Supplemental Digital Content is available in the text.
Keywords: aorta, thoracic, atherosclerosis, B-lymphocytes, mice, phosphorylcholine
Objective—
The V1 (VHS107.1.42) immunoglobulin heavy chain gene is thought to be critical in producing IgM natural antibodies of the T15-idiotype that protect against both atherosclerosis and infection from Streptococcus pneumoniae. Our aim was to determine whether genetic loss of the V1 gene increased atherosclerotic plaque burden in vivo because of a reduction in the T15-idiotype or other atheroprotective antibodies.
Approach and Results—
We crossed VHS107.1.42-deficient mice with the atherosclerosis-prone Apoe−/− and Ldlr−/− strains. Although these double knockout strains manifested no defects in B-cell development, we did observe a substantial reduction in early immune responses against phosphocholine after immunization. However, the titers of plasma antibodies reacting against defined atherosclerotic antigens such as oxidized low-density lipoprotein, as well as the T15-idiotype, were unaffected by loss of the VHS107.1.42 gene in hypercholesterolemic mice. Furthermore, we observed no increase in atherosclerotic lesion formation, either within the aortic arch or aortic root. Robust deposition of IgM within atherosclerotic plaques could also be readily observed in both control and experimental mice.
Conclusions—
Our data indicate that IgM-dependent protection against atherosclerosis is unlikely to be dependent on antibodies that use the VHS107.1.42 gene, in contrast to the acute immune response conferred by this heavy chain in the response to phosphocholine and in providing resistance against lethal S pneumoniae infection.
There is a general consensus that the pathology of atherosclerotic plaque formation includes a strong inflammatory component and that selective amelioration of this inflammation could be of therapeutic benefit.1 One of the immune cell subsets implicated in atherosclerosis disease progression are B lymphocytes, which as antibody-secreting cells form the humoral arm of immunity. B cells have been shown to have ambivalent functions in atherosclerotic plaque formation. Splenectomy2,3 or genetic ablation of B cells4 resulted in an overall increase in atherosclerosis in susceptible animal models, whereas acute B-cell depletion via anti-CD20 therapy indicated possible proatherogenic functions.5,6 However, a major humoral function of B cells is the secretion of the so-called natural antibodies that have been proposed to protect against atherosclerosis plaque formation.7 Natural antibodies are generally germline encoded and of the IgM isotype and are thought to be predominately produced by the B-1 type of mature B cells. Mice unable to secrete IgM are more prone to diet-induced atherosclerosis.8 In addition, transfer of B-1 cells that cannot secrete IgM fails to protect against the increase in atherosclerosis in Apoe−/− mice that is observed after splenectomy, whereas transfer of wild-type B-1 cells fully restored plasma IgM levels and prevented accelerated lesion formation in these mice.9
Many antigens have been implicated in the process of atherosclerotic lesion formation. Prominent among these is the modification of low-density lipoprotein (LDL) into oxidized low-density lipoprotein (OxLDL).10,11 This modification unmasks the phosphocholine epitope of oxidized phospholipids that is subsequently recognized by the natural antibody identified by the T15-idiotype (T15-id).12 The recognition of OxLDL by T15-id antibodies is thought to mediate protection from atherosclerosis because T15-id IgM antibodies have been shown to limit proinflammatory cytokine secretion by oxidized phospholipids in macrophages, block OxLDL-induced foam cell formation, and promote apoptotic cell clearance.7,12,13
The T15-id was originally discovered as a phosphocholine binding antibody of the IgA subtype from hybridomas obtained after pristane injection.14–16 The production of the T15-id is thought to be absolutely dependent on a successful V(D)J recombination event between the VHS107.1.42 (also known as V117), DHFL16.1 and the JH1 gene segments.18,19 The immune reaction to phosphocholine in a hapten form is characterized by an early response dominated by T15-id B cell clones. The secondary immune response against phosphocholine differs as the humoral response consists of antibodies that have incorporated VHS107.1.42 immunoglobulin heavy chains in their rearrangement but are T15-id negative or alternatively uses other VH genes in a productive V(D)J rearrangement but nevertheless bind phosphocholine.20,21
A well-characterized protective physiological role of T15-id antibodies is the recognition of phosphocholine present in the capsular polysaccharide of Streptococcus pneumoniae.22 The T15-id is produced in both germfree and conventional mice from ≈1 week after birth, thereby providing protection against S pneumoniae infection as a part of the natural immunoglobulin repertoire.23 Nonimmunized mice deficient for the VHS107.1.42 gene are highly susceptible to death after S. pneumonia infection.24 This is thought to be because of the lack of T15-id antibodies induced in the early immune response that would normally prevent binding of S pneumoniae to the platelet-activating factor receptor and subsequent transport across host cell membranes. Immunization of Ldlr−/− mice with heat killed S pneumoniae increases the titers of T15-id IgM antibodies that bind OxLDL and decreases the extent of atherosclerosis.22 Moreover, infusion of T15-id IgM antibodies reduces plaque formation within grafted veins of Apoe−/− mice, whereas this was not sufficient to affect the extent of native atherosclerosis in the aortic origin.25 In addition, infusion of T15-Id IgM had no effect on accelerated atherosclerosis in a carotid cuff model in Apoe−/− mice fed an atherogenic diet.26 However, the duration of the T15-id IgM intervention or the levels achieved by the infusion strategy may not have been sufficient to mediate a protective effect in these studies.
