Abstract
Introduction
As the flows of immigrant populations increase worldwide, their heterogeneity becomes apparent with respect to the differences in the prevalence of chronic physical and mental disease. Multimorbidity provides a new framework in understanding chronic diseases holistically as the consequence of environmental, social, and personal risks that contribute to increased vulnerability to a wide variety of illnesses. There is a lack of studies on multimorbidity among immigrants compared to native-born populations.
Methodology
This nationwide multi-register study in Norway enabled us i) to study the associations between multimorbidity and immigrant origin, accounting for other known risk factors for multimorbidity such as gender, age and socioeconomic levels using logistic regression analyses, and ii) to identify patterns of multimorbidity in Norway for immigrants and Norwegian-born by means of exploratory factor analysis technique.
Results
Multimorbidity rates were lower for immigrants compared to Norwegian-born individuals, with unadjusted odds ratios (OR) and 95% confidence intervals 0.38 (0.37–0.39) for Eastern Europe, 0.58 (0.57–0.59) for Asia, Africa and Latin America, and 0.67 (0.66–0.68) for Western Europe and North America. Results remained significant after adjusting for socioeconomic factors. Similar multimorbidity disease patterns were observed among Norwegian-born and immigrants, in particular between Norwegian-born and those from Western European and North American countries. However, the complexity of patterns that emerged for the other immigrant groups was greater. Despite differences observed in the development of patterns with age, such as ischemic heart disease among immigrant women, we were unable to detect the systematic development of the multimorbidity patterns among immigrants at younger ages.
Conclusions
Our study confirms that migrants have lower multimorbidity levels compared to Norwegian-born. The greater complexity of multimorbidity patterns for some immigrant groups requires further investigation. Health care policies and practice will require a holistic approach for specific population groups in order to meet their health needs and to curb and prevent diseases.
Introduction
Multimorbidity is highly prevalent among older people, women and those with lower socioeconomic levels, but not exclusive to these groups [1–3]. Patients with multimorbidity often present with lower function levels, higher levels of polypharmacy, poorer quality of life, increased health care utilization and mortality rates over and above the risk attributable to individual diseases [2, 4–7].
Multimorbidity provides a holistic framework in understanding chronic diseases as the consequence of environmental, social, and personal risks that contribute to increased vulnerability to a wide variety of illnesses as opposed to studying mental and physical health diseases one by one [8–10]. Several studies have attempted to disentangle the different multimorbidity patterns; i.e. the non-random positive association of specific diseases and health problems, also called associative multimorbidity [9]. Despite the varying populations and methodologies, three common patterns of multimorbidity are observed in these studies: cardiovascular-metabolic, mental health and musculoskeletal [2, 9, 11]. In total up to one hundred different multimorbidity patterns have been identified [9].
The proportion of immigrants is growing in Europe [12], but they are heterogeneous, both in their origins, status and migration histories. Many different theories can contribute to hypothesise an association between migration and multimorbidity. According to the “healthy immigrant theory”, some immigrants are healthier than the host population of their new country because they represent a selected and healthier subgroup of their country of origin population [13]. On the other hand, even in the presence of better health at arrival, the health of immigrants worsens quicker than non-immigrants after arrival [14, 15]. Although the theories related to migration are still evolving, migration itself can increase vulnerability to environmental, social, behavioural and psychological risks [12] during and/or after the migration process for all immigrants [16–19]. However, the healthy immigrant theory may not apply to refugees and asylum seekers, who are forced into migration and therefore have generally poorer mental and physical health compared to the host population [20]. A study on multimorbidity among young asylum seekers in Switzerland showed a relatively high prevalence of multimorbidity [16], thus concurring with this theory. Also, in a recently published study of immigrants in Norway [21], multimorbidity was significantly lower among labour and education immigrants, but higher among refugees, compared to family reunification immigrants.
Compared to the Norwegian-borns, a lower percentage of immigrants use primary care services but once they are in contact with health care, they often become frequent users [22, 23]. Although significant differences regarding use of services for both psychological [24] and physical [22] diagnoses for immigrants and Norwegian- borns have been observed, none of the published studies have assessed the patterns of the global burden of disease, leaving the health picture of immigrants rather fragmented. Two recent international reviews have highlighted the scarcity of knowledge about multimorbidity for patients from lower and middle-income countries [1], and for immigrants [25].
Beside the effects of the reasons for migration on health, country of origin of the migrants must also be taken into consideration due the global variance in the prevalence of specific diseases, in addition to interactions between genetic and migration factors [26]. Particularly relevant are the high prevalence of diabetes mellitus among immigrants from South Asian and some African countries [27, 28] or of cardiovascular diseases among immigrants from South Asians and Eastern countries [29]. However, to the best of our knowledge, no study to date has studied multimorbidity and its patterns among immigrants from different geographical regions compared to a native-born population.
