My awareness of the importance of glutathione in health began with the brilliant commentary on oceanic disease (IMCJ 7.1) by associate editor Sid Baker, md.1 Since then, as I have studied detoxification, mitochondrial function, and healthy aging, the critical role of adequate glutathione to health has become ever more apparent. I have now mentioned glutathione in several previous editorials: protection from oxidative stress (IMCJ 8.3),2 protection from mercury and other toxic metals (IMCJ 8.2, 9.3, 10.4),3–5 protection from alcohol (IMCJ 11.6),6 and protection from persistent organic pollutants (POPs) (IMCJ 12.2).7 This resulted in my creating a 60-slide lecture on glutathione, which I gave for the first time at the October 2013 Restorative Medicine Conference in San Diego, California. As several attendees told me it was one of the most important lectures they had ever heard, I decided to make glutathione the topic of this editorial.
Glutathione Physiology, Production, and Recycling
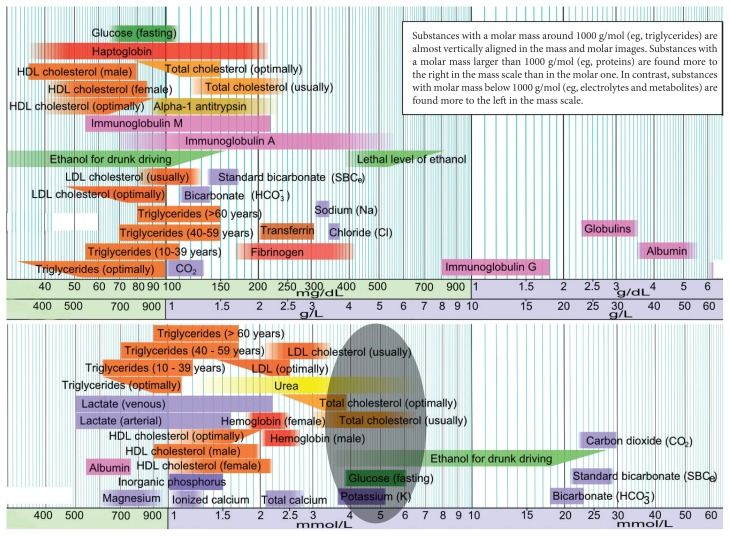
Glutathione is a tripeptide (cysteine, glycine, and glutamic acid) found in surprisingly high levels—5 millimolar—concentrations in most cells. As can be seen in Figure 1, this is the same concentration in cells as glucose, potassium, and cholesterol! Considering the high level of metabolic activity required to produce glutathione, such a high level underlines its importance.
Figure 1.
Concentration of Molecules in Cells
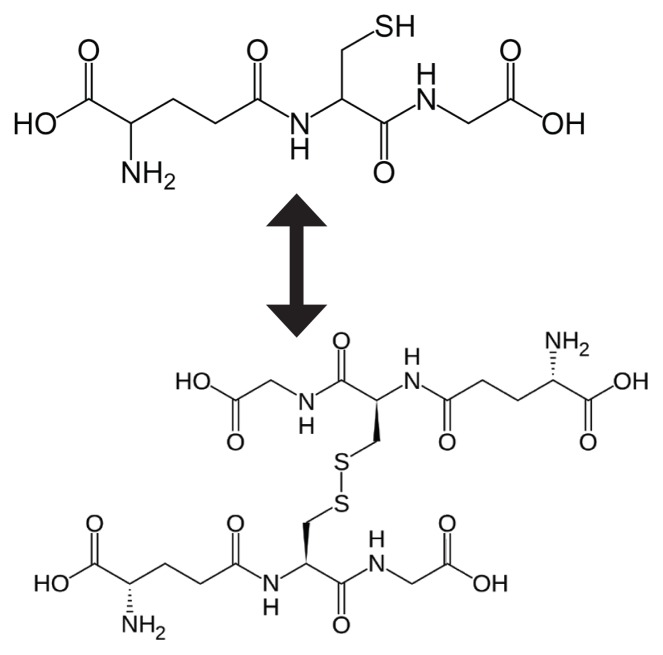
Glutathione exists in cells in 2 states: reduced (GSH) and oxidized (GSSG). As can be seen in Figure 2, oxidized glutathione is actually 2 reduced glutathiones bound together at the sulfur atoms.
