Supplemental Digital Content is available in the text.
Keywords: hemodynamics, septic, shock, survival rate, vasopressor agents
Objective:
Selective vasopressin V1A receptor agonists may have advantages over arginine vasopressin in the treatment of septic shock. We compared the effects of selepressin, a selective V1A receptor agonist, arginine vasopressin, and norepinephrine on hemodynamics, organ function, and survival in an ovine septic shock model.
Design:
Randomized animal study.
Setting:
University hospital animal research laboratory.
Subjects:
Forty-six adult female sheep.
Interventions:
Fecal peritonitis was induced in the anesthetized, mechanically ventilated, fluid-resuscitated sheep, and they were randomized in two successive phases. Three late-intervention groups (each n = 6) received IV selepressin (1 pmol/kg/min), arginine vasopressin (0.25 pmol [0.1 mU]/kg/min), or norepinephrine (3 nmol [0.5 μg]/kg/min) when mean arterial pressure remained less than 70 mm Hg despite fluid challenge; study drugs were thereafter titrated to keep mean arterial pressure at 70–80 mm Hg. Three early-intervention groups (each n = 7) received selepressin, arginine vasopressin, or norepinephrine at the same initial infusion rates as for the late intervention, but already when mean arterial pressure had decreased by 10% from baseline; doses were then titrated as for the late intervention. A control group (n = 7) received saline. All animals were observed until death or for a maximum of 30 hours.
Measurements and Main Results:
In addition to hemodynamic and organ function assessment, plasma interleukin-6 and nitrite/nitrate levels were measured. In the late-intervention groups, selepressin delayed the decrease in mean arterial pressure and was associated with lower lung wet/dry weight ratios than in the other two groups. In the early-intervention groups, selepressin maintained mean arterial pressure and cardiac index better than arginine vasopressin or norepinephrine, slowed the increase in blood lactate levels, and was associated with less lung edema, lower cumulative fluid balance, and lower interleukin-6 and nitrite/nitrate levels. Selepressin-treated animals survived longer than the other animals.
Conclusions:
In this clinically relevant model, selepressin, a selective V1A receptor agonist, was superior to arginine vasopressin and to norepinephrine in the treatment of septic shock, especially when administered early.
Septic shock, defined as sepsis with persistent arterial hypotension despite adequate fluid resuscitation, affects about 15% of ICU patients and is an important cause of morbidity and mortality (1). Optimal vasopressor support represents an important aspect in the management of patients with septic shock (2). Arginine vasopressin (AVP) may have a place in the treatment of septic shock when administered in addition to norepinephrine (NE) (2). The addition of AVP may exert beneficial effects directly or by reducing the need for catecholamines, thus limiting their adverse effects, including immune suppression, stimulation of cellular metabolism, and myocardial injury (3). Some recent studies have suggested that AVP administration may also be of benefit in less severe forms of septic shock or as an early intervention to limit edema formation and organ damage, rather than as rescue therapy (4–7), but the effects of this approach on outcomes have not been fully investigated.
AVP stimulates several types of receptors (V1A, V1B, V2, oxytocin receptors) and thus has effects other than just V1A receptor-mediated vasopressor actions (8, 9). The agonist potency of AVP at the V1A and V2 receptors is of the same magnitude (i.e., AVP is a mixed V1A/V2 receptor agonist) (8, 10, 11). Because V2 and oxytocin receptor stimulation may aggravate sepsis-induced vasodilation and V2 receptor stimulation may promote fluid accumulation and increase the risk of microvascular thrombosis (12, 13), it is interesting to ask whether a selective V1A receptor agonist would be preferable. In animal models of pneumonia-induced sepsis and septic shock, selective V1A receptor agonists have been reported to reverse hypotension more effectively than other vasopressors (14, 15) and also to reduce vascular/capillary leakage (16), improve heart, lung, and kidney function, and prolong survival (14, 15).
We have developed a clinically relevant ovine model of peritonitis-induced septic shock with fever, tachycardia, hyperdynamic state, decreased vascular tone, early capillary leakage, and multiple organ failure, which has been used to test the effects of new therapeutic interventions (17, 18). In the present study, the effects on hemodynamics, oxygen consumption, organ function, and survival time of a selective V1A receptor agonist, selepressin (FE 202158) (10, 11), were compared with those of AVP and NE when administered early and later in the course of sepsis. We hypothesized that selepressin would have more favorable effects on these variables than AVP and NE, especially when administered early. Some of the results of the current study have been previously reported in the form of an abstract (19).
METHODS
The study was approved by our institutional review board for animals. Care and handling of the animals were in accordance with National and International Guidelines (20).
We studied 46 healthy female sheep weighing 25–35 kg, premedicated with intramuscular midazolam (Dormicum; Roche SA, Brussels, Belgium) (0.25 mg/kg) and ketamine hydrochloride (Imalgine; Merial, Lyon, France) (20 mg/kg). After IV injection of fentanyl (Fentanyl; Janssen, Berchem, Belgium) (30 μg/kg) and rocuronium (Esmeron; Organon, Oss, The Netherlands) (0.1 mg/kg) and endotracheal intubation, mechanical ventilation was started in volume-controlled mode (Servo ventilator 300 C; Siemens, Upplands Väsby, Sweden) with a tidal volume of 10 mL/kg, a positive end-expiratory pressure (PEEP) of 5 cm H2O, an inspiratory time/expiratory time of 1:2, and a square wave pattern. The stomach was emptied with an orogastric tube and a balloon catheter (14F; Teleflex Medical, Research Triangle Park, NC) was inserted into the bladder for collecting urine. Under aseptic conditions, a PiCCO arterial catheter (4F; Pulsion Medical Systems, Wayne, NJ) was placed in the right femoral vein. A venous introducer was placed in the right femoral artery and used to insert a 7F pulmonary artery catheter (Edwards Lifesciences, Irvine, CA). The catheters were connected to pressure transducers (Edwards Lifesciences). Through a midline laparotomy, ultrasonic Doppler flow probes were placed around the superior mesenteric artery and the left renal artery and the cecum was punctured to collect feces. After collection of 1.5 g/kg body weight of feces, the puncture site was disinfected and closed. A plastic tube was inserted through the abdominal wall into the abdominal cavity, and the abdomen was closed.
