Abstract
BACKGROUND
Vagal hyperactivity promotes atrial fibrillation (AF), which has been almost exclusively attributed to acetylcholine. Vasoactive intestinal polypeptide (VIP) and acetylcholine are neurotransmitters co-released during vagal stimulation. Exogenous VIP has been shown to promote AF by shortening action potential duration (APD), increasing APD spatial heterogeneity, and causing intra-atrial conduction block.
OBJECTIVE
The purpose of this study was to investigate the effects of neuronally released VIP on atrial electrophysiologic properties during vagal stimulation.
METHODS
We used a specific VIP antagonist (H9935) to uncover the effects of endogenous VIP released during vagal stimulation in canine hearts.
RESULTS
H9935 significantly attenuated (1) the vagally induced shortening of atrial effective refractory period and widening of atrial vulnerability window during stimulation of cervical vagosym-pathetic trunks (VCNS) and (2) vagal effects on APD during stimulation through fat-pad ganglion plexus (VGPS). Atropine completely abolished these vagal effects during VCNS and VGPS. In contrast, VGPS-induced slowing of local conduction velocity was completely abolished by either VIP antagonist or atropine. In pacing-induced AF during VGPS, maximal dominant frequencies and their spatial gradients were reduced significantly by H9935 and, more pronouncedly, by atropine. Furthermore, VIP release in the atria during vagal stimulation was inhibited by atropine, which may account for the concealment of VIP effects with muscarinic blockade.
CONCLUSION
Neuronally released VIP contributes to vagal effects on atrial electrophysiologic properties and affects the pathophysiology of vagally induced AF. Neuronal release of VIP in the atria is inhibited by muscarinic blockade, a novel mechanism by which VIP effects are concealed by atropine during vagal stimulation.
Keywords: Vagal stimulation, Atrial fibrillation, Vasoactive intestinal polypeptide
Introduction
The intrinsic cardiac neural network is an integral part of the cardiac autonomic network and includes neurons that release noncholinergic and nonadrenergic neural transmitters such as polypeptide Y and vasoactive intestinal polypeptide (VIP).1–5 VIP is a 28-base polypeptide that is co-released with acetylcholine (ACh) during vagal stimulation.6,7 We recently reported that exogenous VIP affects multiple ionic channels and causes shortening of atrial effective refractory periods (AERPs) and action potential duration (APD), with 1353 increased spatial heterogeneity, and slowing of intra-atrial conduction that precipitates conduction block.8 Furthermore, VIP perfusion increases the inducibility of atrial fibrillation (AF) by programmed stimulation.8 VIP also prolongs AF duration.9 However, data are lacking regarding the effects of neuronally released VIP on the atria during vagal hyper-activity. Our current study aimed to test the hypothesis that, consistent with previously described ionic mechanisms of exogenous VIP effects,8 neuronally released endogenous VIP may alter the atrial electrophysiologic properties and thereby contributes to the increased vulnerability to AF during vagal stimulation.
Methods
Animal preparation
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Texas Heart Institute in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Research Council). Mongrel dogs (weight 30–35 kg) were randomly assigned among the in vivo and ex vivo groups (see Expanded Methods in Online Supplementary Material).
Vagal stimulation was performed either through decentralized bilateral cervical vagosympathetic trunks (VCNS) or through ganglionated plexuses (GPs) in the pericardial fat-pad (VGPS). The strength of VCNS, 2-ms pulse width at 20 Hz, was set to achieve a 20% drop in heart rate or second-degree atrioventricular block. The strength of VGPS, 2-ms pulse width at 20 Hz, was set to achieve at least a 20-ms increase in PR interval. To assess the contributions of VIP and ACh effects during vagal stimulation, we used a specific competitive antagonist (H9935) of VIP receptors10 and atropine to block the receptor bindings of the respective transmitters.
Electrophysiologic experiments
During in vivo experiments, AERP was defined as the longest coupling interval (S1–S2) of the extrastimulus that failed to result in atrial capture, with the pacing output set at twice diastolic threshold. Atrial vulnerability window was defined as the range of the coupling intervals, during programmed stimulation from the proximal left inferior pulmonary vein, which resulted in AF lasting > 2 seconds.11
During ex vivo experiments with optical mapping in isolated atrial preparations with intact coronary supply,8 APD was quantified as APD75, which was defined as the interval between the time of local activation and the time when the optical signal had recovered by 75% from the peak value of upstroke. Local conduction velocity (CV) was calculated as previously described.12 AF dominant frequencies (DFs) were derived by fast Fourier transform (FFT) on 2-second recordings of optical during AF.13 The experimental protocol and methods are detailed in the Expanded Methods in the Online Supplementary Material.
Statistical analysis
All data are reported as mean ± SEM. Repeated measure analysis of variance was used for comparisons among multiple groups. A general linear mixed effect model was used to evaluate the effects of VIP antagonist and atropine. All statistical analyses were performed using SAS (version 9.3 for Windows, SAS Institute, Cary, NC). P ≤.05 was considered significant.