Figure 2.
Balance Between GSH and GSSG
The ratio of GSH to GSSG determines cell redox status of cells. Healthy cells at rest have a GSH/GSSG ratio >100 while the ratio drops to 1 to 10 in cells exposed to oxidant stress. Glutathione is also recognized as a thiol buffer maintaining sulfhydryl groups of many proteins in their reduced form. Glutathione is produced exclusively in the cytosol and actively pumped into mitochondria. GSH is made available in cells in 3 ways:
De novo synthesis via a 2-step process catalyzed by the enzymes glutamate cysteine ligase (GCL) and glutathione synthetase (requires ATP).
Regeneration of oxidized GSSG to reduced GSH by glutathione reductase (requires NADPH).
Recycling of cysteine from conjugated glutathione via GGTP (requires NADPH).
Notice that all 3 require energy. The rate of synthesis, regeneration, and recycling is determined primarily by 3 factors8:
De novo glutathione synthesis is primarily controlled by the cellular level of the amino acid cysteine, the availability of which is the rate-limiting step.
GCL activity is in part regulated by GSH feedback inhibition.
If GSH is depleted due to oxidative stress, inflammation, or exposure to xenobiotics, de novo synthesis of GSH is upregulated primarily by increasing availability of cysteine through recycling of GSSG.
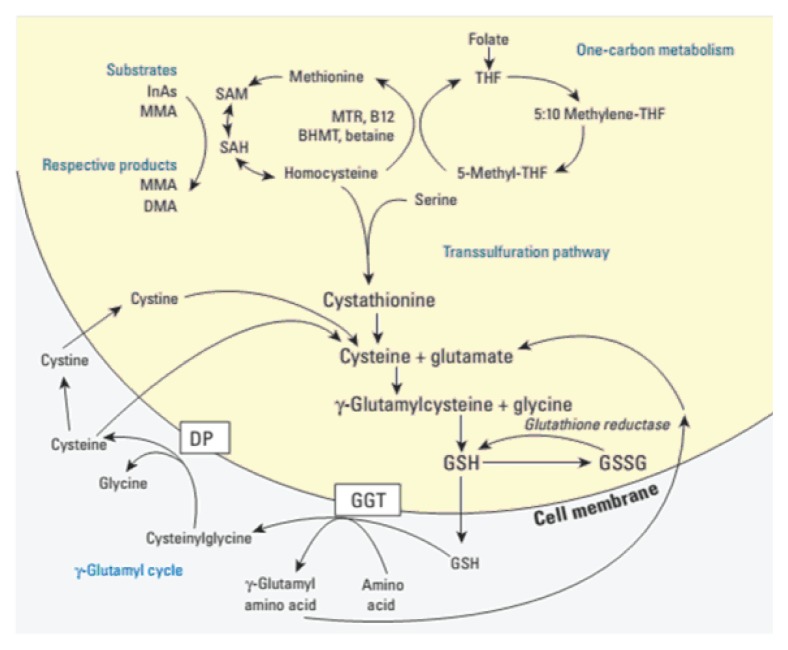
These 3 methods for producing glutathione can be seen in Figure 3.
Figure 3.
Synthesis and Recycling of Glutathione9
Critical Role of Glutathione in Detoxification, Inflammation, and So Much More
It is hard to overstate the importance of glutathione, key roles of which are summarized in Table 1. It plays a crucial role in shielding cellular macromolecules from endogenous and exogenous reactive oxygen and nitrogen species. While it directly quenches some free radicals, of perhaps greater importance is that it deals directly with the causes of oxidative stress such as mercury and POPs.
Table 1.
