Abstract
Spices are used worldwide, particularly, in the Asian and Middle-Eastern countries and considered protective against degenerative diseases, including cancer. Here, we report the efficacy of aqueous and non-aqueous extracts of eleven Apiaceae spices for free radical-scavenging activity and to inhibit cytochrome P450s in two separate reactions involving: i) 4-hydroxy-17β-estradiol (4E2), DNA and CuCl2 and ii) 17β-estradiol, rat liver microsomes, co-factors, DNA and CuCl2. Oxidative DNA adducts resulting from redox cycling of 4E2 were analyzed by 32P-postlabeling. Aqueous (5 mg/ml) and non-aqueous extracts (6 mg/ml) substantially inhibited (83% – 98%) formation of DNA adducts in the microsomal reaction. However, in non-microsomal reaction, only aqueous extracts showed the inhibitory activity (83% – 96%). Adduct inhibition was also observed at 5-fold lower concentrations of aqueous extracts of cumin (60%) and caraway (90%), and 10-fold lower concentrations of carrot seeds (76%) and ajowan (90%). These results suggests the presence of two groups of phytochemicals - polar compounds that have free radical-scavenging activity, and lipophilic compounds that selectively inhibit P450 activity associated with estrogen metabolism. Because most of these Apiaceae spices are used widely with no known toxicity, the phytochemicals from the Apiaceae spices used in foods may be potentially protective against estrogen-mediated breast cancer.
Keywords: Apiaceae spices, Antioxidant activity, Free radical scavenging activity, Redox activity, Oxidative DNA adducts
Introduction
Epidemiological data suggest that an elevated level of free radicals is associated with various diseases including cancer (1). Free radicals formed in cell metabolism and through various environmental factors like pollution, radiation or cigarette smoke are highly reactive and cause DNA damage which possibly leads to aging, cardiac diseases and cancer (2). The examples of important reactive oxygen species (ROS) include super oxide, hydroxyl radical and nitric oxide radicals etc. (2, 3). The imbalance between the free radicals and antioxidants that quenches them leads to cellular damage termed as oxidative stress (4–6).
Antioxidants and enzymes such as catalase play an important role in balancing biological systems by suppressing the formation of active oxygen species (ROS) by reducing hydroperoxides (ROO•) and H2O2, and scavenging free radicals among others (7). Thus, it is important to maintain equilibrium between free radical generation and antioxidants supply. When endogenous antioxidants are insufficient to engage the highly reactive free radicals, it is beneficial to provide antioxidants, e.g. dietary to further mitigate damaging free radicals. The antioxidant potential of fruits and vegetables has been established by in vitro and in vivo studies (8, 9). Bioactive phytochemicals in fruits and vegetable are highly diverse yet commonly demonstrate similar bioactivities including antioxidant, antiproliferative, and anti-angiogenic properties as well as blocking of cell cycle and/or by induction of apoptosis that contribute to demonstrated anticancer properites (10, 11). Further, consumption of fruits and vegetables is associated with reduced risk of cancer, heart disease, and memory loss (12, 13).
Historically, herbs and spices have enjoyed a rich tradition of use for their flavor enhancement characteristics and for their medicinal properties. Spices provide a rich array of phytochemicals that may reduce the risk of certain cancers (14, 15). In-vitro studies indicate that bioactive components of herbs and spices can inhibit pathways that regulate cell division, cell proliferation, and detoxification as well as inflammatory and immune response (15). Moreover, spices are rich in phytochemicals like essential oils, polyphenolics and phenolic terpenes; all of which have been shown to inhibit or attenuate cancer initiation or progression (16).
DNA damage has been associated with cancer as well as aging processes (17). There are several pathways identified in the development of cancer. One of them is oxidative DNA damage contributed by redox activity of endogenous and exogenous species. To assess the DNA damage caused by ROS, 32P-postlabeling followed by 2-dimensional polyethyleneimine-cellulose thin-layer chromatography (PEI-cellulose TLC) is commonly used (18). This method has an advantage of detecting a variety of polar adducts in addition to the oxidative DNA lesion 8-oxo-2’-deoxyguonosine (8-oxodG) (19) .
In this study, we compared 11 commercially available organic spices (Fig. 1) belonging to Apiaceae family for their antioxidant capacity using the redox activity of 4-hydroxy- 17β-estradiol (4E2). Chemopreventive activity of the spices extracts was assessed by the inhibition of cytochrome P450s associated with metabolism of 17β-estradiol.
Figure 1.