This nationwide multi-register study in Norway enabled us i) to study the associations between multimorbidity and immigrant status as classified by area of origin, accounting for other known risk factors for multimorbidity, and ii) to identify patterns of multimorbidity in Norway for immigrants and Norwegian-born at different ages. Based on the previously described existing theories and on our earlier studies on use of health care services in Norway [22, 23, 30], our hypothesis was that immigrant groups would have lower rates of multimorbidity compared to Norwegian-born. Associations between length of stay in Norway and use of health services in our previous studies indicate that health worsens quicker for migrants and therefore we hypothesize that they develop multimorbidity patterns at a younger age.
Methods
This register-based study relies on merged data from the National Population Register and the Norwegian Health Economics Administration database (HELFO). The personal identification number assigned to Norwegian citizens and to legal immigrants staying in Norway for at least six months was used to link the registries. Irregular immigrants without legal residence and regular migrants staying for shorter periods were not included in the study.
All 15 year old or older Norwegians (n = 3,349,721), defined as born in Norway with both parents from Norway, and immigrants (n = 389,807), defined as born abroad with both parents from abroad, registered in Norway in 2008 were included in the study. Other categories like born in Norway with one or both parents from abroad and adopted children were excluded because of their low numbers among immigrants older than 15 years old. Information on gender, age, personal income level and country of origin was obtained from the National Population Register for all study subjects. Age was categorized into three groups: 15–44, 45–64 and 65+ years. Income level was categorized in four levels: low (under 50,000 Norwegian Crowns (NOK)), medium (50,001 to 200,000 NOK), high (200,001 to 400,000 NOK) and very high (over 400,000 NOK). According to Statistics Norway, countries of origin were classified into six broad areas: North America and Oceania, Nordic countries, West Europe excluding Turkey, Eastern Europe and Africa, Asia including Turkey and Latin America together [31]. We conducted analyses for each of these regions but, for the sake of parsimony and in order to have enough persons in all age categories, we recoded areas with similar characteristics into North America and Western Europe excluding Turkey (named as “Western countries”, n = 109,428), Eastern Europe (n = 99,301) and Africa, Asia including Turkey and Latin America together (named as “Other non-Western countries”, n = 181,068). A list with the major countries represented in each of the areas is presented as supplemental material in S1 Table.
The HELFO-database contains claims for all patient contacts within the public primary health care services including both consultations with general practitioners (GPs) and emergency room (ER) services. Each claim contains at least one medical diagnosis based on the International Classification of Primary Care (ICPC-2) registered by the physician. These ICPC-2 diagnoses originally sampled in 2008 for reimbursement and administrative purposes were grouped according to the Expanded Diagnostic Clusters (EDC) of the Johns Hopkins University Adjusted Clinical Groups (ACG®) Case-Mix System [5]. The EDC methodology assigns ICPC-2 codes found in claims to one of 269 EDCs. As broad groupings of diagnosis codes, EDCs help to remove differences in coding behaviour between practitioners. The 114 chronic EDCs included in the study were selected based on the list published by Salisbury et al in 2011 [32].
Analyses
Descriptive analyses were conducted. Morbidity level was presented as the proportion of patients with none, one, or two or more chronic EDCs registered during the year 2008. A dichotomous multimorbidity variable based on the total number of the selected EDCs registered for each person was created, defined by two or more different chronic diagnoses [10]. For this dependent variable, binary logistic regression analyses were conducted in four steps. Firstly, the independent variables age, gender, immigrant area of origin and income level were included one at the time. Secondly, the first three variables were included together in Model 1. Thirdly, all four independent variables were included simultaneously in Model 2. Lastly, Model 3 also included the number of visits to primary care in 2008. Analyses were also conducted with the multimorbidity variable being three or more chronic EDCs, obtaining similar results, not shown in the article.
To determine multimorbidity patterns, an exploratory factor analysis technique was applied by gender and age category, and for Norwegian-borns and the three defined immigrant groups separately. This methodology has been thoroughly described by Prados-Torres et al [33], and includes only EDCs with prevalence equal to or greater than 1% for each age and gender subgroup studied. Due to the dichotomous nature of the EDC variables, tetra-choric correlation matrices were conducted to determine which EDCs were included in each factor, with no restriction regarding the number of patterns in which each EDC could be included. The factors resulting from these matrices were interpreted as multimorbidity patterns (chronic EDCs related to each other), scoring between -1 and 1 depending on the strength of the association of each of the EDCs to the disease pattern. To determine the number of factors to extract, a scree plot representing the eigenvalues of the correlation matrix in descending order was utilized, extracting the number of factors that corresponded to the sequence number of the eigenvalue that produced the inflection point of the curve. When a clear solution was not obtained by this method, a clinical approach based on the authors’ expertise was used to determine the patterns with the most plausible pathophysiologic explanation. The same strategy was used when factor scores were greater than 1 (Heywood phenomenon). An oblique rotation (Oblimin) was applied, allowing the factors to be correlated with one another, and EDCs with scores equal to or greater than 0.25 for each factor were selected for the relevant multimorbidity patterns. The adequacy of the sample used to perform the factor analysis was measured using the Kaiser-Meyer-Olkin (KMO). This parameter takes values between 0 and 1, which are closer to 1 with a greater goodness of fit. Analyses were conducted in SPSS 20.0 and Stata 13.0.
