In vivo, mouse leukemia |
 CD3 CD3 CD19 CD19 CD11b CD11b Liver weight Liver weight Spleen weight Spleen weight NK activity NK activity |
3 or 6 mg/kg PO × 3 wk; hot water extract | Lin et al, 201216 |
| Reishi | In vivo, human, late-stage cancer; prospective, nonplacebo-controlled trial with 34 participants |
 CD56 CD56 NK cells NK cells |
1800 mg PO TID × 3 mo; Ganopoly, hot water extraction, then 75% ethanol extraction, then purified by gel filtration | Gao et al, 200318 |
| Reishi | In vivo, mouse cancer cell line CT26 |
 Phagocytosis via TLR4 Phagocytosis via TLR4 |
50, 100, 200 mg/kg IP; standardized PSG-1 polysaccharide | Zhang et al, 201319 |
| Reishi | In vivo, mouse lung cancer |
 NK cell activity NK cell activity |
50, 100, 200 mg/kg IP × 10 d; kg of ganoderic acid-Me, purified from Ganoderma lucidum | Wang et al, 200720 |
| Reishi | In vivo, mouse sarcoma 180 |
 NK NK Spleen lymphocytes Spleen lymphocytes CD8+ T cells CD8+ T cells CD4+ T Cells CD4+ T Cells |
50, 100, 200 mg/kg IP; Ganoderma polysaccharides | Wang et al, 201221 |
| Reishi | In vivo, mouse leukemia |
 CD3 CD3 CD19 CD19 CD11b CD11b |
3 mg/kg/d or 6 mg/kg/d IP; crude extract | Chang, Yang, and Yang, 200939 |
| Reishi | In vitro, human colon-cancer line HCT-116 |
 Cell growth Cell growth Cell adhesion Cell adhesion MMP-9 MMP-9 NF-κB NF-κB iNOS iNOS |
Varied dose; ganoderic acid | Chen et al, 201040 |
| Reishi | In vitro, human hepatoma HepG2 cell line |
 MMP-9 MMP-9 NF-κB NF-κB ERK ERK |
10, 25, 50, 75, 100 mM; purified lucidenic acid | Weng, Chau, Hsieh, 200841 |
| Reishi | In vitro, MAD-MB-231 human breast cancer cell line |
 Akt Akt NF-κB NF-κB |
0.25, 0.5, 1 mg/mL; standardized powdered extract (20:1) with spores to 13.5% polysaccharides and 6% triterpenes | Jiang et al, 200442 |
| Reishi | In vitro, human prostate cancer cell line |
 VEGF VEGF TGF-β1 TGF-β1 |
0.25, 0.5 or 1.0 mg/mL × 24 h; ReishiMax brand | Stanley et al, 200543 |
| Reishi | In vitro, MAD-MB-231 human breast cancer cell line |
 AP-1 AP-1 NF-κB NF-κB CDK4 CDK4 uPA uPA |
0.1, 0.25, 0.5 mM; purified ganoderic acid A, F, and H | Jiang et al, 200844 |
| Reishi | In vitro, human inflammatory breast cancer line |
 MMP-9 MMP-9 |
0.5, 1.0 mg/mL every 48 h of 96 h; extract of fruiting body and cracked spores | Martinez-Montemayor et al, 201125 |
| Maitake | In vivo, mouse colon cancer |
 Tumor-specific CD8+ and CD4+ T cells Tumor-specific CD8+ and CD4+ T cells NK cells NK cells T-cell infiltration T-cell infiltration Treg cells Treg cells |
20 or 80 mg/kg PO for 20 d; MD-fraction | Masuda et al, 201345 |
| Maitake | In vivo, BALB/c mice implanted with colon 26 carcinoma cells |
 CD8+ and CD4+ T cells CD8+ and CD4+ T cells |
7.8 mg/kg/d IP; D-fraction | Harada et al, 200333 |
| Cordyceps | In vitro, human bladder cancer cell lines 5637 and T-24 |
 MMP-9 MMP-9 NF-κB NF-κB |
50, 100, 200 μg/mL; cordycepin | Lee, Kim, and Moon, 201046 |
| Turkey Tail | In vivo, human breast cancer, phase I clinical trial |
 Lymphocyte count Lymphocyte count NK activity NK activity CD8+ T cells CD8+ T cells CD19+ B cells CD19+ B cells |
6 or 9 g PO daily for 6 wk | Torkelson et al, 201247 |
Abbreviations: QD = every day; PO = by mouth; TID = 3 ×/d; PSG-1 = Ganoderma atrum polysaccharide; IP = intraperitoneal.