During the experiment, anesthesia was maintained with a continuous IV infusion of a mixture of ketamine (10 mg/kg/hr), morphine (0.5 mg/kg/hr), midazolam (0.5 mg/kg/hr), and rocuronium (0.1 mg/kg/hr). Ringer’s lactate (RL) solution and 6% hydroxyethyl starch (HES) solution (Voluven; Fresenius Kabi, Schelle, Belgium) were initially infused at a rate of 2 mL/kg/hr each. Fluid resuscitation was titrated to prevent hypovolemia, indicated by a stable pulmonary arterial occlusion pressure (PAOP) during the study period. When mean arterial pressure (MAP) remained less than 70 mm Hg despite fluid boluses (100 mL RL plus 100 mL HES given over 6 min), the fluid infusion was gradually reduced to 2 mL/kg/hr to avoid rapid development of refractory respiratory failure. Hypokalemia (< 3.5 mEq/L) and hypoglycemia (< 40 mg/dL) were corrected during the experiment.
Experimental Protocol
After baseline measurements, 1.5 g/kg/body weight feces were injected into the abdominal cavity to induce peritonitis, and the animals were then randomized into different groups (see below). Vasopressors were continuously infused via the cephalic vein as used for clinical administration. The doses of vasopressors were selected on the basis of previous experience from exploratory research in sheep (10). Animals were observed until death or for a maximum of 30 hours, after which they were killed by potassium chloride injection.
This study was performed in two successive phases (Fig. 1): late intervention, to compare the effects of selepressin, AVP, and NE in established shock; and early intervention, to compare the effects of selepressin, AVP, and NE early in the development of shock.
Figure 1.

Schematic diagram of the experimental protocol. SEL = selepressin, AVP= arginine vasopressin, NE = norepinephrine, MAP = mean arterial pressure.
Late Intervention.
The study vasopressor was administered when MAP remained less than 70 mm Hg despite fluid challenge (defined as “refractory hypotension”) and titrated to target an MAP of 70–80 mm Hg (target MAP). Each titration step was maintained for at least 15 minutes to determine whether the infusion rate was of sufficient magnitude to meet the requirements of the animal. Animals were randomized into three groups (each n = 6).
Selepressin ([Phe2,Ile3,Hgn4,Orn(iPr)8]vasopressin; Ferring Pharmaceuticals A/S, Copenhagen, Demark) infusion was started at a dose of 1 pmol/kg/min and increased stepwise by 1 pmol/kg/min to a maximum of 10 pmol/kg/min to maintain the target MAP.
AVP (American Regent, Shirley, NY) infusion was started at a dose of 0.25 pmol/kg/min (0.1 mU/kg/min) and increased stepwise by 0.25 pmol/kg/min to a maximum of 2.5 pmol/kg/min (1 mU/kg/min) to maintain the target MAP.
NE (Levophed; Hospira Benelux BVBA, Brussels, Belgium) infusion was started at a dose of 3 nmol/kg/min (0.5 μg/kg/min) and increased stepwise by 3 nmol/kg/min to a maximum of 30 nmol/kg/min (5 μg/kg/min) to maintain the target MAP.
Early Intervention.
The study vasopressor was infused if MAP decreased by 10% from baseline. The initial infusion rate was fixed until MAP decreased to less than 70 mm Hg despite fluid challenge. Thereafter, the vasopressor doses were titrated to maintain MAP at 70–80 mm Hg as in the previous experiments, each titration step being maintained for at least 15 minutes. Animals were randomized into three groups (each n = 7).
Selepressin: animals received an initial dose of 1 pmol/kg/min selepressin; when MAP decreased less than 70 mm Hg despite fluid challenge, selepressin doses were increased stepwise by 1 pmol/kg/min to a maximum of 10 pmol/kg/min to maintain target MAP.
AVP: animals received an initial dose of 0.25 pmol/kg/min (0.1 mU/kg/min); when MAP decreased less than 70 mm Hg despite fluid challenge, vasopressin doses were then increased stepwise by 0.25 pmol/kg/min to a maximum of 2.5 pmol/kg/min (1 mU/kg/min) to maintain MAP.
NE: animals received an initial dose of 3 nmol/kg/min (0.5 μg/kg/min); when MAP decreased less than 70 mm Hg despite fluid challenge, NE doses were increased stepwise by 3 nmol/kg/min to a maximum of 30 nmol/kg/min (5 μg/kg/min) to maintain target MAP.
A control group of seven animals received 0.9% saline solution with no vasopressors.
Measurements
Heart rate, MAP, and mean pulmonary artery pressure (MPAP) (Sirecust 404; Siemens, Erlangen, Germany), cardiac output and core temperature (Vigilance monitor; Edwards Lifesciences), renal and superior mesenteric arterial blood flow (T208 flowmeter; Transonic Systems, Ithaca, NY), and ventilator parameters were monitored continuously; right atrial pressure, PAOP, arterial and mixed-venous blood gases, hemoglobin concentration, blood lactate, and electrolyte concentrations (ABL725 and OSM3; Radiometer Medical A/S, Brønshøj, Denmark) were measured hourly; urine output and infused volume were all recorded hourly. The time to develop oliguria (defined as urine < 0.5 mL/kg/hr) was also recorded. Extravascular lung water (EVLW) (PiCCO; Pulsion Medical Systems) was determined every 2 hours. Arterial plasma and urine were collected at baseline and at 6, 12, 18, 24, and 30 hours after feces injection and sent to the central laboratory department in Erasme Hospital, Brussels, Belgium, for biochemical measurements, including protein, creatinine, coagulation parameters, and colloid oncotic pressure. A set of plasma samples was stored at –70° before shipping to the Bioanalytical Laboratory of Ferring Pharmaceuticals A/S for measurement of selepressin concentration.
Derived variables, such as cardiac index, stroke volume index, systemic and pulmonary vascular resistance index, left ventricular stroke work index (LVSWI), oxygen delivery index (Do2I), and oxygen consumption index (Vo2I) were calculated using standard formulas as in previous studies (18). Dynamic lung compliance was calculated as tidal volume/(peak inspiratory pressure – PEEP) (21). The cumulative fluid balance was estimated as the difference between cumulative infusion volume and cumulative urine output.