Results
Neuronally released VIP contributes to vagal effects on atrial electrophysiologic properties
In vivo experiment
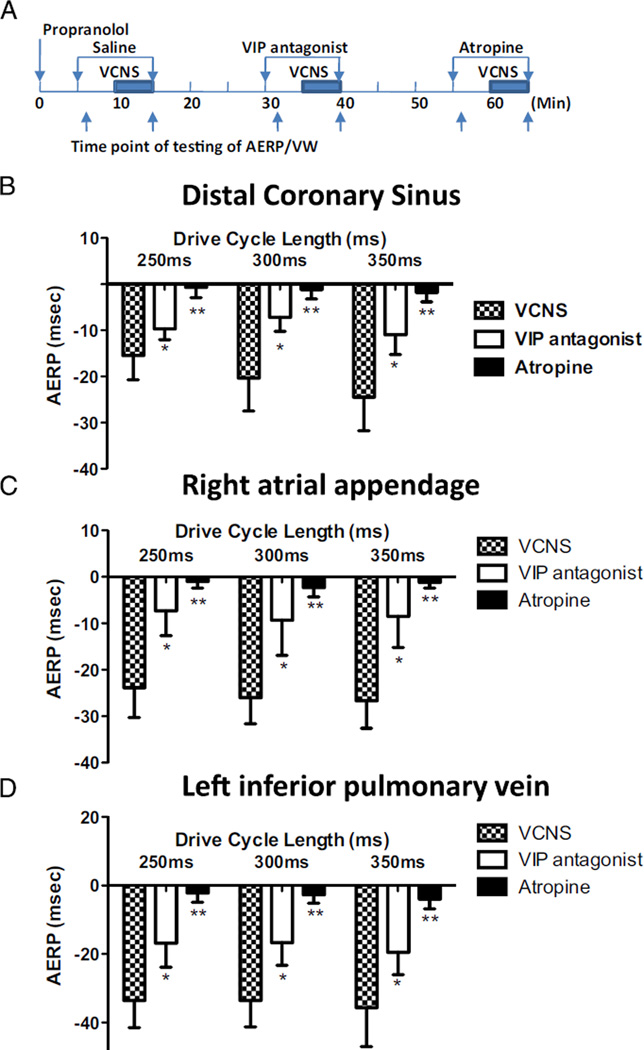
The effects of vagal stimulation of the AERP were quantified by measuring the difference between the AERP values determined immediately before and during VCNS (ΔAERP). ΔAERP was measured at 3 drive cycle lengths of 250, 300, and 350 ms at 3 sites: distal coronary sinus, right atrial appendage, and proximal left inferior pulmonary vein (n = 6; Figure 1). ΔAERP was determined during 3 episodes: (1) with infusion of normal saline (VCNS only), (2) with VIP antagonist (VCNS and H9935), and (3) with muscarinic blockade atropine (VCNS and atropine). H9935 significantly attenuated vagally induced ΔAERP at all 3 sites and 3 drive cycle lengths (P < .01), whereas atropine completely abolished ΔAERP (P < .001). Without VCNS, H9935 had no effect on AERP (see Online Supplementary Results, Table 1).
Figure 1.
Neuronally released vasoactive intestinal polypeptide (VIP) contributes to atrial effective refractory period (AERP) shortening during vagal stimulation through cervical vagosympathetic trunks (VCNS). A: Schematic representation of experiment protocol. Changes of atrial effective refractory periods (ΔAERP) were defined as the difference between AERP determined before and during vagal stimulation VCNS at cycle lengths of 250,300, and 350 ms from the coronary sinus (B), right atrial appendage (C), and left inferior pulmonary vein (D), respectively. Propranolol was used to block sympathetic effects. *P < .05, **P < .01 vs VCNS only. The absolute AERP values are presented in Online Supplementary Material, Table 2.
Ex vivo experiment
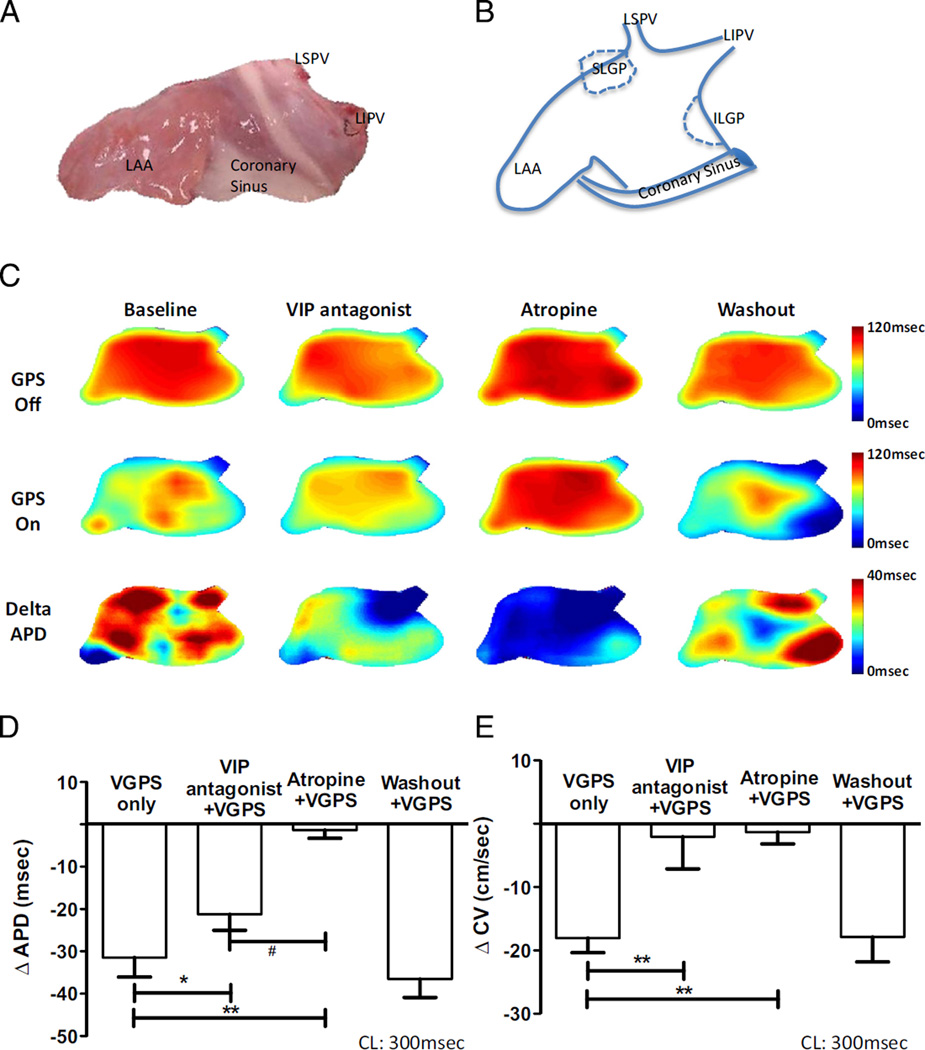
VGPS effect was quantified by ΔAPD75, that is the difference between APD75 determined with and without VGPS at a drive cycle length of 300 ms (n = 8; Figures 2A–2C). H9935 reduced ΔAPD75 during VGPS by about 30%, from 31.49 ± 4.6 ms to 21.24 ± 3.8 ms (P <.05 vs VGPS only), whereas atropine completely eliminated VGPS-induced ΔAPD75 (1.34 ± 1.91 ms, P <.001 vs VGPS only, P < .05 vs VPGS with VIP antagonist; Figure 2D). H9935 eliminated VGPS-induced reduction of CV (ΔCV: 2.04 ± 5.01 cm/s vs 18.04 ± 2.33 cm/s with VPGS only, P <.001) as well as atropine did (1.31 ± 1.89 cm/s, P < .001 vs VPGS only; Figure 2E). In addition, VGPS increased both standard deviations of APD (APD-SD) and CV (CV-SD), as indexes of spatial heterogeneity, which was significantly attenuated by H9935 and essentially eliminated by atropine (see Online Supplementary Results, Figure 1).