Photographs of selected spices used in the study.
Materials and Methods
Chemicals and spices
17β-estradiol (E2) and 4-hydroxy-17β-estradiol were purchased from Steraloids, Inc., (Newport, RI). Acetonitrile and HPLC-grade water were purchased from Sigma-Aldrich. Anise (Pimpinella anisum), coriander (Coriandrum sativum var. vulgare), cumin (Cuminum cyminum), dill (Anethum graveolens), fennel (Foeniculum vulgare var. vulgare), carrot seeds (Daucus carota), celery (Apium graveolens) and parsley (Petroselinum crispum) were purchased from local stores, angelica (Angelica archangelica) seeds from Mountain Rose Herbs (Eugene, OR), caraway (Carum carvi) from Frontier Natural Products Corp. (Norway, IA), and ajowan (Carum copticum) from Zamouri Spices (Olathe, KS). All enzymes, chemicals and solvents used for 32P-postlabeling assay were as described elsewhere (20). All other chemicals were of analytical grade.
Preparation of the extracts
Test spices were powdered and sieved to obtain fine powder. Spices (0.5 g each) were extracted individually three times with 10 vol. of water or acetonitrile separately for 2 h in a shaking water bath at room temperature (22 °C). Samples were centrifuged at 3,000x g for 15 min and supernatant was collected. Pooled extracts were concentrated under reduced pressure in SpeedVac (Savant, Thermo Scientific) and reconstituted in 1 ml 50% dimethyl sulfoxide (DMSO) and passed through 0.22 μm filter. Ajowan, carrot seeds, cumin and dill extracts were further diluted to 2.5, 1, 0.5, 0.25, 0.01 mg/ml for dose-response studies. The effect of temperature and time on the extraction efficiency was investigated by extracting 1 g of fennel with 10 vol of water at 22, 40, 60, 80 and 100 °C for 6, 4, 2, 1 and 0.5 h, respectively, and extracts were concentrated and reconstituted in 1 ml 50% DMSO.
Effect of the spice extracts on redox activity of 4E2/CuCl2
Salmon testis (st)-DNA (300 μg/ml) in 10 mM Tris-HCl (pH 7.4) was pre-incubated with either vehicle alone (DMSO) or aqueous (0.01 to 5 mg/ml) and non-aqueous extracts (0.012 to 6 mg/ml) for 15 min at 37 ºC. After adding 4E2 (50 μM; 2% ethanol) and CuCl2 (50 μM), the reaction mixture was further incubated at 37 °C for 4 h, and the DNA was isolated by solvent- extraction and ethanol precipitation as described (18, 20). The isolated DNA was tested for oxidative DNA adducts.
Effect of the spice extracts on microsomal activation of E2
st-DNA (300 μg/ml) was pre-incubated in 1 ml reactions containing 50 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 2.5 mM glucose-6-phosphate, 1 U/ml G6PDH, 0.5 mM NADP+, and α-naphthoflavone- induced rat liver microsomal proteins (1 mg/ml) in the presence of vehicle alone or aqueous and non-aqueous extracts (0.01 to 5 mg/ml) in a shaker water bath at 37 °C for 15 min. E2 (50 μM; 2% ethanol) and CuCl2 (50 μM) were added to the reaction mixture and further incubated for 4 h. Reactions were terminated by the addition of EDTA followed by centrifugation (9,000x g; 10 min). DNA was isolated from the supernatant by removal of RNA and proteins by digesting with RNases A and T1 and proteinase K and a series of extractions with phenol, phenol: Sevag (chloroform:isoamyl alcohol, 24:1), and Sevag, followed by precipitation of the DNA with ethanol (5). The DNA concentration was estimated spectrophotometrically.
Analysis of oxidative DNA adducts by 32P-postlabeling
Oxidative DNA adducts were analyzed by nuclease P1-mediated 32P-postlabeling/TLC (22). Briefly, 10 μg of DNA was digested with micrococcal nuclease and spleen phosphodiesterase (enzyme: substrate, 1:5, 37 °C, 5 h). Before further treatment with nuclease P1 (enzyme: substrate, 1:2.5; 37 °C; 1 h) to enrich DNA adducts, an aliquot was removed for evaluation of normal nucleotide levels. Enriched adducts and normal nucleotides were labeled with [γ-32P] ATP (60 μCi, 0.4 μM; 6,000 Ci/mmol) and T4 polynucleotide kinase. Labeled oxidative adducts were resolved by 2-D PEI-cellulose TLC (D1 = 1 M formic acid/40 mM sodium phosphate, pH 6; and D2 = isopropanol: 4 M ammonium hydroxide, 1:1). Normal nucleotides were labeled in parallel and resolved in 180 mM sodium phosphate, pH 6.0, by 1-D PEI-cellulose TLC. Adduct and normal nucleotides were detected and quantitated by Packard InstantImager. Adduct levels were expressed as adducts/106 nucleotides.