In addition to the T15-id encoding VHS107.1.42 gene segment, many other VH genes can be incorporated into antiphosphocholine-binding specificities.27 Noticeably, mice that are deficient for DHFL16.1 lose T15-id IgM production and have increased susceptibility to S pneumoniae infection, yet have normal levels of serum anti-OxLDL antibodies.28 Therefore, we wished to determine whether the loss of the VHS107.1.42 gene is similarly critical against the development of atherosclerosis as it is for the protection against lethal S pneumoniae infection. We present evidence that the atheroprotective effects of IgM do not depend on serum immunoglobulins that require the VHS107.1.42 gene.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Normal B-Cell Development in the Absence of the VHS107.1.42 Gene
The production of T15-id antibodies is thought to be critically dependent on the VHS107.1.42 gene.18,24,29 However, the in vivo function of VHS107.1.42 with regard to B-cell development and atherosclerosis has to date not been determined. We first quantified the expression levels of the VHS107.1.42 gene compared with those of the neighboring VH genes in splenic B-1 cells using previously published mRNA-seq analysis of sorted cell populations from the spleen30 (Figure 1A). When compared with the most highly expressed B-1 VH gene, namely VH11.2.53, VHS107.1.42 is expressed 77-fold lower (Figure 1B) and is only the 61st most highly expressed functional VH gene of the 113 measured in splenic B-1 lymphocytes. Next, we crossed VHS107.1.42−/− mice onto the Apoe−/− background, so that effects of VHS107.1.42 in the context of hyperlipidemia and spontaneous atherosclerotic plaque formation could be ascertained. We confirmed these mice were deficient for the VHS107.1.42 gene by designing PCR primers specific for this gene segment. Whereas we could readily detect a positive PCR band in control Apoe–/– VHS107.1.42+/– mice for both the VHS107.1.42 and the related VHS107.3.62 genes, we could only detect the presence of VHS107.3.62 in Apoe–/– S107.1.42–/– experimental mice (Figure 1C), consistent with the fact that the VHS107.1.42 has been deleted in experimental mice. Noticeably, we could also confirm the presence of the most 5′ and 3′ proximal genes to VHS107.1.42, namely VHQ52.13.40 and the VHS107.2pg.43 pseudogene, respectively (Figure 1C). Therefore, the Apoe−/− VHS107.1.42−/− experimental mice are deleted for the VHS107.1.42 gene in a minimally intrusive manner that has not lead to spurious deletions within the neighboring immunoglobulin heavy chain environment.
Figure 1.
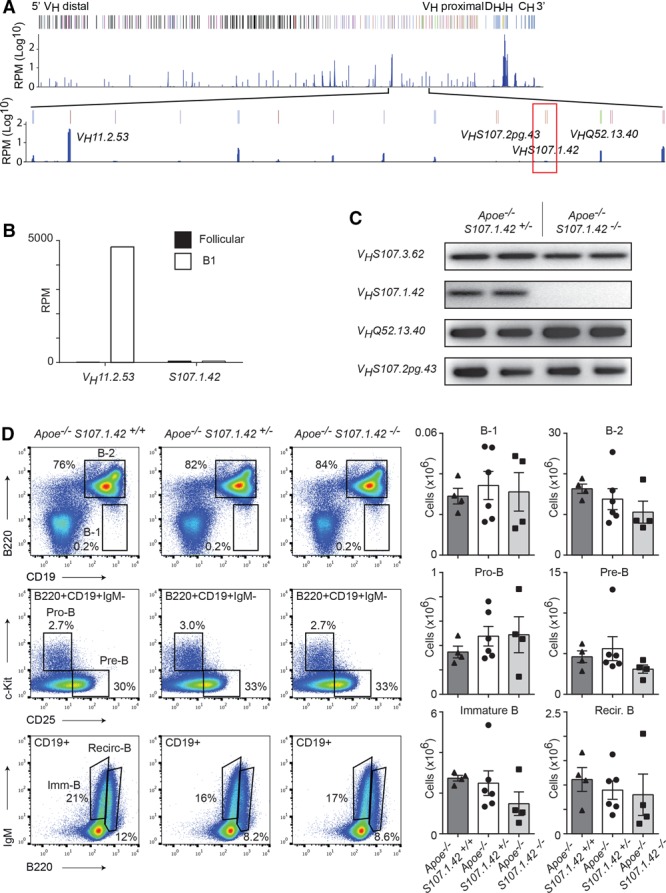
Spleen B-cell development in the absence of the VHS107.1.42 gene. A, Schematic representation of the immunoglobulin heavy chain with selected VH genes annotated and the region around VHS107.1.42 enlarged. Previously published mRNA-seq reads are indicated and displayed logarithmically as reads per million (RPM). The VHS107.1.42 gene is outlined with a red box. B, Expression levels in RPM of VHS107.1.42 and VH11.2.53 genes from spleen B1 and follicular B cells. C, Polymerase chain reaction amplification from genomic DNA of the VHS107.3.62, VHS107.1.42, VHS107.2pg.43, and VHQ52.13.40 genes from Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice as indicated. D, Fluorescence-activated cell sorter analysis of bone marrow for B-cell differentiation in Apoe−/− VHS107.1.42+/+, Apoe−/− VHS107.1.42+/−, and Apoe−/− VHS107.1.42−/− mice aged 4 to 6 wk. Cells were gated to calculate absolute cell numbers for B1 (CD19+B220lo), B-2 (CD19+B220+), pro-B (CD19+B220+c-Kit+CD25−IgM−), pre-B (CD19+B220+c-Kit−CD25+IgM−), immature B (CD19+B220loIgM+), and recirculating (CD19+B220+IgM+) populations as shown in the graphs on the right. Each point represents an individual mouse, and error bars represent SEM.
In the mouse, bone marrow lymphocytes commit to the B-cell lineage at the pro-B stage of development (defined CD19+c-Kit+) with VH–DJH recombination of the immunoglobulin heavy chain also occurring at this developmental stage. Successful recombination of one of the IgH alleles results in the formation of the pre–B-cell receptor at the pre–B-cell stage (CD19+CD25+IgM−) followed by immunoglobulin light chain recombination and progression to the immature stage. Finally, B lymphocytes exit from the bone marrow to the spleen for the final maturation stages but can return as recirculating B cells. It has previously been reported that between 32% and 64% of hybridomas obtained from aged Apoe−/− mice on a hybrid C57BL/129 background specifically recognize OxLDL,31 and that some of these secrete antibodies of the T15-id.12 Therefore, it was possible that loss of the VHS107.1.42 encoded T15-id repertoire may impact B-cell development in the context of an Apoe−/− background even though, as described above, this VH gene is not highly expressed. Therefore, we analyzed early B-cell development in VHS107.1.42-deficient mice through fluorescence-activated cell sorter analysis of the bone marrow from 4- to 6-week-old Apoe−/− VHS107.1.42+/+, Apoe−/− VHS107.1.42+/−, and Apoe−/− VHS107.1.42−/− mice (Figure 1D). We found no significant differences in the ratios or absolute cell numbers of any B-cell examined stages in the 3 cohorts of mice. Therefore, we conclude that loss of a single or both copies of the VHS107.1.42 gene does not affect early B-cell development in the bone marrow.