Mushrooms can affect cancer through immunomodulation resulting in tumor destruction or can have an effect on the tumor directly. Studies that measure direct tumor markers may be indirectly measuring the end result of immunomodulation or directly measuring other factors, such as cell cycle arrest influenced by mushrooms. In Table 5, the effects of mushrooms on tumor volume, angiogenesis, apoptosis, and survival are presented. Of particular note, a derivative of turkey tail mushroom, polysaccharide K (PSK), when administered to stage II/III colorectal patients, was found to be effective.14 PSK was given at 3 g/d for 2 years in conjunction with standard therapy and survival was assessed. The researchers found that the control group had a 60% survival rate compared to 86.8% in the PSK treatment group, a finding that was statistically significant.
Table 5.
Markers of Immune Cell Activation
| Mushroom | In vivo/vitro/model | Measure of Immune Activation | Dose | Reference |
|---|---|---|---|---|
| Agaricus | In vitro, human hepatocarcinoma cell line |
 % apoptotic cells % apoptotic cells Cell growth inhibited Cell growth inhibited Intracellular accumulation of doxorubicin Intracellular accumulation of doxorubicin |
5–100 μg/mL dose-dependent response; Agaricus hot-water extraction with ethanol precipitations and gel chromatography fractionation | Lee and Hong, 201148 |
| Agaricus | In vitro, osteosarcoma cell line |
 Cell growth Cell growth |
100, 200, 400 μg; purified polysaccharide | Wu et al, 201249 |
| Reishi | In vivo, mouse sarcoma 180 |
 Cell proliferation Cell proliferation |
50, 100, 200 μg/kg IP; Ganoderma polysaccharides | Wang et al, 201221 |
| Reishi | In vivo, mouse lung cancer model |
 Splenocyte proliferation Splenocyte proliferation Tumor size Tumor size Tumor growth Tumor growth Tumor metastasis Tumor metastasis |
50, 100, 200 mg/kg IP × 10 d; ganoderic acid-Me purified from Ganoderma lucidum | Wang et al, 200720 |
| Reishi | In vivo, mouse cancer cell line CT26 |
 Tumor growth Tumor growth |
50, 100, 200 mg/kg IP; PSG-1 polysaccharide | Zhang et al, 201319 |
| Reishi | In vivo, Lewis lung carcinoma model in mice |
 Tumor growth Tumor growth |
28 mg/kg IP QD × 7 d; ganoderic acid | Chen et al, 201050 |
| Reishi | In vivo, mouse leukemia model |
 Phagocytosis from PBMC Phagocytosis from PBMC |
3 mg/kg/d or 6 mg/kg/d; crude extract | Chang, Yang, and Yang, 200939 |
| Reishi | In vivo, S-180 sarcoma mouse model |
 Sarcoma size Sarcoma size |
100, 200, 400 mg/kg IP; hot water extraction of fruiting body, stipe, and sporoderm broken spores | Yue et al, 200822 |
| Reishi | Ehrlich’s ascites carcinoma in mice |
 Tumor volume by 80.8% Tumor volume by 80.8% |
100 mg/kg administered IP 24 h after tumor induction | Joseph et al, 201151 |
| Reishi | In vitro, human prostate-cancer cell line |
 Angiogenesis Angiogenesis |
0.25, 0.5 or 1.0 mg/mL × 24 h; ReishiMax proprietary extract | Stanley et al, 200543 |
| Reishi | In vitro, human breast-cancer cell line MDA-MB-231 |
 Cell proliferation Cell proliferation |
0.1, 0.25, 0.5 mM; purified ganoderic acid A, F, and H | Jiang et al, 200844 |
| Reishi | In vitro, human MAD-MB-231 breast cancer cells |
 Cell proliferation; complete inhibition at highest dosage Cell proliferation; complete inhibition at highest dosage |
0.25, 0.5, 1.0 mg/mL; standardized powdered extract (20:1) with spores to 13.5% polysaccharides and 6% triterpenes | Jiang et al, 200442 |
| Reishi | In vitro, human inflammatory breast-cancer line |
 Cell viability Cell viability Apoptosis Apoptosis BCL-2 BCL-2 TERT TERT PDGFB PDGFB |
0.5, 1.0 mg/mL every 48 h for 96 h; extract of fruiting body and cracked spores | Martinez-Montemayor et al, 201125 |
| Reishi | In vitro, human colon-cancer cell line |
 Cell growth Cell growth Cell adhesion Cell adhesion |
Varied doses; purified ganoderic acid | Chen et al, 201040 |
| Maitake | In vivo, carcinoma-bearing BALB/c mice |
 Tumor volume Tumor volume |
7.8 mg/kg/d IP for 19 d; D-fraction | Kodama et al, 200226 |
| Maitake | In vivo, colon cancer mouse model |