Statistical analysis
All calculations were performed with GraphPad Prizm statistical software (version 4.03; La Jolla, CA). One way analysis of variance (ANOVA) was done to compare the dose response. Student’s t-test was used to compare for parameters between treatment groups. We chose a p-value of 0.05 to declare significant results.
Results
Preparation of aqueous and non-aqueous extracts
The dry spices were powdered and extracted with 10 vol. of water or acetonitrile separately in order to extract polar or lipophilic compounds, respectively. Water is preferred as a solvent to determine its ability to extract bioactives as most of the folklore formulations or household consumption of spices are water based. Both aqueous and organic extracts were concentrated to dryness and stored at −80 °C until use and then reconstituted in 50% DMSO prior to use.
Inhibition of redox activity of 4E2
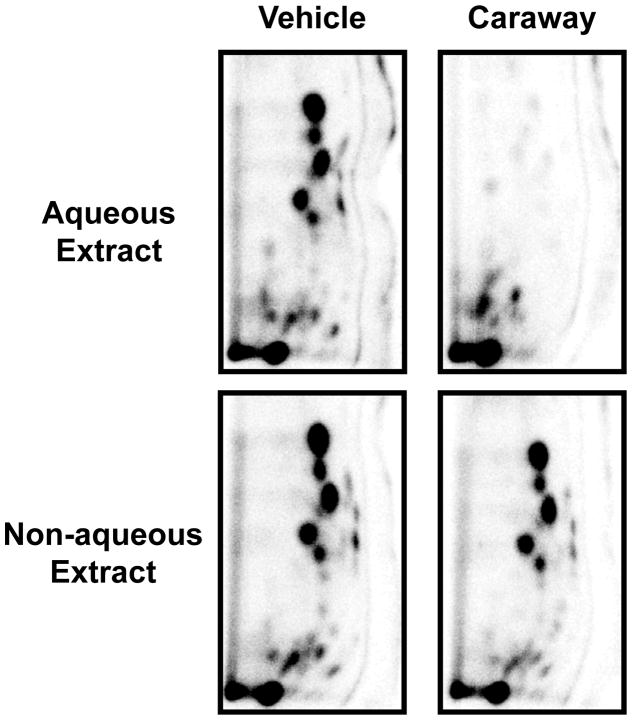
The antioxidant activity of the spice extracts was determined by incubating the aqueous or non-aqueous extracts with st-DNA damaged with oxygen free radicals from redox cycling of 4E2 by CuCl2. Analysis of oxidative DNA adducts by 32P-postlabeling assay revealed several oxidative DNA adducts (Fig. 2). These adducts have been assigned as polar DNA adducts formed by attack of hydroxyl radicals (23).
Figure 2.
Representative autoradiographs of 32P-labeled oxidative DNA adducts resulting from Cu(II)-mediated activation of 4E2 in the presence of either vehicle (2% ethanol) or aqueous and non-aqueous spice of caraway extracts. Labeled oxidative DNA adducts were resolved by two-directional PEI-cellulose TLC (D1 = 1 M formic acid/40 mM sodium phosphate, pH 6.0 onto a 6 cm Whatman no. 17 paper wick; D2 = isopropanol: 4 M ammonium hydroxide, 1:1) and detected by Packard InstantImager.
Aqueous extracts of fennel prepared under various conditions (22 °C for 6h, 40°C for 4 h, 60°C for 2 h, and 80°C for 1 h) showed essentially the same degree of adduct inhibition (94–97%) indicating that the length of extraction can be offset by increase in temperature. However, extracts prepared at 100 °C for 0.5 h showed somewhat lower adduct inhibition (81%) indicating some bioactives were lost at this temperature. Subsequent extractions for the remaining studies employed three extractions at room temperature for 2 h each.