Role of the VHS107.1.42 Gene in the Formation of Mature B-Cell Compartments and the Response to Phosphocholine-KLH Immunization
Mature B-1 cells can be readily isolated in the spleen and peritoneal cavity of the mouse. Therefore, we quantified the total number of B-1 (CD19+B220lo) and B-2 (CD19+B220+) cells at these 2 sites. Fluorescence-activated cell sorter analysis of the spleen from 10- to 14-week-old Apoe−/− VHS107.1.42+/+, Apoe−/− VHS107.1.42+/−, and Apoe−/− VHS107.1.42−/− mice demonstrated consistent numbers of B-1 and B-2 lymphocytes across all 3 genotypes (Figure 2A), strongly arguing against a critical function of the VHS107.1.42 gene in the generation of the peripheral B-1 cell population. Furthermore, these CD19+B220lo B-1 cells were uniformly CD43+ and could be further subdivide into CD5+ (B-1a) and CD5− (B-1b) populations. We also repeated this analysis on the peritoneal cavity of 10- to 14-week-old mice (Figure 2B), and again we could not detect any significant differences in the mature B-1 and B-2 cell populations regardless of the presence or absence of the VHS107.1.42−/− immunoglobulin heavy chain gene.
Figure 2.
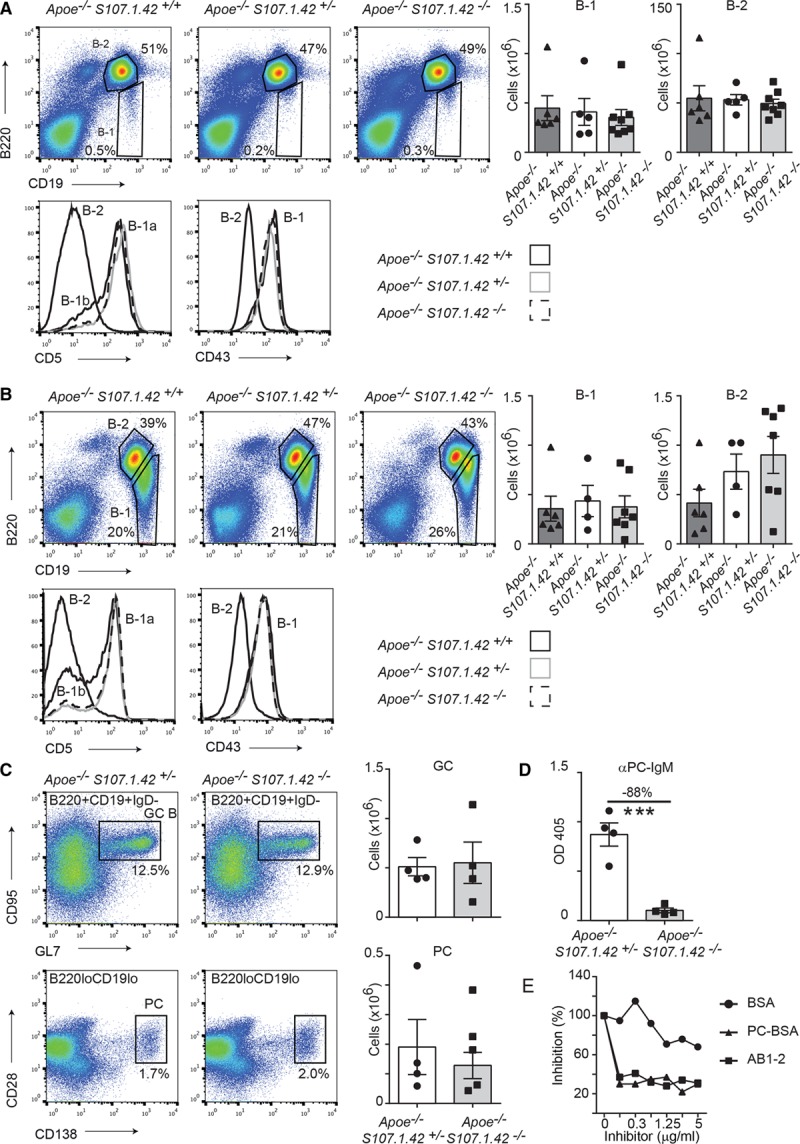
The dependence of peripheral B-cell development and immune responses on the VHS107.1.42 gene. A, Representative fluorescence-activated cell sorter (FACS) analysis of splenic B-cell populations in Apoe−/− VHS107.1.42+/+, Apoe−/− VHS107.1.42+/− mice and Apoe−/− VHS107.1.42−/− mice aged 10 to 14 wk shown in the left. Cells were gated to calculate absolute cell numbers for B-1 (CD19+B220lo) and B-2 (CD19+B220+) populations. Each point represents an individual mouse, and error bars represent SEM. Spleen B-1 and B-2 cells from 6-wk-old mice were further analyzed for CD5 and CD43 expression as shown in the histograms. B, FACS analysis of the peritoneal cavity from 10- to 14-wk-old mice as described in A. C, Spleen analysis from Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice aged 10 to 12 wk injected with phosphocholine (PC)-KLH/alum and analyzed after 6 d. Top, Germinal center B cells (CD19+B220+IgD-CD95+GL7+) and the (bottom) plasma cells (CD19loB220loCD138+CD28+). Absolute cell numbers for the germinal center (GC) and PC cell populations calculated from these gates are depicted in the graphs in the right. Control mice are depicted by white bars and experimental in gray. D, ELISA measurements of plasma antiphosphocholine IgM responses in plasma 6 d after PC-KLH/alum immunization from Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice. E, Competition ELISA with pooled plasma from Apoe−/− VHS107.1.42+/− mice captured with AB1.2 (anti-T15-id) and competed as indicated.
It has been previously reported that VHS107.1.42−/− mice have strongly reduced primary immune responses to the hapten phosphocholine. We repeated this analysis using control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice immunized with phosphocholine-KLH (phosphocholine coupled to keyhole limpet hemocyanin) with Alum adjuvant. Both control and experimental mice robustly formed germinal centers and produced plasma cells 6 days after primary immunization (Figure 2C). However, we observed a significant 88% reduction in IgM antibodies recognizing phosphocholine in experimental mice consistent with the original description of the VHS107.1.42−/− mouse strain (Figure 2D). Finally, we performed competition ELISA assays on control mice using the AB1-2 clone that recognizes the T15-id as a capture antibody and competed with BSA, phosphocholine-BSA, and AB1-2 (Figure 2E). Addition of phosphocholine-BSA and AB1-2, but not BSA, efficiently competed binding indicating that this primary antiphosphocholine response consists of the T15-id. Taken together, control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice have indistinguishable B-cell compartments under steady state conditions; however, the Apoe−/− VHS107.1.42−/− strain manifests a highly specific defect in the primary immune response to the phosphocholine hapten.
