Abstract
Objectives
We determined the expression of matrix metalloproteinase (MMP)–1, MMP-9, collagen types I and III, and fibronectin from rabbit vocal folds after injury.
Methods
Thirty rabbits were involved in this study. Five animals were assigned to each time period. Noninjured vocal fold specimens were collected as a control. Gene expression was analyzed at 2, 4, 8, 24, 48, and 72 hours after injury by use of real-time polymerase chain reaction.
Results
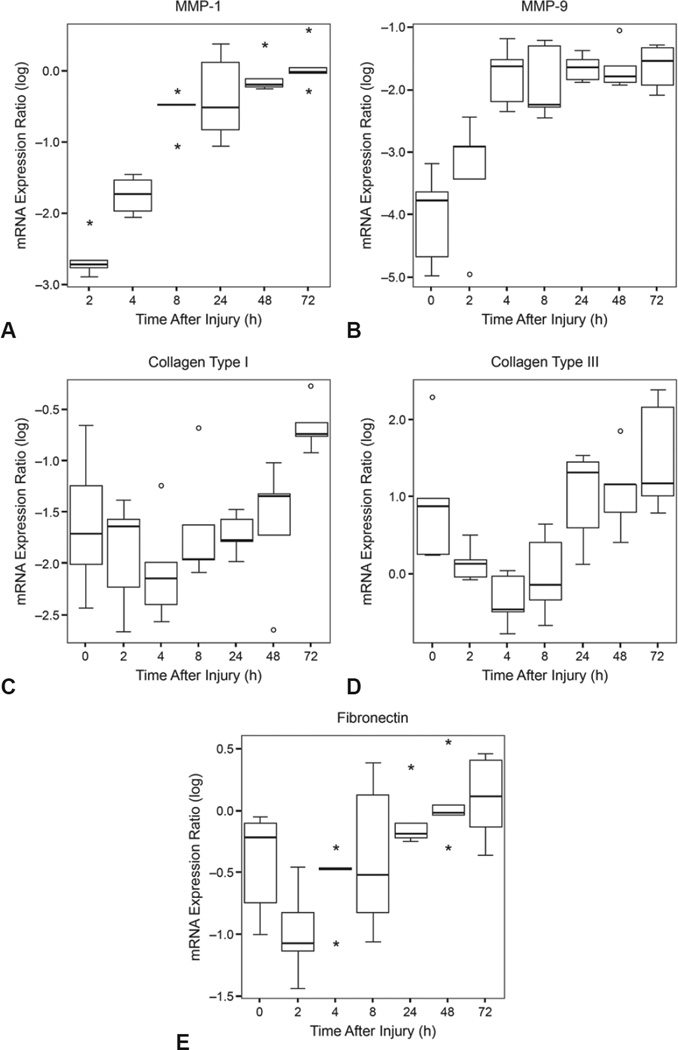
Compared to 2 hours after injury, MMP-1 expression was increased at 4, 8, 24, 48, and 72 hours. Compared to 4 hours, MMP-1 expression was increased at 8, 24, 48, and 72 hours. Compared to the control specimens, MMP-9 expression was increased at 4, 8, 24, 48, and 72 hours. Compared to 2 hours, MMP-9 expression was increased at 4, 8, 24, 48, and 72 hours. Compared to 2 and 4 hours, collagen type I expression was increased at 72 hours. Collagen type III expression was increased at 72 hours compared to 2, 4, and 8 hours. Compared to 2 hours, fibronectin expression was increased at 24, 48, and 72 hours.
Conclusions
Results revealed time-dependent changes in expression of MMP-1, MMP-9, collagen types I and III, and fibronectin from rabbit vocal folds after injury. Future experiments are planned to investigate the effects of phonation on expression of these genes after injury.
