Abstract
Context
Curcumin has a number of beneficial effects, such as functioning as a potent antioxidant,1 anti-inflammatory, 2 and anticancer agent. Because of its poor oral bioavailability, very high oral doses and repeated dosing have been used to obtain effective plasma levels, with mixed results. High doses of curcumin may cause gastric disturbance, often resulting in poor patient compliance.
Objective
The objective of this study was to compare the relative bioavailability of MicroActive Curcumin—an advanced, micronized formulation of curcumin that is 25% curcuminoids in a sustained release matrix—with that of an unformulated, 95% pure curcumin powder.
Design
A dissolution study compared the solubility of the formulated and the unformulated curcumin. The research team also performed a single-dose, 12-h, crossover uptake study with 10 participants and a high-dose tolerability and accumulation study with 3 participants, comparing the 2 forms of curcumin.
Setting
The study was done in MAZE Laboratories (Purchase, NY, USA).
Participants
Ten healthy male and female volunteers, aged 21–66 y, took part in the single-dose study. Three participants, 2 female and 1 male aged 40–55 y, took part in the tolerability and accumulation study. The participants were people from the community.
Intervention
For the dissolution study, the research team filled hard gelatin capsules with unformulated 95% curcumin powder and the MicroActive Curcumin powder to the equivalent of 25 mg curcuminoids. For the single-dose study, participants received 500 mg of curcumin in 2 forms. MicroActive Curcumin capsules were administered after breakfast, and blood samples were drawn at 1, 2, 4, 8, and 12 h postdose. After a 7-d washout period, the protocol was repeated for unformulated, 95% curcumin powder capsules. For the tolerability study, the unformulated, 95% curcumin powder was given at a dose that provided 2 g of curcumin for 7 d followed by 5 g of curcumin for an additional 7 d. After a washout period of 14 d, the protocol was repeated with MicroActive Curcumin. Participants then continued to take the MicroActive Curcumin for >3 mo.
Outcome Measures
For the dissolution study, the curcumin was quantified at room temperature using reverse-phase, high-performance liquid chromatography (HPLC) with a Phenomenex Luna column (150 × 4.6 mm, 5 μm) (Phenomenex Inc, Torrance, CA, USA). For the single-dose and the tolerability studies, hydrolysis of conjugates and extraction of curcuminoids from the plasma were performed. The curcuminoids were quantified using reverse-phase HPLC with an ultraviolet-visible detector as described above.
Results
The dissolution study indicated that the sustained-release curcumin had greater dissolution for 12 h at all points tested, compared with the unformulated curcumin. Very little of the unformulated curcumin powder had been released at the end of the 12 h. The results of the single-dose uptake study indicated that the sustained-release formula was 9.7 × more bioavailable than the unformulated powder (P < .001, paired t test). Additionally, all participants showed uptake from the sustained-release formulation. That formulation also resulted in significant increases in the plasma demethoxylated curcuminoids, but the research team did not observe the same increases for the unformulated curcumin powder. The sustained-release formulation was well tolerated, without adverse effects in the high-dose tolerability study.
Conclusions
Formulation of micronized curcumin in a combination of surfactants, oils, and polymers improves the absorption of curcumin. In addition, the unique plasma demethylated curcuminoid profile may enhance the therapeutic effects of MicroActive Curcumin not observed with unformulated curcumin at moderate and well-tolerated doses. MicroActive Curcumin was well tolerated, without any adverse effects in a high-dose tolerability study. These properties have the potential to make high-dose curcumin supplementation more accessible through simplified incorporation into food and beverage preparations.