When the animal died, the bloodless wet weight of the central lobe of the right lung was determined. Its dry weight was also measured after the lobe had been dehydrated for 24 hours in an oven at 200°C. The wet/dry weight ratio was used as an estimate of pulmonary edema severity.
Interleukin-6 and Nitrite Measurements
Plasma interleukin (IL)-6 was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) assay. Ninety-six well plates were coated with mouse antiovine IL-6 monoclonal antibody (MCA 1659; Serotec, Oxford, United Kingdom) diluted to 1 μg/mL and incubated at 4°C overnight. After aspirating the coating solution, blocking solution was added to the wells for 2 hours and then the coated plates were washed three times. Diluted samples were transferred into wells to incubate for 1 hour at 37°C, and then the plates were washed three times. Diluted rabbit antiovine IL-6 polyclonal antibody (AHP424; Serotec) was added to incubate for 1 hour at room temperature. The contents of each well were discarded and the plate rinsed three times; the substrate for sheep anti-rabbit immunoglobulin G:horseradish peroxidase (STAR54; Serotec) was then added to the plate and allowed to react for color development for 10 minutes. The optical density of the plate wells was then read on an ELISA plate reader at 450 nm.
Nitrite levels, as an index of nitric oxide (NO) concentration (22), were measured with a commercial kit (Parameter Total NO/Nitrite/ Nitrate, SKGE001; R&D Systems, Minneapolis, MN).
Statistical Analysis
Data were checked for normality using the Kolmogorov-Smirnov test. As no deviation from normality was detected, data are described as mean ± sd. Data were analyzed using linear mixed models with group, time, and group × time interactions as fixed effects, subjects (sheep) as a random effect, and vasopressor dose as a covariate. Each time point difference between selepressin and other groups was compared with a least significant difference adjustment in case of a significant group effect and/or of a significant time × group interaction. One-way analysis of variance with repeated measures followed by a least significant difference post hoc multiple comparisons was used to compare the time to develop oliguria, the time to develop a 10% decrease in MAP or hypotension, and the lung wet/dry ratio. Plasma selepressin concentration and survival times are expressed as median with interquartile ranges (25th–75th). Kaplan-Meier survival curves were constructed and analyzed using the log-rank test. Data were analyzed using IBM SPSS Statistics 19 for Windows (IBM, Somers, NY). All reported p values are two-sided, and a p value of less than 0.05 was considered to be statistically significant.
RESULTS
Late Intervention (Comparing the Effects of Selepressin, AVP, and NE in Established Shock)
Systemic Hemodynamics.
After induction of peritonitis, all sheep developed fever, tachycardia, and hypotension with an increased cardiac output and decreased systemic vascular resistance (SVR). The initial time to develop refractory hypotension was similar in the three vasopressor groups and the control group (Table 1). Titration of the three vasopressors delayed the decrease in MAP (Fig. 2A) and prolonged the time to develop hypotension similarly (Table 1), but the increases in heart rate and MPAP (Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/B479) were less marked in the selepressin group than in the NE group. There were no significant differences in cardiac index, SVR index (SVRI), or LVSWI among the groups (Fig. 2A; and Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/B479).
TABLE 1.
Time to Develop Refractory Hypotensiona
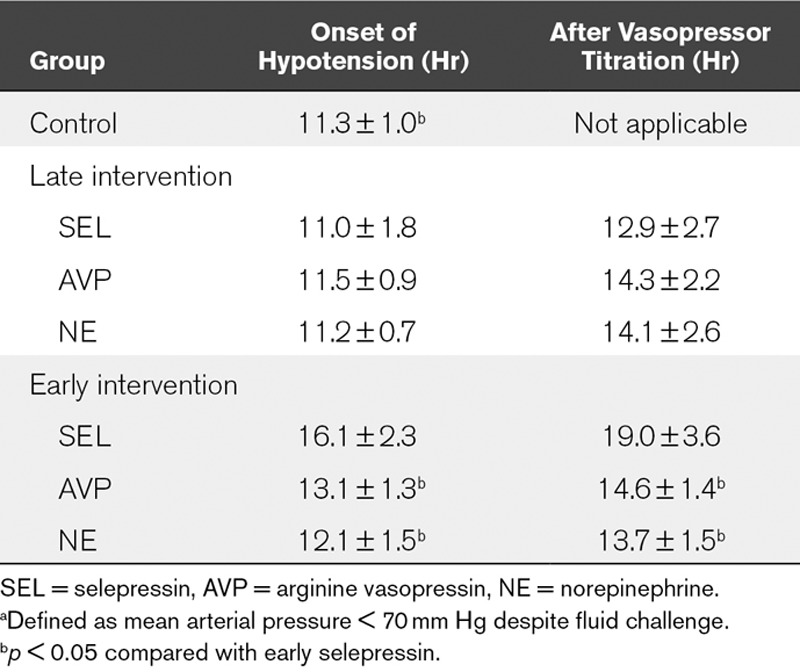
Figure 2.
Evolution of cardiac index (CI), mean arterial pressure (MAP), and blood lactate concentration in late-intervention group (A) and early-intervention group (B). Time effects were significant. For the late intervention, group effects were nonsignificant except for MAP (p < 0.05), and group × time interaction effects were nonsignificant except for MAP. For the early intervention, group and group × time interaction effects were significant. */$/#p < 0.05 control group (C), arginine vasopressin (AVP), or norepinephrine (NE) compared with selepressin (SEL), respectively.
Organ Function and Regional Perfusion.