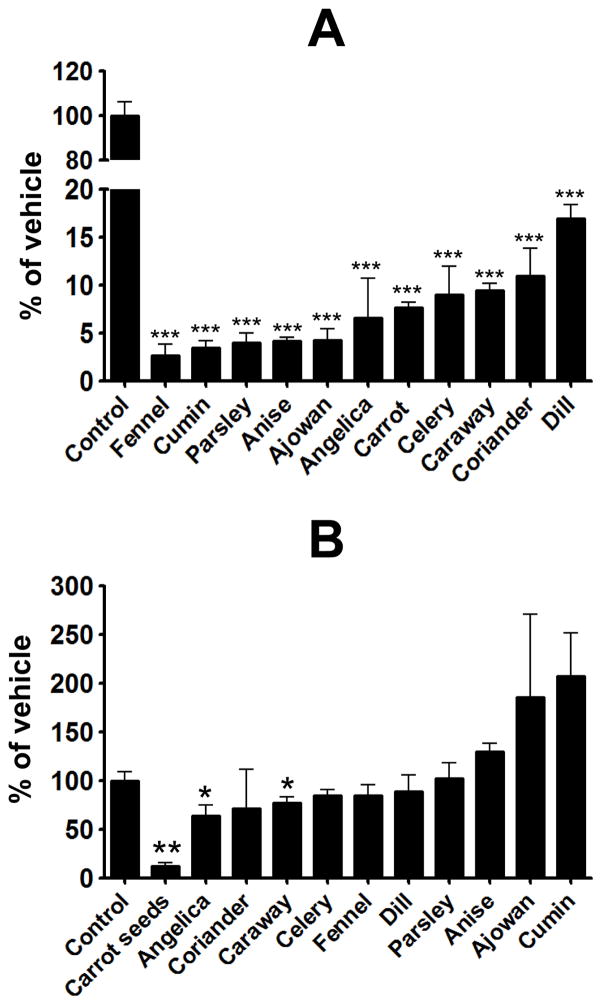
Aqueous extracts from fennel, cumin, parsley, anise, ajowan, angelica, carrot seeds, celery, caraway, coriander and dill exhibited 83–97% inhibition of the oxidative DNA adducts at a concentration of 0.4 mg/ml (Fig. 3A). Aqueous extracts of ajowan, carrot seeds and cumin were also tested at lower doses. Ajowan extract showed the highest antioxidant activity, with 40% and 75% inhibition at 0.01 and 0.05 mg/ml, respectively, followed by carrot seed extract which showed about 70% inhibition at 0.2 mg/ml, and cumin extract which showed about 20% inhibition at 0.2 mg/ml. With the exception of carrot seeds (85%), angelica (35%) and caraway (22%) (Fig. 3B) the non-aqueous extracts of these spices failed to show any significant protection. .
Figure 3.
Effect of aqueous extracts (A) and non-aqueous extracts of indicated spices on Cu(II)-mediated oxidatively generated DNA damage by 4E2. Experimental conditions are described in Materials and Methods. Levels of DNA adducts were determined as adducts/106 nucleotides and represented as % of control. Data represent average ± SD of four samples and a p-value of <0.05 was considered significant.
Inhibition of P450 activity by aqueous extracts
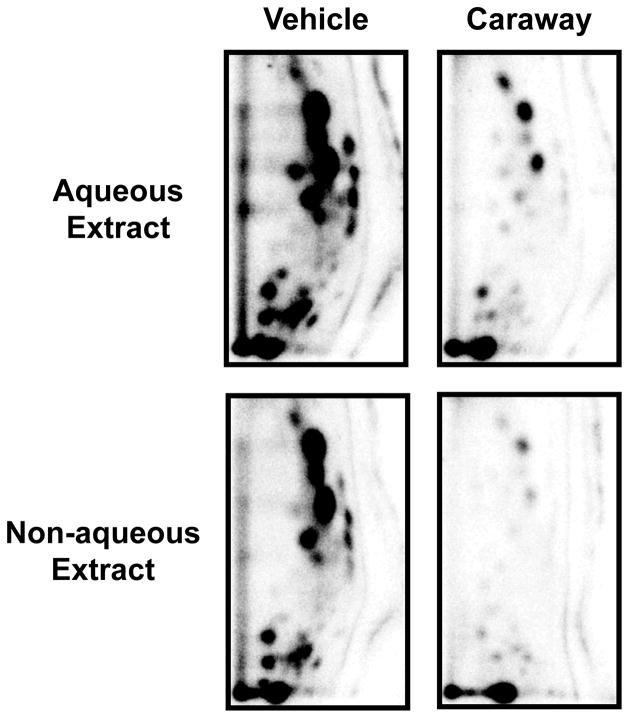
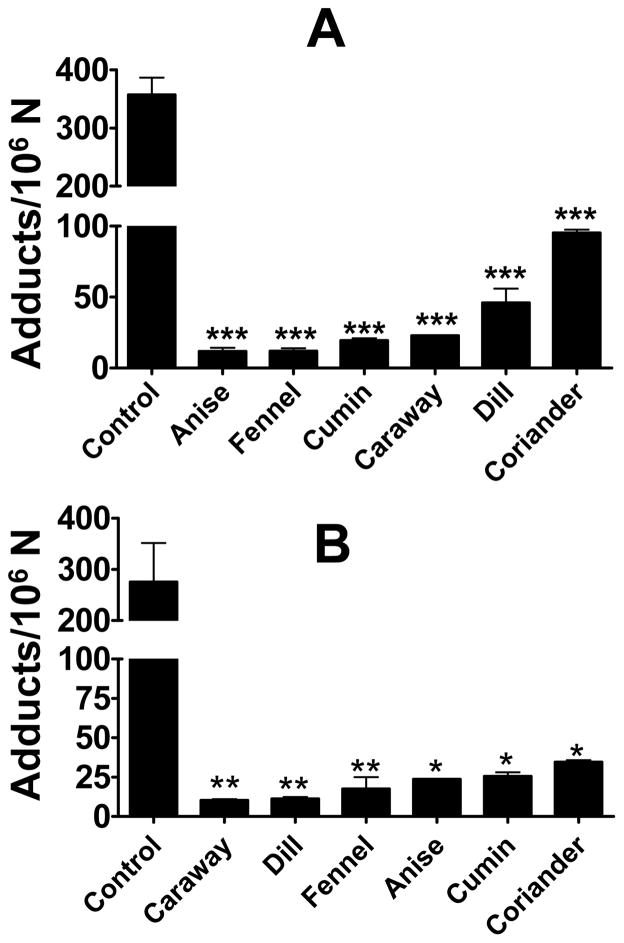
4E2 is formed by P450-mediated activation of E2 and represents a presumptive carcinogenic metabolite. In this study we used microsomal activation of E2, Cu2+-mediated activation of the resulting 2E2/4E2, and oxidative DNA adduct formation to determine effects of the spice extracts on metabolic activation of E2. Analysis of the purified DNA by 32P-postlabeling showed essentially the same adduct profile (Fig. 4) as found by Cu2+-4E2 reaction (Fig. 2). No qualitative differences were found in the absence or presence of the