Curcumin is a lipophilic, phenolic compound isolated from the turmeric (Curcuma longa) rhizome that is widely used in traditional medicine, such as in ayurveda in India. Commercially available 95% curcumin is a mixture of 3 curcuminoids, typically containing approximately 77% curcumin, 17% demethoxycurcumin, and 6% bisdemethoxycurcumin. Research has shown that curcumin has a number of beneficial effects, such as functioning as a potent antioxidant,1 anti-inflammatory,2 and anticancer agent in cell-based and animal studies.3 However, curcumin is also practically insoluble in water and is rapidly eliminated from the digestive tract, having shown poor oral bioavailability in human and animal studies.4 Because of that poor bioavailability, very high oral doses and repeated dosing have been used to obtain effective plasma levels, with mixed results.5,6,7 High doses of curcumin may cause gastric disturbance, often resulting in poor patient compliance.5,6
Several methods of formulation have been developed to improve the bioavailability of curcumin, including using combinations of surfactants, cosurfactants, oils, and/or organic solvents to form microemulsions8 or self-emulsifying systems9 and nanoparticle colloidal dispersions in water-soluble carriers.10,11 These formulations have many disadvantages for commercial production. Curcumin is fully solubilized in a microemulsion or self-emulsifying formulation, and the amount dissolved is limited by the solubility of curcumin in the surfactant-oil mixtures. Hence, the concentration of curcumin generally is lower than 20% in such systems. For example, Hu et al8 reported on an optimal microemulsion system where curcumin solubility was up to 32.5 mg/mL (3.25% curcumin).
When converted further into a powder, the concentration of curcumin in such formulations is reduced even more, resulting in solid dosage forms. These solid dosage forms, including tablets or capsules, have to be larger—and a greater number of tablets or capsules is needed—to provide the therapeutic dose, which reduces patient compliance. Additionally, if the microemulsions or self-emulsifying formulations are not converted into powders, the liquid formulations are limited to incorporation into soft-gelatin capsules, which makes the final products more expensive. Preparation of nanoparticle, colloidal, solid dispersions of curcumin generally involve use of organic solvents or colloidal milling to reduce the particle size. These preparations also involve a drying step, such as spray-drying or freeze-drying with an excipient. These procedures add to production costs, making the final dosage form more expensive.
In the present study, the research team has developed a formulation containing a dispersion of micronized curcuminoids in a sustained-release matrix to improve the bioavailability and residence time of the curcuminoids. The formulation, named MicroActive Curcumin, is available as a free-flowing powder containing 25% curcuminoids that are suitable for use in solid-dosage forms, powder formulations, and as bulk powder. The current study compares the dissolution and bioavailability of MicroActive Curcumin with unformulated, 95% curcumin.
Materials
High-performance liquid chromatography (HPLC)-grade solvents and other chemicals used in the buffers of the sustained-release formulation were obtained from VWR International (Radnor, PA, USA). Purified standard curcumin, demethoxycurcumin, and bisdemethoxycurcumin (>98%) purity by HPLC, were provided by Chromadex (Irvine, CA, USA). Polysorbate 80, β-glucuronidase, and sulfatase were obtained from Sigma-Aldrich (St Louis, MO, USA). Unformulated, 95% curcumin powder was obtained from Maypro Industries (Purchase, NY, USA).
Methods: Dissolution Test
Procedures
The in vitro dissolution study was performed using the Varian 7020 dissolution tester (Varian Inc, Cary, North Carolina, USA) with the basket configuration at 37°C and 100 rpm. The method was based on the US Pharmacopeia (USP) dissolution test for extended-release dosage forms.12 The research team filled hard gelatin capsules with the unformulated 95% curcumin powder and the MicroActive Curcumin powder, to the equivalent of 25 mg curcuminoids. The capsules were introduced into 750 mL of 0.1N hydrochloric acid (0.1N HCl)—simulated gastric fluid without enzymes, maintained at at 37°C. At the end of the studies, which lasted 1 and 2 hours, 3 mL of the sample were withdrawn and filtered through a 10-μ filter. The reduced volume was replaced each time with a fresh medium. At the end of 2 hours, the pH of the medium was adjusted to 6.5—simulating intestinal fluid without enzymes, with 195 mL of 0.2M tribasic sodium-phosphate solution equal to 37°C. Polysorbate 80 dissolved in 55 mL of water was added to a concentration of 0.25% to simulate intestinal fluid. Aliquots were withdrawn at 4, 6, 9, and 12 h for analysis, as described previously, and the removed volume was replaced with fresh medium each time. The aliquots were diluted with methanol for HPLC analysis.