Contribution of the VHS107.1.42 Encoded Antibodies to Humoral Immune Responses Against Common Atherosclerosis Antigens
We next wished to determine how the loss of the VHS107.1.42 gene segment would affect the titers of selected immunoglobulins by using ELISA assays on plasma from male (Figure 3A) and female (Figure 3B) mice aged 10 or 20 weeks. Total plasma IgM levels in control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice were similar. Surprisingly, however, reactivity of plasma IgM with the AB1-2 monoclonal antibody, which specifically recognizes the heavy and light chain of T15-id,32 was unaffected by the loss of the VHS107.1.42 gene. Multiple antibody specificities, including the VHS107.1.42 encoded T15-id, react against phosphocholine (phosphocholine) and to antigens present in Cu2+ OxLDL. We next measured plasma IgM antibodies reacting against these antigens as well as another model of OxLDL, malondialdehyde modified LDL (MDA-LDL). Similar levels of reactivity against phosphocholine, Cu2+ OxLDL, and malondialdehyde modified LDL were present in both control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice. We next repeated these experiments using Ldlr−/− VHS107.1.42+/+ and Ldlr−/− VHS107.1.42−/− mice and again found similar responses to these antigens (Figure 3C). In summary, the loss of the VHS107.1.42−/− gene does not reduce the plasma IgM titers against common atherosclerosis-associated antigens.
Figure 3.
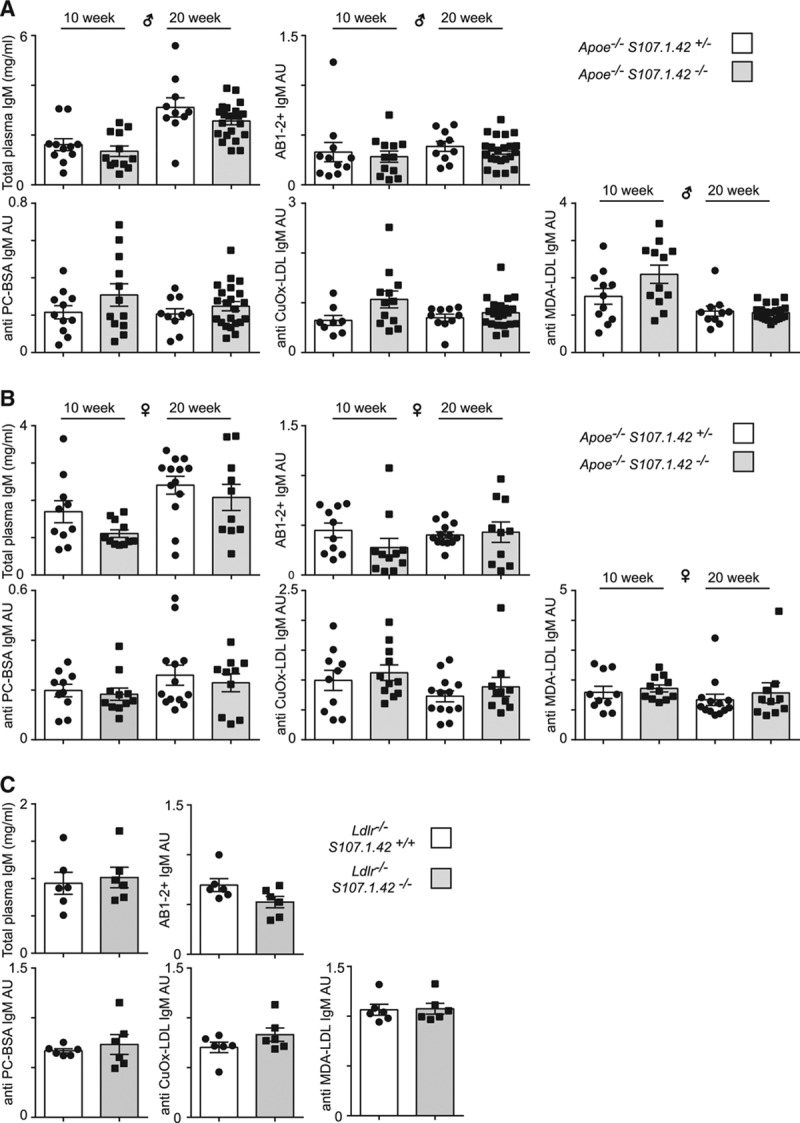
The loss of the VHS107.1.42 gene and humoral immune responses in atherosclerosis. ELISA analysis of 10- and 20-wk-old (A) male and (B) female Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice. Indicated are the total plasma IgM levels, titers of plasma IgM recognized by the AB1-2 antibody normalized to total IgM levels and plasma IgM levels against the common atherosclerosis-associated antigens phosphocholine (PC)-BSA, Cu2+ oxidized low-density lipoprotein (CuOx-LDL) and malondialdehyde modified (MDA)-LDL all normalized to total plasma IgM levels. Each point represents an individual mouse, and error bars represent SEM. Control mice are depicted by white bars, and experimental are by gray. C, Analysis of male Ldlr−/− VHS107.1.42+/+ control and Ldlr−/− VHS107.1.42−/− experimental mice.
