Abstract
Objectives
We describe a method for eliciting phonation in an in vivo rabbit preparation using low-frequency, bipolar pulsed stimulation of the cricothyroid muscles with airflow delivered to the glottis.
Methods
Ten New Zealand White breeder rabbits weighing 3 to 5 kg were used in this study. The cricothyroid muscles were isolated bilaterally, and separate pairs of anode-cathode hooked-wire electrodes were inserted into each muscle. A Grass S-88 stimulator and 2 constant-current PSIU6 isolation units were used to deliver bipolar square wave pulses to each cricothyroid muscle, with airflow delivered to the glottis through a cuffed endotracheal tube.
Results
Phonation was evoked with a 50-Hz, 4-mA stimulus train of 1-ms pulses delivered to each cricothyroid muscle. The pulse trains were on for 2 seconds and were repeated every 5 seconds over a period of 180 minutes. Airflow was delivered at 143 cm3/s, producing phonation measuring 71 to 85 dB sound pressure level.
Conclusions
Evoked phonation is feasible in rabbits by use of bipolar stimulation of the cricothyroid muscles with airflow delivered to the glottis. The in vivo rabbit preparation described may provide a useful small animal option for studies of evoked phonation. From the level and consistency of the adduction observed, we hypothesize that current spreading to the underlying adductor muscles and nerves resulted in neural pathway involvement beyond discrete activation of the cricothyroid muscle, providing sufficient approximation of the vocal folds for phonation.
Keywords: in vivo model, phonation intensity, rabbit
INTRODUCTION
Neuromuscular activation of muscles via electrical stimulation has been shown to be relatively safe in animals and humans with use of pulsatile currents at low amperages.1,2 Neuromuscular stimulation of laryngeal muscles alters the size and shape of the glottis.3–6 Transtracheal and transesophageal stimulation of the recurrent laryngeal nerve and transmucosal electrical stimulation of individual laryngeal muscles has been found to be an effective approach for eliciting vocal fold motion.1,4 Animal models provide a useful approach for investigating the effects of phonation on vocal fold tissue properties.7,8 Previously, Gray and Titze7 studied the histology of hyperphonated vocal folds using an evoked canine phonation model. The dog was chosen because of its size and for being a species that was relatively easy to work with. Despite the advantages and convenience of the in vivo canine preparation, some of its disadvantages included the propensity for kennel cough, a predisposition toward developing laryngeal edema and nodular formations, and a layered vocal fold structure characteristically different from that of humans, which may provide dogs with a different capacity for resistance to phonotrauma.7 Our laboratory has developed an in vivo rabbit preparation to investigate the effects of phonation on vocal fold homeostasis.8 Our long-term goal is to systematically investigate phonation-induced gene regulation, synthesis and degradation of the extracellular matrix, and the role of mechanical forces on phonation-dependent tissue remodeling. From a tissue standpoint, the rabbit shares similarities with humans in terms of vocal fold microarchitectural properties and extracellular matrix components. These favorable tissue properties have led to its use as a model for vocal fold wound healing in recent years.9–12 The rabbit is also an inherently quiet animal, providing increased control over non-experimental vocalization, without the need for ancillary procedures to suppress voice use.13 In the present study, we describe a method for eliciting phonation in an in vivo rabbit preparation using low-frequency, bipolar pulsed stimulation of the cricothyroid muscles with airflow delivered to the glottis.
METHODS
Ten New Zealand White breeder rabbits weighing 3 to 5 kg were used in this study. The animals were anesthetized with ketamine hydrochloride 35 mg/kg, xylazine hydrochloride 5 mg/kg, and acepromazine maleate 0.75 mg/kg administered by intramuscular injection with subsequent intramuscular injection of ketamine hydrochloride (17.5 mg/kg) and acepromazine maleate (0.375 mg/kg) as needed to maintain a surgical plane of anesthesia. The heart rate and spot oxygen saturation (SpO2) were monitored throughout the experiment to monitor the animal’s state of anesthesia and general well-being. The animals were placed supine on an operating platform, and the larynx and trachea were exposed by a midline neck incision extending from the hyoid bone to the sternal notch. The larynx was suspended with a custom-designed laryngoscope. The cricothyroid muscles were isolated bilaterally, and separate pairs of anode-cathode hooked-wire electrodes were inserted into each cricothyroid muscle. The anode was inserted along the superior edge of each cricothyroid muscle in a lateral-to-medial direction. The cathode was inserted along the inferior edge of each cricothyroid muscle in a medial-to-lateral direction. A Grass S-88 stimulator (SA Instrumentation, Encinitas, California) and 2 direct current PSIU6 isolation units (1 isolation unit per muscle) were used to deliver square wave pulses to the cricothyroid muscles. Pulse trains were delivered every 5 seconds (2 seconds on; 3 seconds off).