Methods: Clinical Study
Participants
The study was reviewed by the Sterling Institutional Review Board (Atlanta, GA, USA). The study was conducted in the contract research organization, MAZE Laboratories (Purchase, NY, USA). For the single-dose, crossover, bioavailability study, the research team recruited 10 healthy male and female volunteers, aged 21–66 years, who were not using turmeric powder in their food preparations or consuming turmeric or curcumin-containing supplements. The participants were people from the community recruited by advertising in the local media. The participants were not taking any other medications. Written informed consent was obtained from all participants.
Individuals were excluded based on the following conditions: (1) known malabsorption or maldigestion, diabetes, hypertension, gallbladder disease, or any condition that the principal investigator believed could put the participant at undue risk; (2) a body mass index (BMI) < 18 kg/m2 or > 30 kg/m2; (3) chronic intake of medications; (4) a history or current abuse of drugs, medication, or alcohol or intake of >2 alcoholic beverages per day; (5) known hypersensitivity to the study’s products or to any ingredient in the products’ preparations; (6) pregnancy or lactation in women; and (7) participation in another clinical trial within the 4 weeks prior to the current study or concurrent participation in another clinical trial.
Intervention
The single-dose bioavailability study compared the 2 forms of curcumin powder, both in hard gelatin capsules: (1) a commercially available, unformulated curcumin standardized to contain 95% curcuminoids and (2) MicroActive Curcumin—a micronized, sustained-release formulation containing 25% curcuminoids. MicroActive Curcumin is a proprietary mixture of polyglycerol esters of fatty acids, medium-chain triglycerides, hydroxypropylmethylcellulose, sodium alginate, and microcrystalline cellulose. Participants received 500 mg of curcumin in each of the 2 forms.
Procedures
Participants were instructed not to consume any foods or supplements containing turmeric or curcumin for at least 1 week before the study. They were also instructed not to consume any food for at least 12 hours prior to a blood draw at baseline. Following the overnight fasting, 7 mL of blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes to permit establishment of a baseline value of curcumin. On the day of the study, a standard breakfast, lunch (approximately 500 kcal, 15% protein, 30% fat, and 55% carbohydrate), and a snack were offered to the participants. MicroActive Curcumin capsules were administered after breakfast, and blood samples were drawn at 1, 2, 4, 8, and 12 hours postdose. After a 7-day washout period, the protocol was repeated for the unformulated, 95% curcumin powder capsules. The participants were instructed not to consume any curcumin- or turmeric-containing foods or supplements during the washout period. The samples were stored on ice and protected from light, and the plasma was separated by centrifugation within 1 hour of collection and stored at −80°C until analysis.
Methods: Tolerability and Accumulation Study
Participants
The tolerability study was performed with 3 participants—2 female and 1 male, aged 40–55 years—also recruited from the community. All the protocol used for the study such as selection of participants, review, monitoring, and inclusion exclusion criteria were as described previously.
Intervention
In the first phase of the study, the unformulated, 95% curcumin powder was given at a dose that provided 2 g of curcumin for 7 days, followed by 5 g of curcumin for an additional 7 days. After a washout period of 14 days, the protocol was repeated with MicroActive Curcumin, providing the same dose of curcumin until day 14. Participants then continued to take MicroActive Curcumin providing 5 g curcumin for 3 more months.