Normal Development of Atherosclerosis in the Absence of VHS107.1.42 Encoded Immunoglobulins
To gain insight into the role of the VHS107.1.42 gene in atherosclerosis development, we examined the extent of atherosclerotic lesion formation in our cohort of control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− mice. We measured plaque development by en face staining of aortas in male and female mice at 10 and 20 weeks fed a normal chow diet. We found no significant difference in lesion formation between control and experimental mice at either 10 (Figure 4A) or 20 (Figure 4B) weeks regardless of sex. To evaluate whether VHS107.1.42 could help protect against atherosclerosis in the context of a Western diet, 10-week-old control male Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice were placed on a Western diet for 10 to 12 weeks. Analysis of the aortas by en face staining again revealed no significant difference in atherosclerosis plaque formation (Figure 4C). Finally, we determined atherosclerotic plaque formation in a separate strain of atherosclerotic prone mice. It has previously been shown that Ldlr−/− mice develop atherosclerosis in a diet-dependent manner.33 Therefore, we analyzed Ldlr−/− VHS107.1.42+/+and Ldlr−/− VHS107.1.42−/− mice to determine whether the VHS107.1.42 gene protects against atherosclerosis in a strain-dependent manner. Male mice aged 12 weeks were placed on a Western diet for a further 8 weeks before analysis. Similar to the Apoe−/− strain, induction of atherosclerosis in Ldlr−/− mice through Western diet was not affected by the loss of the VHS107.1.42 gene (Figure 4D). In summary, mice deficient for VHS107.1.42 develop atherosclerotic plaques similar to control mice independently of age, sex, diet, or genetic background.
Figure 4.
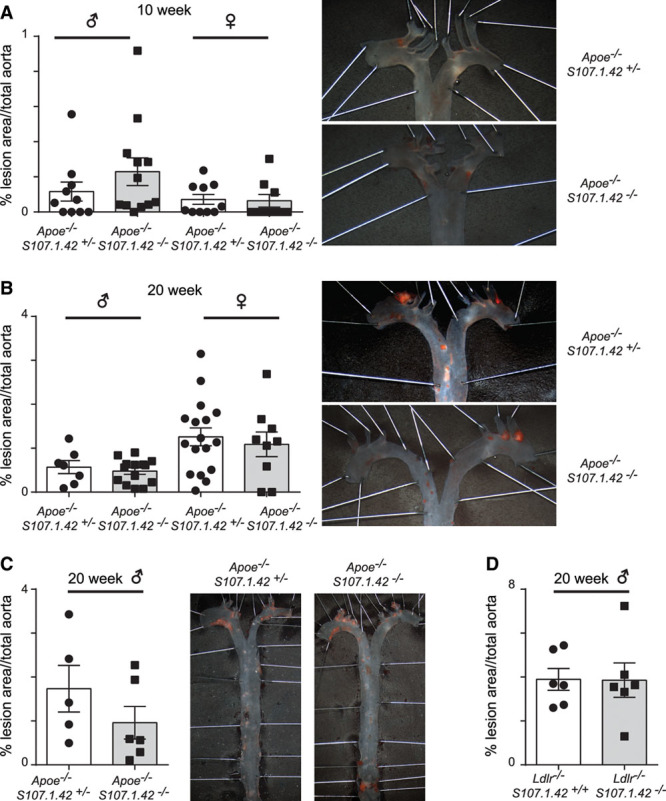
The role of the VHS107.1.42 gene in the initiation and maintenance of aortic atherosclerosis plaque formation. A, Sudan IV en face staining of control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/−experimental mice at 10 wk fed on conventional chow diet. Quantification of the total percentage lesion area in the aorta for male and female mice at 10 wk are shown with a representative picture from female mice depicted in the right. Each data point represents an individual mouse, and error bars indicate SEM. B, As above, but with mice at 20 wk on conventional chow diet. C, Atherosclerosis in male Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice fed a Western diet for 10 to 12 wk. D, Aortic lesion development in male Ldlr−/− VHS107.1.42+/+ control and Ldlr−/− VHS107.1.42−/− experimental mice fed a Western diet for 8 wk.
Function of VHS107.1.42 Antibodies on Plasma Lipids
T15-id antibodies have been shown to form circulating immune complexes with ApoB100-carrying particles and have been suggested to function in preventing the uptake of OxLDL by macrophages.13,34 Therefore, we determined the plasma concentrations of cholesterol and triglycerides in control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice at 20 weeks on both a normal and a Western diet, as well as Ldlr−/− VHS107.1.42+/+ and Ldlr−/− VHS107.1.42−/− mice maintained on a Western diet. Although plasma cholesterol levels were not altered in control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice on either normal or Western diet, we did observe a significant decrease (26%) in plasma cholesterol levels in experimental Ldlr−/− VHS107.1.42−/− versus Ldlr−/− VHS107.1.42+/+control mice (Figure 5A). This pattern was repeated when plasma triglycerides were measured, with no effect seen on loss of the VHS107.1.42 gene on the Apoe−/− background, but a significant 47% drop in experimental Ldlr−/− VHS107.1.42 −/− versus Ldlr−/− VHS107.1.42+/+ control mice (Figure 5B). We next determined the relative association of cholesterol with different lipoproteins by plasma high-performance liquid chromatography fractionation. Analysis of pooled serum revealed a small increase in cholesterol associated with high-density lipoprotein in experimental Ldlr−/− VHS107.1.42 −/− versus Ldlr−/− VHS107.1.42+/+control mice (Figure 5C). Interestingly, loss of the VHS107.1.42 gene correlated with small but significant decreases in body weight on both the Apoe−/− and Ldlr−/− genetic backgrounds (Figure 5D). These experiments would indicate that any humoral immune-mediated effects on lowering blood cholesterol and triglyceride levels are likely to be independent of the VHS107.1.42 T15-id antibody specificity.
Figure 5.