spice extracts, however, significant quantitative differences occurred (Fig. 4). Compared with the vehicle treatment (358 ± 58 adducts/106 nucleotides), the aqueous extracts of anise, fennel, cumin, caraway and dill inhibited the adduct formation almost completely (85–95%); coriander extract showed over 70% inhibition (Fig. 5A).
Figure 4.
Representative autoradiographs of 32P-labeled oxidative DNA adducts resulting from rat liver microsomes/Cu(II)-mediated activation of E2 in the presence of vehicle (2% ethanol) or caraway extracts. Labeled adducts were resolved by two-directional PEI-cellulose TLC (D1 = 1 M formic acid/40 mM sodium phosphate, pH 6.0 onto a 6 cm Whatman no. 17 paper wick; D2 = isopropanol: 4 M ammonium hydroxide, 1:1) and detected by Packard InstantImager.
Figure 5.
Effect of aqueous (A) and non- aqueous extracts (B) on rat liver microsome/Cu(II)-mediated oxidatively generated DNA damage by E2. Data represent average ± SD of four replicates, and a p-value of <0.05 was considered significant. Levels of oxidative DNA adducts were determined as adducts/106 nucleotides.
The non-aqueous extracts of all these six spices also showed essentially the same degree of adduct inhibition as observed with the aqueous extracts, i.e., 90–96% inhibition with extracts of anise, fennel, cumin, caraway and dill, and 83% inhibition with coriander extract (Fig. 5B).
Discussion
Antioxidants have attracted much interest for their protective effect against free radical damage associated with many degenerative diseases including cancer. We previously demonstrated by co-chromatography that the profile of polar oxidative adducts generated in vitro using either 4E2/CuCl2 or H2O2/CuCl2 were similar (23), indicating that DNA adducts resulting from redox activity of 4E2 in the presence of CuCl2 originated from oxidative mechanisms.