This study is part of the project “Immigrants’ health in Norway”, which was approved by the Regional Committee for Medical and Health Research Ethics, the Norwegian Data Inspectorate, the Norwegian Labour Welfare Service and the Norwegian Directorate of Health. The Norwegian Social Science Data Service prepared the final data file.
Results
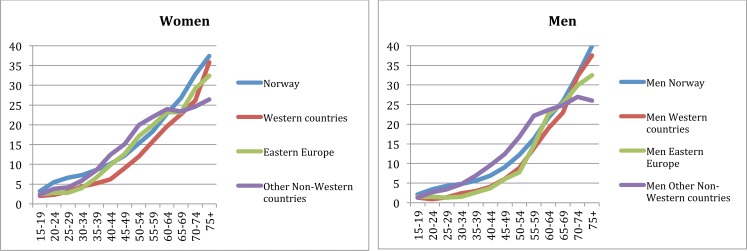
Demographic characteristics of Norwegians and immigrants are presented in Table 1. With few exceptions, women were underrepresented across immigrant groups. While immigrants from Western countries had higher income levels than Norwegians, income levels were lower for the rest of immigrants. Norwegians had more chronic conditions registered compared to immigrants except for those younger than 65 years from other non-Western countries. Fig 1 depicts the proportion of Norwegians and immigrants with multimorbidity by age and gender. Male and female 30 to 60 years old immigrants from other non-Western countries showed higher multimorbidity compared to Norwegians of the same age, but this was the group of origin with lowest global prevalence of multimorbidity among the older age groups.
Table 1. Demographic and health information for natives and immigrants in Norway.
| Norwegian-born | Western Countries (West Europe & N. America) | Eastern Europe | Other Non-Western (Asia, Africa & Latin America) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 15–44 | 45–64 | 65+ | 15–44 | 45–64 | 65+ | 15–44 | 45–64 | 65+ | 15–44 | 45–64 | 65+ |
| Numbers | 1,557,485 | 1,086,136 | 706,100 | 56,564 | 35,508 | 17,366 | 73,425 | 21,957 | 3,919 | 131,929 | 42,848 | 6,291 |
| Women, % | 48.8 | 49.5 | 56.7 | 45.8 | 44.9 | 62.5 | 43.4 | 43.5 | 56.0 | 53.2 | 46.5 | 50.9 |
| Income level (in 1000 NOK per year), % | ||||||||||||
| Low (<50) | 23.6 | 20.9 | 89.8 | 19.5 | 20.8 | 86.2 | 25.3 | 27.6 | 91.3 | 41.2 | 45.0 | 92.4 |
| Medium (50–200) | 17.3 | 10.0 | 5.2 | 16.3 | 10.8 | 6.1 | 23.4 | 15.1 | 4.0 | 19.9 | 11.9 | 3.3 |
| High (200–400) | 32.2 | 32.1 | 2.8 | 34.1 | 29.4 | 3.8 | 39.5 | 39.8 | 2.5 | 27.1 | 26.5 | 2.7 |
| Very high (>400) | 26.9 | 37.0 | 2.1 | 30.1 | 39.0 | 3.9 | 11.8 | 17.5 | 2.2 | 11.7 | 16.6 | 1.6 |
| Number of chronic conditions registered in 2008, % | ||||||||||||
| None | 76.4 | 56.3 | 33.0 | 83.6 | 64.3 | 38.1 | 86.1 | 68.3 | 40.7 | 76.4 | 54.8 | 44.1 |
| One | 17.8 | 27.7 | 33.0 | 13.0 | 23.3 | 31.7 | 10.7 | 19.2 | 30.5 | 17.7 | 27.5 | 30.7 |
| Two or more | 5.8 | 16.0 | 34.0 | 3.4 | 12.4 | 30.2 | 3.2 | 12.5 | 28.8 | 5.9 | 17.6 | 25.2 |
| Number of visits to GP or ER in 2008 | ||||||||||||
| Mean (SD) | 2.4(3.5) | 3.0(3.9) | 4.2 (4.8) | 1.8(2.9) | 2.5(3.5) | 3.9(4.7) | 1.7(3.1) | 2.6 (3.9) | 3.4 (4.3) | 2.9(3.9) | 3.9(4.6) | 3.5(4.4) |
Fig 1. Multimorbidity by age. Norwegian-born and immigrants by gender.