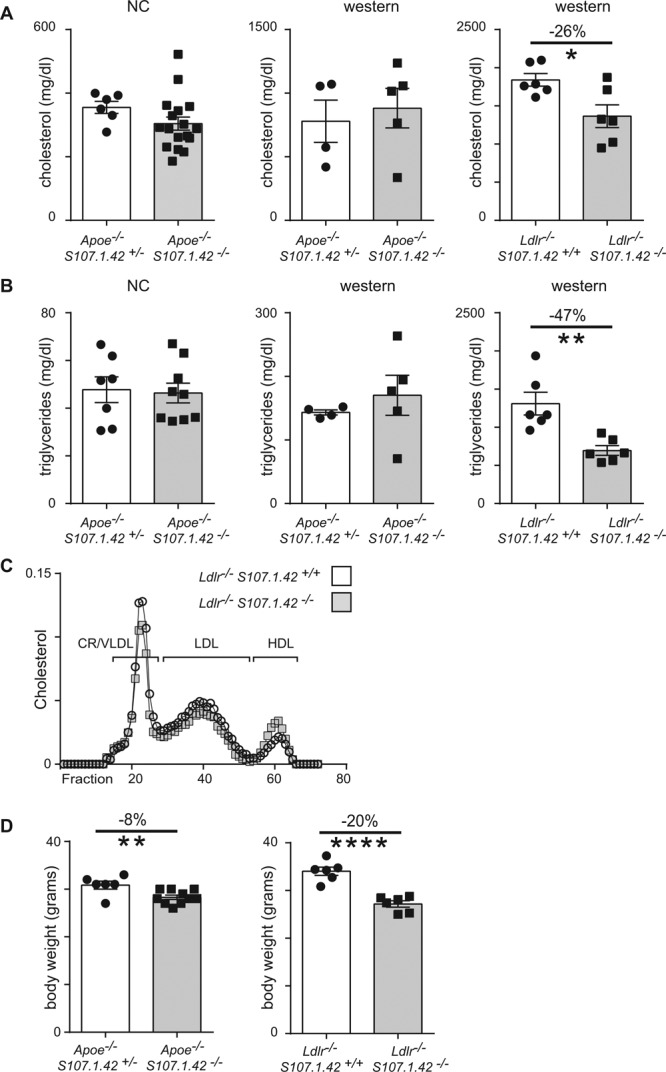
Body weight and plasma lipid levels in the absence of the VHS107.1.42 gene. A, Plasma from 20-wk-old male Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/−experimental mice (left and middle) or Ldlr−/− VHS107.1.42+/+ control and Ldlr−/− VHS107.1.42−/− experimental mice (right) were assayed to quantify total blood cholesterol levels. Apoe−/− mice were either maintained on a conventional diet throughout or were first placed on conventional diet until 10 wk followed by 10 to 12 wk on Western diet. The Ldlr−/− strain was placed on a conventional diet for 12 wk followed by 8 wk on a Western diet. Each point represents an individual mouse, and error bars show SEM. Control mice are depicted by white bars, and experimental are by gray. *P<0.05 vs control. B, Plasma triglycerides levels from mice cohorts as described above. **P<0.01 vs control. C, High-performance liquid chromatography fractionation of plasma lipoproteins to determine relative cholesterol content from Ldlr−/− VHS107.1.42+/+ control and Ldlr−/− VHS107.1.42−/− experimental mice. D, Body weight from mice maintained on a Western diet as described above. ****P<0.0001 vs control. CR indicates chylomicron remnant; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NC, normal chow; and VLDL, very low-density lipoprotein.
Atherosclerotic Plaque Formation in the Aortic Root Is Independent of VHS107.1.42
Atherosclerotic plaque formation in the aortic root is associated with deposition of IgM that has previously been shown to contain the T15-id. Therefore, we quantify the extent of plaque formation in the absence of the VHS107.1.42 encoded T15-id. Serial aortic root sections from female control Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− experimental mice aged 20 weeks maintained on normal chow diet were stained with Oil Red O, and the total lesion area was quantified. We did not detect any significant differences in plaque formation between control and experimental mice similar to the results from the en face analysis of the aorta. Female Apoe−/− VHS107.1.42+/+ had a similar extent of atherosclerosis development in the aortic root as Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− mice, indicating that that plaque formation proceeds similarly regardless of the status of the VHS107.1.42 gene (Figure 6A). In addition, subjecting male mice to 8 weeks of Western diet approximately doubled the extent of atherosclerosis but again quantification revealed no difference between control and experimental mice (Figure 6B). Finally, using immunofluorescence, we were also able to robustly observe widespread IgM deposition in the atherosclerotic plaque of 20-week-old female mice maintained on a normal chow diet even in the absence of the VHS107.1.42 gene encoded T15-id antibodies (Figure 6C).
Figure 6.
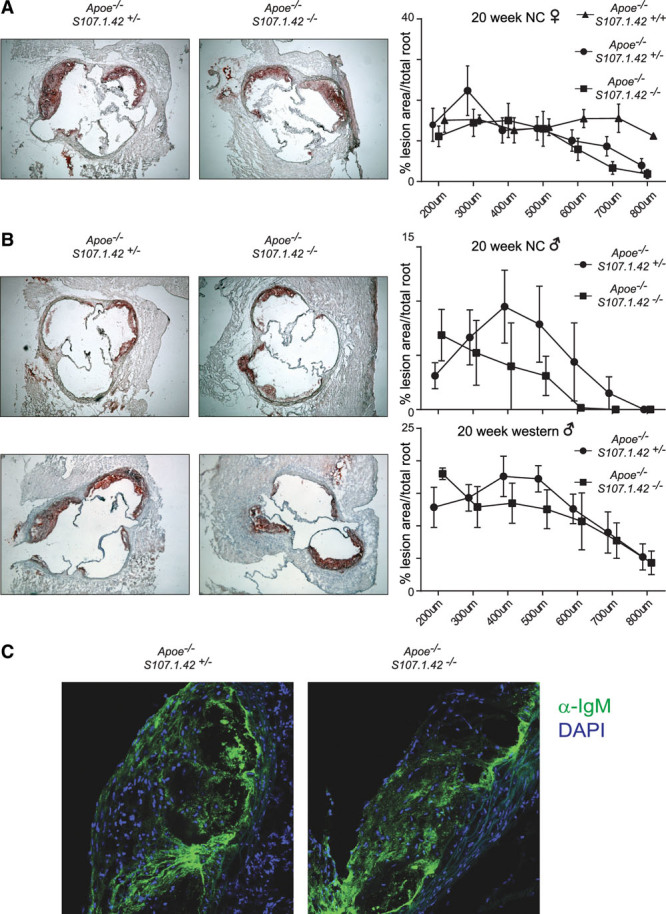
Aortic root atherosclerosis and IgM deposition in VHS107.1.42-deficient mice. A, Oil-red-O–stained aortic roots of 20-wk female Apoe−/− VHS107.1.42+/+, Apoe−/− VHS107.1.42+/−, and Apoe−/− VHS107.1.42−/− mice maintained on a conventional chow diet. Quantification of the mice is shown on the right. The areas under the curves did not differ significantly between experimental and control groups. B, Male Apoe−/− VHS107.1.42+/− and Apoe−/− VHS107.1.42−/− mice fed conventional diet (top) or a Western diet for 8 wk (bottom). C, Confocal microscopy of total IgM (green) staining in aortic root sections from 20-wk-old female Apoe−/− VHS107.1.42+/− control and Apoe−/− VHS107.1.42−/− experimental mice on normal chow (DAPI stained in blue). NC indicates normal chow.
