Abstract
BACKGROUND
Our objective was to evaluate the accuracy of cardiovascular disease (CVD) risk score classification by direct LDL cholesterol (dLDL-C), calculated LDL cholesterol (cLDL-C), and non–HDL cholesterol (non–HDL-C) compared to classification by reference measurement procedures (RMPs) performed at the CDC.
METHODS
We examined 175 individuals, including 138 with CVD or conditions that may affect LDL-C measurement. dLDL-C measurements were performed using Denka, Kyowa, Sekisui, Serotec, Sysmex, UMA, and Wako reagents. cLDL-C was calculated by the Friedewald equation, using each manufacturer’s direct HDL-C assay measurements, and total cholesterol and triglyceride measurements by Roche and Siemens (Advia) assays, respectively.
RESULTS
For participants with triglycerides <2.26 mmol/L (<200 mg/dL), the overall misclassification rate for the CVD risk score ranged from 5% to 17% for cLDL-C methods and 8% to 26% for dLDL-C methods when compared to the RMP. Only Wako dLDL-C had fewer misclassifications than its corresponding cLDL-C method (8% vs 17%; P <0.05). Non–HDL-C assays misclassified fewer patients than dLDL-C for 4 of 8 methods (P < 0.05). For participants with triglycerides ≥2.26 mmol/L (≥200 mg/dL) and <4.52 mmol/L (<400 mg/dL), dLDL-C methods, in general, performed better than cLDL-C methods, and non–HDL-C methods showed better correspondence to the RMP for CVD risk score than either dLDL-C or cLDL-C methods.
CONCLUSIONS
Except for hypertriglyceridemic individuals, 7 of 8 dLDL-C methods failed to show improved CVD risk score classification over the corresponding cLDL-C methods. Non–HDL-C showed overall the best concordance with the RMP for CVD risk score classification of both normal and hypertriglyceridemic individuals.
LDL cholesterol (LDL-C),9 a major risk factor for cardiovascular disease (CVD), is the primary target of lipid-lowering therapy, and is used to classify patients into various CVD risk categories (1). Lipoproteins comprise a heterogeneous group of particles of varying size and lipid and protein composition (2), making the development of specific methods for LDL-C challenging. The reference measurement procedures (RMPs) for LDL-C (rLDL-C) and HDL-C (rHDL-C) are based on ultracentrifugation to remove VLDL and chylomicrons, followed by heparin-manganese precipitation to remove LDL (3). Although rLDL-C is impractical for routine use, it has been validated as a CVD biomarker in large clinical studies (4, 5) and is the standard to which all routine methods are compared (6). Until recently, LDL-C was not usually directly measured but was instead estimated from total cholesterol (TC), HDL cholesterol (HDL-C), and triglyceride (TG) using the Friedewald equation (7). It is known, however, that calculated LDL-C (cLDL-C) becomes progressively less accurate with increasing TGs, is not valid for type III hyperlipoproteinemia, and requires fasting samples (7). In addition, bias and imprecision from the 3 separate measurements used in the calculation can adversely affect cLDL-C accuracy (8).
To address these limitations, various homogeneous reagents for the direct measurement of LDL-C (dLDL-C) have been developed and are now widely adopted (2). An advantage of these methods is that they do not depend on the measurement of TGs, and therefore are less influenced by nonfasting samples. Another advantage is that they are fully automated on various platforms and hence have relatively good precision (9). Nevertheless, previous studies of dLDL-C methods have shown that they may not show complete specificity toward LDL-C and may not always offer a significant practical advantage over cLDL-C (2, 8, 10, 11). These earlier studies, however, were sometimes limited, because they often examined only 1 direct method, and many did not test dyslipidemic populations or compare the results to rLDL-C (2, 8, 10).
Recently, we completed a study comparing all the current dLDL-C methods on the market to rLDL-C (9). We observed that dLDL-C methods frequently failed to meet National Cholesterol Education Program (NCEP) total error goals on dyslipidemic samples when compared to the β-quantification ultracentrifugation RMP for LDL-C. Using the same population, we examined in this study the concordance of CVD risk score classification by the various dLDL-C and cLDL-C methods, using the direct HDL-C (dHDL-C) method from each manufacturer in the calculation, to the CVD risk score obtained by rLDL-C. In addition, apolipoprotein (apo)-B and apoA-I, the main protein structural components of LDL and HDL, respectively, as well as non–HDL-C, were also assessed for CVD risk score classification.
Materials and Methods
PATIENT SAMPLES
Participants were recruited at the Virginia Commonwealth University Medical Center and NIH, with the approval of institutional review boards. Details of the population (n = 175), which included 37 healthy controls, with the majority of the remaining participants recruited from a lipid or CVD clinic, have been previously described (9). A total of 104 participants fasted >12 h, 24 fasted 10–12 h, 11 fasted 8–10 h, and 36 fasted <8 h. Sera were stored at 4 °C, and all measurements were completed within 48 h of collection.
LIPID AND LIPOPROTEIN ANALYSIS
Results for rLDL-C, rHDL-C, dLDL-C, dHDL-C, TG, and TC from the previous study (9 ) were used. Ultracentrifugation reference measurement procedures for LDL-C and HDL-C were performed at the CDC. Direct LDL-C and HDL-C methods [Denka Seiken, Kyowa Medex, Sekisui Medical (formerly Daiichi), Serotec, Sysmex International Reagents, UMA, Wako Pure Chemical Industries, and Roche Diagnostics (distributor of Kyowa Medex reagents with Roche calibrator and controls)] were performed on a Hitachi 917 analyzer (Roche Diagnostics), using parameters recommended by each manufacturer. TC was measured by using Roche reagents adapted for a Siemens Advia 1650 analyzer. Total TG was measured, without glycerol blanking, using Siemens Advia reagents on an Advia 1650 analyzer. Method performance for TC and TG was verified by participation in the CDC Lipid Standardization Program (12 ), and the mean biases compared to the CDC-RMPs were 0.2% (range −0.3% to 0.8%) for TC and −0.1% (range −3.0% to 2.5%) for TG.