The Critical Roles of Glutathione
|
Glutathione is involved in the detoxification of both xenobiotic and endogenous compounds. It facilitates excretion from cells (Hg), facilitates excretion from body (POPs, Hg) and directly neutralizes (POPs, many oxidative chemicals). Glutathione facilitates the plasma membrane transport of toxins by at least 4 different mechanisms, the most important of which is formation of glutathione S-conjugates. Low levels of glutathione and/or transferase activity are also associated with chronic exposure to chemical toxins and alcohol, cadmium exposure, AIDS/HIV, macular degeneration, Parkinson’s disease, and other neurodegenerative disorders.
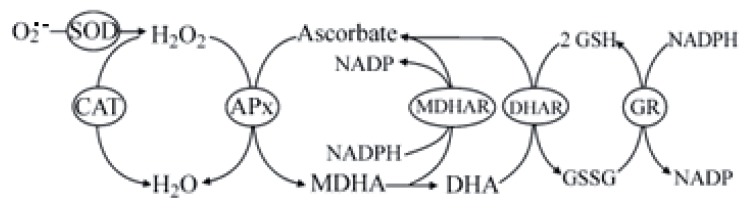
Glutathione directly scavenges diverse oxidants: superoxide anion, hydroxyl radical, nitric oxide, and carbon radicals. Glutathione catalytically detoxifies: hydroperoxides, peroxynitrites, and lipid peroxides.11 Another way glutathione protects cells from oxidants is through recycling of vitamins C and E as shown in Figure 4.10
Figure 4.
Glutathione Protection via Recycling10
Abbreviations: APx = ascorbate peroxidase; CAT = catalase; DHA = dehydroascorbate; DHAR = dehydroascorbate reductase; MDHA = monodehydroascorbate; MDHAR = monodehydroascorbate reductase; GR = glutathione reductase; GSH = reduced glutathione; GSSG = glutathione disulphide; SOD = superoxide dismutase.
Another indication of the key roles of glutathione in health is that the accumulation of GSSG due to oxidative stress is directly toxic to cells, inducing apoptosis by activation of the SAPK/MAPK pathway.12 Glutathione depletion triggers apoptosis, although it is unclear whether it is mitochondrial or cytosol pools of GSH that are the determining factor.13
Perhaps the best indicator of the importance of glutathione is that its cellular and mitochondrial levels directly are highly associated with health and longevity.
Clinical Applications
As shown in Table 2, depletion of GSH has been implicated in many chronic degenerative diseases.
Table 2.
Diseases Associated with GSH Depletion14
|
GSH depletion has been strongly associated with the diseases and loss of function with aging. A representative study of community-dwelling elderly found that higher glutathione levels were associated with higher levels of physical health, fewer illnesses, and higher levels of self-rated health.15 As might be expected, then, GSH status has been found to parallel telomerase activity, an important indicator of lifespan.16 This depletion of GSH also shows up as progressive loss of mitochondrial function due to accumulation of damage to mtDNA.17 The ability of animal species to protect their mtDNA is directly proportional to longevity.18
GGT as Measure of Glutathione Need
GGT (gamma-glutamyl transferase) is upregulated in proportion to the need for glutathione such as for the detoxification of POPs.19 It provides the rate-limiting cysteine through a catabolic “salvage pathway.” Increases in GGT correlate with many diseases: metabolic syndrome, both fatal and nonfatal coronary heart disease (CHD) events, atherosclerosis, fatty liver, diabetes, cancer, hypertension, and carotid intima-media thickness.20–22 Of particular note, these are elevations of GGT within the supposedly “normal” range. For example, men with a GGT of 40 to 50 have a 20-fold increased risk of diabetes.23 Research also shows a GGT 30 to 40—well within the normal range—is associated with a doubling of the risk of all-cause mortality.24 (For a more comprehensive discussion of the remarkable correlations between GGT and disease risks, please see my editorial in IMCJ 8.3).2
Ways to Increase Intracellular Glutathione
Considering how important glutathione is to health, many researchers have looked for ways to increase intracellular and intramitochondrial levels. The good news is that there are several effective strategies. The first, of course, is to decrease the need for glutathione, which means decreasing toxic load. The most obvious is limiting alcohol consumption (see my editorial in IMCJ 11.6).6,25 Less obvious is decreasing exposure to POPs, the primary source of which are conventionally grown foods. (See my editorial in IMCJ 12.2.)7 Another strategy is to provide other antioxidants to decrease oxidative stress. A good example is α-lipoic acid, supplementation of which increases mitochondrial glutathione levels even though ALA is not used in the synthesis or recycling of glutathione.26