Discussion
Our current understanding of the pathology of both atherosclerotic plaque formation and protection from S pneumoniae infection strongly implicates a protective function for T15-id natural antibodies secreted by the B-1 subset of mature B cells. In this study, we have crossed mice that are deleted for the VHS107.1.42 gene, which was thought to be required to produce the T15-id, with well-characterized atherosclerosis-prone mouse strains to determine the in vivo function of this humoral entity on plaque formation. In contrast to the protection from lethal infection from S pneumoniae that the VHS107.1.42 confers, the development of atherosclerosis seems to be unaffected by the loss of the VHS107.1.42 gene.
The 2 major VH genes expressed in mature B-1 cells are the VH11.2.53 and VH12.1.78 segments.30 Interestingly, these genes when combined with the correct κ light chain are known to bind phosphatidylcholine, but their function in atherosclerosis has not been determined. In contrast, VHS107.1.42 is only expressed in a minor fraction of B-1 cells, and unsurprisingly deletion of VHS107.1.42 did not elicit a detectable defect in B-1 lymphopoiesis.
We were unable to observe any differences in the humoral responses to common atherosclerosis antigens after crossing VHS107.1.42−/− mice with either the Apoe−/− or the Ldlr−/− strains. This is in agreement with previous experiments that revealed normal titers of antibodies against modified LDL in the VHS107.1.42−/− strain when compared with control mice.35 Furthermore, loss of VHS107.1.4224 still permitted humoral responses to phosphocholine in otherwise nonhyperlipidemic mice. However, we did not observe the expected decrease in T15-id reactivity in our hyperlipidemic strains. Although VHS107.1.42 is thought to be an essential component of the T15-id, many studies have shown that alternative immunoglobulin heavy chains can participate in a T15-id+ positive V(D)J rearrangement. Notably this seems to be strain specific. In the BALB/c strain, >99% of the T15-id incorporates VHS107.1.42, whereas in BALB/c×CBA F1 hybrids, this ratio drops to 32%.32 Interestingly, the majority of peritoneal B-1a cells from the BALB/c strain that react against both phosphocholine and AB1-2 seem to use a VH11 immunoglobulin heavy chain gene.28 Crucially, however, it is the canonical T15-id incorporating VHS107.1.42 that provides a protective immune response against S pneumoniae. In this regard, it is noticeable that the BALB/c strain is the most resistant to lethal S pneumoniae infection,36 and it could be speculated that its almost total incorporation of VHS107.1.42 in its T15-id may confer this serological protection. The BALB/c strain is also among the most resistant to diet-induced atherosclerosis,37 and the relative contribution of VH genes to its atheroprotective humoral immune repertoire, not least within the T15-id, may in part contribute to atherosclerosis resistance. Noticeably, the Apoe−/− VHS107.1.42−/− experimental mice used in this study still had a strongly impaired acute response to phosphocholine, as measured by antiphosphocholine IgM titers in the primary immune response to phosphocholine-KLH immunization. This is consistent with the original description of VHS107.1.42−/− mice, which also showed a highly impaired primary immune response, whereas secondary immune responses were less affected because of the production of heteroclitic antibodies.24 Similarly, we demonstrate that in aged mice, the antibody titers against phosphocholine remained constant in the absence of VHS107.1.42. Therefore, we conclude that the acute VHS107.1.42-dependent antiphosphocholine IgM primary immune response, which is critical against S pneumoniae infection, is dispensable for the protection against atherosclerosis. Alternatively, hypercholesterolemia in atherosclerosis-prone mice fails to robustly trigger this primary response in marked contrast to the vigorous reaction after immunization or bacterial infection. This suggests that hypercholesterolemia affects T15-id IgM responses differently or to a lesser extent. However, this may not be the case for other T15-id responses, produced independently of VHS107.1.42. In line with a protective role of T15-id IgM responses on immunization, expansion of T15-id IgM by pneumococcal immunization has been shown to decrease atherosclerotic lesion formation.38
In addition, multiple antigenic epitopes on OxLDL are important in the immune response to atherosclerosis and likely these function in a redundant manner or elicit a large number of minor responses that are not easily distinguished. Notably, we observed normal deposition of IgM within aortic root plaques even in the absence of VH S107.1.42-containing T15-id antibodies.
In summary, we have used 2 different mouse strains that are susceptible to atherosclerosis and rendered these strains unable to produce VHS107.1.42-containing antibodies. Under all the conditions we tested, we were unable to detect an effect on atherosclerosis, regardless of sex, diet, age, or anatomic location of the plaque. Therefore, we conclude that the VHS107.1.42 gene is dispensable for atherosclerosis initiation and maintenance, which is in strong contrast to the protection against S pneumoniae that this immunoglobulin heavy chain gene segment confers. We further speculate that protection against atherosclerosis mediated by IgM is likely to be mediated by many different VH genes with multiple specificities, for example, for epitopes of OxLDL.
Acknowledgments
M. Centa performed most experiments using the Apoe−/−. S. Gruber performed the experiments using the Ldlr−/− strain and performed ELISA. D.K. Johansson contributed to the initial atherosclerosis analysis. D. Nilsson prepared aortas together with M. Centa. M. Centa, S. Gruber, G.K. Hansson, C.J. Binder, and S. Malin devised experiments and interpreted results. S. Malin and C.J. Binder initiated the study. S. Malin wrote the article with contributions from M. Centa, S. Gruber, G.K. Hansson, D.F.J. Ketelhuth, and C.J. Binder.
Sources of Funding
This research was supported by the Swedish Medical Research Council (Vetenskapsrådet) to the Linné Center CERIC (Center of Excellence for Research on Inflammation and Cardiovascular disease), the EU FP7-Health-2013-innovation-1 programme Athero-B-Cell, the EU FP7 programme VIA, Groschinskys Stiftelse, Nanna Svartz Fond, Stiftelsen Apotekare Hedbergs prize, Stockholm County Council, Åke Wiberg Stiftelsen, and the Karolinska Institute.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- OxLDL
- oxidized low-density lipoprotein
- T15-id
- T15-idiotype
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.115.305990/-/DC1.