Figure 2.
Neuronally released vasoactive intestinal polypeptide (VIP) contributes to shortening of action potential duration (APD) and slowing of local conduction velocity (CV) during vagal stimulation through the ganglionated plexus (VGPS). A: Anatomic photograph of recording areas from the left atria. B: Schematic illustration of the recording area. C: Optical recordings from a representative experiment. Top row: APD maps recorded without VGPS in the following sequence (from the left to the right): at baseline, with VIP antagonist perfusion, with atropine perfusion, and after washout, respectively. Middle row: Same recordings as in the top row except VGPS was turned on. Bottom row: Pixel-by-pixel difference in APD by subtracting the middle row from the top row, indicating the amplitude and spatial distribution of APD changes in response to VGPS. Color bars indicate the range of APD in milliseconds. D: Composite data from 8 canine left atria indicating the effects of vagal stimulation on changes of APD (ΔAPD). Note VIP antagonist only partially blunted the effect of vagal stimulation on APD. E: Composite data from 8 canine left atria indicating the effects of vagal stimulation on changes in local conduction velocity (ΔCV) determined during pacing at 300 ms. Note both VIP antagonist and atropine could abolish vagal effect on CV. ILGP = inferior left ganglionated plexus; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; SLGP = superior left ganglionated plexus. *P < .05, **P <.01 vs baseline (VGPS only); P < .05 vs VIP antagonist plus VGPS. N = 8 dogs. CL = cycle length.
Both ΔAPD and ΔCV recovered toward their baseline values after 15-minute washout (ΔAPD: 36.51 ± 4.38 ms, P = .95; ΔCV: 17.83 ± 4.01 cm/s, P = .95 compared with initial recording during initial VGPS without VIP antagonist or atropine). Without VGPS, H9935 had no effect on APD or CV (see Online Supplementary Results, Tables 2 and 3).
Muscarinic modulation of neuronal VIP release during vagal stimulation
In vivo experiment
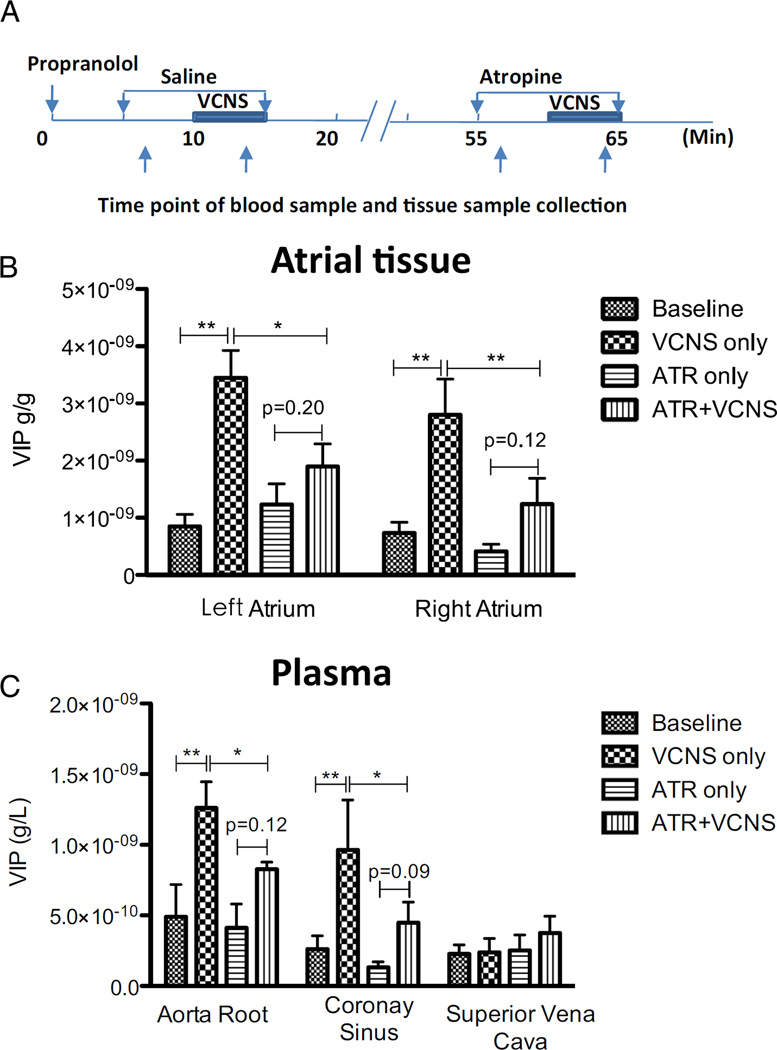
VCNS significantly increased VIP tissue content in both the
left atrium (LA) by 2.60 ± 0.57 ng/g (P < .01) and the right atrium (RA) by 2.07 ± 0.60 ng/g (P < .01; Figures 3A and 3B). However, with atropine, VCNS failed to significantly increase VIP tissue content (only by 0.67 ± 0.27 ng/g in LA, P = .20; and by 0.83 ± 0.48 ng/g in RA, P = .12). Atropine had no effect on baseline VIP tissue content without VCNS.
Figure 3.