The obvious strategy is to directly administer glutathione. This can be done orally, topically, intravenously, intranasally, or in nebulized form. Glutathione administered intravenously, inhaled, and ingested intranasally increases systemic levels.27 IV glutathione has a short half-life but has shown at least short-term efficacy in several diseases. Oral administration is controversial; while most research shows that oral glutathione does not increase RBC glutathione, there are a few studies that show efficacy.28 My opinion is that unmodified oral glutathione is unlikely to consistently elevate cellular levels. Oral and transdermal liposomal glutathione show promise, but research is early.29
Finally, we can provide specific nutrients to promote glutathione production. As noted above, cysteine availability is the rate-limiting step in the de novo production of glutathione. While oral cysteine does not make it through the digestive track, supplemental cysteine in the form of whey or N-acetylcysteine (NAC) is effective at raising levels. While there is substantial variation, 1000 mg/d of NAC will substantially increase glutathione in virtually all patients.30 For the rare patient who reacts to NAC, SAMe can be used.31 Do not use methionine as it will increase homocysteine. Interestingly, supplementing with NAC (600 mg/d for 4 wk) decreases GGT 25%, suggesting that increasing de novo synthesis decreases the need for GGT recycling.32
For those looking for a nonsupplemental solution, 500 mL of alcohol-free beer per day raises RBC glutathione 29%!33 There are many other examples of foods that increase glutathione. For example, 83 g/d of almonds increases glutathione in smokers by 16% and decreases their DNA damage by 29%.34
Finally, there is meditation—practitioners have 20% higher levels of glutathione.35
Clinical Application
Direct administration and promotion of production of glutathione have been used effectively in a wide range of diseases: Parkinson’s, peripheral obstructive arterial disease, cystic fibrosis, emphysema, COPD, preterm infants autism, contrast-induced nephropathy, chronic otitis media, lead exposure, nail biting(!), nonalcoholic fatty liver disease, exercise-induced fatigue—the list is long and surprisingly diverse.36–46
Summary
Clearly, adequate availability of glutathione is critical for maintaining health, protecting the body from toxins, and promoting longevity. Fortunately, there is much we can do to optimize glutathione levels: primarily decrease toxin exposure (including alcohol) and promote production with regular consumption of whey or NAC. I think we are just scratching the surface of the clinical benefits that can be achieved through enhancing intracellular and intramitochondrial glutathione. (I hope you, my dear reader, enjoy these editorials as much as do in writing them.)
In This Issue
I have been intrigued for quite some time by the surprising efficacy and diversity of mushrooms in the promotion of health and treatment of disease. Why would mushrooms produce substances helpful for humans? What was our adaptation as we evolved as a species that facilitated this benefit? The questions are many. Alena G. Guggenheim, nd; Kirsten M. Wright, bs; and Heather L. Zwickey, phd, provide us an excellent review article on how immune modulation by mushrooms can be beneficial in integrated cancer care. I invited Paul Stamets, dsc, and Heather Zwickey, phd, to write a commentary on mushrooms to help us better understand these very interesting agents.
Our original research this issue is a randomized, controlled trial of the use of pranayam for treatment of chronic obstructive pulmonary disease. Anupama Gupta, md; Rajesh Gupta, md; Sushma Sood, md; and Mohammad Arkham, bnys, show us that even chronic conditions respond to nonpharmacological interventions.