LDL-C was calculated by the Friedewald equation: [cLDL-C (mmol/L) = TC (mmol/L) − HDL-C (mmol/L) −TG (mmol/L)/2.22] (7 ), using dHDL-C from each manufacturer and TC and TG, as described above. Non–HDL-C was calculated by the following equation: (non–HDL-C = TC − HDL-C), using either dHDL-C from each manufacturer or rHDL-C and TC as described above. The reference values for VLDL cholesterol (rVLDL-C) were calculated by the following equation, using TC and RMPs for LDL-C and HDL-C: (rVLDL-C = TC − rLDL-C − rHDL-C. For dLDL-C values <0.08 mmol/L (3 mg/dL) or when cLDL-C was <0, a value of 0.05 mmol/L (2 mg/dL) was assigned.
apoA-I and apoB were measured on frozen samples stored at −70° C between 6 and 12 months and were performed in singleton in 1 analytical run, using a nephelometric method on the Dimension Vista® System (Siemens Healthcare Diagnostics). To verify traceability of results, apoB IFCC/WHO standard (SP3-08) and apoA-I IFCC/WHO standard (SP1-01) were measured in quadruplicate and yielded results close to their assigned values [SP3-08 apoB: 118 mg/dL vs mean (SD) 117 (2.2) mg/dL; SP1-01 apoA-1: 150 mg/dL vs 155 (3.7) mg/dL].
STATISTICAL ANALYSIS
JMP Statistical Software (SAS Institute) and Analyze-it for Microsoft Excel (Analyze-it Software) were used. Performance of dLDL-C and cLDL-C compared to rLDL-C was assessed by use of coefficients of determination and weighted Deming regression analysis. Performance of LDL-C methods was evaluated in participants with TG concentrations <2.26 mmol/L (200 mg/dL) and between 2.26 and 4.52 mmol/L (200 and 400 mg/dL) and included both diseased and nondiseased individuals. Participants were classified into CVD risk categories on the basis of NCEP criteria (1 ) as described in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol57/issue3. The McNemar test was used to assess whether the rate of misclassification of participants with dLDL-C or cLDL-C differed significantly from the reference measurement procedure. The nominal data used for the McNemar analysis were misclassification rates for dLDL-C, cLDL-C, and non–HDL-C compared to their RMPs. For each method, misclassification rates were compared to their RMP as previously described (13). For example, we considered a null hypothesis that any given dLDL-C method does not mis-classify patients either more or less frequently than its corresponding cLDL-C method. If both parts of this hypothesis are rejected, we assert equivalence in the rate of misclassification.
Results
COMPARISON OF DIRECT AND CALCULATED LDL-C METHODS FOR SAMPLES WITH TG <2.26 mmol/L (200 mg/dL)
Coefficients of determination (R2) with rLDL-C ranged from 0.85 to 0.99 for dLDL-C assays and from 0.96 to 0.98 for cLDL-C assays (Table 1). All the assays also showed a relatively small proportional and fixed bias. The dLDL-C and cLDL-C results from each method were used to classify CVD risk score, according to NCEP risk categories and compared to the risk scoreclassification obtained by using rLDL-C. The overall misclassification rate of CVD risk score classifications ranged between 5% and 17% for cLDL-C methods and was lower than that observed for 5 of the 8 corresponding dLDL-C methods, which had misclassification rates between 8% and 26%. Statistically, there were significantly (P < 0.05) more misclassifications with Roche and Serotec dLDL-C methods compared to their corresponding cLDL-C methods (Roche dLDL-C 20% vs cLDL-C 6%; Serotec dLDL-C 27% vs cLDL-C 7%). Only the Wako cLDL-C method showed significantly more misclassifications than its corresponding dLDL-C method (17% vs 8%) (P < 0.05).
Table 1.
dLDL-C and cLDL-C vs rLDL-C.
| Denka | Kyowa | Roche | Sekisui | Serotec | Sysmex | UMA | Wako | |
|---|---|---|---|---|---|---|---|---|
| dLDL-C vs rLDL-C [TGs < 2.26 mmol/L (200 mg/dL)] (n = 145) | ||||||||
| R2 | 0.97 | 0.98 | 0.98 | 0.99 | 0.97 | 0.97 | 0.85 | 0.99 |
| Sy|x mmol/L (mg/dL) | 0.08 (3.09) | 0.08 (3.09) | 0.09 (3.48) | 0.06 (2.32) | 0.08 (3.09) | 0.12 (4.64) | 0.18 (6.96) | 0.05 (1.93) |
| Slope (95% CI) | 0.99 (0.89 to 1.10) | 1.00 (0.88 to 1.12) | 0.93 (0.77 to .09) | 0.98 (0.92 to 1.05) | 0.91 (0.81 to 1.01) | 0.91 (0.63 to 1.20) | 0.99 (0.90 to 1.07) | 0.99 (0.98 to 1.01) |
| Intercept (95% CI), mmol/L, mg/dL | −0.02 (−0.25 to 0.22), −0.77 (−9.67 to 8.51) | −0.06 (−0.33 to 0.22), −2.32 (−12.76 to 8.51) | −0.02 (−0.38 to 0.35), −0.77 (−14.69 to 13.53) | −0.02 (−0.16 to 0.13), −0.77 (−6.19 to 5.03) | −0.01 (−0.25 to 0.22), −0.39 (−9.67 to 8.51) | 0.11 (−0.65 to 0.65), 0.00 (−25.14 to 25.14) | −0.04 (−0.23 to 0.15), −1.55 (−8.89 to 5.80) | 0.05 (0.02 to 0.09), 1.93 (0.77 to 4.38) |