Keywords: gene expression, injury, phonation, rabbit model, scar, vocal fold
INTRODUCTION
Wound healing is a dynamic process involving a complex interplay of various cells, extracellular matrices, and soluble mediators. From a wound healing perspective, the vocal fold lamina propria is an interesting tissue exposed to considerable amounts of stress and strain during phonation.1 The contribution of these mechanical forces during remodeling of the vocal fold extracellular matrix is poorly understood. Recently, our laboratory developed an in vivo rabbit phonation model to understand the role of mechanical forces on expression and turnover of the vocal fold extracellular matrix.2 Using the evoked phonation model, we found evidence of alterations in matrix metalloproteinase (MMP)–1 gene expression from normal rabbit vocal folds exposed to experimentally induced phonation.2
Because the extracellular matrix contributes to the viscoelastic properties of the vocal fold lamina propria, cellular responses to injury and phonotrauma are physiologic events with a potentially important impact on vocal fold reparative mechanisms. These processes may play an important role in maintenance of the vocal fold and remodeling of the extracellular matrix after injury. As an extension of our work, the current experiment was performed to determine the expression of MMP-1, MMP-9, collagen types I and III, and fibronectin from nonphonated rabbit vocal folds after injury. Our eventual goal will be to investigate alterations in the expression of these genes following experimentally induced phonation initiated after vocal fold injury. In the current experiment, we utilized real-time (RT) polymerase chain reaction (PCR) to measure the messenger RNA (mRNA) expression of MMP-1, MMP-9, collagen types I and III, and fibronectin in noninjured rabbit vocal folds and injured rabbit vocal folds at 2, 4, 8, 24, 48, and 72 hours after injury.
MATERIALS AND METHODS
Surgical Procedures
Thirty male New Zealand White breeder rabbits weighing 3 to 5 kg were involved in this study. The animals were anesthetized with intramuscular injections of ketamine (35 mg/kg), xylazine (5 mg/kg), and acepromazine (0.75 mg/kg) with subsequent intramuscular injections of ketamine (17.5 mg/kg) and acepromazine (0.375 mg/ kg) as needed to maintain an adequate plane of anesthesia. After induction of anesthesia, the animals were placed on an operating platform in a supine position. A custom laryngoscope was inserted transorally to visualize the larynx. After adequate visualization, a 1-mm segment of mucosa was removed from the middle one third portion of the vocal fold bilaterally with microforceps to create injury and to obtain control specimens for RT-PCR. Postinjury specimens were collected in the same fashion at 2, 4, 8, 24, 48, and 72 hours after wounding. All mucosa samples were immediately submerged in RNA-later (Qiagen Inc, Valencia, California) and then incubated at 4°C overnight and stored at −80°C for later analysis.
Real-Time RT-PCR
We placed tissue specimens in 120 mg of zirconia/silica beads (1-mm diameter) and homogenized them at 4,800 rpm for 90 seconds using a Mini-Beadbeater homogenizer (BioSpec Products, Inc, Bartlesville, Oklahoma). Messenger RNA was extracted from tissue specimens with the RNeasy Fibrous Tissue Mini Kit (Qiagen Inc) and treated with ribonuclease-free deoxyribonuclease I (Qiagen Inc) to minimize contamination from genomic DNA. The mRNA samples were stored at −80°C. Reverse transcription was performed with the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California) according to the manufacturer’s recommended reaction protocol.
The iQ SyBR Green Supermix Kit was used to perform real-time PCR in a 50 µL volume reaction mixture composed of 500 nmol/L primer one, 500 nmol/L primer two, 25 µL iQ SyBR Green Supermix, and 1.2 µL template complementary DNA ribonuclease-free water. Rabbit-specific primers were used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MMP-1, MMP-9, collagen type I, collagen type III, and fibronectin. The detailed forward and reverse primer sequences and the sizes of the products are summarized in the Table. The PCR was performed under the following conditions: 1 cycle at 95°C for 15 minutes, followed by 50 cycles at 95°C for 30 seconds, 58°C for 60 seconds, and 72°C for 60 seconds, and 1 cycle at 50°C to 95°C in 0.5°C increments to make a melting curve. The PCR measurement was repeated 2 times per specimen. We used the iCycler iQ Real-time PCR Detection System (Bio-Rad) to detect the PCR products.