The potential of test spices (fennel, cumin, parsley, anise, ajowan, angelica, carrot seeds, celery, caraway, coriander and dill) to inhibit 4E2/Cu(II)-induced oxidative DNA adducts was evaluated in this study. Aqueous extracts of these spices showed high antioxidant potency by inhibiting the redox activity of 4E2, whereas non-aqueous extracts were essentially ineffective, except for carrot seeds, angelica and caraway which showed modest protection. It is evident that phytochemicals in aqueous extracts are more potent antioxidant than in non-aqueous extracts and likely to represent different group(s) of compounds. Our results corroborate the finding of Goswami and Chatterjee (24) who demonstrated high antioxidant activity of ajowan (Trachyspermum ammi) and fennel (Foeniculum vulgare) extracts. In another study aqueous extract of dill has been shown to possess higher hydroxyl radical scavenging activity compared to onion, pepper and garlic (25). Jayakumar and Kanthimathi (26) have also reported significant protection of hydrogen peroxide-induced DNA damage analyzing by comet assay with cumin, fennel and caraway extracts.
Several polyphenolics from these spices have been reported to possess antioxidant properties. Anethole is one of the chief components of anise and fennel and its derivative anethole dithiolethione showed antioxidant activity with their ability to suppress tumor necrosis factor and ability to reduce oxidative stress by acting as scavengers of hydroxyl radicals (27, 28). In another study, anethole induced apoptosis, and combinations of anethole with cisplatin and oxaliplatin were highly synergistic (29). There are several other examples of phenolics such as p-cynene (ajowan and cumin), limonene (celery and caraway), thymol (ajowan) and β-pinene (cumin and celery) that have been shown to possess high antioxidant properties (30–32). In addition, the biological properties of polar and non-polar compounds from spices have been reviewed (33–35). A good correlation of phenolic content with high antioxidant activity of has been established by Kim et al (25). However, the concentration of the phenolics and flavonoids in various herbs and spices varies depending on their cultivar and climate (25).
E2 treatment has been reported to enhance polar oxidative DNA adducts (36); however, the exact mechanism for this is unclear. The effect of E2 treatment on the expression of various P450 enzymes involved in its metabolism has been studied. Diethylstilbesterol, an E2 analogue, can induce CYP1A2 levels in mouse liver (37). E2 itself has been shown to increase CYP1A2 expression in hamster liver (38) and it is known that E2 induces the expression of several P450s involved in its metabolism (38). Thus, E2 treatment may induce an environment conducive to the production of its carcinogenic metabolites in the liver.
In this study we also examined the capacity of E2 to induce oxidative DNA adducts in a cell-free system and its modulation by the various spice extracts. Rat liver microsome/Cu(II)-mediated activation of E2 resulted in the formation of oxidative DNA adducts, as analyzed by 32P-postlabeling, and the adduct pattern was identical to the oxidative DNA adducts resulting from CuCl2-4E2 reaction. Further evidence in support of the microsomal E2 adducts being hydroxylated DNA adducts and not related to quinone derivatives of 2-/4-E2 is based on chromatographic properties (39).
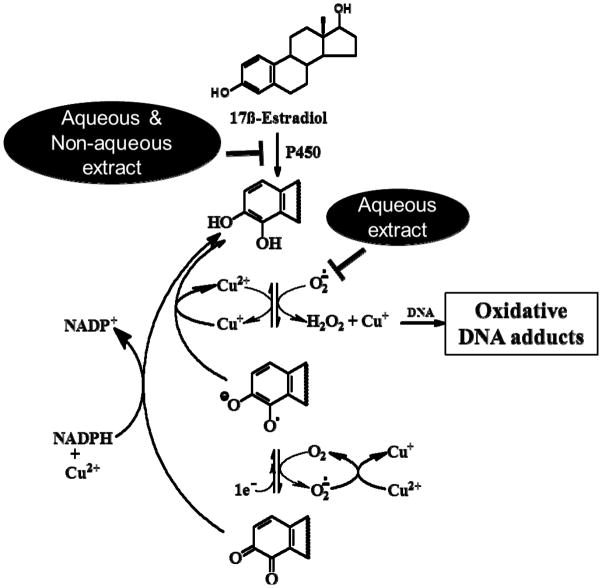
Both aqueous and non-aqueous extracts of all the test spices showed significant inhibition of microsomal E2 DNA adducts. These DNA adducts originate from a two-step process: First, E2 is metabolized by microsomal P450s to 2E2/4E2. Second, the resulting 2E2-/4E2 then produce ROS in the presence of CuCl2. Since the aqueous, not the non-aqueous extracts of the spices inhibited 4E2/CuCl2-generated oxidative adducts, the inhibition of the microsomal DNA adducts by the non-aqueous extracts may be attributed to the inhibition of the P450s, while the inhibition of the microsomal DNA adducts by the aqueous extracts likely resulted from the scavenging of the ROS (Fig. 6).