Neuronal release of vasoactive intestinal polypeptide (VIP) during stimulation through cervical vagosympathetic trunks (VCNS) in vivo. A: Schematic representation of experiment protocol and sample collection time-points. B: Changes of VIP content in atrial tissue from the appendages. C: Changes of VIP concentrations in plasma VIP releases, as reflected by tissue content in left and right atria and plasma concentrations (in aortic root and coronary sinus), were increased by vagal stimulation at the cervical level, and such increased VIP release was inhibited by atropine (ATR) except that vagal stimulation had little effect on VIP concentration sampled from the superior vena cava. No sample was collected with washout because of the prolonged half-life of atropine in vivo. *P < .05; **P < .01.
Blood samples from 3 sites (aorta root, coronary sinus, and superior vena cava) were collected before and during VCNS, with and without atropine perfusion (Figure 3C). Without atropine, VCNS increased plasma VIP concentration by 0.77 ± 0.45 ng/L at the aorta root (P < .01) and by 0.70 ± 0.41 ng/L at the coronary sinus (P < .01) but did not affect the concentration of VIP in the superior vena cava (0.24 ± 0.02 ng/L vs 0.23 ± 0.02 ng/L, P = .45). Atropine did not affect plasma VIP concentration at any of the 3 sites without VCNS but reduced VCNS-induced increase in plasma VIP concentration at the aorta root to 0.43 ± 0.15 ng/L (P < .01 vs VCNS only) and at the coronary sinus to 0.31 ± 0.16 ng/L (P < .01 vs VCNS only).
Ex vivo experiment
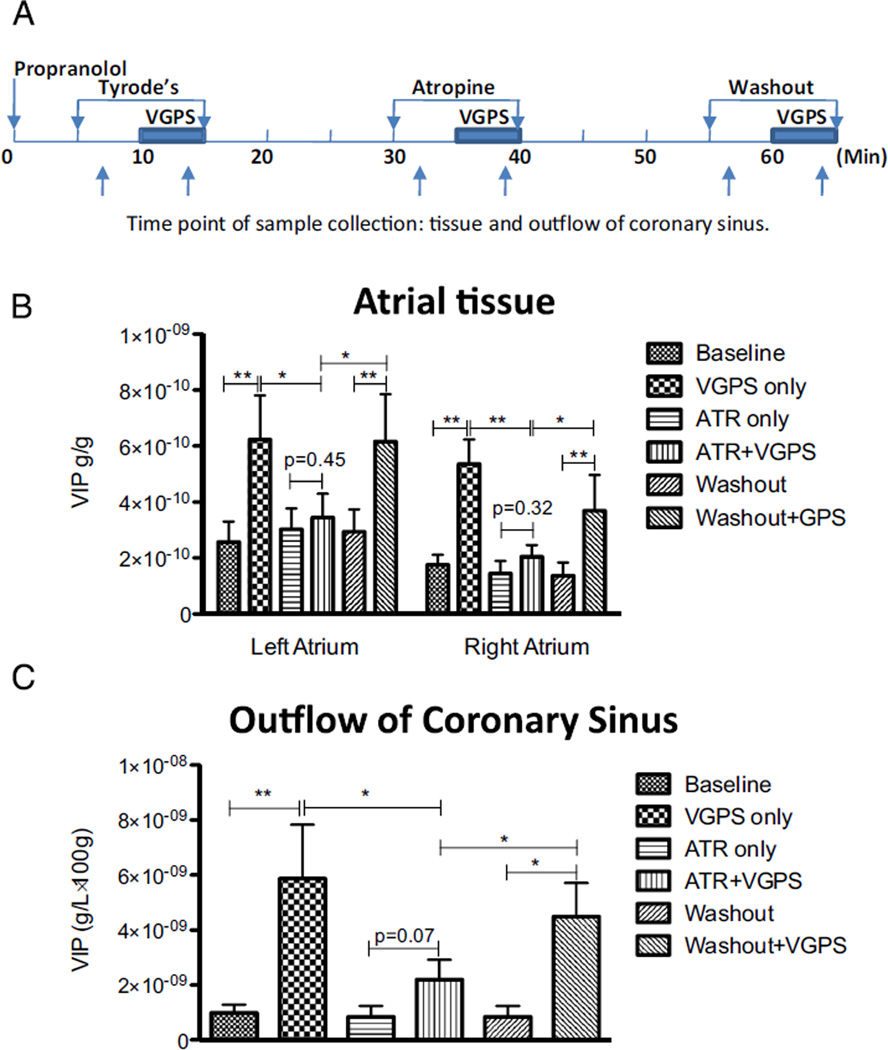
Similarly, tissue VIP contents in LA and RA and VIP concentrations in coronary sinus were compared with and without VGPS in Langendorff-perfused atria (Figure 4A). VGPS increased VIP tissue content by 3.62 ± 01.40 ng/g (P <.01) in LA and by 3.67 ± 0.84 ng/g (P < .01) in RA, which was almost eliminated by atropine (0.33 ± 1.40 ng/g, P = .45, in LA and 0.62 ± 0.41 ng/g, P = .32 in RA, respectively; Figure 4B).
Figure 4.
Neuronal release of vasoactive intestinal polypeptide (VIP) during stimulation through the ganglionated plexus (VGPS) ex vivo. A: Schematic representation of experiment protocol and sample collection time-points. B: Changes of VIP content in atrial tissue. C: Changes of VIP concentrations in plasma. VIP releases, as reflected by tissue content in left and right atria and plasma concentration (coronary sinus), were increased by vagal stimulation at the GP level, but such increased VIP release was inhibited by atropine (ATR). Note that muscarinic inhibition of VIP release seemed to be more complete with vagal stimulation at the GP level compared with preganglionic vagal stimulation (Figure 4) and was reversible once atropine was washed out. *P < .05; **P < .01.
In addition, atropine inhibited VGPS-induced increase of VIP concentration in outflow of coronary sinus from 4.49 ± 2.0 ng/ (L•100 g) to 1.35 ± 5.85 ng/(L•100 g) (Figure 4C).