 Tumor size Tumor size |
20 or 80 mg/kg PO for 20 d; MD-fraction | Masuda et al, 201345 |
| Maitake | In vivo, male C3H/ HeN mice bearing MM-46 carcinoma |
 Tumor size Tumor size |
5mg/kg/d PO QD for 20 d; D-fraction | Inoue, Kodama and Nanba, 200231 |
| Maitake | In vitro, human prostate cancer cell PC-3 |
 Cell growth 65% Cell growth 65% |
50,000 IU/mL; D-fraction | Pyo et al, 200852 |
| Turkey Tail | In vivo/human stage II or III colorectal cancer |
 5-y survival (60% control; 86.7% PSK treatment group) 5-y survival (60% control; 86.7% PSK treatment group) |
3g/d PO × 2 y; PSK | Ohwada et al, 200614 |
Abbreviations: IP=intaperitoneal; PSG-1=Ganoderma atrum polysaccharide; QD=every day; PBMC=peripheral blood mononuclear cell; BCL-2=B cell lymphoma 2; TERT=telomerase reverse transcription factor; PDGFB=platelet-derived growth factor-B polypeptide; PO=by mouth; PSK=polysaccharide K.
Few studies examining immunological outcomes have been conducted within the clinical trial framework. That framework is the key to moving the knowledge of mushroom immunology out of the lab and animal models and into both physically well and diseased human populations. A recent phase 1, dose-escalation, clinical trial of turkey tail evaluated dosing safety and immune function in women with breast cancer.47 Turkey tail extract was well-tolerated and was immunomodulatory at higher doses (6 g or 9 g) by increasing CD8+ T cells and CD19+ B cells. The researchers also found that the radiation-induced decline in NK cells was improved by a 6-gram dosing per day of turkey tail.
Agaricus has also been tested by Ohno et al in a phase I clinical study of safety with participants in cancer remission.53 At all doses—1.8, 3.6, and 5.4 g/d for 6 months, Agaricus was well-tolerated, with a 12% rate of adverse events that were digestive in nature, such as nausea. While Agaricus was deemed safe, the study did not follow immune outcomes for the enrolled patients.
Gao et al studied the use of reishi polysaccharides in late-stage cancer patients and late-stage, lung cancer patients.17,18 In participants with late-state lung cancer treated with 5.4 g/d of a proprietary reishi extract (Ganopoly), IL-2, IL-6, and IFN-γ increased. Great variability in patients’ responses occurred, with some participants having a very significant increase while others had minimal changes. This finding suggests that subgroups of patients may respond more favorably to reishi, although the mechanisms of such a difference have not been studied at this time. When Ganopoly was studied in late-stage cancer patients, it was found that a dose of 5.4 g/d increased IL-2, IL-6, and IFN-γ and decreased TNF-α and IL-1. This dosage also increased NK cells (CD56+ cells) and NK activity.
The immune-stimulating impact that mushrooms can exert on NK cells, macrophages, and T cells can also provide a protective effect against chemotherapeutic myelosuppression, one of the most serious deleterious effects of chemotherapy. Because severe myelosuppression neutropenia often truncates treatment and requires hospitalization before full therapeutic effects can be achieved, reducing myelosuppression would allow for better response to chemotherapy.54,55 One promising study examined the effect of the MD-fraction from the maitake mushroom on cisplatin-induced myelosuppression in a mouse model. Mice given 8 mg/kg/d while treated with cisplatin did not experience a decrease in NK cells, DCs, and macrophages. These mice also maintained body weight and spleen weight compared to those treated with cisplatin alone.28 Another study demonstrated that mice that had been immunosuppressed with cyclophosphamide and then subsequently treated with a water-soluble extract from reishi had an increase in red blood cells (RBCs), white blood cells (WBCs), NK T cells, splenic NK cells, and a number of bone marrow cells.56 Given the need to find treatments for this difficult side effect, human studies are needed at this time that examine whether mushrooms are protective against myelosuppression during chemotherapy.