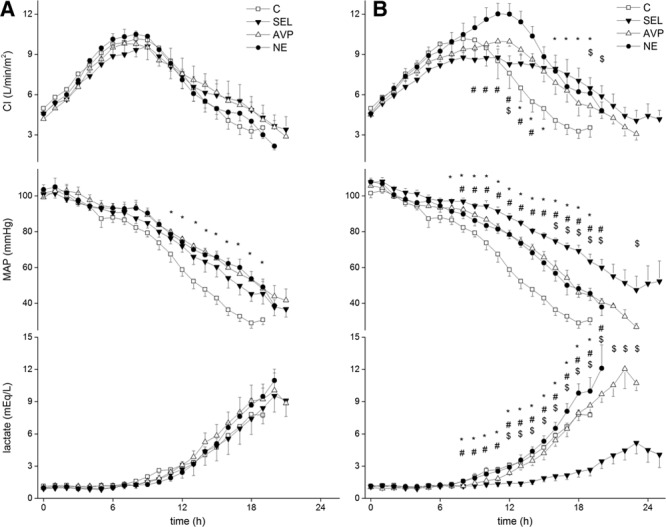
Despite similar evolutions in the Pao2/Fio2 ratio, lung compliance, pH, and extravascular lung water index (EVLWI) in all groups (Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/B479), selepressin-treated animals had a lower postmortem lung wet/dry ratio than the other animals (Fig. 3A). There was no significant difference in the cumulative fluid balance (Fig. S1A, Supplemental Digital Content 1, http://links.lww.com/CCM/B479), Do2I, mixed venous oxygen saturation (Svo2) (Table S1, Supplemental Digital Content 1, http://links.lww.com/CCM/B479), or blood lactate level (Fig. 2A). Renal blood flow, mesenteric blood flow, urine output, creatinine clearance, and time to develop oliguria (Table S1 and Figs. S1A and S2A, Supplemental Digital Content 1, http://links.lww.com/CCM/B479) were similar in the treated groups.
Figure 3.
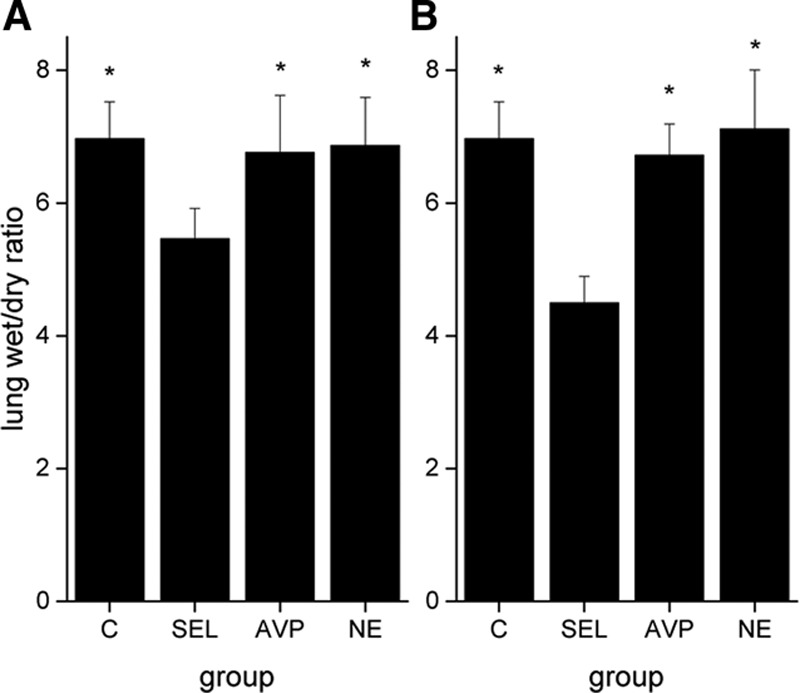
Postmortem lung wet/dry weight ratio in late-intervention group (A) and early-intervention group (B). The differences in late or early intervention were significant (p < 0.005). *p < 0.005 compared with selepressin (SEL). C = control group, AVP = arginine vasopressin, NE = norepinephrine.
Biological Mediators and Plasma Selepressin Concentration.
Plasma IL-6 and plasma nitrite/nitrate concentrations increased in all groups (Fig. 4A). Plasma IL-6 concentration increased less in the selepressin-treated animal group than in the NE and control groups. Plasma nitrite/nitrate concentrations increased similarly in the selepressin group and the control group, but less than in the NE group. Plasma selepressin concentrations were 0.19 nmol/L (0.19–2.28) and 4.04 nmol/L (0.62–9.79) at 12 and 18 hours after initiation of selepressin infusion, respectively.
Figure 4.
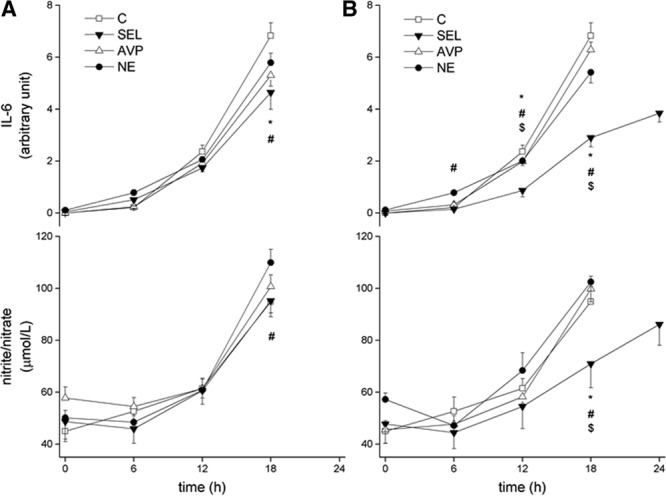
Evolution of plasma interleukin (IL)-6 and nitrite/nitrate concentration in late-intervention group (A) and early-intervention group (B). Time effects and group × time interaction effects were significant. Group effects with the late intervention were nonsignificant, but, with the early intervention, they were significant for IL-6 but not for nitrite/nitrate. */$/#p < 0.05 control group (C), arginine vasopressin (AVP), or norepinephrine (NE) compared with selepressin (SEL), respectively
Coagulation.
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) increased progressively in all groups and fibrinogen concentration and platelet counts decreased (Table S2, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). There were no significant differences in these parameters among groups.
Outcome.
The median survival time in the selepressin group (21.0 hr [21.0–23.0]) was significantly longer than in the control (19.0 hr [18.5–21.0]) and NE (20.0 hr [20.0–20.5]) groups but similar to the AVP group (21.5 [21.0–23.0]) (overall log-rank chi-square = 15.53; p < 0.005) (Fig. 5A).
Figure 5.