Procedures
Blood samples were drawn at baseline, day 7, and day 14 for both the unformulated and formulated versions of curcumin. Participants also filled out an adverse-events questionnaire during their visits for blood draws. They were asked to indicate whether they had had any symptoms, such as abdominal pain, bloating, gas, burping, loose stool, or diarrhea, and what the severity of the symptoms was. For the extended period during which participants took the formulated version, additional blood samples were drawn at 30, 60, and 90 days, and again participants filled out adverse-events questionnaires.
Outcome Measures
Dissolution Study
Curcumin was quantified at room temperature using reverse-phase HPLC with a Phenomenex Luna column (Phenomenex Inc, Torrance, CA, USA) (150 × 4.6 mm, 5μm). The samples were eluted using an isocratic mobile phase consisting of 45% acetic acid (5%) and 55% acetonitrile. The flow rate was 1 mL/min and the detection wavelength was 420 nm. Standard curcumin was used for quantification. The retention time for bisdemethoxycurcumin, demethoxycurcumin, and curcumin was 4.4, 4.81, and 5.2 minutes, respectively, under these conditions.
Single-dose and Tolerability Studies
Hydrolysis of conjugates and extraction of curcuminoids from the plasma were performed based on the method described by Vareed et al.13 One mL of plasma was incubated with 2000 units of β-glucuronidase and 260 units of sulfatase at 37°C for 3.5 hours. The samples were extracted 3 times with ethyl acetate/methanol (95:5), and the solvents were evaporated under nitrogen protected from light. The residue was dissolved in methanol-1% acetic acid (0.2 mL) for HPLC analysis. The curcuminoids were quantified using reverse-phase HPLC with an ultraviolet-visible detector as described above.
Statistical Analysis
Single-dose and Tolerability Studies
The amount of curcuminoids in the blood samples for experimental and control groups was determined using the area under the curve (AUC) calculated with the trapezoid rule. The amount of curcuminoids absorbed by participants under each condition was compared using the student t test (repeated measures). Calculations and analysis were made using Prism 4 for Macintosh (GraphPad, La Jolla, CA, USA).
Results
Dissolution Test
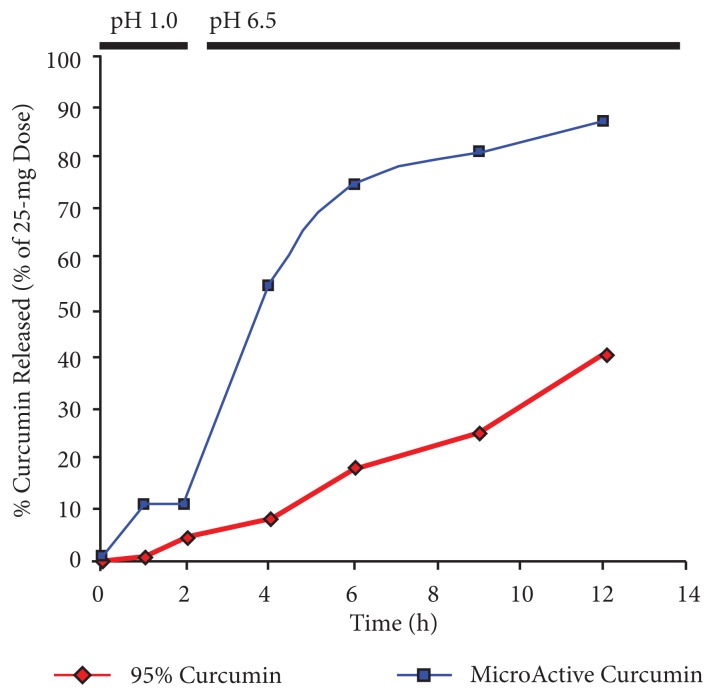
Figure 1 presents the dissolution profiles of MicroActive Curcumin and unformulated 95% curcumin powder. The MicroActive formula showed a higher dissolution at all time points tested, with sustained release up to 12 hours. Nearly 10% of the dose was released in the gastric pH by 2 hours. Between 4 and 12 hours, approximately 50% to 88% of the dose was in solution. With the 95% curcumin powder, very little was released in the gastric pH by 2 hours. Between 4 and 12 hours, 10% to 40% of the dose was in solution.