The logistic regression analyses performed showed that multimorbidity was significantly associated with female gender, with a clear dose-response association for age and income level in all models. Immigrants had a significantly lower probability of multimorbidity compared to Norwegian-borns, with Eastern Europeans showing the lowest odds. Although the probability of multimorbidity was significantly higher for immigrants from other non-Western countries when adjusting for age and gender, this difference disappeared when adjusting for income level. Also the associations between multimorbidity, age and gender were moderated by the inclusion of income level in the model (Table 2). To study potential interactions, analyses were conducted separately by gender, showing associations in the same direction, although men from Eastern European countries had even lower adjusted odds ratio (OR) for multimorbidity (0.49, 95% CI 0.47–0.51) compared to women from the same origin (0.70, 95% CI 0.67–0.73) in Model 2. The number of visits to primary health care included in Model 3 increased the goodness of fit of the model, but it did not change the direction of the results.
Table 2. Associations between multimorbidity and immigrant status. Binary logistic regression analyses.
| Unadjusted | Adjusted Model 1 | Adjusted Model 2 | Adjusted Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age in years | ||||||||
| 15–44 (ref) | 1 | 1 | 1 | 1 | ||||
| 45–64 | 3.17 | 3.14–3.19 | 3.14 | 3.12–3.17 | 3.44 | 3.41–3.47 | 3.21 | 3.18–3.24 |
| 65+ | 8.57 | 8.50–8.64 | 8.37 | 8.30–8.44 | 5.14 | 5.10–5.19 | 4.58 | 4.53–4.63 |
| Gender | ||||||||
| Men (ref) | 1 | 1 | 1 | 1 | ||||
| Women | 1.29 | 1.29–1.30 | 1.19 | 1.18–1.20 | 1.06 | 1.05–1.06 | 0.90 | 0.89–0.90 |
| Immigrant area of origin | ||||||||
| Norwegian-born (ref) | 1 | 1 | 1 | 1 | ||||
| Western Europe & North America | 0.67 | 0.66–0.68 | 0.74 | 0.72–0.75 | 0.75 | 0.74–0.77 | 0.81 | 0.79–0.83 |
| Eastern Europe | 0.38 | 0.37–0.39 | 0.65 | 0.64–0.67 | 0.59 | 0.57–0.61 | 0.64 | 0.63–0.66 |
| Asia, Africa & Latin America | 0.58 | 0.57–0.59 | 1.02 | 1.01–1.04 | 0.83 | 0.82–0.85 | 0.68 | 0.66–0.69 |
| Income level | ||||||||
| Low (ref) | 1 | - | - | 1 | 1 | |||
| Medium | 0.34 | 0.34–0.35 | - | - | 0.60 | 0.59–0.61 | 0.59 | 0.58–0.60 |
| High | 0.30 | 0.29–0.30 | - | - | 0.48 | 0.48–0.49 | 0.47 | 0.46–0.47 |
| Very high | 0.21 | 0.21–0.21 | - | - | 0.31 | 0.31–0.31 | 0.39 | 0.39–0.40 |
| Number of visits to primary health care services | ||||||||
| Number of visits | 1.29 | 1.28–1.29 | - | - | - | - | 1.27 | 1.27–1.28 |
| Nagelkerke R Square | - | 0.148 | 0.171 | 0.332 | ||||
Model 1: gender, age and immigrant area of origin; Model 2: Model 1 plus income level; Model 3: Model 2 plus number of visits
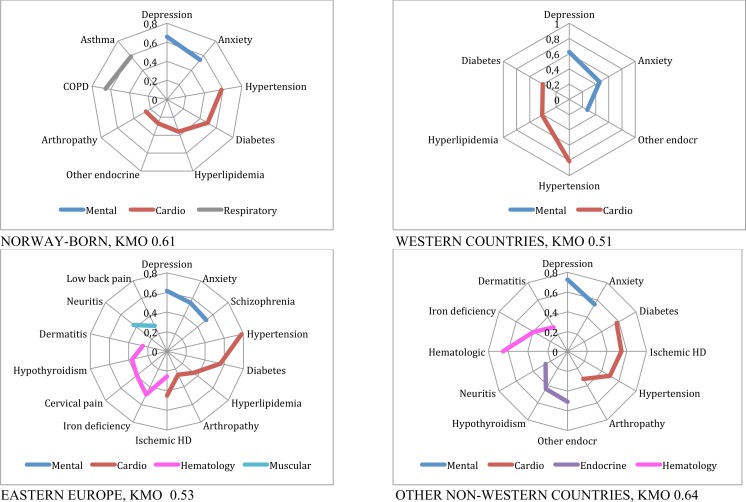
The multimorbidity patterns for Norwegians and immigrants based on the described factor analyses are presented in Table 3 and Figs 2 to 5, and summarised in Table 4. In the Figures, EDCs belonging to the same pattern are linked through a continuous line in different colours depending on the type of pattern, for example blue for mental or red for cardiovascular patterns. For all groups studied but one, KMO was higher than 05, indicating an acceptable goodness of fit.