After atropine washout, the VGPS-induced increases in VIP tissue contents and concentration in coronary sinus were recovered (Figures 4B and 4C). Atropine had no effect on VIP tissue content or concentration in coronary sinus outflow without VGPS.
Neuronally released VIP contributes to vagal effects in atrial fibrillation
In vivo experiment
VCNS increased the atrial vulnerability window (AVW) from 3.3 ± 1.7 ms to 38.0 ± 5.5 ms (ΔAVW: 34.2 ± 6.8 ms, P < .001, n = 6). H9935 significantly attenuated such vagally induced increase in AVW (ΔAVW decreased to 16.7 ± 5.1 ms, P < .05). In contrast, atropine completely eliminated such increase in AVW (ΔAVW: −1.7 ± 2.1 ms, P < .001). Without VCNS, H9935 had no effects on AVW (ΔAVW: 0.0 ± 2.2 ms, P = .92).
Ex vivo experiment
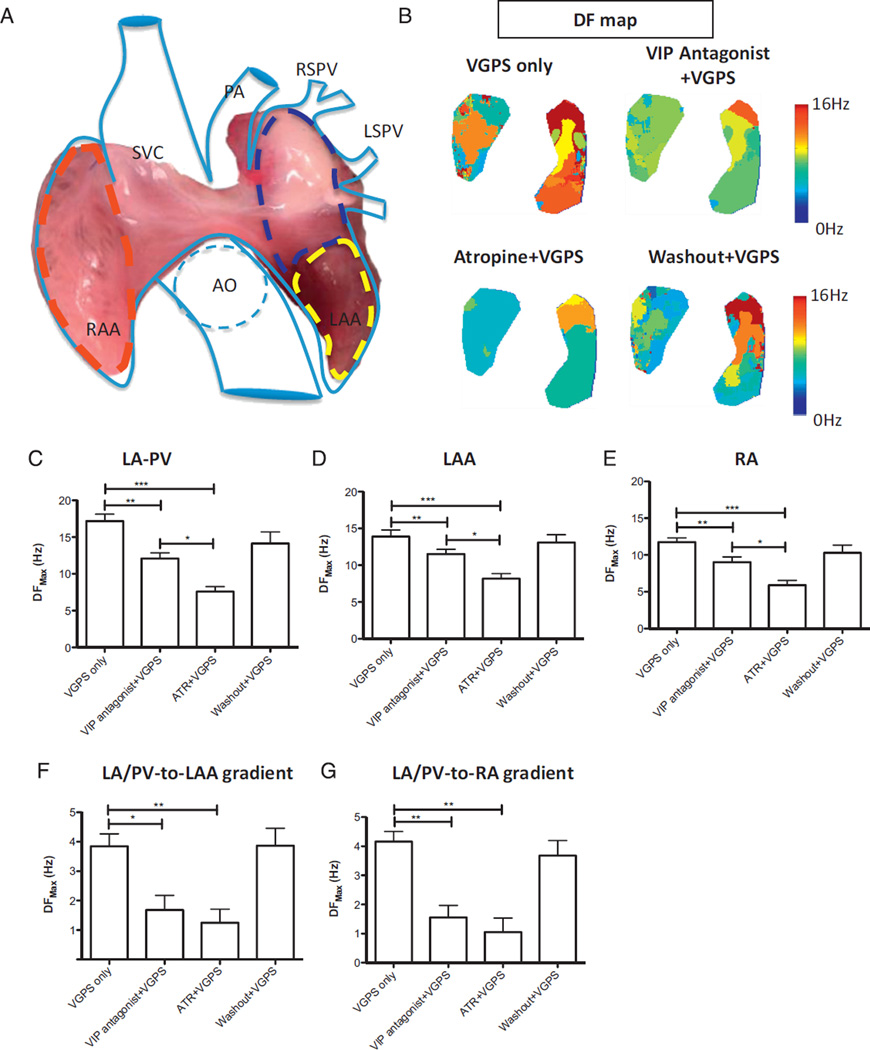
Sustained AF was induced with rapid pacing from the proximal left inferior pulmonary vein in 6 dogs during VGPS. No sustained AF was induced without VGPS. Continued VGPS was required for AF maintenance during which the highest values of DFmax were seen in the LA–PV region (17.2 ± 0.94 Hz; Figure 5A). DFmax was lower in the left atrial appendage (LAA; 13.9 ± 0.89 Hz) and the lowest in the RA (11.8 ± 0.57 Hz; Figure 5B). H9935 decreased DFmax to 12.1 ± 0.78 Hz in the LA–PV region, 11.5 ± 0.64 Hz in the LAA, and 9.0 ± 0.72 Hz in the RA (P < .01 vs VGPS only). Atropine decreased DFmax dramatically to 7.6 ± 0.68 Hz in LA–PV, 8.2 ± 0.67 Hz in LAA, and 5.9 ± 0.63 Hz in RA (P < .001 vs VGPS only, and P < .05 vs VGPS plus H9935). After washout, DFmax of AF recovered toward baseline (14.2 ± 1.56 Hz in LA–PV, 13.1 ± 1.06 Hz in LAA, and 10.3 ± 1.04 Hz in RA, P = NS vs VGPS only; Figures 5C–5E)
Figure 5.
Neuronally released vasoactive intestinal polypeptide (VIP) affects the dominant frequency (DF) of induced atrial fibrillation (AF) during vagal stimulation through the ganglionated plexus (VGPS). A: Anatomic photograph and schematic illustration of recording and analysis area in biatrial preparation. B: Maps of DFs in pacing-induced AF during VGPS only, VGPS with VIP antagonist perfusion, VGPS with atropine perfusion, and VGPS after washout of atropine obtained in a representative experiment. Note that both VIP antagonist and atropine reduced DFs, although the effect of atropine was quantitatively more pronounced than that of VIP antagonist. Color bar indicates the range of DFs. C, D: Composite data showed similar results in all 3 regions of the atria. C: DF distribution in the left atrium and its junction with pulmonary veins (LA–PV; encircled by blue dashed line in A). D: DF distribution in the left atrial appendage (LAA; encircled by yellow dashed line in A). E: DF distribution in the right atrium (RA; encircled by red dashed line in A). The gradient of DFmax between LA–PV and RA and that between LA–PV and RA also was decreased by either VIP antagonist or atropine as shown in F and G, respectively). *P < .05; **P < .01; ***P < .001. N = 6 dogs. AO = aorta; LAA = left atrial appendage; LSPV = left superior pulmonary vein; PA = pulmonary vein; RAA = right atrial appendage; RSPV = right superior pulmonary vein; SVC = superior vena cava.