My first introductions to the importance of nutrition to health and the destructiveness of the Western diet was reading the works of Weston A. Prince, dds, and Francis M. Pottenger, Jr, md. If you have not had the chance to see the Pottenger cats movie—please do so.47 It is available through the Price-Pottenger Nutrition Foundation. The results are stunning. After my daughter Raven earned her MS/RD in nutrition she was considering a PhD in anthropology. I suggested she make her PhD thesis repeating Price’s work 100 years later. She decided to go into clinical nutrition practice instead, so I hope someone else will follow up this insightful work. IMCJ is delighted to be providing an interview highlighting the important Foundation.
As nutritional medicine becomes more established with research, clinical training, and public acceptance, the reactionary forces have become ever more strident. Books, magazine and newspaper articles, and television and radio interviews—we seem to see some critique of nutritional medicine every week. We all need to be proactive to help ensure the public is not misinformed. An excellent example is Thomas G. Guilliams, phd, who addresses the many deficiencies in “The Case is Closed: Editorial Bias Prevents Reasonable Evaluation of Dietary Supplements.” I fully support one of his key points that evaluating supplements with drug study protocols as single agents is not scientifically valid for most nutrients. Virtually all nutrients are a part of complex systems. Research designs for agents that poison single enzymes make little sense when studying nutrients.
We continue with our new feature interviewing a keynote speaker from an upcoming conference of one of our affiliate professional associations. This issue features my long time friend, Patrick Hanaway, md, who will be emcee at the Institute for Functional Medicine (IFM) conference on food and nutrition May 29–31, 2014, in San Francisco. These annual IFM educational programs are in my top 5 conferences each year. Congratulations to Patrick on his new position as director of medical education for the Institute for Functional Medicine.
Apparently living in a distant land has not impaired John Weeks’s connection to everything integrative medicine. One of the few (very few) positive provisions of the Affordable Care Act (aka, Obamacare) is that it included health care provider nondiscrimination. It is frustrating to see the efforts to repeal this provision. Perhaps one of the worst aspects of the Affordable Care Act is that the individual mandate has not only eliminated health care plans for millions of Americans, but it has also eliminated the high-deductible plans that are so favored by our patients who choose to prioritize integrative medicine for their primary care. Yet another “unintended consequence” of government overreach. On the other hand, good to see Tracy Gaudet, md, providing such innovative leadership for the VA. Our troops truly need fully integrated medicine. Very interesting to see the American Herbal Products Association calling for a voluntary national standard for the disclosure of GMOs. I shudder to think that our medicinal herbs might be becoming so modified without our even knowing about it.
BackTalk by Bill Benda, md—ouch, this one hurt.
Joseph Pizzorno, nd, Editor in Chief
drpizzorno@innovisionhm.com
References
- 1.MacDonald Baker S. The metaphor of oceanic disease. Integrative Med Clin J. 2008;7(1):40–45. [Google Scholar]
- 2.Pizzorno J. The path ahead: measuring oxidative stress. Integrative Med Clin J. 2009;8(3):8–10. [Google Scholar]
- 3.Pizzorno J. The path ahead: is mercury toxicity an epidemic? (Part II) Integrative Med Clin J. 2009;8(2):8–12. [Google Scholar]
- 4.Pizzorno J. The path ahead: vitamin D: still learning about dosage. Integrative Med Clin J. 2010;9(3):8–11. [Google Scholar]
- 5.Pizzorno J. The path ahead: clinical experience in decreasing mercury load. Integrative Med Clin J. 2011;10(4):10–13. [Google Scholar]
- 6.Pizzorno J. The path ahead: what should we tell our patients about alcohol? Integrative Med Clin J. 2012;11(6):8–11. [Google Scholar]