Mushrooms With Antineoplastic Agents
In addition to treating chemotherapeutic myelosuppression, studies have shown that medicinal mushrooms can be used in conjunction with antineoplastic agents to increase the efficacy of chemotherapeutic agents and radiation, the mainstay treatments for most cancers.
Chemotherapy must penetrate the tumor and accumulate within each cell to induce cell cycle arrest and apoptosis. Each of the mushrooms discussed within this review has been shown to increase the effects of chemotherapy, usually by increasing the dose of chemotherapeutic agent that accumulates within a cell (Table 6). For example, when an Agaricus extract high in β-glucan is used in conjunction with doxorubicin, a chemotherapeutic agent, the effectiveness of the drug is increased.48 Doxorubicin combined with Agaricus is accumulated at higher doses within hepatocellular carcinoma cells and increases apoptosis compared to doxorubicin alone.
Table 6.
Mushrooms and Chemotherapeutic Agents
| Chemotherapeutic Agent | Indicated Mushroom | Reference |
|---|---|---|
| Trastudzumab | PSK (turkey tail) | Lu et al, 2011b37 |
| Cyclophosphamide | Reishi | Zhu et al, 200756 |
| Cisplatin | Maitake, Cordyceps, reishi | Masuda et al, 200928; Yao et al, 201257 |
| Docetaxel | PSK (turkey tail) | Kinoshita et al, 200958; Wenner et al, 201259 |
| Doxorubicin | Agaricus | Lee and Hong, 201148 |
Abbreviations: PSK = polysaccharide K.
Similarly, PSK extracted from turkey tail increases the efficacy of the drug docetaxel in the treatment of human gastric carcinoma. Within an in vitro and an in vivo model, Kinoshita et al found that PSK inhibited NF-κB, and survivin, an antiapoptotic molecule.58 The researchers were able to use a lower dose of the drug to induce similar levels of apoptosis. Other studies confirm this observation in a human prostate cancer model.59 Extracts from reishi in the form of ganoderic acid A were recently found to increase accumulation of the chemotherapeutic agent cisplatin inside tumor cells. Specifically, ganoderic acid A sensitized the cancer cell line HepG2 to cisplatin by suppressing Janus kinase/signal transducers and activators of transcription (JAK/STAT3), allowing cisplatin to amplify the apoptosis rate.57
Akin to the effects of reishi, cytotoxicity from cisplatin also increased significantly when Cordyceps extract was added.60 To understand the mechanism of this increased cytotoxicity, researchers can examine a study in which Cordyceps was used in an in vitro model of nonsmall-cell lung cancer (NSCLC), a treatment resistant form of cancer that accounts for 80% of that cancer. Cordyceps extract decreased vascular endothelial growth factor (VEGF) and basic fibrogrowth factor (bFGF) in vitro. Thus, Cordyceps can decrease blood supply to the cancer cell and increase the ability of cisplatin to exert cytotoxic effects.
Some anticancer therapies are dependent on NK-cell function to induce apoptosis. One such drug is trastuzumab, a HER2-targeted monoclonal antibody therapy. When PSK from turkey tail was given with trastuzumab, cell-mediated cytotoxicity was greatly increased.37 Interestingly, when PSK and trastuzumab were used alone, they had similar rates of tumor inhibition. Combined, these 2 treatments decreased cell growth in tumors by 96%.
In addition to chemotherapy, researchers are seeking to improve the deleterious side effects of radiation therapy using mushrooms. β-Glucan isolated from reishi significantly improves mouse survival postradiation. Pillai and Devi studied mouse survival, hematology, liver GSH (reduced glutathione), liver malondialdehyde (MDA) and bone marrow chromosomal aberrations in mice exposed to a 4-Gy or 8-Gy radiation dose with or without β-glucan.61 They found that β-glucan rescued 66% of mice from death, compared to 100% mortality when no radioprotective agent was used. When combined with the radioprotective drug amifostine, survival increased to 83%. They also found a significant decrease in bone marrow aberrations in mice pretreated with β-glucan.