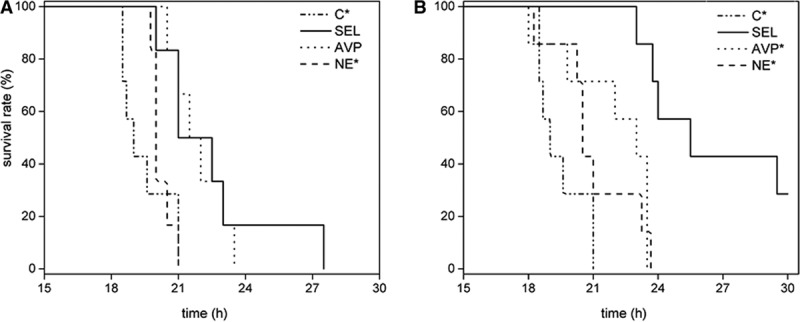
Kaplan-Meier survival curves in late-intervention group (A) and early-intervention group (B). *p < 0.05 compared with selepressin (SEL) group. C = control group, AVP = arginine vasopressin, NE = norepinephrine.
Early Intervention (Comparing the Effects of Selepressin, AVP, and NE Early in the Development of Shock)
Systemic Hemodynamics.
The mean time to develop a 10% decrease in MAP was similar in all groups (2.5 ± 0.3 hr, 2.6 ± 0.2 hr, 2.6 ± 0.1 hr, and 2.8 ± 0.7 hr in the selepressin, AVP, NE, and control groups, respectively; p=0.627). Selepressin blunted the increase in heart rate and notably delayed the decrease in cardiac index compared with the control group (Fig. 2B; and Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). MAP was better maintained with selepressin than with AVP or NE, and SVRI and LVSWI were maintained better with selepressin than with NE (Fig. 2B; and Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). The time to develop refractory hypotension in the selepressin group was significantly prolonged compared with the other groups (Table 1), despite a lower fluid balance.
Organ Function and Regional Perfusion.
Selepressin delayed the decreases in Pao2/Fio2 ratio, lung compliance, and pH (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). The lung wet/dry ratio was lower in the selepressin group than in the other groups (Fig. 3B), as was the EVLWI (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). The selepressin group also had a less positive cumulative fluid balance (Fig. S1B, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). Less edema formation was also suggested indirectly by the higher colloid oncotic pressure in the selepressin group than in the other groups (Table S4, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). Selepressin-treated animals had higher Do2I and Svo2 than the NE-treated animals and control animals (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). Blood lactate concentrations were also lower in the selepressin-treated animal group than in animals in the other groups (Fig. 2B).
Renal blood flow and creatinine clearance were higher in the selepressin group than in the NE and control groups (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). Oliguria occurred later in the selepressin group than in the other groups (Fig. S2B, Supplemental Digital Content 1, http://links.lww.com/CCM/B479). Changes in mesenteric blood flow were similar among groups (Table S3, Supplemental Digital Content 1, http://links.lww.com/CCM/B479).
Biological Mediators and Plasma Selepressin Concentration.
The increases in plasma IL-6 and nitrite/nitrate concentrations were significantly less in the selepressin group than in the other groups (Fig. 3B). Plasma selepressin concentrations were 0.19 nmol/L (0.19–0.39), 0.38 nmol/L (0.19–0.40), 0.67 nmol/L (0.40–2.67), and 2.24 nmol/L (1.41–9.03) at 6, 12, 18, and 24 hours, respectively.
Coagulation.
Selepressin blunted the increases in PT and aPTT compared with the AVP and control groups. The effects on the evolution of these parameters were greater in the selepressin group (Table S4, Supplemental Digital Content 1, http://links.lww.com/CCM/B479).
Outcome.
The median survival time was significantly longer in the selepressin group (25.5 hr [23.8–25.5]) than in the other groups (AVP, 23.0 hr [19.8–23.5]; NE, 20.5 hr [20.3–23.3]; control, 19.0 hr [18.5–21.0]) (log-rank chi-square = 22.75, p < 0.01) (Fig. 5B).
DISCUSSION
The main findings of the present study are that 1) administered when septic shock was already established, the selective V1A receptor agonist, selepressin, maintained MAP to a similar extent as did NE and the mixed V1A/V2 receptor agonist, AVP, but was associated with reduced lung edema compared with these two vasopressor agents and with prolonged survival compared with NE; 2) administered early (i.e., when MAP had decreased by just 10% from baseline), the effects of selepressin were more pronounced, with better hemodynamic stabilization, preserved lung and renal function, reduced cumulative fluid balance, attenuated coagulation disorders, and prolonged survival. In the present model, selepressin was, therefore, superior to AVP or NE in terms of hemodynamic resuscitation, kidney perfusion, and survival; this was associated with reduced systemic inflammation and vascular/capillary leakage.
AVP and its analogs have been shown to exert strong vasopressor effects in several experimental and clinical studies of septic shock (5–7, 23–25). Unlike NE, which increases arterial tone by stimulating vascular α-adrenergic receptors on the peripheral arterioles, AVP and its analogs induce vasoconstriction by stimulating the vascular V1A receptors (9). Although when compared with AVP and NE alone, the AVP analog, terlipressin, a prodrug of lysine vasopressin, also a mixed V1A/V2 receptor agonist like AVP (26), was associated with better maintained MAP in porcine and ovine models of fecal peritonitis-induced septic shock (5, 27) and in an ovine model of IV endotoxin-induced septic shock (28), as well as in patients with septic shock (25), other studies have suggested that selective stimulation of V1A receptors may be more beneficial than V1A + V2 receptor costimulation in septic shock. [Phe2,Orn8]vasotocin, a selective V1A receptor agonist (11), was superior to AVP when added to NE in an ovine model of fecal peritonitis-induced septic shock (14) or infused alone in an ovine model of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia-induced sepsis (15). In the present study, the highly selective V1A receptor agonist, selepressin, a full V1A receptor agonist like AVP but with virtually no V2 receptor agonist activity (10, 29), had stronger vasopressor effects than AVP or NE. Similar observations were made by Maybauer et al (16) in an ovine model of sepsis induced by Pseudomonas aeruginosa pneumonia. Because V2 receptor stimulation can cause vasodilation by inducing NO production from endothelial cells (30), the higher selectivity of selepressin for the V1A receptor may result in less NO being generated, leading to less formation of nitrite and nitrate, which are oxidative metabolites of NO. This effect was demonstrated by Rehberg et al (15) with the selective V1A receptor agonist, [Phe2,Orn8]vasotocin, in an ovine model of MRSA pneumonia-induced sepsis. In the present study, we noted lower plasma nitrite/nitrate concentrations in the selepressin group compared with the other groups.