Figure 1.
Comparison of Dissolution Profiles for Curcumin
Single-dose Study
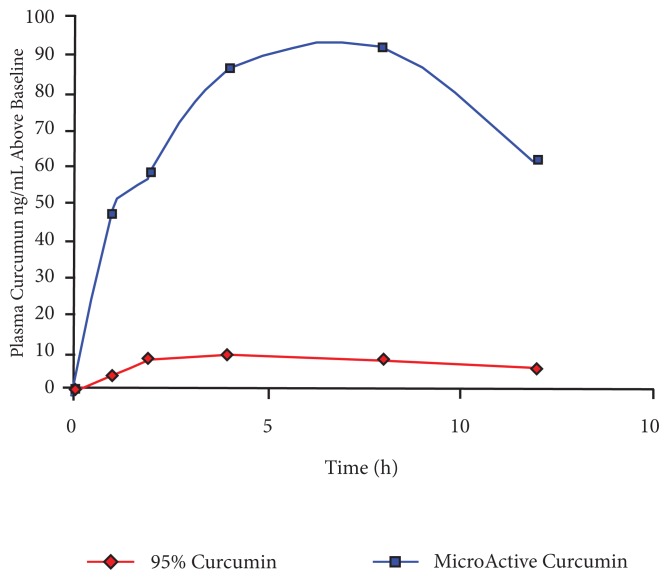
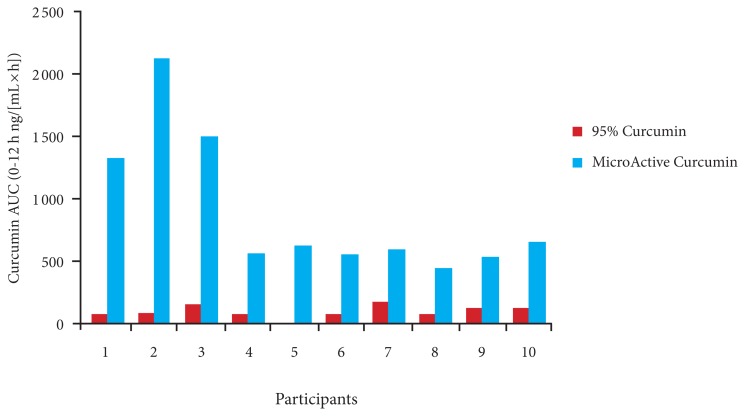
Figure 2 presents the average uptake of curcumin from MicroActive Curcumin and 95% curcumin powder for the 10 participants in the single-dose study. MicroActive Curcumin showed higher absorption at all time points tested, with a Tmax of 4 hours, followed by sustained release during the remaining hours. The plasma levels remained high at 12 hours, indicating sustained release for more than 12 hours. The average area under the curve (AUC 0–12 h ng/([mL × h])) was 887.48 ± 549.98 for MicroActive Curcumin and 91.82 ± 50.00 for 95% curcumin. The ratio of MicroActive to unformulated 95% curcumin AUC was 9.7. The results were highly significant (P < .001; paired t test). Figure 3 presents the comparison of AUC of curcumin for individual participants. All the participants showed higher absorption when taking MicroActive Curcumin compared with 95% curcumin.
Figure 2.
Comparison of Uptake of Curcumin for the 10 Participants in the Single-dose Study
Figure 3.
Comparison of the AUC of Curcumin for Individual Participants in the Single-dose Study
Abbreviations: AUC = area under the curve.