Vocal fold position was monitored with a 2.7-mm 5° telescope during stimulation (Panoview, Richard Wolf, Vernon Hills, Illinois). The threshold of vocal fold motion was recorded by increasing the stimulation current until a minimal vocal fold twitch was observed. Recordings of vocal fold adduction were made by further increasing the stimulation current and frequency in a stepwise fashion to observe the resulting glottal closure patterns. Airflow was delivered with a flowmeter (GF8522-1700, Barnant, Barrington, Illinois) and a humidifier (Concha Therm III, Hudson RCI, Temecula, California) providing compressed, humidified air (37°C) to the glottis through a cuffed endotracheal tube with an internal diameter of 3.0 mm, positioned 2 cm below the glottal aperture. The cuff of this tube was inflated to seal off the trachea. A separate endotracheal tube with an internal diameter of 4.0 mm was inserted through a tracheostomy 3.5 cm below the cricoid cartilage to allow for spontaneous respiration.
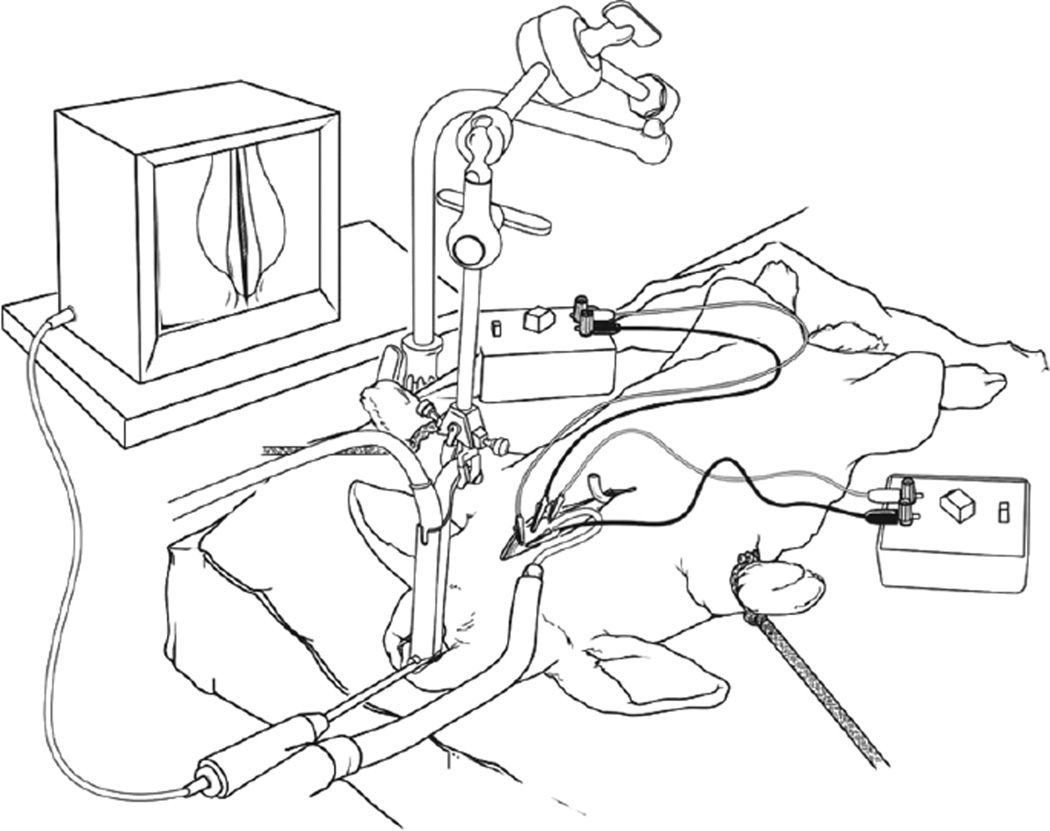
The airflow rate for the phonation experiment was determined by performing an upward sweep of airflow on the flowmeter in combination with electrical stimulation until continuous audible phonation was obtained. Measurements of phonation intensity were collected at 5, 30, 90, and 180 minutes after the onset of phonation with a sound level meter (RadioShack, Fort Worth, Texas). The sound level meter was positioned at the proximal end of the laryngoscope used to visualize the vocal folds, for an approximate distance of 15 cm from the larynx. The experiments were performed in a small animal procedure room. The average background noise level in the laboratory was 37.89 dB sound pressure level (SPL). Figure 1 shows a schematic of the in vivo rabbit preparation.