Table 3. Patterns of multimorbidity and contributing diseases for men and women 15 to 44 years old across groups. Results of factor analyses applying oblique rotation (Oblimin). a .
| Norwegian-born | Western Countries (West Europe & N. America) | Eastern Europe | Other Non-Western countries (Asia, Africa & Latin America) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men 15–44 | ||||||||||||
| Diseases | Mental health | Respiratory | Diseases | Mental health | - | Diseases | Mental health | - | Diseases | Mental health | Respiratory | - |
| Score | Score | Score | Score | Score | Score | |||||||
| Depression | 0.68 | - | Depression | 0.55 | - | Depression | 0.67 | - | Depression | 0.89 | - | - |
| Anxiety | 0.72 | - | Anxiety | 0.89 | - | Anxiety | 0.93 | - | Anxiety | 0.55 | - | - |
| Substance use | 0.67 | - | Asthma | - | 0.29 | - | ||||||
| Dermatitis | - | 0.36 | Cervical pain | - | 0.76 | - | ||||||
| Asthma | - | 0.45 | ||||||||||
| KMO 0.6776 | KMO 0.5451 | KMO 0.5317 | KMO 0.5929 | |||||||||
| Women 15–44 | ||||||||||||
| Diseases | Mental health | Endocrine | Diseases | Mental health | Respiratory | Diseases | Mental health | Endocrine | Diseases | Mental health | Endocrine | Haematology |
| Score | Score | Score | Score | Score | Score | Score | Score | Score | ||||
| Depression | 0.66 | - | Depression | 0.70 | - | Depression | 0.91 | - | Depression | 0.77 | - | - |
| Anxiety | 0.66 | - | Anxiety | 0.63 | - | Anxiety | 0.57 | - | Anxiety | 0.68 | - | - |
| Other endocr. | - | 0.58 | Dermatitis | - | 0.63 | Cervical pain | 0.34 | - | Cervical pain | 0.26 | - | - |
| Hypothyroid. | - | 0.56 | Asthma | - | 0.33 | Other endocr. | - | 0.94 | Asthma | 0.28 | - | - |
| Hypertension | - | 0.29 | Hypothyroid | - | 0.45 | Other endoc. | - | 0.42 | - | |||
| Hypothyroid | - | 0.78 | - | |||||||||
| Iron defic. | - | - | 0.77 | |||||||||
| Hematology | - | - | 0.51 | |||||||||
| KMO 0.637 | KMO 0.5327 | KMO 0.6118 | KMO 0.6704 | |||||||||
a Expanded Diagnostic Clusters with scores equal to or greater than 0.25 for each factor were selected for the relevant multimorbidity patterns.
KMO: Kaiser-Meyer-Olking measure of sampling adequacy
Fig 2. Patterns of multimorbidity among men living in Norway. Men 45 to 64 years old.
Fig 5. Patterns of multimorbidity among women living in Norway. Women 65 or older.
Table 4. Expanded Diagnostic Clusters (EDCs*) included in the Patterns of multimorbidity for natives and immigrants by age and gender.