Discussion
The evidence base for using mushrooms in cancer treatment has greatly increased in the past 5 years. Many researchers are working to purify and study individual constituents of mushrooms to understand their effects on apoptosis, cell cycle arrest, and immune modulation.62 This research is allowing researchers to move from lab bench to bedside. As this review has demonstrated, mushrooms show great promise as adjunctive treatment used in conjunction with typical care for patients with cancer, as well as treatment to stimulate the immune response to cancer. Research to date has shown a high safety profile of for mushrooms and a lack of negative interactions. As the science continues to emerge, it is likely that the efficacy and safety will justify medicinal mushrooms as an adjunct treatment. Table 7 summarizes potential clinical applications.
Table 7.
Summary of Potential Clinical Applications
| Type of Cancer | Indicated Mushroom |
|---|---|
| Nonsmall-cell lung cancer | Cordyceps |
| Lung cancer | Reishi |
| Gastric cancer | PSK (turkey tail) |
| Hepatocellular carcinoma | Agaricus, reishi |
| Leukemia | Agaricus, reishi |
| Lymphoma | Cordyceps |
| Breast cancer | Reishi, maitake, turkey tail |
| Colon cancer | Maitake, reishi, turkey tail |
| Prostate cancer | Reishi |
| Sarcoma | Reishi |
Abbreviations: PSK = polysaccharide K.
The mushrooms discussed in this review elicit effects on cytokine production. The authors know that immune stimulation during cancer can be beneficial in terms of tumor regression and patients’ survival.2 Upon diagnosis, most patients are treated with antineoplastic therapy and are immunosuppressed. Emerging evidence suggests that mushrooms may reverse myelosuppression, which makes them a promising adjunct therapy to optimize overall treatment outcomes.
Anytime an adjunct therapy is added to a conventional therapy, drug-botanical interaction must be addressed. Interestingly, mushrooms appear to increase the effects of chemotherapy. This important finding must be considered when patients are using mushrooms for myelosuppression or other symptoms.
While the immunological findings are promising, ultimately this information must be applied to patients and clinical outcomes, as the goal when working with any patient with cancer is to improve quality of life and ultimately improve survival. To that end, the meta-analysis of turkey tail by Eliza et al demonstrated an increased rate of survival for cancer patients who took this mushroom, especially participants with breast, gastric, and colorectal cancers.63 The articles examined in this meta-analysis did not obtain immunologic outcomes and were thus not included in the current article. Similarly, a retrospective case series of patients who were treated for hepatocellular carcinoma with a combination of 11 different integrative therapies, which included Cordyceps and β-glucan from Agaricus, showed a significant correlation between the number of treatments used and survival. Patients given ≥4 agents had a survival of 40.2 vs 6.4 months for those given ≤3 agents (P < .001). Of these individuals, participants whose combination therapy included Cordyceps had the longest survival.64
Conclusions
As the treatment of various cancers continues to evolve, mushrooms should be considered as an adjunct therapy. As with any phytochemical, the dose, concentration, absorption, and extraction methods play a role in the pharmacological effects, and these factors will be important in future studies. With more research and a better understanding of how different mushrooms elicit varied effects, it will be increasingly important that integrative clinicians work with oncologists to determine the appropriate treatment for each individual. Research into underlying mechanisms of mushrooms will continue to help in devising new strategies for treating cancer, preventing its long-term complications, and increasing survival.