In our study, the three vasopressors did not reduce cardiac index, stroke volume index, or LVSWI, and the early administration of these vasopressors even resulted in increases in these variables. All pure vasopressors can reduce cardiac output (6, 23, 25). AVP and its V1A receptor agonist analogs can reduce myocardial function due to reduced coronary blood flow (31), and the decrease in stroke volume may be associated with a marked increase in afterload (32); this effect is dose-dependent. In studies in porcine fecal peritonitis-induced septic shock (6), ovine endotoxin-induced septic shock (33) and healthy dogs (34), in which AVP or a V1A receptor agonist was infused at low vasopressor doses, myocardial injury was not detected. In a clinical trial comparing terlipressin and AVP, neither intervention significantly affected stroke volume (25). In addition, early administration of selepressin, as in the present study, might mitigate the negative inotropic effects and myocardial injury associated with sepsis by reducing excessive NO and/or cytokine levels (35). This effect was also demonstrated in an ovine model of MRSA pneumonia-induced sepsis, in which administration of [Phe2,Orn8]vasotocin was associated with reduced NO concentrations and increased left ventricular contractility (15).
AVP can decrease proinflammatory cytokine release via V1A receptor stimulation (36). Conversely, down-regulation of V1A receptor expression in different tissues can contribute to increases in proinflammatory cytokine release (37). In this context, selective V1A receptor agonism in our study was associated with attenuation of systemic inflammation, especially when given early. Unfortunately, we could not determine the concentrations of many cytokines because there are no specific monoclonal antibodies available in sheep. The anti-inflammatory effects likely contributed to the maintenance of cardiopulmonary function, the limitation of edema formation, and possibly the mitigation of mitochondrial injury (38). As a result, tissue oxygen metabolism was improved, as reflected by lower blood lactate concentrations. The attenuation of coagulopathy by selepressin was also remarkable because selective V1A receptor agonism not only limits the expression of tissue factor induced by IL-6 (39) but also does not induce V2-receptor-related procoagulant effects mediated by von Willebrand factor release and reduction in fibrinogen levels (13, 29).
Our model is characterized by marked early vascular/capillary leakage, as reflected by a very positive fluid balance and a 50% decrease in total plasma protein concentration already by 2 hours after sepsis induction. A positive fluid balance has been shown to be an independent risk factor for mortality in patients with septic shock (40). In ovine models of sepsis and septic shock induced by fecal peritonitis (14) or MRSA pneumonia (15), administration of the selective V1A receptor agonist, [Phe2,Orn8]vasotocin, resulted in reduced vascular leakage and hence less lung edema and better gas exchange than did AVP or NE. Likewise, in the present study, selepressin-treated animals had less lung edema, as reflected by the lower EVLW and postmortem lung wet/dry weight ratio, compared with the AVP, NE, or control groups, particularly when the drug was administered early.
Renal function was better preserved by selepressin, particularly when administered early. In experimental and clinical studies of septic shock, AVP, terlipressin, or [Phe2,Orn8]vasotocin was shown to increase urine output and creatinine clearance (14, 41, 42). Because selepressin cannot induce V2 receptor-mediated antidiuresis, due to its very high selectivity for the V1A receptor (8, 10), restoration of arterial pressure may have contributed to the preserved renal perfusion and the increased urine output. Improved glomerular filtration, as indicated by better preserved creatinine clearance, could also be related to inhibition of NO generation by selepressin because excessive NO may injure the glomerular endothelium and its metabolism (43). The benefit of the reduction in NO generation on renal function was also observed in ovine sepsis models using [Phe2,Orn8]vasotocin (14, 15). Improved renal function may potentially contribute to a less positive fluid balance.
The beneficial effects of AVP analogs on outcome are not definitely proven (4), although a meta-analysis of nine randomized controlled trials (44) suggested that AVP and terlipressin may reduce mortality when compared with NE in patients with septic shock. In our study, early administration of selepressin was associated with much longer survival than early administration of AVP. Early administration of [Phe2,Orn8]vasotocin also prolonged survival in an ovine model of peritonitis-induced septic shock similar to that used in the present study (14).
Our study extends the previous observations by Rehberg et al (14, 15) and Maybauer et al (16) and further explores the best timing of administration in the course of sepsis. The effects of selepressin were clearly superior when administration was started early. The advantage of earlier onset of administration has also been suggested in previous studies. A first-line treatment regimen of AVP or terlipressin had better effects than NE alone in ovine (5) and porcine (6) models of fecal peritonitis-induced septic shock and in a predefined subgroup of patients with mild septic shock in the Vasopressin and Septic Shock Trial (4).
The superiority of AVP and V1A receptor agonist analogs over NE may be partly associated with the adverse effects of NE, including stimulation of cellular metabolism, arrhythmias, and myocardial injury. AVP and V1A receptor agonist analogs can also induce vasoconstriction with risks of reduced organ blood flow (34, 45–47). In the present study, we did not observe such adverse effects. Three factors may have contributed to this finding: first, we used generous fluid resuscitation to prevent any fluid deficit; second, the upper limit of the dose ranges of selepressin, AVP, and NE was relatively low in terms of vasopressor action; and third, the animals were previously healthy, so the present observations may not fully apply to patients with comorbidities, including coronary or vascular disease. In addition, antibiotics were not administered in the present study to avoid a confounding factor that may have improved survival.
This study could not determine the precise mechanisms underlying the potential advantages of selepressin, and future studies should focus on this aspect, including measurements of AVP levels, additional biomarkers, and histologic and immunohistochemical examination for evaluation of organ injury, coagulopathy, and vascular barrier dysfunction. Furthermore, as in the clinical setting, some sheep may be highly responsive and others less responsive to vasopressors, and it may be interesting to explore this possibility in future studies.