| Norwegian-born | Western countries (West Europe & North America) | Eastern Europe | Other Non-Western (Asia, Africa & Latin America) | |||||
|---|---|---|---|---|---|---|---|---|
| Patterns | EDCs | Patterns | EDCs | Patterns | EDCs | Patterns | EDCs | |
| Men, 15–44 | Mental health | 7 | Mental health | 4 | Mental health | 3 | Mental health | 7 |
| Respiratory/atopic | Respiratory | |||||||
| Men, 45–64 | Mental health | 17 | Mental health | 16 | Mental health | 9 | Mental-psychiatry | 15 |
| Cardiovascular | Cardiovascular | Cardio-endocrine | Cardiovascular | |||||
| Cardio-endocrine | Cardio-endocrine | Cardio-endocrine | ||||||
| Respiratory | Respiratory | Respiratory | ||||||
| Men, 65+ | Mental-geriatric | 32 | Mental health | 30 | Mental-psychosomatic | 28 | Cardiovascular | 23 |
| Cardiovascular | Mental-geriatric | Cardiovascular + | Cardio-endocrine | |||||
| Cardio-endocrine | Cardiovascular | Cardio-endocrine | Malignant + | |||||
| Respiratory | Cardio-endocrine | Malignant + | Musculoskeletal + | |||||
| Muscular | Complex endocrine | |||||||
| Malignant | ||||||||
| Women, 15–44 | Mental health | 10 | Mental health | 6 | Mental health | 8 | Mental health | 11 |
| Endocrine | Respiratory/atopic | Endocrine | Endocrine | |||||
| Haematological | ||||||||
| Women, 45–64 | Mental health | 17 | Mental health | 13 | Mental-psychiatry | 16 | Mental health | 16 |
| Cardio-endocrine | Cardio-endocrine | Cardio-endocrine | Cardio-endocrine | |||||
| Respiratory | Musculoskeletal | Endocrine | ||||||
| Haematological | Haematological | |||||||
| Women, 65+ | Mental-geriatric | 29 | Mental-geriatric | 29 | Mental health | 25 | Mental-psychosomatic | 23 |
| Cardiovascular | Cardiovascular | Cardio-endocrine | Cardiovascular | |||||
| Cardio-endocrine | Cardio-endocrine | Haematological + | Haematological + | |||||
| Musculoskeletal | Respiratory | Other + | ||||||
| Respiratory | Malignant | |||||||
*Number of chronic EDCs with a prevalence of 1% or higher included in the analyses.
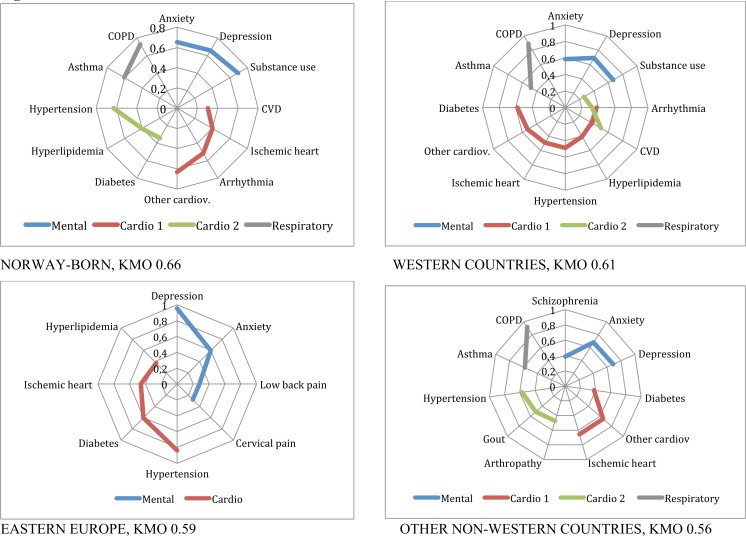
Men 15 to 44: A mental health pattern of depression and anxiety was present in all groups, and included abuse of substances for Norwegians. Both Norway and other non-Western countries showed a respiratory pattern of asthma (Table 3).
Men 45 to 64: The mental health pattern persisted, and included substance abuse for Norway and Western countries, but was related to pain in Eastern European countries and to schizophrenia in other non-Western countries. Two cardiovascular patterns, called Cardio 1 and Cardio 2 in the Figures, one of them including diabetes in a cardio-endocrine pattern, emerged for Norway, Western and other non-Western countries. Eastern European countries only showed one cardio-endocrine pattern. A respiratory (asthma-chronic obstructive pulmonary disease (COPD)) pattern appeared in most groups with the exception of Eastern countries (Fig 2).
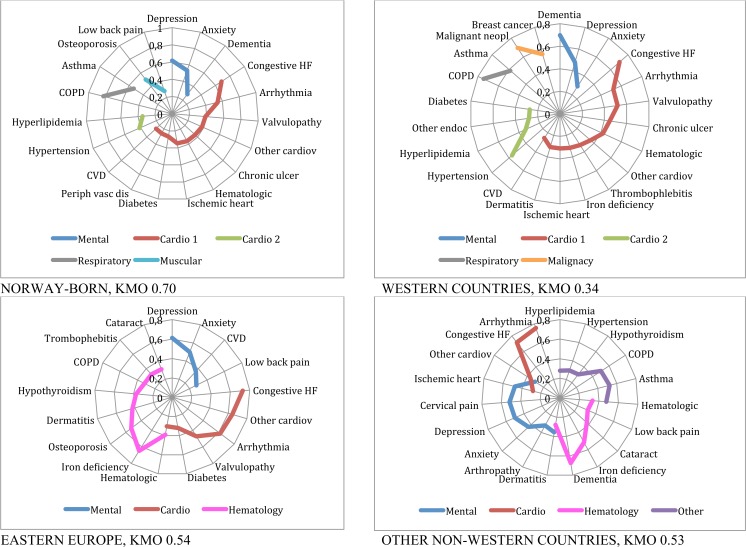
Men 65 years and older: Anxiety and depression were associated at this stage to dementia in a mental-geriatric pattern for Norwegians and Western patients, who also presented another mental health pattern combined with cardiovascular disease. For Eastern Europeans, anxiety and depression were associated to pain as in the younger group, but also to other diagnoses in a more complex (psychosomatic) mental health pattern. Depression was not included in any pattern among other non-Western immigrants in this age group. Two cardiovascular patterns emerged for all groups, one of them being a cardio-endocrine one always including diabetes. The cardiovascular pattern included congestive heart failure (CHF), arrhythmia, valvulopathy and other cardiac conditions for most of the groups, but varied widely for the different groups as shown in Fig 3. Another complex pattern for Eastern Europeans also included diabetes, iron deficiency, hypothyroidism, low back pain and prostatic hypertrophy. The respiratory pattern emerged as a singular pattern for Norway, while asthma and COPD where associated to other patterns for Eastern and other non-Western countries. A pattern of malignancy emerged in all but Western countries, with prostate cancer in combination with haematological disease for all groups. These malignant patterns were more complex for Eastern Europeans and for other non-Western countries. Last, a musculoskeletal pattern containing respiratory disease, cataract and dermatitis appeared in this group (Fig 3).