| % Observed agreement (95% CI), κ | 87% (80% to 92%), 0.83 | 90% (84% to 95%), 0.87 | 80% (73% to 86%), 0.74 | 91% (85% to 95%), 0.88 | 74% (66% to 81%), 0.66 | 82% (75% to 88%), 0.76 | 86% (79% to 91%), 0.81 | 92% (86% to 96%), 0.89 |
| % In lower/higher risk category | 6%/8% | 8%/2% | 19%/1% | 7%/2% | 265%/1% | 17%/1% | 9%/6% | 3%/5% |
| % Exceeding total error goal | 13% | 8% | 19% | 6% | 35% | 26% | 15% | 4% |
| cLDL-Ca vs rLDL-C [TGs < 2.26 mmol/L (200 mg/dL)] (n = 145) | ||||||||
| R2 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.96 | 0.97 |
| Sy|x mmol/L (mg/dL) | 0.07 (2.71) | 0.08 (3.09) | 0.07 (2.71) | 0.08 (3.09) | 0.07 (2.71) | 0.08 (2.97) | 0.12 (4.64) | 0.05 (1.93) |
| Slope (95% CI) | 0.97 (0.86 to 1.08) | 0.95 (0.78 to 1.13) | 1.00 (0.89 to 1.12) | 0.99 (0.86 to 1.13) | 0.99 (0.90 to 1.08) | 1.00 (0.89 to 1.10) | 0.93 (0.71 to 1.16) | 0.93 (0.52 to 1.33) |
| Intercept (95% CI), mmol/L, mg/dL | −0.02 (−0.26 to 0.23), −0.77 (−10.05 to 8.89) | −0.02 (−0.42 to 0.39), −0.77 (−16.24 to 15.08) | −0.06 (−0.33 to 0.20), −2.32 (−12.76 to 7.73) | −0.02 (−0.34 to 0.29), −0.77 (−13.15 to 11.21) | −0.02 (−0.22 to 0.19), −0.77 (−8.51 to 7.35) | 0.01 (−0.23 to 0.25), 0.49, (−8.80 to 9.74) | 0.04 (−0.48 to 0.55), 1.55 (−18.56 to 21.27) | −0.02 (−0.93 to 0.89), −0.77 (−35.96 to 34.42) |
| % Observed agreement (95% CI), κ | 91% (85% to 95%), 0.88 | 88% (82% to 93%), 0.85 | 95% (89% to 98%), 0.93 | 92% (87% to 96%), 0.90 | 93% (88% to 97%), 0.91 | 90% (80% to 93%), 0.87 | 84% (77% to 90%), 0.79 | 83% (76% to 89%), 0.78 |
| % In lower/higher risk category | 7%/2% | 10%/1% | 3%/3% | 3%/5% | 2%/5% | 1%/9% | 10%/6% | 15%/1% |
| % Exceeding total error goal | 12% | 14% | 10% | 9% | 8% | 10% | 15% | 25% |
| dLDL-C vs rLDL-C [TGs ≥ 2.26 mmol/L (200 mg/dL) and <4.52 mmol/L (400 mg/dL)] (n = 20) | ||||||||
| R2 | 0.97 | 0.83 | 0.82 | 0.99 | 0.82 | 0.84 | 0.74 | 0.98 |
| Sy|x mmol/L (mg/dL) | 0.07 (2.71) | 0.13 (5.03) | 0.13 (5.03) | 0.04 (1.55) | 0.14 (5.41) | 0.13 (5.03) | 0.16 (6.19) | 0.05 (1.93) |
| Slope (95% CI) | 1.07 (0.98 to 1.15) | 1.10 (0.92 to 1.28) | 1.06 (0.88 to 1.24) | 1.06 (1.00 to 1.11) | 1.04 (0.83 to 1.24) | 1.12 (0.93 to 1.31) | 1.07 (0.85 to 1.35) | 0.97 (0.91 to 1.04) |
| Intercept (95% CI), mmol/L, mg/dL | −0.12 (−0.31 to 0.07), −4.64 (−11.99 to 2.71) | −0.01 (−0.34 to 0.32), −0.39 (−13.15 to 12.37) | −0.04 (−0.37 to 0.29), −1.55 (−14.31 to 11.21) | −0.09 (−0.24 to 0.06), −3.48 (−9.28 to 2.32) | −0.30 (−0.75 to 0.16), −11.60 (−29.00 to 6.19) | −0.32 (−0.72 to 0.08), −12.37 (−27.84 to 3.09) | 0.14 (−0.29 to 0.56), 5.41 (−11.21 to 21.66) | 0.27 (0.12 to 0.43), 10.44 (4.64 to 16.63) |
| % Observed agreement (95% CI), κ | 75% (51% to 91%), 0.69 | 60% (36% to 81%), 0.52 | 65% (41% to 85%), 0.57 | 90% (68% to 99%), 0.87 | 70% (46% to 88%), 0.62 | 80% (56% to 94%), 0.75 | 45% (23% to 69%), 0.33 | 70% (46% to 88%), 0.62 |
| % In lower/higher risk category | 10%/15% | 5%/35% | 15%/20% | 0%/10% | 25%/5% | 15%/5% | 10%/45% | 5%/25% |
| % Exceeding total error goal | 5% | 30% | 15% | 0% | 45% | 10% | 45% | 30% |
| cLDL-Ca vs rLDL-C [TGs ≥ 2.26 mmol/L (200 mg/dL) and <4.52 (400 mg/dL)] (n = 20) | ||||||||
| R2 | 0.84 | 0.85 | 0.85 | 0.83 | 0.85 | 0.83 | 0.83 | 0.82 |
| Sy|x mmol/L (mg/dL) | 0.16 (6.19) | 0.15 (5.80) | 0.15 (5.80) | 0.16 (6.19) | 0.15 (5.80) | 0.15 (5.80) | 0.16 (6.19) | 0.16 (6.19) |
| Slope (95% CI) | 1.15 (0.94 to 1.36) | 1.15 (0.96 to 1.33) | 1.16 (0.97 to 1.35) | 1.15 (0.95 to 1.35) | 1.17 (0.99 to 1.35) | 1.15 (0.96 to 1.34) | 1.15 (0.96 to 1.35) | 1.18 (0.96 to 1.41) |
| Intercept (95% CI), mmol/L, mg/dL | −0.53 (−1.00 to −0.06), −20.49 (−38.67 to −2.32) | −0.46 (−0.86 to −0.07), −53.75 (−33.26 to −2.71) | −0.44 (−0.85 to −0.03), −17.01 (−32.87 to −12.76) | −0.45 (−0.87 to −0.03), −17.40 (−33.64 to −1.16) | −0.47 (−0.85 to −0.09), −18.17 (−32.87 to −3.48) | −0.40 (−0.79 to −0.02), −15.47 (−30.55 to −0.77) | −0.41 (−0.82 to −0.00), −15.85 (−31.71 to 0.00) | −0.66 (−1.17 to −0.15), −25.52 (−45.24 to −5.80) |
| % Observed agreement (95% CI), κ | 50% (27% to 73%), 0.38 | 55% (32% to 77%), 0.43 | 65% (41% to 85%), 0.57 | 45% (23% to 69%), 0.31 | 60% (36% to 81%), 0.50 | 55% (32% to 77%), 0.45 | 50% (27% to 73%), 0.38 | 50% (27% to 73%), 0.38 |
| % In lower/higher risk category | 35%/15% | 35%/10% | 20%/15% | 35%/20% | 20%/20% | 25%/20% | 25%/25% | 35%/15% |
| % Exceeding total error goal | 40% | 40% | 40% | 45% | 40% | 35% | 40% | 50% |
cLDL-C was calculated using direct HDL-C from each indicated manufacturer.