PRIMER SEQUENCES
| Gene | Primer Sequence | Product (bp) |
|---|---|---|
| Matrix metalloproteinase–1 | Forward: 5'-TCA GTT CGT CCT CAC TCC AG-3' Reverse: 5'-TTG GTC CAC CTG TCA TCT TC-3' |
322 |
| Matrix metalloproteinase–9 | Forward: 5'-TGC CAG GAG TAC CTG TTC CGC TAT G-3' Reverse: 5'-TGC CAC TTG AGG TCA CCC TCG AA-3' |
218 |
| Collagen type I | Forward: 5'-CCT GGC ACC CCA GGT CCT CA-3' Reverse: 5'-TCG CTC CCA GGG TTG CCA TC-3' |
227 |
| Collagen type III | Forward: 5'-AAG CCC CAG CAG AAA ATT G-3' Reverse: 5'-TGG TGG AAC AGC AAA AAT CA-3' |
160 |
| Fibronectin | Forward: 5'-CTC ACC CGA GGC GCC ACC TA-3' Reverse: 5'-TCG CTC CCA CTC CTC TCC AAC G-3' |
186 |
| Glyceraldehyde-3-phosphate dehydrogenase | Forward: 5'-TCG GCA TTG TGG AGG GGC TC-3' Reverse: 5'-TCC CGT TCA GCT CGG GGA TG-3' |
177 |
We determined the relative quantitative gene expression using the ratio of target gene concentration to the internal control gene GAPDH. For PCR negative control, primers were not added during PCR. Polymerase chain reaction products were separated by electrophoresis in 2.5% agarose gels containing 0.5 µg/mL ethidium bromide to verify PCR products by fragment size.
Statistical Analysis
Separate 1-way analysis of variance (ANOVA) tests were used to investigate differences in gene expression levels across time. Bilateral vocal fold specimens were averaged for each animal before we did the group analyses with 5 animals per time period. A randomized and equally matched number of noninjured vocal fold specimens were used to establish a control for RT-PCR. The expression ratios were log-transformed to better meet the assumptions of ANOVA. An adjusted α level of 0.010 was used to investigate main effects. If the F test revealed a significant main effect, pairwise comparisons between time points were examined (Fisher’s protected least significant difference). An adjusted α level of 0.0023 was used to account for multiple pairwise comparisons.
RESULTS
Results from the ANOVA revealed a significant main effect for MMP-1 expression across time (p = 0.000; see Figure, A). Matrix metalloproteinase 1 was not expressed in control specimens, but was significantly expressed in the vocal fold after injury. Pairwise comparisons revealed that compared to 2 hours after injury, expression of MMP-1 was significantly increased at 4 hours (p = 0.001), 8 hours (p = 0.000), 24 hours (p = 0.000), 48 hours (p = 0.000), and 72 hours (p = 0.000). Compared to 4 hours after injury, expression of MMP-1 was significantly increased at 8 hours (p = 0.000), 24 hours (p = 0.000), 48 hours (p = 0.000), and 72 hours (p = 0.000).
Log-transformed messenger RNA (mRNA) expression ratios of A) matrix metalloproteinase (MMP)–1 and B) MMP-9, C) collagen type I, D) collagen type III, and E) fibronectin from noninjured rabbit vocal fold (0 hours) and various times after injury (2, 4, 8, 24, 48, and 72 hours). Error bars display standard deviation from mean. Median is indicated by thick black line. Outlier and extreme variables are denoted by asterisks and circles, respectively.
Results from the ANOVA revealed a significant main effect for MMP-9 expression across time (p = 0.000; see Figure, B). Pairwise comparisons revealed that compared to control (0 hours), expression of MMP-9 was increased at 4 hours (p = 0.000), 8 hours (p = 0.000), 24 hours (p = 0.000), 48 hours (p = 0.000), and 72 hours (p = 0.000). Compared to 2 hours after injury, expression of MMP-9 was significantly increased at 4 hours (p = 0.000), 8 hours (p = 0.001), 24 hours (p = 0.000), 48 hours (p = 0.000), and 72 hours (p = 0.000).
Results from the ANOVA revealed a significant main effect for collagen type I expression across time (p = 0.007; see Figure, C). Pairwise comparisons revealed that compared to 2 hours after injury, collagen type I was significantly increased at 72 hours (p = 0.001). Collagen type I was also significantly increased at 72 hours (p = 0.000) compared to 4 hours after injury. Collagen type I was nonsignificantly increased at 72 hours (p = 0.007) compared to control (0 hours).