Fig 1.
Schematic of in vivo rabbit preparation.
RESULTS
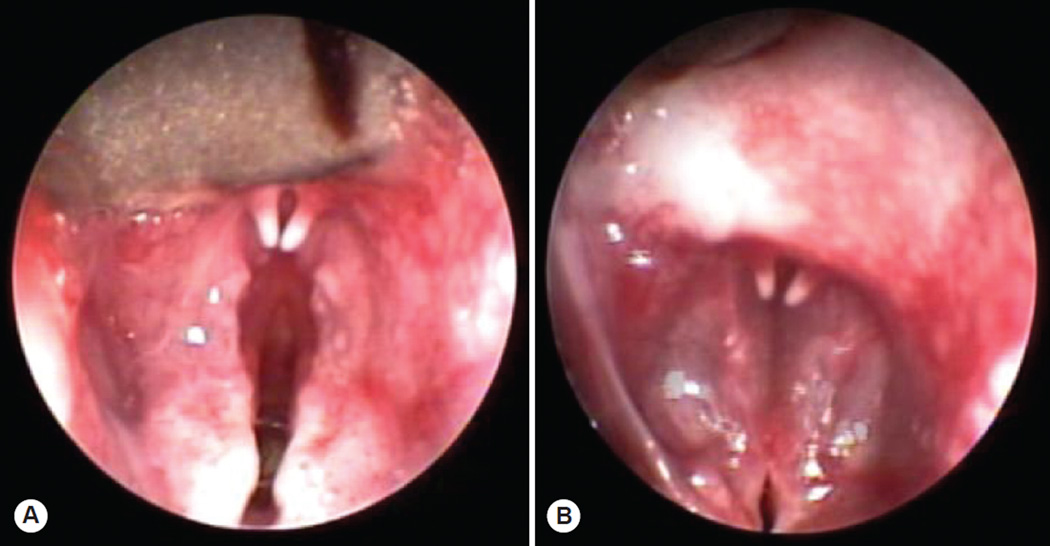
The threshold of vocal fold motion, defined as the level of current resulting in a minimal vocal fold twitch, was observed at 1 mA. Further increasing the stimulation current and frequency resulted in graded vocal fold adduction, until complete vocal fold adduction was observed (Fig 2). The stimulation settings resulting in complete glottal closure ranged from 50 to 60 Hz and from 3 to 4 mA. An airflow rate of 143 cm3/s was selected for the phonation trials based on an airflow sweep with stimulation set to 50 Hz and 4 mA. This flow value represented the flow rate between phonations. Phonation was evoked with a 50-Hz, 4-mA stimulus train of 1-ms pulses delivered to each cricothyroid muscle. The pulse trains were on for 2 seconds and were repeated every 5 seconds over a period of 180 minutes. Airflow was delivered at 143 cm3/s, producing phonation measuring 71 to 85 dB SPL (Fig 3).
Fig 2.
Endoscopic images of rabbit larynx in A) abducted and B) phonatory positions.
Fig 3.
Mean phonation intensity measured 5, 30, 90, and 180 minutes after onset of phonation. Error bars represent standard deviation from mean.
Figure 4 displays alterations in the mean phonation intensity at various stimulation frequencies (20 to 60 Hz) with airflow delivered at 143 cm3/s and stimulation current delivered at 4 mA from a subset of study animals. The phonation intensity ranged from 69 to 85 dB SPL. Pearson product moment correlations were used to investigate the relationship between stimulation frequency and phonation intensity. The correlation coefficients (r) were 0.719 (animal 839), 0.828 (animal 841), 0.526 (animal 856), and 0.846 (animal 858).