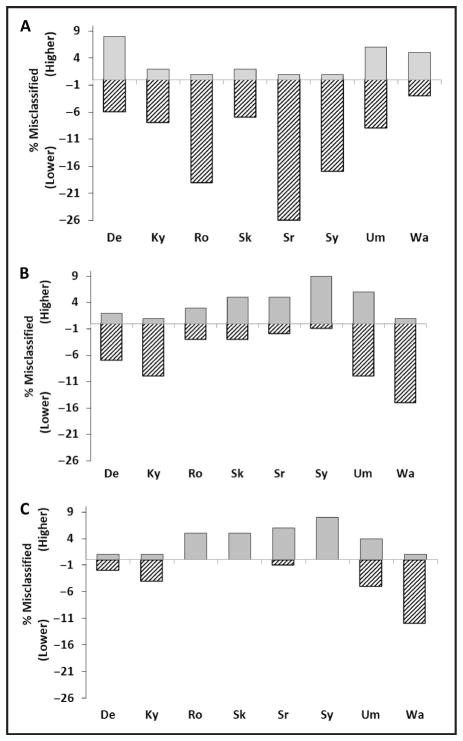
The percentage of individuals classified by the dLDL-C methods into a lower risk category compared to rLDL-C ranged between 3% and 26%, whereas only 1%–8% of individuals were misclassified into a higher risk category (Fig. 1). Except for Denka and Wako dLDL-C methods, dLDL-C methods misclassified more patients into a lower rather than a higher risk category. Only in 2 cases, which both occurred with the UMA dLDL-C method, was any individual misclassified by more than 2 risk categories. In the case of cLDL-C methods, no consistent pattern was observed in terms of the direction of misclassifications (Fig. 1); 3 cLDL-C methods had a positive bias and 4 had a negative bias.
Fig. 1. Misclassification rate for CVD risk for those participants with TG levels <2.26 mmol/L (200 mg/dL), n = 145.
Percent of test results that were classified into either a higher (shaded bar) or lower (hatched bar) CVD risk category compared to RMPs are shown for dLDL-C (A), cLDL-C (B), or non–HDL-C (C). De, Denka; Ky, Kyowa; Ro, Roche; Sr, Serotec; Sk, Sekisui, Sy, Sysmex; Um, UMA; Wa, Wako.
COMPARISON OF DIRECT AND CALCULATED LDL-C METHODS ON HYPERTRIGLYCERIDEMIC SAMPLES
This analysis (Table 1) was limited to 20 individuals with TG concentrations ≥2.26 mmol/L (200 mg/dL) and <4.52 mmol/L (400 mg/dL), because of the known limitation of the Friedewald equation for hypertriglyceridemic samples. In general, the dLDL-C methods performed better than their corresponding cLDL-C methods in this population when assessed by total error or the percent observed agreement with rLDL-C for cardiovascular risk score classification. The cLDL-C methods also appeared to show a bias for categorizing individuals into lower risk categories compared to dLDL-C methods (Table 1 and online Supplemental Fig. 1).
EXAMINATION OF FACTORS CONTRIBUTING TO ERROR IN cLDL-C
In Table 2, we present data for the contribution of errors from the dHDL-C assays in calculating LDL-C. For those patients with TG concentrations <2.26 mmol/L (200 mg/dL), residual SDs (Sy|x) for dHDL-C were relatively low [range 0.06–0.08 mmol/L (2.3–3.1 mg/dL)], except for the UMA assay (0.22 mmol/L, 8.5 mg/dL). Between 6% and 20% of values for dHDL-C methods exceeded total error goals in patients with TG concentrations <2.26 mmol/L (200 mg/dL). When compared to rHDL-C for CHD risk score classification, fewer misclassifications were observed for dHDL-C assays (Table 2) than were observed with dLDL-C assays (Table 1). All dHDL-C assays, however, except Sekisui, showed a substantial increase in the number of results that exceeded total error goals, in patients with TG concentrations ≥2.26 mmol/L (200 mg/dL).
Table 2.
dHDL-C vs rHDL-C.
| Denka | Kyowa | Roche | Sekisui | Serotec | Sysmex | UMA | Wako | |
|---|---|---|---|---|---|---|---|---|
| dHDL-C vs rHDL-C [TGs < 2.26 mmol/L (200 mg/dL)] (n = 146) | ||||||||
| R2 | 0.97 | 0.99 | 0.98 | 0.98 | 0.97 | 0.97 | 0.85 | 0.98 |
| Sy|x mmol/L (mg/dL) | 0.08 (3.09) | 0.06 (2.32) | 0.06 (2.32) | 0.07 (2.71) | 0.08 (3.09) | 0.08 (3.09) | 0.22 (8.51) | 0.07 (2.71) |
| Slope (95% CI) | 1.07 (1.02 to 1.12) | 1.12 (1.08 to 1.15) | 1.06 (1.02 to 1.09) | 1.01 (0.97 to 1.05) | 1.09 (0.99 to 1.19) | 1.01 (0.94 to 1.07) | 1.29 (1.21 to 1.37) | 0.98 (0.93 to 1.04) |
| Intercept (95% CI), mmol/L, mg/dL | −0.08 (−0.13 to −0.02), −3.09 (−5.03 to −0.77) | −0.11 (−0.15 to −0.07), −4.25 (−5.80 to −2.71) | −0.10 (−0.14 to −0.61), −3.87 (−5.41 to −2.32) | −0.06 (−0.11 to −0.02), −2.32 (−4.25 to −0.77) | −0.16 (−0.28 to −0.03), −6.19 (−10.83 to −1.16) | −0.09 (−0.17 to −0.01), −3.48 (−6.57 to −0.39) | −0.33 (−0.41 to −0.26), −12.76 (−15.85 to −10.05) | 0.10 (0.03 to 0.17), 3.87 (1.16 to 6.57) |