Results from the ANOVA revealed a significant main effect for collagen type III expression across time (p = 0.000; see Figure, D). Pairwise comparisons revealed that compared to 2 hours after injury, collagen type III was significantly increased at 72 hours (p = 0.001). Compared to 4 hours after injury, collagen type III was significantly increased at 24 hours (p = 0.001), 48 hours (p = 0.001), and 72 hours (p = 0.000). Collagen type III expression was significantly increased at 72 hours (p = 0.000) compared to 8 hours after injury. Expression of collagen type III was nonsignificantly decreased at 4 hours after injury (p = 0.002) compared to control (0 hours).
Results from the ANOVA revealed a significant main effect for fibronectin expression across time (p = 0.002; see Figure, E). Pairwise comparisons revealed that compared to 2 hours after injury, fibronectin expression was significantly increased at 24 hours (p = 0.001), 48 hours (p = 0.000), and 72 hours (p = 0.000) after injury.
DISCUSSION
In order to better understand the process of vocal fold wound healing, research scientists have used animal models to characterize the histologic changes of the vocal fold extracellular matrix.3–9 More recently, studies have been performed to investigate mRNA expression of inflammatory factors from vocal folds after injury.10,11 Although these studies have been helpful in providing an appreciation of changes in the levels and expression of various extracellular matrix components, the influence of phonation on vocal fold reparative processes remains unknown.
To help elucidate the role of phonation in the expression and turnover of the vocal fold extracellular matrix, our laboratory developed an in vivo rabbit phonation model.2 We chose the rabbit as a model to investigate cellular responses to injury and phonotrauma because it is an inherently quiet animal, permitting increased control over nonexperimental vocalization, and it shares tissue microarchitectural similarities with humans.3,7,9,12 As an extension of our previous work to a reparative setting, the purpose of the current experiment was to investigate the mRNA expression of MMP-1, MMP-9, collagen types I and III, and fibronectin from rabbit vocal fold after injury, with the eventual goal of investigating phonation-dependent changes in the expression of these genes.
Matrix metalloproteinases belong to a family of enzymes involved in the turnover of the extracellular matrix.13 Matrix metalloproteinase 1 is an interstitial collagenase involved in the turnover of collagen types I and III. The expression of MMP-1 is stable in normal resting tissues; however, expression is upregulated during physiologic and pathologic tissue repair.14 In the current study, MMP-1 was not expressed in the control specimens. However, expression was significantly up-regulated at 4, 8, 24, 48, and 72 hours after injury. Previously, we discovered increased MMP-1 gene expression in normal rabbit vocal folds exposed to 3 hours of experimentally induced phonation.2 Because MMP-1 is involved in the turnover of collagen in the extracellular matrix, it will be necessary in future experiments to examine how experimentally induced phonation influences MMP-1 gene expression after injury. Additionally, MMP-1 transcription is suppressed by transforming growth factor β, and de novo synthesis can be induced by various cytokines; thus, it will also be necessary to investigate the role of inflammatory cytokines in the expression of MMP in the vocal fold after injury.13
Matrix metalloproteinase–9 plays an important role during extracellular matrix remodeling. Its substrates include denatured collagens, type IV collagen, type V collagen, elastin, and fibronectin.15 In skin, MMP-9 expression is maximally up-regulated 24 to 72 hours after injury.16,17 In the current experiment, MMP-9 expression was increased significantly from 4 to 72 hours after injury, compared to control. Matrix metalloproteinase–9 expression did not return to preinjury levels during any of the time points investigated. In dermal wounds, MMP-9 expression returns to near baseline levels after reepithelialization.16,17 Thus, the stabilization of MMP-9 expression may coincide with the process of reepithelialization, which begins around day 5 in injured rabbit vocal folds.9 However, it is not clear whether phonation initiated before this time prolongs reepithelialization and/or the return of MMP-9 expression to baseline. It is also unclear how recovery from phonotrauma influences transient MMP expression. In a previous study, we found MMP-9 gene expression to be nonsignificantly increased in normal rabbit vocal folds after 3 hours of experimentally induced phonation followed by 1 hour of recovery. However, we could not conclude whether MMP-9 mRNA levels were returning to baseline or increasing away from baseline.2 Further studies are needed to examine longer postinjury time points for investigating stabilization of MMP-1 and MMP-9 mRNA to preinjury levels. Studies are also needed to investigate the influence of phonation on stabilization of MMP-1 and MMP-9 expression in the vocal fold after injury.