- 7.Pizzorno J. The path ahead: persistent organic pollutants (POPs)—a serious clinical concern. Integrative Med Clin J. 2013;12(2):8–11. [Google Scholar]
- 8.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30(1–2):60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall MN, Niedzwiecki M, Liu X, et al. Chronic arsenic exposure and blood glutathione and glutathione disulfide concentrations in bangladeshi adults. Environ Health Perspect. 2013;121(9):1068–1074. doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira FK, Menezes-Benavente L, Galvão VC, Margis-Pinheiro M. Multigene families encode the major enzymes of antioxidant metabolism in Eucalyptus grandis L. Genet Mol Biol. 2005;28(3):529–538. [Google Scholar]
- 11.Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11(11):2685–2700. doi: 10.1089/ARS.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filomeni G, Aquilano K, Civitareale, et al. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic Biol Med. 2005;39(3):345–354. doi: 10.1016/j.freeradbiomed.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Marí Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11(11):2685–2700. doi: 10.1089/ARS.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390(3):191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julius M, Lang CA, Gleiberman L, Harburg E, DiFranceisco W, Schork A. Glutathione and morbidity in a community-based sample of elderly. J Clin Epidemiol. 1994;47(9):1021–1026. doi: 10.1016/0895-4356(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 16.Borrás C, Esteve JM, Viña JR, Sastre J, Viña J, Pallardó FV. Glutathione regulates telomerase activity in 3T3 fibroblasts. J Biol Chem. 2004;279(33):34332–34335. doi: 10.1074/jbc.M402425200. [DOI] [PubMed] [Google Scholar]
- 17.Wei YH, Ma YS, Lee HC, Lee CF, Lu CY. Mitochondrial theory of aging matures—roles of mtDNA mutation and oxidative stress in human aging. Zhonghua Yi Xue Za Zhi (Taipei) 2001;64(5):259–270. [PubMed] [Google Scholar]
- 18.Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14(2):312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 19.Pompella A, Emdin M, Franzini M, Paolicchi A. Serum gamma-glutamyltransferase: linking together environmental pollution, redox equilibria and progression of atherosclerosis? Clin Chem Lab Med. 2009;47(12):1583–1584. doi: 10.1515/CCLM.2009.350. [DOI] [PubMed] [Google Scholar]
- 20.Jo SK, Lee WY, Rhee EJ, et al. Serum gamma-glutamyl transferase activity predicts future development of metabolic syndrome defined by 2 different criteria. Clin Chim Acta. 2009;403(1–2):234–240. doi: 10.1016/j.cca.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Van Hemelrijck M, Jassem W, Walldius G, et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47(13):2033–2041. doi: 10.1016/j.ejca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Eroglu S, Sade LE, Polat E, Bozbas H, Ulus T, Muderrisoglu H. Association between serum gamma-glutamyltransferase activity and carotid intima-media thickness. Angiology. 2011;62(2):107–110. doi: 10.1177/0003319710386471. [DOI] [PubMed] [Google Scholar]
- 23.Jo SK, Lee WY, Rhee EJ, et al. Serum gamma-glutamyl transferase activity predicts future development of metabolic syndrome defined by 2 different criteria. Clin Chim Acta. 2009;403(1–2):234–240. doi: 10.1016/j.cca.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Van Hemelrijck M, Jassem W, Walldius G, et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer. 2011;47(13):2033–2041. doi: 10.1016/j.ejca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Eroglu S, Sade LE, Polat E, Bozbas H, Ulus T, Muderrisoglu H. Association between serum gamma-glutamyltransferase activity and carotid intima-media thickness. Angiology. 2011;62(2):107–110. doi: 10.1177/0003319710386471. [DOI] [PubMed] [Google Scholar]