| % Observed agreement (95% CI), κ | 93% (88% to 97%), 0.88 | 91% (85% to 95%), 0.86 | 93% (88% to 97%), 0.89 | 93% (88% to 97%), 0.89 | 91% (85% to 95%), 0.86 | 88% (81% to 93%), 0.81 | 86% (80% to 91%), 0.79 | 87% (80% to 92%), 0.80 |
| % In lower/higher risk category | 5%/3% | 1%/8% | 5%/1% | 7%/0% | 8%/1% | 12%/0% | 5%/8% | 1%/12% |
| % Exceeding total error goal | 6% | 8% | 6% | 8% | 12% | 16% | 17% | 20% |
| dHDL-C vs rHDL-C [TGs ≥ 2.26 mmol/L (200 mg/dL)] (n = 28) | ||||||||
| R2 | 0.93 | 0.97 | 0.96 | 0.98 | 0.78 | 0.9 | 0.62 | 0.8 |
| Sy|x mmol/L (mg/dL) | 0.10 (3.87) | 0.11 (4.25) | 0.11 (4.25) | 0.07 (2.71) | 0.26 (10.05) | 0.38 (14.69) | 0.21 (8.12) | 0.18 (6.96) |
| Slope (95% CI) | 1.10 (0.91 to 1.29) | 0.93 (0.58 to 1.27) | 0.85 (0.51 to 1.21) | 1.07 (0.90 to 1.23) | 0.58 (0.37 to 0.79) | 1.07 (0.02 to 2.11) | 0.91 (0.82 to 1.00) | 0.62 (0.00 to 1.24) |
| Intercept (95% CI), mmol/L, mg/dL | −0.02 (−0.18 to 0.13), −0.77 (−6.96 to 5.03) | 0.08 (−0.22 to 0.38), 3.09 (−8.51 to 14.69) | 0.10 (−0.20 to 0.41), 3.87 (−7.73 to 15.85) | −0.07 (−0.20 to 0.07), −2.71 (−7.73 to 2.71) | 0.38 (0.19 to 0.57), 14.69 (−7.35 to 22.04) | −0.12 (−1.05 to 0.80), −4.64 (−40.60 to 30.94) | 0.06 (0.02 to 0.09), 2.32 (−0.77 to 3.48) | 0.45 (−0.12 to 1.03), 17.40 (−4.64 to 39.83) |
| % Observed agreement (95% CI), κ | 89% (72% to 98%), 0.76 | 89% (72% to 98%), 0.73 | 86% (67% to 96%), 0.63 | 86% (67% to 96%), 0.63 | 82% (63% to 94%), 0.55 | 79% (59% to 92%), 0.40 | 79% (59% to 92%), 0.56 | 75% (55% to 89%), 0.52 |
| % In lower/higher risk category | 4%/7% | 11%/0% | 14%/0% | 14%/0% | 14%/4% | 21%/0% | 14%/7% | 0%/25% |
| % Exceeding total error goal | 25% | 11% | 14% | 7% | 25% | 25% | 29% | 43% |
The term TG (mmol/L)/2.22, used in the Friedewald equation, provides an estimate of VLDL cholesterol and is another source of error for cLDL-C. Part of the error is due to imprecision and bias from the TG measurement, including whether endogenous glycerol is subtracted. In addition, TG has a relatively large biologic variability of approximately 20% CV, which also contributes to errors in calculating LDL-C (14). In our population, TG (mmol/L)/2.22 and VLDL cholesterol (n =144, after exclusion of one outlier) showed a relatively weak relationship (R2 = 0.65) and relatively large residual SD (Sy|x) [0.12 mmol/L (4.9 mg/dL)], even for those individuals with TG <2.26 mmol/L (<200 mg/dL) (online Supplemental Fig. 2), which is approximately twice the amount of error contributed from the dHDL-C methods.
NON–HDL-C FOR CVD RISK SCORE CLASSIFICATION
Non–HDL-C, which is a measure of cholesterol associated with all apoB-containing particles, was examined as an alternative for CVD risk score classification (Table 3 and online Supplemental Table 2). Non–HDL-C is unaffected by errors related to estimating VLDL cholesterol and is also unaffected by issues related to the lipoprotein specificity of dLDL-C methods toward the various apoB-containing lipoproteins. For patients with TG concentrations <2.26 mmol/L (200 mg/dL), non–HDL-C calculated by using dHDL-C methods showed a strong relationship (R2 ≥ 0.97) to non–HDL-C calculated with rHDL-C. The percent of individuals classified by the non–HDL-C methods into a lower risk category compared to the reference non–HDL-C method ranged between 0% and 11%, whereas 1%–8% were misclassified into a higher risk category. Except for the Wako dLDL-C, non–HDL-C methods showed overall less misclassifications than dLDL-C methods or cLDL-C methods (Fig. 1).
Table 3.
Comparison of results and classification based on direct non–HDL-C vs RMP non–HDL-C.