Figure 6.
Proposed mechanism of inhibition of estrogen mediated oxidative DNA adducts by aqueous and non-aqueous extracts. Partially adapted from Spencer et. al. (39).
Some of the spices investigated in this study such as ajowan, caraway, fennel, cumin and anise are common in the Asian and Middle-Eastern cuisines as well as in American eateries and are used to varying degrees in home recipes. It is estimated that daily intake of each of these spices may be 2–20 g in Indian subcontinent. Thus, human consumption of these spices in some parts of the world is already established at relatively high levels. Establishing in vivo efficacy of the aqueous and non-aqueous extracts of these spices will provide a simple and effective strategy for preventing breast and perhaps other cancers.
Acknowledgments
This work was supported from the USPHS grant CA-125152, and Agnes Brown Duggan Endowment. R.C.G. holds the Agnes Brown Duggan Chair in Oncological Research.
References
- 1.Toyokuni S. Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB life. 2008;60:441–7. doi: 10.1002/iub.61. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn MA. Oxygen free radicals and antioxidants. Am J Nurs. 2003;103:58–62. doi: 10.1097/00000446-200304000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991;69:965–8. doi: 10.1007/BF01645140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H, Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–31. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 6.Sies H, Mehlhorn R. Mutagenicity of nitroxide-free radicals. Arch Biochem Biophys. 1986;251:393–6. doi: 10.1016/0003-9861(86)90087-1. [DOI] [PubMed] [Google Scholar]
- 7.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–93. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 8.Araujo KL, Magnani M, Nascimento JA, Souza AL, Epaminondas PS, et al. Antioxidant activity of co-products from guava, mango and barbados cherry produced in the brazilian northeast. Molecules. 2014;19:3110–9. doi: 10.3390/molecules19033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubio L, Motilva MJ, Romero MP. Recent advances in biologically active compounds in herbs and spices: a review of the most effective antioxidant and anti-inflammatory active principles. Critical reviews in food science and nutrition. 2013;53:943–53. doi: 10.1080/10408398.2011.574802. [DOI] [PubMed] [Google Scholar]
- 10.Kausar H, Jeyabalan J, Aqil F, Chabba D, Sidana J, et al. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer letters. 2012;325:54–62. doi: 10.1016/j.canlet.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–7. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Toh JY, Tan VM, Lim PC, Lim ST, Chong MF. Flavonoids from fruit and vegetables: a focus on cardiovascular risk factors. Current atherosclerosis reports. 2013;15:368. doi: 10.1007/s11883-013-0368-y. [DOI] [PubMed] [Google Scholar]
- 13.Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–34. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- 14.Low Dog T. A reason to season: the therapeutic benefits of spices and culinary herbs. Explore. 2006;2:446–9. doi: 10.1016/j.explore.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–61. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahle KW, Brown I, Rotondo D, Heys SD. Plant phenolics in the prevention and treatment of cancer. Adv Exp Med Biol. 2010;698:36–51. doi: 10.1007/978-1-4419-7347-4_4. [DOI] [PubMed] [Google Scholar]
- 17.Best BP. Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 2009;12:199–208. doi: 10.1089/rej.2009.0847. [DOI] [PubMed] [Google Scholar]
- 18.Ravoori S, Vadhanam MV, Davey DD, Srinivasan C, Nagarajan B, et al. Modulation of novel DNA adducts during human uterine cervix cancer progression. Int J Oncol. 2006;29:1437–43. [PubMed] [Google Scholar]
- 19.Gupta RC, Arif JM. An improved (32)P-postlabeling assay for the sensitive detection of 8-oxodeoxyguanosine in tissue DNA. Chem Res Toxicol. 2001;14:951–7. doi: 10.1021/tx000131d. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RC, Spencer-Beach G. Natural and endogenous DNA adducts as detected by 32P-postlabeling. Regul Toxicol Pharmacol. 1996;23:14–21. doi: 10.1006/rtph.1996.0003. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RC. 32P-postlabeling for detection of DNA adducts. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. Plenum Press; New York: 1996. pp. 45–61. [Google Scholar]