- 26.M, Ingersoll RT, Lykkesfeldt J, et al. (R)-alpha-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999;13(2):411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 27.Buhl R, Vogelmeier C, Critenden M, et al. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc Natl Acad Sci U S A. 1990;87(11):4063–4067. doi: 10.1073/pnas.87.11.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen J, Bradley RD. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Altern Complement Med. 2011;17(9):827–833. doi: 10.1089/acm.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, Geier MR. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011;17(12):CR677–CR682. doi: 10.12659/MSM.882125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pendyala L, Creaven PJ. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a phase I trial. Cancer Epidemiol Biomarkers Prev. 1995;4(3):245–251. [PubMed] [Google Scholar]
- 31.Liber CS, Packer L. S-Adenosylmethionine: molecular, biological, and clinical aspects—an introduction. Am J Clin Nutr. 2002;76(5):1148S–1150S. doi: 10.1093/ajcn/76/5.1148S. [DOI] [PubMed] [Google Scholar]
- 32.Pamuk GE, Sonsuz A. N-acetylcysteine in the treatment of non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2003;18(10):1220–1221. doi: 10.1046/j.1440-1746.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- 33.Martínez Alvarez JR, Bellés VV, López-Jaén AB, Marín AV, Codoñer-Franch P. Effects of alcohol-free beer on lipid profile and parameters of oxidative stress and inflammation in elderly women. Nutrition. 2009;25(2):182–187. doi: 10.1016/j.nut.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Jia X, Chen CY, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007;137(12):2717–2722. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- 35.Sharma H, Datta P, Singh A, et al. Gene expression profiling in practitioners of Sudarshan Kriya. J Psychosom Res. 2008;64(2):213–218. doi: 10.1016/j.jpsychores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Hauser RA, Lyons KE, McClain T, Carter S, Perlmutter D. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson’s disease. Mov Disord. 2009;24(7):979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 37.Arosio E, De Marchi S, Zannoni M, Prior M, Lechi A. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2002;77(8):754–759. doi: 10.4065/77.8.754. [DOI] [PubMed] [Google Scholar]
- 38.Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127(1):308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 39.Stav D, Raz M. Effect of N-acetylcysteine on air trapping in COPD: a randomized placebo-controlled study. Chest. 2009;136(2):381–386. doi: 10.1378/chest.09-0421. [DOI] [PubMed] [Google Scholar]
- 40.Cooke RW, Drury JA. Reduction of oxidative stress marker in lung fluid of preterm infants after administration of intra-tracheal liposomal glutathione. Biol Neonate. 2005;87(3):178–180. doi: 10.1159/000082623. [DOI] [PubMed] [Google Scholar]
- 41.Kern JK, Geier DA, Adams JB, Garver CR, Audhya T, Geier MR. A clinical trial of glutathione supplementation in autism spectrum disorders. Med Sci Monit. 2011;17(12):CR677–CR682. doi: 10.12659/MSM.882125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitoh T, Satoh H, Nobuhara M, et al. Intravenous glutathione prevents renal oxidative stress after coronary angiography more effectively than oral N-acetylcysteine. Heart Vessels. 2011;26(5):465–472. doi: 10.1007/s00380-010-0078-0. [DOI] [PubMed] [Google Scholar]
- 43.Testa B, Testa D, Mesolella M, D’Errico G, Tricarico D, Motta G. Management of chronic otitis media with effusion: the role of glutathione. Laryngoscope. 2001;111(8):1486–1489. doi: 10.1097/00005537-200108000-00028. [DOI] [PubMed] [Google Scholar]
- 44.Kasperczyk S, Dobrakowski M, Kasperczyk A, Ostałowska A, Birkner E. The administration of N-acetylcysteine reduces oxidative stress and regulates glutathione metabolism in the blood cells of workers exposed to lead. Clin Toxicol (Phila) 2013;51(6):480–486. doi: 10.3109/15563650.2013.802797. [DOI] [PubMed] [Google Scholar]
- 45.Ghanizadeh A, Derakhshan N, Berk M. N-acetylcysteine versus placebo for treating nail biting, a double blind randomized placebo controlled clinical trial. Antiinflamm Antiallergy Agents Med Chem. 2013;12(3):223–228. doi: 10.2174/1871523011312030003. [DOI] [PubMed] [Google Scholar]
- 46.Medved I, Brown MJ, Bjorksten AR, et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004;97(4):1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 47.Price-Pottenger Nutrition Foundation. Pottenger Cat Studies [DVD] Lemon Grove, CA: PPNF; [Google Scholar]