Fig 3. Patterns of multimorbidity among men living in Norway. Men 65 or older.
Women 15 to 44: The mental health pattern was also present among young women in all groups, and included cervical pain for Eastern and other non-Western countries and asthma for the latter. With the exception of Western countries, all presented an endocrine pattern including hypothyroidism and other endocrine disorders. Western countries presented an atopic-respiratory pattern of dermatitis and asthma while other non-Western countries presented a haematological pattern of iron deficiency and other haematological disorders (Table 3).
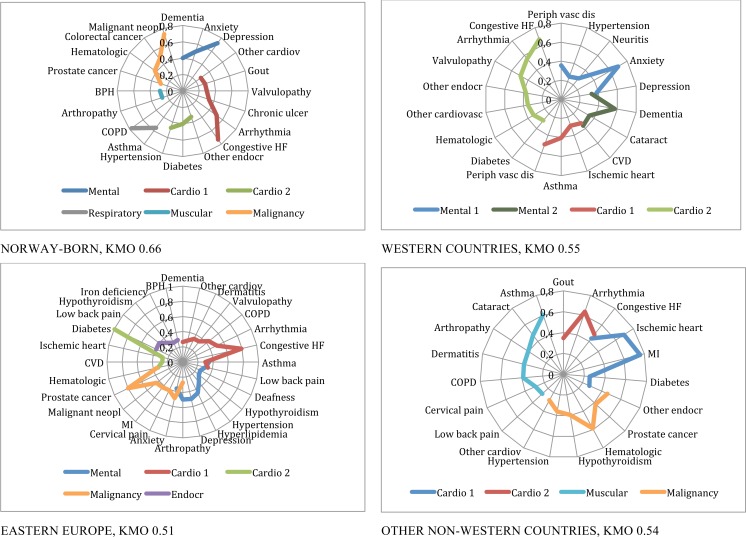
Women 45 to 64: A mental health and a cardiovascular pattern were common to all countries in this age category. The mental health pattern included other endocrine disorders for Western countries and comprised schizophrenia in Eastern European countries. For all groups, hypertension and diabetes were included in the emerging cardio-endocrine pattern, and hyperlipidemia in all but other non-Western countries. Arthropathy was also included in this pattern, except for Western countries. Ischemic heart disease was associated to this pattern for Eastern Europeans and other non-Western countries. The respiratory pattern appeared only among Norwegians. A musculoskeletal pattern combining low-back pain and peripheral neuropathy emerged for Eastern countries. An endocrine pattern of hypothyroidism was revealed for non-Western countries. Last, for Eastern Europe and non-Western countries, a haematological pattern comprised iron deficiency and dermatitis, in addition to cervical pain, hypothyroidism and ischemic heart disease for the former, and other endocrine disorders for the latter (Fig 4).
Fig 4. Patterns of multimorbidity among women living in Norway. Women 45 to 64 years.
Women 65 and older: As for men, a mental-geriatric pattern appeared for Norway and Western countries, while depression and anxiety were associated to CVD and pain for Eastern Europeans and to a more complex (psychosomatic) mental pattern for non-Western women. Women from Norway and Western countries presented two cardiovascular patterns, one of them including diabetes, while only one emerged for East Europeans and other non-Western. The main cardiovascular pattern for elderly included CHF, arrhythmia and other cardiac conditions in all groups, and was more complex for Norwegians and women from Western countries compared to Eastern Europeans and non-Western women. However, the psychosomatic pattern for this last group was also related to cardiovascular diseases as explained. A second, lighter, cardiovascular pattern of hyperlipidemia and hypertension alone appeared for Norwegians. For Western countries these risk factors were associated in a cardio-endocrine pattern with diabetes and other endocrine disorders. Among non-Western women, the combination was even more complex. A simple respiratory pattern was present for Norwegians and women from Western countries. A musculoskeletal pattern was present for Norwegians, and a malign one including breast cancer crystallised only for Western Europe. Non-Western and Eastern European women presented complex haematological patterns (Fig 5).