CONCLUSION
In this clinically relevant model of fecal peritonitis-induced septic shock, the selective V1A receptor agonist, selepressin, was superior to AVP and to NE in the treatment of septic shock. In particular, early administration of selepressin improved the systemic and pulmonary circulations and pulmonary and renal function, attenuated the sepsis-associated coagulation disorder and systemic inflammatory response, and prolonged survival. These observations support the early administration of selective V1A agonists in septic shock as first-line vasopressors, an approach that warrants investigation in clinical trials.
ACKNOWLEDGMENT
We are grateful to Hassane Njimi, BSc, PhD, for his help with the statistical analyses.
Supplementary Material
Footnotes
*See also p. 234.
Current address for Dr. Laporte: Laporte & Associates, LLC, Biotech & Pharma R&D Consultants, San Diego, CA.
Dr. He conducted the experiment, acquired and analyzed the data, and drafted the article. Dr. Su designed and directed the protocol. Dr. Taccone helped perform the experiments, interpret the data, and draft the article. Drs. Laporte, Kjølbye, and Reinheimer participated in the protocol design and critically revised the article. Dr. Zhang carried out the biochemical measurements. Drs. Xie and Moussa helped perform the experiments and acquire the data. Dr. Vincent was involved in the study design and in critically revising the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by a research grant from the International PharmaScience Center, Ferring Pharmaceuticals A/S. The sponsor participated in the protocol design and provided the selepressin used in the study. The sponsor was not involved in performing the experiments or in analyzing the data but did review the article for critical content.
Dr. Laporte received funding from Ferring Research Institute and Ferring International PharmaScience Center and was employed by Ferring Research Institute at the time of the study. Dr. Kjølbye is employed by Ferring Pharmaceuticals A/S. Dr. Reinheimer is employed by Ferring Pharmaceuticals A/S. Dr. Vincent reports that a research grant was paid to the department by International PharmaScience Center, Ferring Pharmaceuticals A/S, during the conduct of the study. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 3.Richard C. Stress-related cardiomyopathies. Ann Intensive Care. 2011;1:39. doi: 10.1186/2110-5820-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell JA, Walley KR, Singer J, et al. VASST Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 5.Rehberg S, Ertmer C, Köhler G, et al. Role of arginine vasopressin and terlipressin as first-line vasopressor agents in fulminant ovine septic shock. Intensive Care Med. 2009;35:1286–1296. doi: 10.1007/s00134-009-1470-z. [DOI] [PubMed] [Google Scholar]
- 6.Simon F, Giudici R, Scheuerle A, et al. Comparison of cardiac, hepatic, and renal effects of arginine vasopressin and noradrenaline during porcine fecal peritonitis: A randomized controlled trial. Crit Care. 2009;13:R113. doi: 10.1186/cc7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luckner G, Dünser MW, Jochberger S, et al. Arginine vasopressin in 316 patients with advanced vasodilatory shock. Crit Care Med. 2005;33:2659–2666. doi: 10.1097/01.ccm.0000186749.34028.40. [DOI] [PubMed] [Google Scholar]
- 8.Koshimizu TA, Nakamura K, Egashira N, et al. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL, Su F. Physiology and pathophysiology of the vasopressinergic system. Best Pract Res Clin Anaesthesiol. 2008;22:243–252. doi: 10.1016/j.bpa.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Laporte R, Kohan A, Heitzmann J, et al. Pharmacological characterization of FE 202158, a novel, potent, selective, and short-acting peptidic vasopressin V1a receptor full agonist for the treatment of vasodilatory hypotension. J Pharmacol Exp Ther. 2011;337:786–796. doi: 10.1124/jpet.111.178848. [DOI] [PubMed] [Google Scholar]
- 11.Wisniewski K, Galyean R, Tariga H, et al. New, potent, selective, and short-acting peptidic V1a receptor agonists. J Med Chem. 2011;54:4388–4398. doi: 10.1021/jm200278m. [DOI] [PubMed] [Google Scholar]
- 12.Rehberg S, Ertmer C, Lange M, et al. Role of selective V2-receptor-antagonism in septic shock: A randomized, controlled, experimental study. Crit Care. 2010;14:R200. doi: 10.1186/cc9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann JE, Oksche A, Wollheim CB, et al. Vasopressin-induced von Willebrand factor secretion from endothelial cells involves V2 receptors and cAMP. J Clin Invest. 2000;106:107–116. doi: 10.1172/JCI9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehberg S, Ertmer C, Vincent JL, et al. Role of selective V1a receptor agonism in ovine septic shock. Crit Care Med. 2011;39:119–125. doi: 10.1097/CCM.0b013e3181fa3898. [DOI] [PubMed] [Google Scholar]
- 15.Rehberg S, Yamamoto Y, Sousse L, et al. Selective V(1a) agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am J Physiol Heart Circ Physiol. 2012;303:H1245–H1254. doi: 10.1152/ajpheart.00390.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maybauer MO, Maybauer DM, Enkhbaatar P, et al. The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis. Crit Care Med. 2014;42:e525–e533. doi: 10.1097/CCM.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su F, Huang H, Akieda K, et al. Effects of a selective iNOS inhibitor versus norepinephrine in the treatment of septic shock. Shock. 2010;34:243–249. doi: 10.1097/SHK.0b013e3181d75967. [DOI] [PubMed] [Google Scholar]
- 18.He X, Su F, Velissaris D, et al. Administration of tetrahydrobiopterin improves the microcirculation and outcome in an ovine model of septic shock. Crit Care Med. 2012;40:2833–2840. doi: 10.1097/CCM.0b013e31825b88ba. [DOI] [PubMed] [Google Scholar]
- 19.Su F, He X, Taccone FS, et al. Early administration of the selective v1a receptor agonist selepressin is superior to arginine vasopressin or norepinephrine in a sheep model of sepsis. Abstr. Crit Care Med. 2012;40:123. [Google Scholar]
- 20.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th Edition. Washington, DC: The National Academies Press; 2015. [Google Scholar]