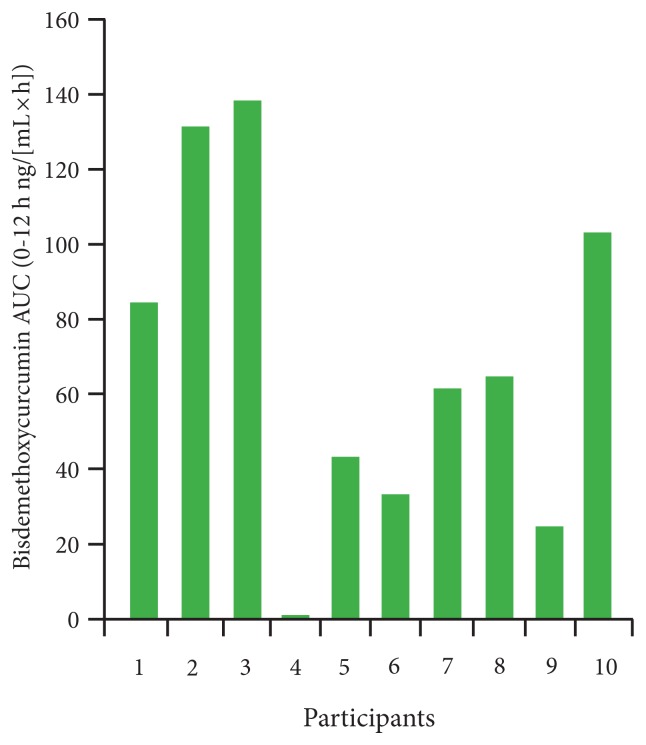
Taking a single dose of the MicroActive Curcumin also resulted in detectable amounts of demethoxycurcumin and bisdemethoxycurcumin, which was not true for the 95% curcumin. Figure 4 presents the AUC of bisdemethoxycurcumin for individual participants after a single dose of MicroActive Curcumin.
Figure 4.
AUC for Bisdemethoxycurcumin for Individual Participants in the Single-dose Study When Taking MicroActive Curcumin
Abbreviations: AUC = area under the curve.
High-dose Tolerability and Accumulation Study
In the high-dose tolerability study after 14 days on unformulated 95% curcumin, participants complained about 1 or more of the following issues: dislike of the strong taste, bloating, or gas. The adverse incidents were not consistent between participants, complicating a quantification of results on this small sample. While taking MicroActive Curcumin, participants did not experience these issues either during the initial 14 days or during the 3 months of continued use.
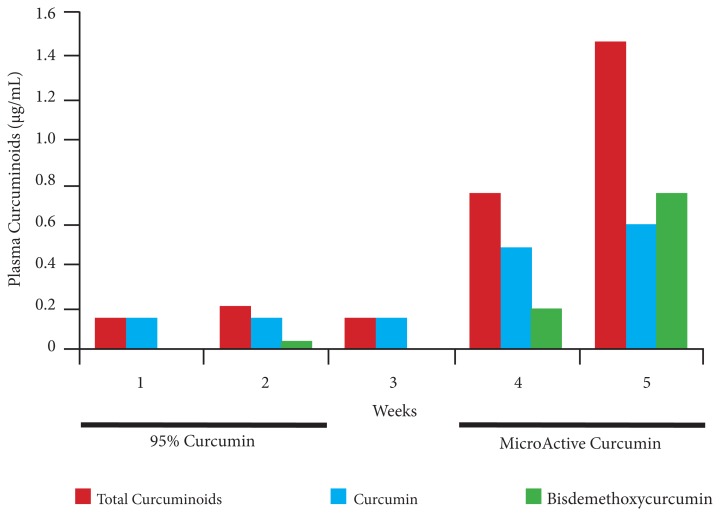
Figure 5 presents the plasma curcuminoids after the 2-week phase of the study. After 14 days of supplementation with 95% curcumin, the average levels of plasma curcumin and bisdemethoxycurcumin were 0.16 μg/mL and 0.022 μg/mL, respectively. After participants started taking MicroActive Curcumin, the levels of curcumin and bisdemethoxycurcumin were 0.60 μg/mL and 0.75 μg/mL, respectively, by the end of 14 days. The relative increases in the levels of plasma curcumin and bisdemethoxycurcumin between week 2 and week 5 were 3.75 × and 34 × the unformulated and the formulated versions, respectively.