References
- 1.Moradali MF, Mostafavi H, Ghods S, Hedjaroude GA. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi) Int Immunopharmacol. 2007;7(6):701–724. doi: 10.1016/j.intimp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atzori M, Garcia-Oscos F, Mendez JA. Role of IL-6 in the etiology of hyperexcitable neuropsychiatric conditions: experimental evidence and therapeutic implications. Future Medicinal Chem. 2012;4(17):2177–2192. doi: 10.4155/fmc.12.156. [DOI] [PubMed] [Google Scholar]
- 4.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38(9):1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain Behav Immun. 2006;20(4):389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 7.Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. 2010;37(3):519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin Neuropharmacol. 2010;33(4):191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013 Nov;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun. 2002;16(6):675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 11.Herskind C, Bamberg M, Rodemann HP. The role of cytokines in the development of normal-tissue reactions after radiotherapy. Strahlenther Onkol. 1998;174(suppl 3):12–15. [PubMed] [Google Scholar]
- 12.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 13.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Ohwada S, Ogawa T, Makita F, et al. Beneficial effects of protein-bound polysaccharide K plus tegafur/uracil in patients with stage II or III colorectal cancer: analysis of immunological parameters. Oncol Rep. 2006;15(4):861–868. [PubMed] [Google Scholar]
- 15.Takimoto H, Kato H, Kaneko M, Kumazawa Y. Amelioration of skewed Th1/ Th2 balance in tumor-bearing and asthma-induced mice by oral administration of Agaricus blazei extracts. Immunopharmacol Immunotoxicol. 2008;30(4):747–760. doi: 10.1080/08923970802279092. [DOI] [PubMed] [Google Scholar]
- 16.Lin JG, Fan MJ, Tang NY, et al. An extract of Agaricus blazei Murill administered orally promotes immune responses in murine leukemia BALB/c mice in vivo. Integr Cancer Ther. 2012;11(1):29–36. doi: 10.1177/1534735411400314. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Tang W, Gao H, et al. Antimicrobial activity of the medicinal mushroom Ganoderma. Food Rev Int. 2005;21(2):211–229. [Google Scholar]
- 18.Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32(3):201–215. doi: 10.1081/imm-120022979. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Nie S, Huang D, Li W, Xie M. Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26 tumor-bearing mice. Food Chem. 2013;136(3–4):1213–1219. doi: 10.1016/j.foodchem.2012.08.090. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Zhao J, Liu J, Huang Y, Zhong JJ, Tang W. Enhancement of IL-2 and IFN-gamma expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo. Int Immunopharmacol. 2007;7(6):864–870. doi: 10.1016/j.intimp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang PY, Zhu XL, Lin ZB. Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum. Front Pharmacol. 2012 Jul;3:135. doi: 10.3389/fphar.2012.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue GG, Fung KP, Leung PC, Lau CB. Comparative studies on the immunomodulatory and antitumor activities of the different parts of fruiting body of Ganoderma lucidum and Ganoderma spores. Phytother Res. 2008;22(10):1282–1291. doi: 10.1002/ptr.2478. [DOI] [PubMed] [Google Scholar]
- 23.Guo L, Xie J, Ruan Y, Zhou L, Zhu H. Characterization and immunostimulatory activity of a polysaccharide from the spores of Ganoderma lucidum. Int Immunopharmacology. 2009;9(10):1175–1182. doi: 10.1016/j.intimp.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Yuen JW, Gohel MD, Ng CF. The differential immunological activities of Ganoderma lucidum on human pre-cancerous uroepithelial cells. J Ethnopharmacol. 2011;135(3):711–718. doi: 10.1016/j.jep.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Montemayor MM, Acevedo RR, Otero-Franqui E, Cubano LA, Dharmawardhane SF. Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr Cancer. 2011;63(7):1085–1094. doi: 10.1080/01635581.2011.601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng G, Lin H, Seidman A, et al. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J Cancer Res Clin Oncol. 2009;135(9):1215–1221. doi: 10.1007/s00432-009-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodama N, Harada N, Nanba H. A polysaccharide, extract from Grifola frondosa, induces Th-1 dominant responses in carcinoma-bearing BALB/c mice. Jpn J Pharmacol. 2002;90(4):357–360. doi: 10.1254/jjp.90.357. [DOI] [PubMed] [Google Scholar]