Collagen is an important structural protein that provides tensile strength to tissue. Excessive deposition of disorganized collagen type I has been observed during the remodeling phase of dermal injury.18,19 In the current experiment, collagen type I expression was significantly increased at 72 hours after injury compared to 2 and 4 hours after injury, and nonsignificantly increased at 72 hours after injury compared to control. Collagen type III expression was significantly increased at 72 hours after injury compared to 2, 4, and 8 hours, and significantly increased at 24 and 48 hours after injury compared to 4 hours after injury. The increase in collagen type I and type III expression at 72 hours appears to coincide with the proliferative phase of wound repair in injured rabbit vocal folds. Specifically, massive cellular infiltrate has been observed on day 3 after injury in rabbit vocal folds.9 It has been proposed that the cellular infiltrate present on day 3 is likely a combination of inflammatory cells and fibroblasts, and that these cells are involved in the production of neomatrix.9 Because chronic vocal fold scar has been characterized by excessive collagen deposition,6,7 alteration of collagen production during the proliferative and remodeling phase of wound repair may be an important step toward minimizing eventual scar formation. Increased deposition of tissue collagen has been observed in rat vocal folds as early as 2 weeks after injury, underscoring the notion of a critical time period for intervention.20 Given the unique properties of vocal fold vibration and the dynamic nature of cell-matrix interactions, it is important to identify phonatory influences on cell signaling and extracellular matrix deposition after injury. This knowledge may lead to improved management of patients undergoing phonomicrosurgery.
Fibronectin is a ubiquitous glycoprotein involved in a number of cellular processes, including cell migration, cell differentiation, and cell-matrix adhesion.21 Fibronectin is abundantly expressed in regenerating tissues such as dermis.22 The results of the current experiment revealed that compared to 2 hours after injury, fibronectin expression was significantly increased at 24, 48, and 72 hours after injury. The increase of fibronectin mRNA levels during the early stages of wound repair appears to be consistent with fibronectin’s effects on cell-cell and cellmatrix adhesion. Specifically, fibronectin acts as a cellular scaffold, guiding fibroblasts by chemotaxis to the site of the wound bed during wound healing.21 Fibronectin promotes reepithelialization, cell migration, and wound contraction. The increase in fibronectin gene expression may be expected during the days following injury to the vocal fold lamina propria, when cells are proliferating and the process of reepithelialization and reconstitution of the basement membrane is under way. Fibronectin is secreted into the extracellular matrix by several cell types, including fibroblasts, endothelial cells, and monocytes.21 In rat vocal fold injury, fibronectin deposition is increased 24 hours after injury, and elevated fibronectin levels persist throughout early tissue remodeling.20 The increased fibronectin gene expression at 24, 48, and 72 hours in the current study coincides with observations of cellular infiltrate believed to be inflammatory cells and fibroblasts around day 3 in rabbit vocal folds after injury.9
Fibronectin is necessary for the formation of a substratum for migration and growth of cells during granulation tissue formation, remodeling, and resynthesis of the connective tissue matrix.23 However, if fibronectin expression is up-regulated for a prolonged period of time after injury, excessive deposition of extracellular matrix and fibrosis may occur. Elevated fibronectin levels have been associated with impaired vocal fold viscoelastic properties and decreased mucosal wave scores.6,24,25 It will be necessary in future experiments to determine the influence of phonation on reepithelialization of the injured vocal fold and stabilization of fibronec- tin mRNA after injury. Future investigation of cellular responses to experimentally induced phonation in the rabbit model may help to clarify the role of phonation during vocal fold wound repair and lead to improved management of patients with postoperative dysphonia.