Figure 5.
Comparison of Uptake of Curcuminoids in the Tolerability and Accumulation Study
MicroActive Curcumin resulted in a significant increase in the plasma bisdemethoxycurcumin, which was not observed with administration of 95% curcumin. The results were further confirmed with HPLC analysis. Two participants who continued supplementing with MicroActive Curcumin after the initial 14 days showed sustained high levels of all 3 curcuminoids during a period of 3 months. The third participant did not continue with the study because of problems with compliance.
Discussion
The objective of this study was to compare the relative bioavailability of an advanced, micronized curcumin formulation (MicroActive Curcumin) with that of unformulated 95% curcumin powder. MicroActive Curcumin, a commercially available form, was formulated in a proprietary mixture of surfactants, oil, and sustained-release polymers to form a micronized dispersion of curcumin. In the dissolution study, the micronized curcumin was released in a period of 12 hours, which is indicative of sustained release on oral dosing. In the single-dose study, all participants showed sustained release of MicroActive Curcumin irrespective of gender and age. Also, all participants showed absorption of curcumin from MicroActive Curcumin in the single-dose study, while some of the subjects absorbed very little from the unformulated 95% curcumin. The bioavailability of curcumin from MicroActive Curcumin was 9.7 × greater than that of the unformulated curcumin.
A significant increase also occurred in the plasma bisdemethoxycurcumin with MicroActive Curcumin, but the same result was not observed with the unformulated 95% curcumin. In the single-dose study, 9 participants out of 10 showed an increase in the bisdemethoxycurcumin levels with MicroActive Curcumin. At higher dosage levels in the accumulation study, a significant increase occurred in bisdemethoxycurcumin levels in the plasma. With 95% curcumin, only 1 participant showed detectable levels of bisdemethoxycurcumin in the high-dose accumulation study.
The reasons for the increase in bisdemethoxycurcumin are not clear. No differences existed in the composition of the curcuminoids between the formulated and unformulated curcumin powders, and the dosage of curcumin used in the studies was the same for both products. Cuomo et al14 have reported a significant increase in the plasma demethoxycurcumin from a lecithin formulation of curcumin.14 They had observed that a reductive microbial metabolization of curcumin might be involved in the formation of the demethoxylated curcuminoids.
Biotransformation of curcumin to tetrahydrocurcumin by intestinal bacteria also has been reported.15 Researchers have reported biotransformation of dietary phenolic compounds, such as anthocyanins, by intestinal bacteria.16 The MicroActive formulation stabilized curcumin in the intestinal pH, which may have resulted in a significant curcumin load for the gut microflora, which is known to demethoxylate curcumin reductively, similarly to the lecithin formulation as noted by Cuomo et al.14
The superior bioavailability and distinct plasma profile of MicroActive Curcumin indicated a better clinical efficacy compared with the unformulated 95% curcumin. The demethoxylated curcuminoids are reported to have better clinical effects compared with curcumin. Bisdemethoxycurcumin is reported to have superior neuroprotective and anticancer properties as compared to curcumin.17,18,19 Demethoxycurcumin is reported to have better anticancer and anti-inflammatory activity compared with curcumin.20,21 Further studies are warranted to explore the unexpected increase in the plasma demethoxylated curcuminoids and the therapeutic potential of MicroActive Curcumin.
Our tolerability and accumulation study is limited by small sample size, which limited quantification of adverse effects with 95% unformulated curcumin. Although MicroActive Curcumin was well tolerated, the limitation in the study design was the use of a high dose of curcumin. The results do not indicate the effects of lower doses for longer-term use on plasma levels.