| Denka | Kyowa | Roche | Sekisui | Serotec | Sysmex | UMA | Wako | |
|---|---|---|---|---|---|---|---|---|
| Non–HDL-C vs RMP non–HDL-C [TGs < 2.26 mmol/L (200 mg/dL)] (n = 146) | ||||||||
| R2 | 0.997 | 0.997 | 0.997 | 0.997 | 0.996 | 0.995 | 0.973 | 0.997 |
| Sy|x mmol/L (mg/dL) | 0.04 (1.55) | 0.03 (1.16) | 0.03 (1.16) | 0.03 (1.16) | 0.05 (1.93) | 0.04 (1.55) | 0.09 (3.48) | 0.04 (1.55) |
| Slope (95% CI) | 1.00 (0.90 to 1.10) | 1.00 (0.95 to 1.04) | 1.01 (0.96 to 1.07) | 1.00 (0.96 to 1.05) | 1.04 (0.90 to 1.17) | 1.00 (0.93 to 1.08) | 1.00 (0.93 to 1.08) | 0.99 (0.90 to 1.07) |
| Intercept (95% CI), mmol/L, mg/dL | −0.01 (−0.28 to 0.27), −0.39 (−10.83 to 10.44) | −0.02 (−0.13 to 0.10), −0.77 (−5.03 to 6.19) | 0.00 (−0.15 to 0.16), 0.00 (−5.80 to 6.19) | 0.05 (−0.06 to 0.16), 1.93 (−2.32 to 6.19) | −0.05 (−0.42 to 0.32), −1.93 (−16.24 to 12.37) | 0.09 (−0.11 to 0.28), 3.48 (−4.25 to 10.83) | 0.09 (−0.11 to 0.28), 3.48 (−4.25 to 10.83) | −0.05 (−0.28 to 0.18), −1.93 (−10.83 to 6.96) |
| % Observed agreement (95% CI), κ | 97% (92% to 99%), 0.95 | 95% (90% to 98%), 0.93 | 95% (90% to 98%), 0.93 | 95% (90% to 98%), 0.94 | 93% (88% to 97%), 0.91 | 93% (87% to 96%), 0.90 | 90% (84% to 95%), 0.87 | 88% (81% to 93%), 0.83 |
| % In lower/higher risk category | 2%/1% | 4%/1% | 0%/5% | 0%/5% | 1%/6% | 0%/8% | 5%/4% | 12%/1% |
| % Exceeding total error goal | 2% | 1% | 1% | 2% | 2% | 4% | 8% | 3% |
| Non–HDL-C vs RMP non–HDL-C [TGs ≥ 2.26 mmol/L (200 mg/dL)] (n = 28) | ||||||||
| R2 | 0.998 | 0.998 | 0.996 | 0.999 | 0.965 | 0.996 | 0.979 | 0.992 |
| Sy|x mmol/L (mg/dL) | 0.02 (0.77) | 0.01 (0.39) | 0.02 (0.77) | 0.02 (0.77) | 0.04 (1.55) | 0.02 (0.77) | 0.06 (2.32) | 0.03 (1.16) |
| Slope (95% CI) | 0.99 (0.97 to 1.01) | 1.00 (0.97 to 1.02) | 1.00 (0.97 to 1.02) | 1.01 (0.99 to 1.02) | 0.99 (091 to 1.06) | 1.01 (0.98 to 1.04) | 1.01 (0.95 to 1.06) | 0.99 (0.96 to 1.03) |
| Intercept (95% CI), mmol/L, mg/dL | −0.02 (−0.10 to 0.06), −0.77 (−3.87 to 2.32) | 0.00 (−0.08 to 0.09), 0.00 (−3.09 to 3.48) | 0.04 (−0.06 to 0.14), 1.55 (−2.32 to 5.41) | −0.01 (−0.06 to 0.05), −0.39 (−2.32 to 1.93) | 0.06 (−0.22 to 0.34), 2.32 (−8.51 to 13.15) | 0.03 (−0.07 to 0.14), 1.16 (−2.71 to 5.41) | −0.02 (−0.32 to 0.28), −0.77 (−12.37 to 10.83) | −0.10 (−0.26 to 0.06), −3.87 (−10.05 to 2.32) |
| % Observed agreement (95% CI), κ | 100% (88% to 100%), 1.00 | 93% (77% to 99%), 0.91 | 89% (72% to 98%), 0.87 | 93% (77% to 97%), 0.91 | 89% (72% to 98%), 0.87 | 82% (63% to 94%), 0.78 | 79% (59% to 92%), 0.74 | 93% (77% to 99%), 0.91 |
| % In lower/higher risk category | 0%/0% | 0%/7% | 0%/11% | 0%/7% | 0%/11% | 0%/18% | 4%/18% | 7%/0% |
| % Exceeding total error goal | 0% | 0% | 0% | 0% | 4% | 0% | 4% | 7% |
For patients with TG concentrations ≥2.26 mmol/L (200 mg/dL) and <4.52 mmol/L (400 mg/dL), the non–HDL-C methods, in general, showed a better correspondence to their RMP than did dLDL-C or cLDL-C methods (online Supplemental Fig. 1). The percent of individuals misclassified into a lower risk category ranged between 0% and 7%, whereas 0%–18% were misclassified into a higher risk category, which was better than that observed for either dLDL-C or cLDL-C methods.
apoB AND apoA-I FOR CVD RISK SCORE CLASSIFICATION
apoB correlated poorly with all dLDL-C methods, and coefficients of determination (R2) ranged between 0.47 and 0.61 (online Supplemental Table 3). The coefficients of determination between apoB and rLDL-C were also relatively low (R2 =0.56). But the coefficient of determination between apoB and non–HDL-C was better and ranged between 0.83 and 0.84 (online Supplemental Table 4). When reference non–HDL-C was compared to apoB, the coefficient of determination was 0.86 (online Supplemental Fig. 3A).
The relationship between apoA-I and rHDL-C was fairly strong (R2 = 0.81) (online Supplemental Fig. 3B). However, the relationships between apoA-I and the various dHDL-C methods were quite variable, with coefficients of determination ranging between 0.66 and 0.83 (online Supplemental Table 5).
Discussion
A major finding from this study is that dLDL-C methods, in general, did not offer an advantage over cLDL-C in classifying patients into NCEP risk score categories in a dyslipidemic population when compared to rLDL-C. In fact, for patients with TG concentrations <2.26 mmol/L (200 mg/dL), cLDL-C values based on Roche and Serotec dHDL-C methods more closely matched rLDL-C for CVD risk score classification than did their corresponding dLDL-C methods. dLDL-C methods did, however, appear to have an advantage over cLDL-C in CVD risk score classification for those patients with TG concentrations ≥2.26 mmol/L (200 mg/dL) (Table 1 and online Supplemental Table 1), because of the poorer performance of dHDL-C methods on hypertriglyceridemic samples (Table 2) and inaccuracies in VLDL cholesterol estimation (online Supplemental Fig. 2). These results suggest that from a practical and cost perspective, it may be better to use cLDL-C for risk score classification in the subset of patients with TG concentrations <2.26 mmol/L (200 mg/dL), because it does not involve doing the extra measurement for dLDL-C. dLDL-C methods may be best reserved for individuals with TG concentrations ≥2.26 mmol/L (200 mg/dL), in whom these methods usually showed an advantage for correctly classifying patients.
Other factors to consider when evaluating dLDL-C and cLDL-C methods for CVD risk score classification is intraindividual biological variability and the requirement for fasting before sample collection. Although biological variability from all 3 variables, namely TC, TG, and HDL-C, affects cLDL-C, it has been shown that intraindividual variation for cLDL-C is similar to that for dLDL-C [7.3% (0.6%) for cLDL-C and 6.8% (0.5%) for dLDL-C] (8 ). Accurate cLDL-C determination also requires that a patient fast before sample collection (15, 16). A potential advantage, therefore, of dLDL-C methods is their use with non-fasting samples. A recent study of a dLDL-C method (Hitachi 917 analyzer, Roche Diagnostics), however, showed a lack of association of nonfasting dLDL-C with CVD risk, which raises questions about the clinical utility of at least this dLDL-C method in nonfasting patients (17, 18). Another study evaluating a dLDL-C method (Sigma Diagnostics) also showed relatively poor performance in nonfasting patients (19). Other studies have also revealed a physiological postprandial decrease in LDL-C values for some patients (15, 20, 21).