CONCLUSIONS
In the current study, we report the expression of MMP-1, MMP-9, collagen types I and III, and fibronectin in noninjured rabbit vocal folds and in injured rabbit vocal folds 2, 4, 8, 24, 48, and 72 hours after injury. The results revealed time-dependent changes in the expression of these genes after vocal fold injury. Knowledge of these changes will be used to design future experiments to investigate the effects of phonation on extracellular matrix gene expression after vocal fold injury in a rabbit in vivo phonation model.2
Acknowledgments
This research was supported by grant R03 DC 008400 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders and by the Vanderbilt University Medical Center Department of Otolaryngology.
Footnotes
Drs Rousseau and Ge contributed equally to study design, data collection, data analysis, and writing. Drs Ohno and French contributed to data analysis and writing. Dr Thibeault contributed to study design and writing.
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University.
Presented at the meeting of the American Laryngological Association, Orlando, Florida, May 1–2, 2008. Recipient of the 2008 Young Faculty/Practitioner Award of the American Laryngological Association.
REFERENCES
- 1.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521–1529. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase–1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 4.Thibeault SL, Bless DM, Gray SD. Interstitial protein alterations in rabbit vocal fold with scar. J Voice. 2003;17:377–383. doi: 10.1067/s0892-1997(03)00064-x. [DOI] [PubMed] [Google Scholar]
- 5.Thibeault SL, Rousseau B, Welham NV, Hirano S, Bless DM. Hyaluronan levels in acute vocal fold scar. Laryngoscope. 2004;114:760–764. doi: 10.1097/00005537-200404000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Rousseau B, Hirano S, Scheidt TD, et al. Characterization of vocal fold scarring in a canine model. Laryngoscope. 2003;113:620–627. doi: 10.1097/00005537-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–124. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau B, Sohn J, Montequin DW, Tateya I, Bless DM. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann Otol Rhinol Laryngol. 2004;113:767–776. doi: 10.1177/000348940411301001. [DOI] [PubMed] [Google Scholar]
- 9.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:19–24. doi: 10.1177/000348940511400105. [DOI] [PubMed] [Google Scholar]
- 10.Lim X, Tateya I, Tateya T, Muñoz-Del-Rio A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 2006;115:921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 11.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory factor profiles one hour following vocal fold injury. Ann Otol Rhinol Laryngol. 2008;117:145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 12.Hirano S, Bless DM, Rousseau B, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37:283–288. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 15.Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 16.Madlener M. Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res. 1998;290(suppl):S24–S29. doi: 10.1007/pl00007450. [DOI] [PubMed] [Google Scholar]
- 17.Soo C, Shaw WW, Zhang X, Longaker MT, Howard EW, Ting K. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–647. doi: 10.1097/00006534-200002000-00024. [Erratum in Plast Reconstr Surg 2000; 106: 236-7.] [DOI] [PubMed] [Google Scholar]
- 18.Peled ZM, Chin GS, Liu W, Galliano R, Longaker MT. Response to tissue injury. Clin Plast Surg. 2000;27:489–500. [PubMed] [Google Scholar]
- 19.Stephens P, Thomas DW. The cellular proliferative phase of the wound repair process. J Wound Care. 2002;11:253–261. doi: 10.12968/jowc.2002.11.7.26421. [DOI] [PubMed] [Google Scholar]
- 20.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 21.Hirschi SD, Gray SD, Thibeault SL. Fibronectin: an interesting vocal fold protein. J Voice. 2002;16:310–316. doi: 10.1016/s0892-1997(02)00102-9. [DOI] [PubMed] [Google Scholar]
- 22.Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- 23.Grinnell F, Billingham RE, Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981;76:181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- 24.Hirano S, Bless DM, Rousseau B, Welham N, Scheidt T, Ford CN. Fibronectin and adhesion molecules on canine scarred vocal folds. Laryngoscope. 2003;113:966–972. doi: 10.1097/00005537-200306000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Thibeault SL, Gray SD, Li W, Ford CN, Smith ME, Davis RK. Genotypic and phenotypic expression of vocal fold polyps and Reinke’s edema: a preliminary study. Ann Otol Rhinol Laryngol. 2002;111:302–309. doi: 10.1177/000348940211100404. [DOI] [PubMed] [Google Scholar]