Conclusions
We have demonstrated that formulation of micronized curcumin in a combination of surfactants, oils, and polymers improves the absorption of curcumin. In addition, the unique plasma demethylated curcuminoid profile may enhance the therapeutic effects of MicroActive Curcumin not observed with unformulated curcumin at moderate and well-tolerated doses. MicroActive Curcumin was well-tolerated, without any adverse effects in a high-dose tolerability study. MicroActive Curcumin is a free flowing powder that was able to achieve sustained release when presented in a capsule form. The solubility allowed MicroActive Curcumin to be mixed with food and beverages more easily, thereby making varied and higher doses more practical and easy to administer.
References
- 1.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25(15):1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 2.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 3.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–398. [PubMed] [Google Scholar]
- 4.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 5.Lao CD, Ruffin MT, IV, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006 Mar;6:10–13. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 8.Hu L, Jia Y, Niu F, Jia Z, Yang X, Jiao K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J Agric Food Chem. 2012;60(29):7137–7141. doi: 10.1021/jf204078t. [DOI] [PubMed] [Google Scholar]
- 9.Ramshankar YV, Suresh S, Devi K. Novel self-emulsifying formulation of curcumin with improved dissolution, antiangiogenic and anti-inflammatory activity. Clin Res Regul Aff. 2008;25(4):213–234. [Google Scholar]
- 10.Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34(5):660–665. doi: 10.1248/bpb.34.660. [DOI] [PubMed] [Google Scholar]
- 11.Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnology. 2007 Apr;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The United States Pharmacopeia-National Formulary. 23rd ed. Rockville, MD: United States Pharmacopeial Convention; 1995. pp. 1795–1796. [Google Scholar]
- 13.Vareed SK, Kakarala M, Ruffin MT, et al. Pharamcokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod. 2011;74(4):664–669. doi: 10.1021/np1007262. [DOI] [PubMed] [Google Scholar]
- 15.Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc Natl Acad Sci U S A. 2011;108(16):6615–6620. doi: 10.1073/pnas.1016217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. Stability and biotransformation of various dietary anthocyanins in vitro. Eur J Nutr. 2006;45(1):7–18. doi: 10.1007/s00394-005-0557-8. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed T, Gilani AH. A comparative study of curcuminoids to measure their effect on inflammatory and apoptotic gene expression in an Aβ plus ibotenic acid-infused rat model of Alzheimer’s disease. Brain Res. 2011 Jul;1400:1–18. doi: 10.1016/j.brainres.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Liu YL, Yang HP, Zhou XD, Gong L, Tang CL, Wang HJ. The hypomethylation agent bisdemethoxycurcumin acts on the WIF-1 promoter, inhibits the canonical Wnt pathway and induces apoptosis in human non-small-cell lung cancer. Curr Cancer Drug Targets. 2011;11(9):1098–1110. doi: 10.2174/156800911798073041. [DOI] [PubMed] [Google Scholar]
- 19.Boonrao M, Yodkeeree S, Ampasavate C, Anuchapreeda S, Limtrakul P. The inhibitory effect of turmeric curcuminoids on matrix metalloproteinase-3 secretion in human invasive breast carcinoma cells. Arch Pharm Res. 2010;33(7):989–998. doi: 10.1007/s12272-010-0703-6. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LJ, Wu CF, Meng XL, et al. Comparison of inhibitory potency of three different curcuminoid pigments on nitric oxide and tumor necrosis factor production of rat primary microglia induced by lipopolysaccharide. Neurosci Lett. 2008;447(1):48–53. doi: 10.1016/j.neulet.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 21.Lee JW, Hong HM, Kwon DD, Pae HO, Jeong HJ. Demethoxycurcumin, a structural analogue of curcumin, induces apoptosis in human renal carcinoma caki cells through the production of reactive oxygen species, the release of cytochrome C, and the activation of caspase-3. Korean J Urol. 2010;51(12):870–888. doi: 10.4111/kju.2010.51.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]