- 21.Wilson WC, Benumof JL. Physiology of the airway. In: Hagberg CA, Gabel JC, editors. Benumof and Hagberg’s Airway Management. Philadelphia, PA: Elsevier; 2013. pp. 118–158. [Google Scholar]
- 22.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albanèse J, Leone M, Delmas A, et al. Terlipressin or norepinephrine in hyperdynamic septic shock: A prospective, randomized study. Crit Care Med. 2005;33:1897–1902. doi: 10.1097/01.ccm.0000178182.37639.d6. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q, Dimopoulos G, Nguyen DN, et al. Low-dose vasopressin in the treatment of septic shock in sheep. Am J Respir Crit Care Med. 2003;168:481–486. doi: 10.1164/rccm.200205-447OC. [DOI] [PubMed] [Google Scholar]
- 25.Morelli A, Ertmer C, Rehberg S, et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): A randomized, controlled pilot study. Crit Care. 2009;13:R130. doi: 10.1186/cc7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wisniewski K, Alagarsamy S, Taki H, et al. Synthesis and biological activity of terlipressin and its putative metabolites. In: Blondelle SE, editor. Understanding Biology Using Peptides: Proceedings of the Nineteenth American Peptide Symposium. New York, NY: Springer; 2006. pp. 489–490. [Google Scholar]
- 27.Asfar P, Hauser B, Iványi Z, et al. Low-dose terlipressin during long-term hyperdynamic porcine endotoxemia: Effects on hepatosplanchnic perfusion, oxygen exchange, and metabolism. Crit Care Med. 2005;33:373–380. doi: 10.1097/01.ccm.0000152253.45901.fb. [DOI] [PubMed] [Google Scholar]
- 28.Westphal M, Stubbe H, Sielenkämper AW, et al. Terlipressin dose response in healthy and endotoxemic sheep: Impact on cardiopulmonary performance and global oxygen transport. Intensive Care Med. 2003;29:301–308. doi: 10.1007/s00134-002-1546-5. [DOI] [PubMed] [Google Scholar]
- 29.Rehberg S, Enkhbaatar P, Rehberg J, et al. Unlike arginine vasopressin, the selective V1a receptor agonist FE 202158 does not cause procoagulant effects by releasing von Willebrand factor. Crit Care Med. 2012;40:1957–1960. doi: 10.1097/CCM.0b013e31824e0fe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufmann JE, Iezzi M, Vischer UM. Desmopressin (DDAVP) induces NO production in human endothelial cells via V2 receptor- and cAMP-mediated signaling. J Thromb Haemost. 2003;1:821–828. doi: 10.1046/j.1538-7836.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 31.Ryckwaert F, Virsolvy A, Fort A, et al. Terlipressin, a provasopressin drug exhibits direct vasoconstrictor properties: Consequences on heart perfusion and performance. Crit Care Med. 2009;37:876–881. doi: 10.1097/CCM.0b013e31819b8199. [DOI] [PubMed] [Google Scholar]
- 32.Westphal M, Stubbe H, Sielenkämper AW, et al. Effects of titrated arginine vasopressin on hemodynamic variables and oxygen transport in healthy and endotoxemic sheep. Crit Care Med. 2003;31:1502–1508. doi: 10.1097/01.CCM.0000063042.15272.84. [DOI] [PubMed] [Google Scholar]
- 33.Lange M, Morelli A, Ertmer C, et al. Continuous versus bolus infusion of terlipressin in ovine endotoxemia. Shock. 2007;28:623–629. doi: 10.1097/shk.0b013e318050c78d. [DOI] [PubMed] [Google Scholar]
- 34.Boucheix OB, Milano SP, Henriksson M, et al. Selepressin, a new V1A receptor agonist: Hemodynamic comparison to vasopressin in dogs. Shock. 2013;39:533–538. doi: 10.1097/SHK.0b013e31828aac4b. [DOI] [PubMed] [Google Scholar]
- 35.Smeding L, Plötz FB, Groeneveld AB, et al. Structural changes of the heart during severe sepsis or septic shock. Shock. 2012;37:449–456. doi: 10.1097/SHK.0b013e31824c3238. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Brinton RD. Suppression of proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha in astrocytes by a V1 vasopressin receptor agonist: A cAMP response element-binding protein-dependent mechanism. J Neurosci. 2004;24:2226–2235. doi: 10.1523/JNEUROSCI.4922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bucher M, Hobbhahn J, Taeger K, et al. Cytokine-mediated downregulation of vasopressin V(1A) receptors during acute endotoxemia in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R979–R984. doi: 10.1152/ajpregu.00520.2001. [DOI] [PubMed] [Google Scholar]
- 38.Garrabou G, Morén C, López S, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 39.Stouthard JM, Levi M, Hack CE, et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. [PubMed] [Google Scholar]
- 40.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa K, Wan L, Calzavacca P, et al. The effects of terlipressin on regional hemodynamics and kidney function in experimental hyperdynamic sepsis. PLoS One. 2012;7:e29693. doi: 10.1371/journal.pone.0029693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon AC, Russell JA, Walley KR, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 2010;36:83–91. doi: 10.1007/s00134-009-1687-x. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Holthoff JH, Seely KA, et al. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol. 2012;180:505–516. doi: 10.1016/j.ajpath.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serpa Neto A, Nassar AP, Cardoso SO, et al. Vasopressin and terlipressin in adult vasodilatory shock: A systematic review and meta-analysis of nine randomized controlled trials. Crit Care. 2012;16:R154. doi: 10.1186/cc11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klinzing S, Simon M, Reinhart K, et al. Moderate-dose vasopressin therapy may impair gastric mucosal perfusion in severe sepsis: A pilot study. Anesthesiology. 2011;114:1396–1402. doi: 10.1097/ALN.0b013e318219d74f. [DOI] [PubMed] [Google Scholar]
- 46.Krejci V, Hiltebrand LB, Jakob SM, et al. Vasopressin in septic shock: Effects on pancreatic, renal, and hepatic blood flow. Crit Care. 2007;11:R129. doi: 10.1186/cc6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Giantomasso D, Morimatsu H, Bellomo R, et al. Effect of low-dose vasopressin infusion on vital organ blood flow in the conscious normal and septic sheep. Anaesth Intensive Care. 2006;34:427–433. doi: 10.1177/0310057X0603400408. [DOI] [PubMed] [Google Scholar]