Fig 4.
Alterations in mean phonation intensity across various stimulation frequencies (20 to 60 Hz) with airflow fixed at 143 cm3/s and stimulation current delivered at 4 mA.
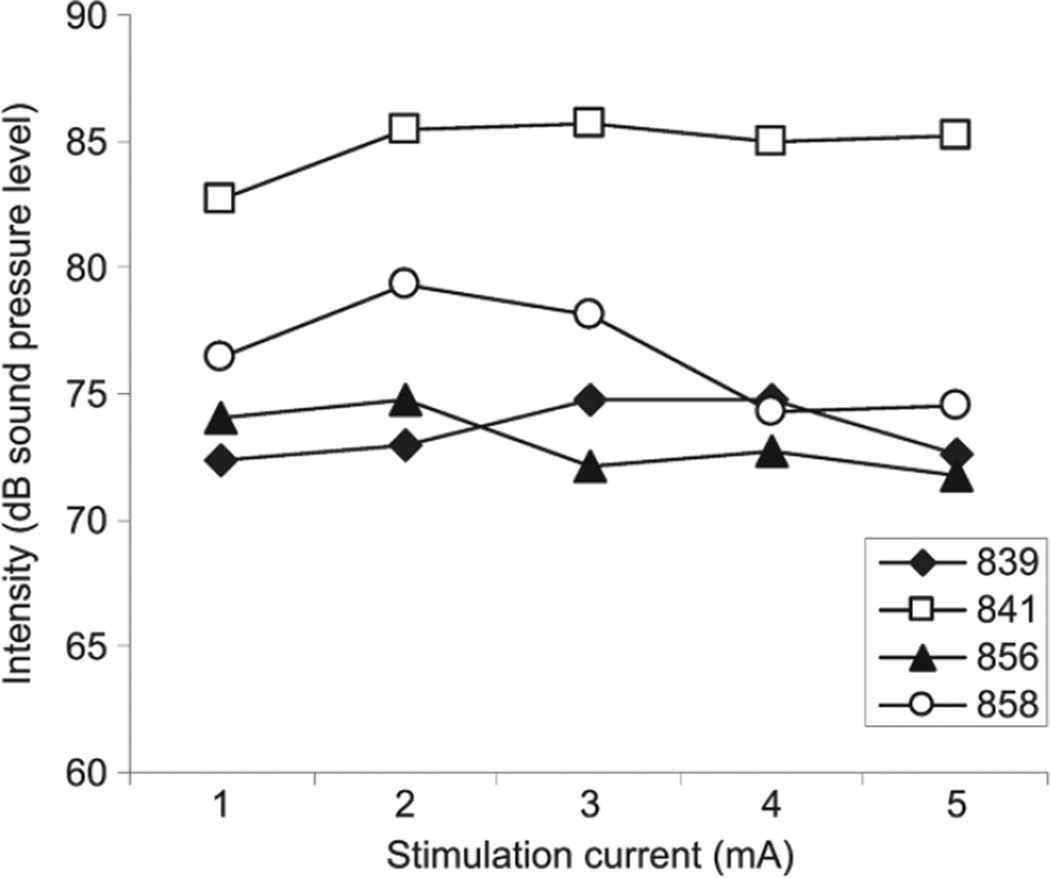
Figure 5 displays alterations in the mean phonation intensity at various stimulation currents (1 to 5 mA) with the airflow delivered at 143 cm3/s and the stimulation frequency delivered at 50 Hz. The phonation intensity ranged from 72 to 86 dB SPL. Pearson product moment correlations were used to investigate the relationship between stimulation current and phonation intensity. The correlation coefficients were 0.478 (animal 841), –0.146 (animal 856), –0.514 (animal 858), and 0.249 (animal 839).
Fig 5.
Alterations in mean phonation intensity across various stimulation currents (1 to 5 mA) with airflow fixed at 143 cm3/s and stimulation frequency delivered at 50 Hz.
DISCUSSION
Animal models provide a useful adjunct to the cadre of techniques used in voice biology. Laryngeal models, including theoretical, ex vivo, and in vivo animal preparations, provide a useful approximation of human laryngeal function and are often necessary for systematic manipulation of parameters that are difficult to control for in a clinical population.14 In vivo animal preparations provide the added advantage of allowing for the study of cells and extracellular matrices in their native 3-dimensional environment.