The gradients of DFmax between LA–PV and LAA (LA/ PV-to-LAA gradient) and between LA–PV and right atrial appendage (RAA; LA/PV-to-RA gradient) were analyzed (Figures 5F and 5G). Compared to VGPS only, H9935 decreased LA/PV-to-LAA gradient from 3.8 ± 0.42 Hz to 1.7 ± 0.5 Hz (P < .05), and LA/PV-to-RA gradient from 4.2 ± 0.34 Hz to 1.6 ± 0.41 Hz (P < .01). Atropine also decreased both gradients significantly (LA/PV-to-LAA gradient: 1.2 ± 0.46 Hz, P < .01 vs VGPS only; LA/PV-to-RAA gradient: 1.1 ± 0.48 Hz, P < .01 vs VGPS only). The decreases in the gradients in response to H3395 and to atropine were not significantly (P > .40). After washout, DFmax gradients were again raised back toward baseline with VGPS only (LA/PV-to-LAA gradient: 3.8 ± 0.59 Hz, P = .39 vs VGPS only; LA/PV-to-RAA gradient: 3.7 ± 0.52 Hz, P = .28 vs VGPS only).
Discussion
Our major findings are as follows. In the presence of endogenous ACh, neuronally released VIP (1) significantly contributes to vagally induced AERP and atrial APD shortening and conduction slowing and (2) may influence AF maintenance by affecting the DFs during AF. These findings confirm our previous report of exogenous VIP effects on ionic currents and support the physiologic relevance of VIP effects on ionic currents.8 Furthermore, we demonstrated that muscarinic blockade inhibits neuronal VIP release in the atria and conceals the VIP effects.
Effects of neuronally released endogenous VIP in the atria
Vagal stimulation results in shortened atrial APD and refractoriness with increased spatial heterogeneity and thereby increases vulnerability to AF, which has been mostly attributed to ACh.3,14–17 Our study provides the first evidence that endogenous VIP synergistically contributes to vagally induced AERP/APD shortening (Figure 1 and 3), which can be accounted for by VIP effect on the slowly activating delayed rectifier potassium current IKs in the presence of ACh (see Online Supplementary Results, Figure 2). VIP increases the spatial heterogeneity of APD distribution, partly due to the uneven expression of VIP receptors among atrial myocytes8,18 and the difference in the expression of repolarizing currents, especially Ito and IKs, among different regions of the atria.8,19
Rosenshtraukh et al20,21 first described vagally induced conduction delay/block. ACh-induced increase in sink-to-source mismatch was proposed to account for the vagally induced conduction block during AF.22 Hirose et al15 demonstrated that intra-atrial conduction delay/block was more evident during vagal stimulation, when both ACh and VIP are released, than during ACh infusion alone. This difference could be explained by the noncholinergic vagal effects mediated through VIP, which suppresses the sodium current INa.8 VIP antagonist could completely abolish the vagal effects on conduction velocity during pacing (Figure 3), supporting the hypothesis that vagally released VIP contributes significantly to the vagal effect on conduction.
Muscarinic receptor-mediated release of VIP in atria during vagal stimulation
Atropine abolishes all cardiac vagal effects with few exceptions, such as VIP effects on accelerating the sinus rhythm.7 However, our data during VCNS and VGPS support that VIP contributes to the vagal effects in atria. We further demonstrated that atropine inhibits neuronal VIP release in atrial tissues during vagal stimulation (Figures 3 and 4). This represents a novel mechanism by which intra-cardiac neuronal VIP release is mediated and helps to explain how atropine could conceal the effects of VIP in the atria during vagal stimulation.23,24 Our data also indicate that a portion of neuronal VIP release may not be mediated by muscarinic receptors as suggested by a trend toward an increased VIP concentration sampled from (1) the aortic root during VCNS, which could be extracardiac as VIP is released from the pulmonary circulation,25 and (2) the coronary sinus during both VCNS and VGPS with muscarinic blockade. Previous reports have shown that release of VIP into the sinoatrial and atrioventricular nodes is not affected by atropine and that VIP innervations in the sinoatrial and atrioventricular nodes are different from those in the atria.2,26,27 Our hypothesis is further corroborated by previous reports that atropine inhibited vagally induced VIP release from the gastrointestinal track in pigs28 and in dogs.29
Furthermore, our data also suggest that VIP-releasing neurons in atria may be governed by a second-order system and, therefore, may be an important component of the local circuit neuron network.30 This is particularly interesting and potentially significant because VIP release is also increased during acute ischemia and heart failure where local circuit neuron network signaling may be activated and even dysfunctional but parasympathetic tone usually is not increased.
Synergistic effects of VIP and ACh in AF
Both atropine and VIP antagonist decreased the DFs of AF and the gradient of DFs between LA and RA during vagal stimulation, although to a lesser degree. Our data indicate that VIP and ACh may synergistically increase DFs and affect AF maintenance.9 This observation may be explained by VIP effects that (1) enhance IKs and abbreviate APD/ AERP and (2) suppress INa and slow intra-atrial conduction.8 Theoretically, slow conduction could result in reduced speed of revolution of rotor(s) and thereby decrease DFs. However, it was shown that DFs are primarily determined by refractoriness of atrial myocardium that limits the maximum frequency of resolution of rotors31.
Study limitations
Uncovering the effects of endogenous neural transmitters in vivo or ex vivo depends on the availability of specific receptor antagonist. In addition to evidence reported in the literature,10,32 use of H9935 as a specific VIP antagonist was supported by the following: (1) it had no direct effects on the atria without vagal stimulation, (2) the results of our current study with H9935 are consistent with the effects of exogenous VIP in both human and canine atria,8 and (3) H9935 completely reversed the effects of exogenous VIP (see Online Supplementary Material, Table 3).