Significance
It is thought that humoral immune responses in the form of natural IgM protects against atherosclerosis. T15 idiotype antibodies have been implicated in the recognition and clearing of oxidized low-density lipoprotein and being essential for protection from Streptococcus pneumoniae infection. Therefore, it has been hypothesized that there is a common genetic determinant in the protection against both atherosclerosis and S pneumoniae infection. We have crossed mice deficient for the VHS107.1.24 gene with both the Apoe−/− and Ldlr−/− strains. We find no evidence that VHS107.1.24 protects against atherosclerosis or is critical to maintaining normal plasma titers of antibodies against antigens implicated in atherosclerosis. Our results indicate that antibody-mediated protection against atherosclerosis by B lymphocytes is likely to require multiple immunoglobulin heavy chain genes.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro S. The influence of thyroidectomy, splenectomy, gonadectomy, and suprarenalectomy upon the development of experimental atherosclerosis in rabbits. J Exp Med. 1927;45:595–607. doi: 10.1084/jem.45.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. doi: 10.1172/JCI7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22:1892–1898. doi: 10.1161/01.atv.0000039169.47943.ee. [DOI] [PubMed] [Google Scholar]
- 5.Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, Kehry M, Dunn R, Agrotis A, Tipping P, Bobik A, Toh B-H. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185:4410–4419. doi: 10.4049/jimmunol.1000033. doi: 10.4049/jimmunol.1000033. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Oufella H, Herbin O, Bouaziz JD, Binder CJ, Uyttenhove C, Laurans L, Taleb S, Van Vré E, Esposito B, Vilar J, Sirvent J, Van Snick J, Tedgui A, Tedder TF, Mallat Z. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B cells and humoral immunity in atherosclerosis. Circ Res. 2014;114:1743–1756. doi: 10.1161/CIRCRESAHA.113.301145. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 10.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palinski W, Miller E, Witztum JL. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc Natl Acad Sci U S A. 1995;92:821–825. doi: 10.1073/pnas.92.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw PX, Hörkkö S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binder CJ, Hartvigsen K, Chang MK, Miller M, Broide D, Palinski W, Curtiss LK, Corr M, Witztum JL. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–437. doi: 10.1172/JCI20479. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter M, Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970;132:737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohn M, Notani G, Rice SA. Characterization of the antibody to the C-carbohydrate produced by a transplantable mouse plasmacytoma. Immunochemistry. 1969;6:111–123. doi: 10.1016/0019-2791(69)90183-9. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PN, Potter M. Induction of plasma cell tumours in BALB-c mice with 2,6,10,14-tetramethylpentadecane (pristane). Nature. 1969;222:994–995. doi: 10.1038/222994a0. [DOI] [PubMed] [Google Scholar]
- 17.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 18.Crews S, Griffin J, Huang H, Calame K, Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981;25:59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- 19.Feeney AJ. Predominance of the prototypic T15 anti-phosphorylcholine junctional sequence in neonatal pre-B cells. J Immunol. 1991;147:4343–4350. [PubMed] [Google Scholar]
- 20.Chang SP, Brown M, Rittenberg MB. Immunologic memory to phosphorylcholine. II. PC-KLH induces two antibody populations that dominate different isotypes. J Immunol. 1982;128:702–706. [PubMed] [Google Scholar]
- 21.Wiens GD, Brown M, Rittenberg MB. Repertoire shift in the humoral response to phosphocholine-keyhole limpet hemocyanin: VH somatic mutation in germinal center B cells impairs T15 Ig function. J Immunol. 2003;170:5095–5102. doi: 10.4049/jimmunol.170.10.5095. [DOI] [PubMed] [Google Scholar]
- 22.Briles DE, Forman C, Hudak S, Claflin JL. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakil M, Briles DE, Kearney JF. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi QS, Zhou L, Schulze DH, Fischer RT, Lustig A, Rezanka LJ, Donovan DM, Longo DL, Kenny JJ. Highly reduced protection against Streptococcus pneumoniae after deletion of a single heavy chain gene in mouse. Proc Natl Acad Sci U S A. 2000;97:6031–6036. doi: 10.1073/pnas.110039497. doi: 10.1073/pnas.110039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faria-Neto JR, Chyu KY, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Cesena FH, Dimayuga PC, Yano J, Zhao X, Kirzner J, Zhou J, Chan LF, Lio WM, Cercek B, Shah PK, Chyu KY. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE-/- mice. Atherosclerosis. 2012;220:59–65. doi: 10.1016/j.atherosclerosis.2011.10.002. doi: 10.1016/j.atherosclerosis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 28.Vale AM, Kapoor P, Skibinski GA, Elgavish A, Mahmoud TI, Zemlin C, Zemlin M, Burrows PD, Nobrega A, Kearney JF, Briles DE, Schroeder HW., Jr The link between antibodies to OxLDL and natural protection against pneumococci depends on D(H) gene conservation. J Exp Med. 2013;210:875–890. doi: 10.1084/jem.20121861. doi: 10.1084/jem.20121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barstad P, Rudikoff S, Potter M, Cohn M, Konigsberg W, Hood L. Immunoglobulin structure: amino terminal sequences of mouse myeloma proteins that bind phosphorylcholine. Science. 1974;183:962–966. doi: 10.1126/science.183.4128.962. [DOI] [PubMed] [Google Scholar]
- 30.Vilagos B, Hoffmann M, Souabni A, Sun Q, Werner B, Medvedovic J, Bilic I, Minnich M, Axelsson E, Jaritz M, Busslinger M. Essential role of EBF1 in the generation and function of distinct mature B cell types. J Exp Med. 2012;209:775–792. doi: 10.1084/jem.20112422. doi: 10.1084/jem.20112422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearney JF, Barletta R, Quan ZS, Quintáns J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur J Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hörkkö S, Bird DA, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner JA, Friedman P, Dennis EA, Curtiss LK, Palinski W, Witztum JL. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Park Y-B, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gingles NA, Alexander JE, Kadioglu A, Andrew PW, Kerr A, Mitchell TJ, Hopes E, Denny P, Brown S, Jones HB, Little S, Booth GC, McPheat WL. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect Immun. 2000;69:426–434. doi: 10.1128/IAI.69.1.426-434.2001. doi: 10.1128/IAI.69.1.426-434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 38.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]