- 22.Gupta RC. 32P-postlabelling analysis of bulky aromatic adducts. IARC Sci Publ. 1993:11–23. [PubMed] [Google Scholar]
- 23.Spencer WA, Vadhanam MV, Jeyabalan J, Gupta RC. Oxidative DNA Damage Following Microsome/Cu(II)-Mediated Activation of the Estrogens, 17β-Estradiol, Equilenin, and Equilin: Role of Reactive Oxygen Species. Chem Res Toxicol. 2012;25:305–314. doi: 10.1021/tx200356v. [DOI] [PubMed] [Google Scholar]
- 24.Goswami N, Chatterjee S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (carom) and Foeniculum vulgare Mill. (fennel) seed extracts. BioMed research international. 2014;2014:582767. doi: 10.1155/2014/582767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IS, Yang M, Goo TH, Jo C, Ahn DU, et al. Radical scavenging-linked antioxidant activities of commonly used herbs and spices in Korea. Int J Food Sci Nutr. 2012;63:603–609. doi: 10.3109/09637486.2011.641942. [DOI] [PubMed] [Google Scholar]
- 26.Jayakumar R, Kanthimathi MS. Dietary spices protect against hydrogen peroxide-induced DNA damage and inhibit nicotine-induced cancer cell migration. Food Chem. 2012;134:1580–1584. doi: 10.1016/j.foodchem.2012.03.101. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, deGraffenried LA. Anethole suppressed cell survival and induced apoptosis in human breast cancer cells independent of estrogen receptor status. Phytomedicine. 2012;19:763–7. doi: 10.1016/j.phymed.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–50. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 29.Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer Res. 2012;32:4843–50. [PubMed] [Google Scholar]
- 30.de Oliveira TM, de Carvalho RB, da Costa IH, de Oliveira GA, de Souza AA, et al. Evaluation of p-cymene, a natural antioxidant. Pharmaceutical biology. 2014:1–6. doi: 10.3109/13880209.2014.923003. [DOI] [PubMed] [Google Scholar]
- 31.de Lima VT, Vieira MC, Kassuya CA, Cardoso CA, Alves JM, et al. Chemical composition and free radical-scavenging, anticancer and anti-inflammatory activities of the essential oil from Ocimum kilimandscharicum. Phytomedicine. 2014;21:1298–302. doi: 10.1016/j.phymed.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Alavinezhad A, Boskabady MH. Antiinflammatory, Antioxidant, and Immunological Effects of Carum copticum L. and Some of Its Constituents. Phytother Res. 2014;28:1739–1748. doi: 10.1002/ptr.5200. [DOI] [PubMed] [Google Scholar]
- 33.Krzyzanowska J, Czubacka A, Oleszek W. Dietary phytochemicals and human health. Adv Exp Med Biol. 2010;698:74–98. doi: 10.1007/978-1-4419-7347-4_7. [DOI] [PubMed] [Google Scholar]
- 34.Kozlowska A, Szostak-Wegierek D. Flavonoids--food sources and health benefits. Roczniki Panstwowego Zakladu Higieny. 2014;65:79–85. [PubMed] [Google Scholar]
- 35.Srinivasan K. Antioxidant Potential of Spices and Their Active Constituents. Crit Rev Food Sci. 2014;54:352–372. doi: 10.1080/10408398.2011.585525. [DOI] [PubMed] [Google Scholar]
- 36.Aiyer HS, Vadhanam MV, Stoyanova R, Caprio GD, Clapper ML, et al. Dietary berries and ellagic acid prevent oxidative DNA damage and modulate expression of DNA repair genes. Int J Mol Sci. 2008;9:327–41. doi: 10.3390/ijms9030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmichael PL, Mills JJ, Campbell M, Basu M, Caldwell J. Mechanisms of hormonal carcinogenesis in the p53+/− hemizygous knockout mouse: studies with diethylstilbestrol. Toxicol Pathol. 2001;29(Suppl):155–60. doi: 10.1080/019262301753178564. [DOI] [PubMed] [Google Scholar]
- 38.Cribb AE, Knight MJ, Dryer D, Guernsey J, Hender K, et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:551–8. doi: 10.1158/1055-9965.EPI-05-0801. [DOI] [PubMed] [Google Scholar]
- 39.Spencer WA, Vadhanam MV, Jeyabalan J, Gupta RC. Oxidative DNA damage following microsome/Cu(II)-mediated activation of the estrogens, 17beta-estradiol, equilenin, and equilin: role of reactive oxygen species. Chem Res Toxicol. 2012;25:305–14. doi: 10.1021/tx200356v. [DOI] [PubMed] [Google Scholar]