Pituitary adenylate cyclase-activating polypeptide (PACAP) is the only known non-VIP neural transmitter that may bind with VIP receptors.33 Previous experiments indicate that PACAP shortens AERP and induces AF by activating postganglionic parasympathetic nerves and, thereby, enhancing ACh release rather than by its direct effect on atrial tissue.34 PACAP does not affect intra-atrial conduction velocity.34
We stimulated vagal hyperactivity by neural stimulation but did not examine the effects of ACh perfusion on VIP release. It is possible that an overwhelming amount of exogenous ACh effects could completely overshadow endogenous VIP effects. Furthermore, neuronal VIP release is modulated by the frequency of vagal impulses and might be affected only minimally by ACh perfusion as tonic stimulation.7 Further studies may be warranted to identify the subtype(s) of muscarinic receptors responsible for modulating neuronal VIP release.
Only the superior and inferior left GPs were simultaneously stimulated in our current study, although there are differences in cardiac response to stimulation of different GPs.35 It will be important to investigate the role of individual GPs in modulating VIP release in the atria in future studies.
Conclusion
Our study provides the first evidence that neuronally released VIP contributes to vagally induced changes in atrial electro-physiologic properties in the presence of concomitantly released ACh. We further demonstrated that neuronally released VIP may participate in the pathophysiology of vagally induced AF, as suggested by previous studies.5,8,9 Neuronal VIP release in the atria during vagal stimulation is mediated by muscarinic receptors, a novel mechanism by which the effects of neuronally released VIP in the atria are concealed by atropine administration.
Supplementary Material
CLINICAL PERSPECTIVES.
Autonomic dysfunctions, including abnormally increased vagal tone, have been shown to promote atrial fibrillation (AF). Vagal effects in AF have been attributed to the effects of acetylcholine. Our study demonstrated that vagally released noncholinergic neural polypeptide (vasoactive intestinal polypeptide [VIP] also contribute to vagal effects in the atria and provided the first evidence that VIP release in the atria is mediated by muscarinic receptors, a novel mechanism that atropine could conceal VIP effects. A full understanding of all the factors, such as those described in our study, that contribute to the pathogenesis of AF is necessary to provide a mechanistic foundation for advancement of AF management and development of new therapeutic innovations. Further studies of the interaction among various components of the cardiac innervations and the molecular basis of VIP effects including signal transduction pathways will be required to identify potential pharmacologic interventions and to facilitate the translation of research results into clinical practice.
Acknowledgments
We thank Drs. Wilsom Lam and Yong-Jian Geng for careful proofreading of the manuscript.
This work was supported in part by American Heart Association Grants 11GRNT8000093 to Dr. Cheng and 13EIA14560061 to Dr. Wehrens; and National Institute of Health Grants R01-HL089598 and R01-HL117641 to Dr. Wehrens.
ABBREVIATIONS
- ACh
acetylcholine
- AERP
atrial effective refractory period
- APD
action potential duration
- AVW
atrial vulnerability window
- CV
conduction velocity
- DF
dominant frequency
- GP
ganglionated plexus
- LA
left atrium
- LAA
left atrial appendage
- RA
right atrium
- RAA
right atrial appendage
- VCNS
vagal stimulation through cervical vagosympathetic trunks
- VGPS
vagal stimulation through ganglionated plexus
- VIP
vasoactive intestinal polypeptide
Footnotes
This work was presented in part at the 34th Annual Scientific Sessions of the Heart Rhythm Society, May 8-11, 2013, Denver, Colorado.
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.hrthm.2015.03.003.
References
- 1.Anderson FL, Kralios AC, Reid B, Hanson GR. Release of vasoactive intestinal peptide and neuropeptide Y from canine heart. Am J Physiol. 1993;265:H959–H965. doi: 10.1152/ajpheart.1993.265.3.H959. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FL, Wynn JR, Kimball J, Hanson GR, Hammond E, Hershberger R, Kralios AC. Vasoactive intestinal peptide in canine hearts: effect of total cardiac denervation. Am J Physiol. 1992;262:H598–H602. doi: 10.1152/ajpheart.1992.262.2.H598. [DOI] [PubMed] [Google Scholar]
- 3.Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marron K, Wharton J, Sheppard MN, Fagan D, Royston D, Kuhn DM, de Leval MR, Whitehead BF, Anderson RH, Polak JM. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 1995;92:2343–2351. doi: 10.1161/01.cir.92.8.2343. [DOI] [PubMed] [Google Scholar]
- 5.Yang D, Xi Y, Ai T, Wu G, Sun J, Razavi M, Delapasse S, Shurail M, Gao L, Mathuria N, Elayda M, Cheng J. Vagal stimulation promotes atrial electrical remodeling induced by rapid atrial pacing in dogs: evidence of a noncholinergic effect. Pacing Clin Electrophysiol. 2011;34:1092–1099. doi: 10.1111/j.1540-8159.2011.03133.x. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg JM, Hokfelt T, Kewenter J, Pettersson G, Ahlman H, Edin R, Dahlstrom A, Nilsson G, Terenius L, Uvnas-Wallensten K, Said S. Substance P-, VIP-, and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology. 1979;77:468–471. [PubMed] [Google Scholar]
- 7.Hill MR, Wallick DW, Martin PJ, Levy MN. Frequency dependence of vasoactive intestinal polypeptide release and vagally induced tachycardia in the canine heart. J Auton Nerv Syst. 1993;43:117–122. doi: 10.1016/0165-1838(93)90348-x. [DOI] [PubMed] [Google Scholar]