The third Adult Treatment Panel of the NCEP currently recommends the use of non–HDL-C, which includes cholesterol from all apoB-containing lipoproteins, as a secondary target of lipid lowering for individuals with TG concentrations ≥2.26 mmol/L (200 mg/dL) (1 ). In this study, non–HDL-C misclassified fewer cases irrespective of TGs than did either dLDL-C or cLDL-C when compared to their corresponding RMPs (Fig. 1). This reduced rate of misclassification was more pronounced for patients with TG concentrations ≥2.26 mmol/L (200 mg/dL), in whom both dLDL-C and cLDL-C methods showed poorer performance (online Supplemental Fig. 1). Non–HDL-C also requires the measurement of only 2 analytes, instead of the 3 used for cLDL-C, thus reducing costs.
Before non–HDL-C can be recommended as a primary screening test, it will be important to establish not only its superior correspondence to its own RMP, but also that non–HDL-C is at least equivalent to LDL-C for predicting CVD. In diabetic patients, with increased TGs, non–HDL-C has indeed been shown in several studies to be superior to LDL-C in predicting CVD risk (22–24). This may be true because apoB-containing lipoproteins other than LDL, such as remnant lipoproteins, also significantly contribute to the pathogenesis of atherosclerosis in diabetic patients. Several large epidemiologic studies also have shown that non–HDL-C in the general population is at least equivalent to or better than LDL-C and apoB in predicting CVD risk (25–28). In the Framingham Heart study, non–HDL-C was found to be superior to LDL-C in individuals who had TGs that were either increased or within the reference interval (29, 30). Furthermore, non–HDL-C was still predictive of CVD in nonfasting individuals (29, 30). A recent metaanalysis of 68 studies that included more than 300 000 individuals found that hazard ratios for CVD were at least equivalent for non–HDL-C and LDL-C, whether LDL-C was calculated or directly measured by several different methods (31).
Recent guidelines from the American Diabetes Association and American College of Cardiology suggest that apoB may be superior to LDL-C as a target for cholesterol therapy (32 ). apoA-I has also been shown in some studies to be equivalent to or superior to HDL-C in CVD risk assessment (33, 34 ). Our data showed that apoB and apoA-I reclassified 17% and 13%, respectively, into a lower CVD risk category and 22% and 5%, respectively, into a higher CVD risk category compared to rLDL-C and rHDL-C (online Supplemental Tables 3 and 5). Because no clinical outcome data were available in this study, we cannot assess the clinical accuracy of the reclassification by the 2 apo methods. Another limitation of this study was that only 1 apoB and apoA-I method was used, although these methods matched closely the values for the apoB (SP3-08) and apoA-I (SP1-01) IFCC/WHO standards. A recent prospective study, using clinical end points, revealed that apoB and apoA-I (Behring Nephelometer, BNII) did not significantly improve CVD risk score reclassification over that based on cLDL-C or HDL-C (RA-1000 analyzer, Bayer Diagnostics) (35 ).
It is important to note that this study had several limitations. The β-quantification procedure used to measure rLDL-C can also be sensitive to cholesterol in intermediate-density lipoproteins and lipoprotein (a) (2). dLDL-C methods that are truly specific for LDL may, therefore, show a negative bias compared to rLDL-C done by β-quantification, as observed for most of the dLDL-C methods in this study. These other apoB-containing lipoprotein fractions, which are also proatherogenic (36, 37 ), however, would contribute to cLDL-C and non–HDL-C values, which may account, at least in part, for the observed improved performance in cardiovascular risk score classification for these markers. Another limitation of this study was that not all patients were fasting (71 participants fasted <12 h), although a separate analysis of this population did not show a significant difference from fasting individuals in terms of the accuracy of CVD risk score by the various methods (online Supplemental Table 6). In addition, TC and TGs were measured using only 1 routine method each, although the methods used were verified for accuracy in the CDC Lipid Standardization Program. Because approximately 80% of the samples in this study came from patients with dyslipidemias, the results from this study may not apply to other populations; however, these are the types of individuals for whom accurate lipid and lipoprotein testing is the most important. Finally, the sample size of the study was relatively small (n = 175), particularly for the subset of individuals with TG ≥2.26 mmol/L (200 mg/dL) and <4.52 mmol/L (400 mg/dL) (n = 20).
In summary, except for hypertriglyceridemic samples, 7 of 8 dLDL-C methods did not improve the accuracy of CVD risk score classification over cLDL-C. This was attributable, at least in part, to the fact that dHDL-C methods, in general, showed greater concordance with their RMP than did dLDL-C methods. Overall, non–HDL-C, using dHDL-C results, showed the best correspondence to its RMP and better harmonization in CVD risk score classification compared to dLDL-C and cLDL-C methods for both low- and high- TG samples. Future studies with clinical end points should be performed to assess the clinical utility of the various direct measurement methods for LDL-C and HDL-C and to resolve the uncertainty about the clinical significance of the lipoprotein fractions that are being excluded or measured in these direct assays compared to the ultracentrifugation RMPs.
Supplementary Material
Acknowledgments
We thank Tonya Mallory for arranging support from the US distributors. We appreciate the assistance of Drs. Todd Gehr, Daniel Carl, Anna Vinnikova, and Velimir Luketic and Carol Sargeant with recruiting patients at Virginia Commonwealth University. We also thank Kara Dobbin at CDC for performing the cholesterol RMP measurements.
Footnotes
Nonstandard abbreviations: LDL-C, LDL cholesterol; CVD, cardiovascular disease; RMP, reference measurement procedure; rLDL-C, reference measurement procedures for LDL-C; rHDL-C, reference measurement procedures for HDL-C; TC, total cholesterol; HDL-C, HDL cholesterol; TG, triglyceride; cLDL-C, calculated LDL cholesterol; dLDL-C, direct measurement of LDL cholesterol; NCEP, National Cholesterol Education Program; dHDL-C, direct HDL cholesterol; apo, apolipoprotein.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Disclaimer: The findings and conclusions in this manuscript are those of the author(s) and do not necessarily represent the views of the CDC/Agency for Toxic Substances and Disease Registry.