The purpose of the present study was to describe a method for eliciting phonation in an in vivo rabbit preparation using low-frequency, bipolar pulsed stimulation of the cricothyroid muscles with airflow delivered to the glottis. It is well known that electrical neuromuscular stimulation produces frequency-dependent vocal fold motion and is an effective approach for modifying the glottal aperture.1,3–6 Sanders et al1 were the first to demonstrate that the recurrent laryngeal nerve could be activated by passing a current across the tracheal or esophageal mucosa and described stimulation to be an effective approach for eliciting vocal fold motion in dogs. In rabbits, a single trunk of the recurrent laryngeal nerve approaches the larynx and inserts into a groove between the esophagus and trachea.15 The posterior cricoarytenoid muscle is essentially innervated by a single branch of the main trunk of the recurrent laryngeal nerve as it crosses over the cricothyroid joint. After entering the posterior cricoarytenoid muscle, this branch breaks off into 2 or 3 smaller intramuscular nerve branches.15 The recurrent laryngeal nerve of rabbits also innervates the interarytenoid muscle and the lateral cricoarytenoid muscle. The interarytenoid muscle is innervated via a nerve branch that passes along the anterior border of the posterior cricoarytenoid muscle and the cricoarytenoid joint, and enters the inferior lateral border of the interarytenoid muscle. The lateral cricoarytenoid branch of the recurrent laryngeal nerve courses the superior border of the cricoid cartilage with 1 or 2 fine twigs branching from the recurrent laryngeal nerve to innervate the lateral cricoarytenoid muscle.15 The remaining fibers of the recurrent laryngeal nerve innervate the pars vocalis and the thyroarytenoid muscle, which are organized into 2 separate neuromuscular compartments. In the present experiment, separate pairs of anode-cathode hooked-wire electrodes were used to deliver bipolar stimulation to the cricothyroid muscles with airflow delivered to the glottis through a cuffed endotracheal tube. On the basis of the level and consistency of adduction observed, we hypothesized that current spreading to the underlying adductor muscles and nerves resulted in neural pathway involvement beyond discrete cricothyroid muscle activation, providing sufficient approximation of the vocal folds for vocalization. Future experiments are planned to investigate the mechanism of the closure response and the effects of modifying the electrode placement on the resulting glottal closure and elicited phonations.
One of the goals of the present experiment was to determine the threshold for eliciting vocal fold motion and the level of stimulation necessary for achieving phonation. The results revealed that the level of current that elicited a minimal vocal fold twitch was around 1 mA. The findings are similar to those in reports of transtracheal and transesophageal, transmucosal, and nerve-elicited neuromuscular activation of laryngeal muscles in canine preparations.1,4,16 Complete glottal closure was achieved with stimulation delivered at 3 to 4 mA and 50 to 60 Hz. Previous reports have cited stimulation currents around 3 mA and stimulation frequencies of 40 to 70 Hz for achieving vocal fold adduction in dogs.1,16 With the electrode configuration used in the present study, audible phonation was achieved with 50-Hz, 4-mA, 1-ms pulsed stimulation with airflow delivered at 143 cm3/s. To further explore the effects of electrical stimulation on phonation, we investigated the effects of stimulation current and frequency on phonation intensity in a subset of study animals. Correlation coefficients revealed positive correlations between stimulation frequency and phonation intensity, whereas stimulation current and phonation intensity appeared to function independently.
Despite the use of fixed stimulation and airflow parameters, phonation intensity did vary across animals. We suspect that differences in rabbit anatomy, depth of anesthesia, and location of the electrodes within stimulated muscles may have resulted in variable glottal configurations and muscle contractile forces, resulting in these differences in phonation intensity. One strategy to control for the variation in phonation output in future studies will be to adjust stimulation and airflow settings to match the intensity, frequency, and quality of elicited phonation across animals.
CONCLUSIONS
Evoked phonation is feasible in rabbits with use of bipolar stimulation of the cricothyroid muscles with airflow delivered to the glottis. In the present experiment, phonations measuring 71 to 85 dB SPL were evoked with a 50-Hz, 4-mA stimulus train of 1-ms pulses to the cricothyroid muscles with airflow delivered at a rate of 143 cm3/s. The in vivo rabbit preparation described may provide a useful small animal option for studies of evoked phonation.