- 28.Masuda Y, Inoue M, Miyata A, Mizuno S, Nanba H. Maitake β-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int Immunopharmacol. 2009;9(5):620–626. doi: 10.1016/j.intimp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Masuda Y, Murata Y, Hayashi M, Nanba H. Inhibitory effect of MD-Fraction on tumor metastasis: involvement of NK cell activation and suppression of intercellular adhesion molecule (ICAM)-1 expression in lung vascular endothelial cells. Biol Pharm Bull. 2008;31(6):1104–1108. doi: 10.1248/bpb.31.1104. [DOI] [PubMed] [Google Scholar]
- 30.Kodama N, Komuta K, Sakai N, Nanba H. Effects of D-Fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull. 2002;25(12):1647–1650. doi: 10.1248/bpb.25.1647. [DOI] [PubMed] [Google Scholar]
- 31.Inoue A, Kodama N, Nanba H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol Pharm Bull. 2002;25(4):536–540. doi: 10.1248/bpb.25.536. [DOI] [PubMed] [Google Scholar]
- 32.Kodama N, Mizuno S, Nanba H, Saito N. Potential antitumor activity of a low-molecular-weight protein fraction from Grifola frondosa through enhancement of cytokine production. J Med Food. 2010;13(1):20–30. doi: 10.1089/jmf.2009.1029. [DOI] [PubMed] [Google Scholar]
- 33.Harada N, Kodama N, Nanba H. Relationship between dendritic cells and the D-fraction-induced Th-1 dominant response in BALB/c tumor-bearing mice. Cancer Lett. 2003;192(2):181–187. doi: 10.1016/s0304-3835(02)00716-4. [DOI] [PubMed] [Google Scholar]
- 34.Svagelj M, Berovic M, Boh B, Menard A, Simcic S, Wraber B. Solid-state cultivation of Grifola frondosa (Dicks: Fr) S.F. Gray biomass and immunostimulatory effects of fungal intra- and extracellular beta-polysaccharides. N Biotechnol. 2008;25(2–3):150–156. doi: 10.1016/j.nbt.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Kawanishi T, Ikeda-Dantsuji Y, Nagayama A. Effects of two basidiomycete species on interleukin 1 and interleukin 2 production by macrophage and T cell lines. Immunobiology. 2010;215(7):516–520. doi: 10.1016/j.imbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Lu H, Yang Y, Gad E, et al. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin Cancer Res. 2011;17(1):67–76. doi: 10.1158/1078-0432.CCR-10-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu H, Yang Y, Gad E, et al. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin Cancer Res. 2011;17(21):6742–6753. doi: 10.1158/1078-0432.CCR-11-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii PL, Prado CK, de Mauro MO, et al. Evaluation of Agaricus blazei in vivo for antigenotoxic, anticarcinogenic, phagocytic and immunomodulatory activities. Regul Toxicol Pharmacol. 2011;59(3):412–422. doi: 10.1016/j.yrtph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Chang YH, Yang JS, Yang JL, et al. Ganoderma lucidum extracts inhibited leukemia WEHI-3 cells in BALB/c mice and promoted an immune response in vivo. Biosci Biotechnol Biochem. 2009;73(12):2589–2594. doi: 10.1271/bbb.90357. [DOI] [PubMed] [Google Scholar]
- 40.Chen WY, Yang WB, Wong CH, Shih DT. Effect of Reishi polysaccharides on human stem/progenitor cells. Bioorg Med Chem. 2010;18(24):8583–8591. doi: 10.1016/j.bmc.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis. 2008;29(1):147–156. doi: 10.1093/carcin/bgm261. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J, Slivova V, Harvey K, Valachovicova T, Sliva D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/ NF-kappaB signaling. Nutr Cancer. 2004;49(2):209–216. doi: 10.1207/s15327914nc4902_13. [DOI] [PubMed] [Google Scholar]
- 43.Stanley G, Harvey K, Slivova V, Jiang J, Sliva D. Ganoderma lucidum suppresses angiogenesis through the inhibition of secretion of VEGF and TGF-beta1 from prostate cancer cells. Biochem Biophys Res Commun. 2005;330(1):46–52. doi: 10.1016/j.bbrc.2005.02.116. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Grieb B, Thyagarajan A, Sliva D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med. 2008;21(5):577–584. [PubMed] [Google Scholar]