- Employment or Leadership: W.G. Miller, Clinical Chemistry, AACC; G.L. Myers, AACC.
- Consultant or Advisory Role: None declared.
- Stock Ownership: None declared.
- Honoraria: None declared.
- Research Funding: G. Miller, principal investigator for the project: Denka Seiken, Kyowa Medex, Sekisui Medical, Serotec, Sysmex International Reagents, UMA, and Wako Pure Chemical Industries donated reagents, calibrators, and controls and contributed financial support to Pacific Biometrics Research Foundation. Genzyme and Pointe Scientific contributed financial support to Pacific Biometrics Research Foundation. Pacific Biometrics Research Foundation provided a grant to Virginia Commonwealth University (funded by the financial contributions noted above). Roche Diagnostics donated reagents, calibrators, controls, and a Hitachi 917 instrument. H.E. van Deventer, Warren Grant Magnuson Clinical Center, Intramural Research Program of the National Institutes of Health. A.T. Remaley, Warren Grant Magnuson Clinical Center, Intramural Research Program of the National Institutes of Health.
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL-cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002;48:236–54. [PubMed] [Google Scholar]
- 3.Nauck M, Wiebe D, Warnick GR. Measurement of high-density-lipoprotein cholesterol. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. 2. Washington, DC: AACC Press; 2000. pp. 227–30. [Google Scholar]
- 4.Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch Intern Med. 1981;141:1128–31. [PubMed] [Google Scholar]
- 5.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, et al. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–72. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 6.Myers GL, Kimberly MM, Waymack PP, Smith SJ, Cooper GR, Sampson EJ. A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements. Clin Chem. 2000;46:1762–72. [PubMed] [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 8.Schectman G, Patsches M, Sasse EA. Variability in cholesterol measurements: comparison of calculated and direct LDL cholesterol determinations. Clin Chem. 1996;42:732–7. [PubMed] [Google Scholar]
- 9.Miller WG, Myers GL, Sakurabayashi I, Bachman LM, Caudill SP, Dziekonski A, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56:977–86. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahu S, Chawla R, Uppal B. Comparison of two methods of estimation of low density lipoprotein cholesterol, the direct versus Friedewald estimation. Indian J Clin Biochem. 2005;20:54– 61. doi: 10.1007/BF02867401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller WG, Waymack PP, Anderson FP, Ethridge SF, Jayne EC. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem. 2002;48:489–98. [PubMed] [Google Scholar]
- 12.Warnick GR, Kimberly MM, Waymack PP, Leary ET, Myers GL. Standardization of measurements for cholesterol, triglycerides, and major lipoproteins. Lab Med. 2008;39:481–90. [Google Scholar]
- 13.Lachenbruch PA, Lynch CJ. Assessing screening tests: extensions of McNemar’s test. Stat Med. 1998;17:2207–17. doi: 10.1002/(sici)1097-0258(19981015)17:19<2207::aid-sim920>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Ricos C, Alvarez V, Cava F, Garcia-Lario J, Hernandez A, Jimenez CMJ, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 15.Cohn JS, McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456–9. [PubMed] [Google Scholar]
- 16.Rifai N, Merrill JR, Holly RG. Postprandial effect of a high fat meal on plasma lipid, lipoprotein cholesterol and apolipoprotein measurements. Ann Clin Biochem. 1990;27(Pt 5):489–93. doi: 10.1177/000456329002700512. [DOI] [PubMed] [Google Scholar]
- 17.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mora S, Rifai N, Buring JE, Ridker PM. Comparison of LDL cholesterol concentrations by Friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women. Clin Chem. 2009;55:888–94. doi: 10.1373/clinchem.2008.117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HH, Ginsburg GS, Harris N, Rifai N. Evaluation and clinical application of a direct low-density lipoprotein cholesterol assay in normolipidemic and hyperlipidemic adults. Am J Cardiol. 1997;80:1295–9. doi: 10.1016/s0002-9149(97)00668-1. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–79. [PubMed] [Google Scholar]
- 21.Lund SS, Petersen M, Frandsen M, Smidt UM, Parving HH, Vaag AA, Jensen T. Sustained postprandial decrease in plasma levels of LDL cholesterol in patients with type-2 diabetes mellitus. Scand J Clin Lab Invest. 2008;68:628– 40. doi: 10.1080/00365510801995736. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M, Grundy SM. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. 2005;28:1916–21. doi: 10.2337/diacare.28.8.1916. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, et al. Non-HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:16–23. doi: 10.2337/diacare.26.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Jiang R, Schulze MB, Li T, Rifai N, Stampfer MJ, Rimm EB, Hu FB. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27:1991–7. doi: 10.2337/diacare.27.8.1991. [DOI] [PubMed] [Google Scholar]
- 25.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776– 85. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–33. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 27.Bittner V, Hardison R, Kelsey SF, Weiner BH, Jacobs AK, Sopko G. Non-high-density lipoprotein cholesterol levels predict five-year outcome in the Bypass Angioplasty Revascularization Investigation (BARI) Circulation. 2002;106:2537–42. doi: 10.1161/01.cir.0000038496.57570.06. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, Bush TL. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–9. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 29.Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101:1003–8. doi: 10.1016/j.amjcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 2006;98:1363–8. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–22. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 33.Kukita H, Hiwada K, Kokubu T. Serum apolipoprotein A-I, A-II and B levels and their discriminative values in relatives of patients with coronary artery disease. Atherosclerosis. 1984;51:261–7. doi: 10.1016/0021-9150(84)90173-4. [DOI] [PubMed] [Google Scholar]
- 34.Maciejko JJ, Holmes DR, Kottke BA, Zinsmeister AR, Dinh DM, Mao SJ. Apolipoprotein A-I as a marker of angiographically assessed coronary-artery disease. N Engl J Med. 1983;309:385–9. doi: 10.1056/NEJM198308183090701. [DOI] [PubMed] [Google Scholar]
- 35.van der Steeg WA, Boekholdt SM, Stein EA, El-Harchaoui K, Stroes ES, Sandhu MS, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: a case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146:640– 8. doi: 10.7326/0003-4819-146-9-200705010-00007. [DOI] [PubMed] [Google Scholar]
- 36.Hackam DG, Anand SS. Emerging risk factors for atherosclerotic vascular disease: a critical review of the evidence. JAMA. 2003;290:932–40. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 37.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III2–7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.