Acknowledgments
The authors thank Erik R. Swanson, MD, and Davood Abdollahian for assistance with manuscript preparation and data interpretation.
This research was supported by NIH grant R03 DC 008400 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University.
Presented at the meeting of the American Broncho-Esophagological Association, Orlando, Florida, May 1–2, 2008.
REFERENCES
- 1.Sanders I, Kraus WM, Aviv JE, Racenstein M, Biller HF. Transtracheal/transesophageal stimulation of the recurrent laryngeal nerve. Laryngoscope. 1987;97:663–667. doi: 10.1288/00005537-198706000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Fall M, Erlandson BE, Nilson AE, Sundin T. Long-term intravaginal electrical stimulation in urge and stress incontinence. Scand J Urol Nephrol Suppl. 1977;44:55–63. [PubMed] [Google Scholar]
- 3.Zealear DL, Dedo HH. Control of paralysed axial muscles by electrical stimulation. Acta Otolaryngol. 1977;83:514–527. doi: 10.3109/00016487709128880. [DOI] [PubMed] [Google Scholar]
- 4.Sanders I, Kraus WM, Morel B, Wu BL, Aviv JE, Biller HF. Transmucosal electrical stimulation of laryngeal muscles. Ann Otol Rhinol Laryngol. 1989;98:339–345. doi: 10.1177/000348948909800505. [DOI] [PubMed] [Google Scholar]
- 5.Zealear DL, Rainey CL, Jerles ML, Tanabe T, Herzon GD. Technical approach for reanimation of the chronically denervated larynx by means of functional electrical stimulation. Ann Otol Rhinol Laryngol. 1994;103:705–712. doi: 10.1177/000348949410300908. [DOI] [PubMed] [Google Scholar]
- 6.Zealear DL, Rainey CL, Herzon GD, Netterville JL, Ossoff RH. Electrical pacing of the paralyzed human larynx. Ann Otol Rhinol Laryngol. 1996;105:689–693. doi: 10.1177/000348949610500904. [DOI] [PubMed] [Google Scholar]
- 7.Gray S, Titze I. Histologic investigation of hyperphonated canine vocal cords. Ann Otol Rhinol Laryngol. 1988;97:381–388. doi: 10.1177/000348948809700410. [DOI] [PubMed] [Google Scholar]
- 8.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase–1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibeault SL, Gray SD, Bless DM, Chan RW, Ford CN. Histologic and rheologic characterization of vocal fold scarring. J Voice. 2002;16:96–104. doi: 10.1016/s0892-1997(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 10.Rousseau B, Hirano S, Chan RW, et al. Characterization of chronic vocal fold scarring in a rabbit model. J Voice. 2004;18:116–124. doi: 10.1016/j.jvoice.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Hirano S, Bless DM, Rousseau B, et al. Prevention of vocal fold scarring by topical injection of hepatocyte growth factor in a rabbit model. Laryngoscope. 2004;114:548–556. doi: 10.1097/00005537-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:19–24. doi: 10.1177/000348940511400105. [DOI] [PubMed] [Google Scholar]
- 13.Milligan SR, Sales GD, Khirnykh K. Sound levels in rooms housing laboratory animals: an uncontrolled daily variable. Physiol Behav. 1993;53:1067–1076. doi: 10.1016/0031-9384(93)90361-i. [DOI] [PubMed] [Google Scholar]
- 14.Berke GS, Moore DM, Hantke DR, Hanson DG, Gerratt BR, Burstein F. Laryngeal modeling: theoretical, in vitro, in vivo. Laryngoscope. 1987;97:871–881. [PubMed] [Google Scholar]
- 15.Ryan S, McNicholas WT, O’Regan RG, Nolan P. Intralaryngeal neuroanatomy of the recurrent laryngeal nerve of the rabbit. J Anat. 2003;202:421–430. doi: 10.1046/j.1469-7580.2003.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paniello RC, Dahm JD. Long-term model of induced canine phonation. Otolaryngol Head Neck Surg. 1998;118:512–522. doi: 10.1177/019459989811800413. [DOI] [PubMed] [Google Scholar]