- 8.Xi Y, Wu G, Ai T, et al. Ionic mechanisms underlying the effects of vasoactive intestinal polypeptide on canine atrial myocardium. Circ Arrhythm Electrophysiol. 2013;6:976–983. doi: 10.1161/CIRCEP.113.000518. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Scherlag BJ, Fan Y, Varma V, Male S, Chaudhry MA, Huang C, Po SS. Inducibility of atrial fibrillation after GP ablations and “autonomic blockade”: evidence for the pathophysiological role of the nonadrenergic and noncholinergic neurotransmitters. J Cardiovasc Electrophysiol. 2013;24:188–195. doi: 10.1111/j.1540-8167.2012.02449.x. [DOI] [PubMed] [Google Scholar]
- 10.Gozes I, McCune SK, Jacobson L, Warren D, Moody TW, Fridkin M, Brenneman DE. An antagonist to vasoactive intestinal peptide affects cellular functions in the central nervous system. J Pharmacol Exp Ther. 1991;257:959–966. [PubMed] [Google Scholar]
- 11.Razavi M, Zhang S, Yang D, Sanders RA, Kar B, Delapasse S, Ai T, Moreira W, Olivier B, Khoury DS, Cheng J. Effects of pulmonary vein ablation on regional atrial vagal innervation and vulnerability to atrial fibrillation in dogs. J Cardiovasc Electrophysiol. 2005;16:879–884. doi: 10.1111/j.1540-8167.2005.50048.x. [DOI] [PubMed] [Google Scholar]
- 12.Bayly PV, KenKnight BH, Rogers JM, Hillsley RE, Ideker RE, Smith WM. Estimation of conduction velocity vector fields from epicardial mapping data. IEEE Trans Biomed Eng. 1998;45:563–571. doi: 10.1109/10.668746. [DOI] [PubMed] [Google Scholar]
- 13.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 14.Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. doi: 10.1111/j.1540-8167.1996.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirose M, Carlson MD, Laurita KR. Cellular mechanisms of vagally mediated atrial tachyarrhythmia in isolated arterially perfused canine right atria. J Cardiovasc Electrophysiol. 2002;13:918–926. doi: 10.1046/j.1540-8167.2002.00918.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 17.Schuessler RB, Rosenshtraukh LV, Boineau JP, Bromberg BI, Cox JL. Spontaneous tachyarrhythmias after cholinergic suppression in the isolated perfused canine right atrium. Circ Res. 1991;69:1075–1087. doi: 10.1161/01.res.69.4.1075. [DOI] [PubMed] [Google Scholar]
- 18.Christophe J, Waelbroeck M, Chatelain P, Robberecht P. Heart receptors for VIP, PHI and secretin are able to activate adenylate cyclase and to mediate inotropic and chronotropic effects. Species variations and physiopathology. Peptides. 1984;5:341–353. doi: 10.1016/0196-9781(84)90232-8. [DOI] [PubMed] [Google Scholar]
- 19.Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL, Kasprowicz K, Bhatta L, Puskas F, Kalifa J, Jalife J. Left versus right atrial difference in dominant frequency, K(þ) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm. 2009;6:1415–1422. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenshtraukh LV, Zaitsev AV, Fast VG, Pertsov AM, Krinsky VI. Vagally induced depression of impulse propagation as a cause of atrial tachycardia. J Mol Cell Cardiol. 1991;23(Suppl 1):3–9. doi: 10.1016/0022-2828(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 21.Rosenshtraukh LV, Zaitsev AV, Fast VG, Pertsov AM, Krinsky VI. Vagally induced block and delayed conduction as a mechanism for circus movement tachycardia in frog atria. Circ Res. 1989;64:213–226. doi: 10.1161/01.res.64.2.213. [DOI] [PubMed] [Google Scholar]
- 22.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 23.Billette J, Tadros R. Concealed autonomic mechanisms underlying atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:196–198. doi: 10.1111/jce.12050. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Scherlag BJ, Niu G, Lu Z, Patterson E, Liu S, Lazzara R, Jackman WM, Po SS. Autonomic elements within the ligament of Marshall and inferior left ganglionated plexus mediate functions of the atrial neural network. J Cardiovasc Electrophysiol. 2009;20:318–324. doi: 10.1111/j.1540-8167.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Lee D, Sung YK. Prospect of vasoactive intestinal peptide therapy for COPD/PAH and asthma: a review. Respir Res. 2011;12:45. doi: 10.1186/1465-9921-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson FL, Hanson GR, Reid B, Thorpe M, Kralios AC. VIP and NPY in canine hearts. Distribution and effect of total and selective parasympathetic denervation. Am J Physiol. 1993;265:H91–H95. doi: 10.1152/ajpheart.1993.265.1.H91. [DOI] [PubMed] [Google Scholar]
- 27.Hill MR, Wallick DW, Mongeon LR, Martin PJ, Levy MN. Vasoactive intestinal polypeptide antagonists attenuate vagally induced tachycardia in the anesthetized dog. Am J Physiol. 1995;269:H1467–H1472. doi: 10.1152/ajpheart.1995.269.4.H1467. [DOI] [PubMed] [Google Scholar]
- 28.Messell T, Harling H, Poulsen SS, Bersani M, Holst JJ. Extrinsic control of the release of galanin and VIP from intrinsic nerves of isolated, perfused, porcine ileum. Regul Pept. 1992;38:179–198. doi: 10.1016/0167-0115(92)90101-y. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Ito S, Ohta T, Asano T, Nakazato Y. Vasoactive intestinal peptide released by acetylcholine in the dog ileum. J Auton Nerv Syst. 1994;48:167–174. doi: 10.1016/0165-1838(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 30.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol. 2013;591:4515–4533. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 32.Moody TW, Zia F, Draoui M, Brenneman DE, Fridkin M, Davidson A, Gozes I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc Natl Acad Sci U S A. 1993;90:4345–4349. doi: 10.1073/pnas.90.10.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 34.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Effects of pituitary adenylate cyclase-activating polypeptide on canine atrial electrophysiology. Am J Physiol Heart Circ Physiol. 2001;281:H1667–H1674. doi: 10.1152/ajpheart.2001.281.4.H1667. [DOI] [PubMed] [Google Scholar]
- 35.Randall DC, Brown DR, McGuirt AS, Thompson GW, Armour JA, Ardell JL. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1066–R1075. doi: 10.1152/ajpregu.00167.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.