- 45.Masuda Y, Inoue H, Ohta H, Miyake A, Konishi M, Nanba H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int J Cancer. 2013;133(1):108–119. doi: 10.1002/ijc.27999. [DOI] [PubMed] [Google Scholar]
- 46.Lee EJ, Kim WJ, Moon SK. Cordycepin suppresses TNF-alpha-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother Res. 2010;24(12):1755–1761. doi: 10.1002/ptr.3132. [DOI] [PubMed] [Google Scholar]
- 47.Torkelson CJ, Sweet E, Martzen MR, et al. Phase 1 clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncol. 2012;2012:251632. doi: 10.5402/2012/251632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JS, Hong EK. Agaricus blazei Murill enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells by NFκB-mediated increase of intracellular doxorubicin accumulation. Int J Oncol. 2011;38(2):401–408. doi: 10.3892/ijo.2010.852. [DOI] [PubMed] [Google Scholar]
- 49.Wu B, Cui J, Zhang C, Li Z. A polysaccharide from Agaricus blazei inhibits proliferation and promotes apoptosis of osteosarcoma cells. Int J Biol Macromol. 2012;50(4):1116–1120. doi: 10.1016/j.ijbiomac.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Chen NH, Liu JW, Zhong JJ. Ganoderic acid T inhibits tumor invasion in vitro and in vivo through inhibition of MMP expression. Pharmacol Rep. 2010;62(1):150–163. doi: 10.1016/s1734-1140(10)70252-8. [DOI] [PubMed] [Google Scholar]
- 51.Joseph S, Sabulal B, George V, Antony KR, Janardhanan KK. Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum. Acta Pharm. 2011;61(3):335–342. doi: 10.2478/v10007-011-0030-6. [DOI] [PubMed] [Google Scholar]
- 52.Pyo P, Louie B, Rajamahanty S, Choudhury M, Konno S. Possible immunotherapeutic potentiation with D-fraction in prostate cancer cells. J Hematol Oncol. 2008 Dec;1:25. doi: 10.1186/1756-8722-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohno S, Sumiyoshi Y, Hashine K, Shirato A, Kyo S, Inoue M. Phase I clinical study of the dietary supplement, Agaricus blazei Murill, in cancer patients in remission. Evid Based Complement Alternat Med. 2011;2011:192381. doi: 10.1155/2011/192381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103(9):1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 55.Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116(23):5555–5563. doi: 10.1002/cncr.25332. [DOI] [PubMed] [Google Scholar]
- 56.Zhu XL, Chen AF, Lin ZB. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J Ethnopharmacol. 2007;111(2):219–226. doi: 10.1016/j.jep.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Yao X, Li G, Xu H, Lü C. Inhibition of the JAK-STAT3 signaling pathway by ganoderic acid A enhances chemosensitivity of HepG2 cells to cisplatin. Planta Med. 2012;78(16):1740–1748. doi: 10.1055/s-0032-1315303. [DOI] [PubMed] [Google Scholar]
- 58.Kinoshita J, Fushida S, Harada S, et al. Local angiotensin II-generation in human gastric cancer: correlation with tumor progression through the activation of ERK1/2, NF-kappaB and survivin. Int J Oncol. 2009;34(6):1573–1582. doi: 10.3892/ijo_00000287. [DOI] [PubMed] [Google Scholar]
- 59.Wenner CA, Martzen MR, Lu H, Verneris MR, Wang H, Slaton JW. Polysaccharide-K augments docetaxel-induced tumor suppression and antitumor immune response in an immunocompetent murine model of human prostate cancer. Int J Oncol. 2012;40(4):905–913. doi: 10.3892/ijo.2011.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji NF, Yao LS, Li Y, He W, Yi KS, Huang M. Polysaccharide of Cordyceps sinensis enhances cisplatin cytotoxicity in non-small cell lung cancer H157 cell line. Integr Cancer Ther. 2011;10(4):359–367. doi: 10.1177/1534735410392573. [DOI] [PubMed] [Google Scholar]
- 61.Pillai TG, Uma Devi P. Mushroom beta glucan: potential candidate for post irradiation protection. Mutat Res. 2013;751(2):109–115. doi: 10.1016/j.mrgentox.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2(1):1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eliza WL, Fai CK, Chung LP. Efficacy of Yun Zhi (Coriolus versicolor) on survival in cancer patients: systematic review and meta-analysis. Recent Pat Inflamm Allergy Drug Discov. 2012;6(1):78–87. doi: 10.2174/187221312798889310. [DOI] [PubMed] [Google Scholar]
- 64.Niwa Y, Matsuura H, Murakami M, Sato J, Hirai K, Sumi H. Evidence that naturopathic therapy including Cordyceps sinensis prolongs survival of patients with hepatocellular carcinoma. Integr Cancer Ther. 2013;12(1):50–68. doi: 10.1177/1534735412441704. [DOI] [PubMed] [Google Scholar]