Discussion
Immigration from all areas of origin was in this study negatively associated to multimorbidity. Regarding the existence of multimorbidity patterns, more similarities than differences were observed among Norwegian-born and immigrants, in particular between Norwegian-born and those from Western countries. Although differences were observed in the development of patterns with age, as it was the case with ischemic heart disease among immigrant women, we could not systematically detect the development of the multimorbidity patterns among immigrants at younger ages.
Our results of lower odds of multimorbidity among immigrants align with a recent study reporting OR of 0.1 (0.0–0.8) and 0.8 (0.7–0.9) for immigrants living in Canada under and over 5 years respectively [34] Including approximately 3,7 million persons, our study is, as far as we know, the largest to date identifying patterns of multimorbidity and the first one that includes immigrants. A picture consistent with previous literature emerged, including cardiovascular-endocrine patterns comprising a variety of cardio-metabolic conditions, sometimes split into two factors, a mental health pattern, and a musculoskeletal pattern [1, 2, 9, 11]. There were, however, some noteworthy differences regarding the mental health pattern, that was associated to schizophrenia in middle aged men and women from other non-Western and East European countries respectively, in line with the literature in the field [35]. For the elderly, the mental health pattern was associated with dementia for Norway, Western countries and women from Eastern European countries. The mental health pattern did not emerge for older men from other non-Western countries and was associated to a more complex psychosomatic pattern for older Easter Europeans of both genders. These differences, concordant with existing studies, could reflect the lower proportion of dementia diagnoses among immigrants from low income countries in Norway [36], higher degrees of somatization among immigrants [37] as well as cultural differences, social stigma attached to some diseases, and communication problems for some groups [38].
Three additional patterns of multimorbidity emerged from our data: malignant, haematological, and respiratory. The malignant pattern appeared at older ages in all groups and included the most common cancer types [39]. However, this pattern was more complex in East European and other non-Western countries. This findings supports Lyratzopoulus et al in 2012 regarding the intricacy of cancer diagnoses and the higher number of consultations needed to refer immigrant patients with cancer to secondary health care [40]. The haematological pattern appeared only in women from Eastern Europe and other non-Western countries. In accordance with the high prevalence of anaemia described among young immigrants [41–43], its main components were iron deficiency and other haematological disorders, but the pattern became more complex with age, especially for Eastern Europeans, with combinations of disease that are hard to explain by classical pathogenesis. These complex patterns might reflect a higher vulnerability to disease as explained by the concept of allostatic overload [44], which has been connected to lower levels of serum erythrocytes and greater mean corpuscular volume [45]. In addition, other explanations include the GPs’ challenges to categorise disease for women from different cultural and linguistic backgrounds [40], and to different presentation of disease among immigrant women compared to Norwegian-born and Western women. Last, the respiratory pattern combining COPD and asthma might reflect idiosyncrasies of the Norwegian system for reimbursement of prescriptions. Until recently, there have been no specific pharmacologic treatments of COPD, and the available therapies are “borrowed” from asthma and adapted to COPD [46]. Because of this, some of the COPD treatments could only be reimbursed for patients with a diagnosis of asthma. This, together with difficulties of labelling COPD versus asthma in the clinics is probably the explanation for this consequent pattern.
The strengths of our register study rely on the nationwide coverage, limiting self-selection bias, a common caveat for immigrants, and providing large numbers to enable classification of immigrants in three different groups, despite large heterogeneity within each group. Although we regrouped areas of origin after exploring each of them separately, there is of course variation in disease patterns and prevalences within groups that our study cannot disentangle. Rarely is information on socioeconomic levels available for the entire population, although income level seemed to be less able to differentiate socioeconomic level among the eldest groups. Last, we used both a single count approach and a more sophisticated study of patterns of morbidity, presenting a more complete view of multimorbidity among Norwegian-born and immigrant groups.
The methods for the study of multimorbidity are still evolving [47]. Multimorbidity can be measured by simple counts of diseases in an individual [1], or using indices to assess morbidity burden, that differentially weight a range of conditions, like the ACG System or the Charlson index [5, 48]. Although the most used definition of multimorbidity includes two or more chronic diseases, the cut-off of three chronic diseases has also been suggested as a valid one [10]. Therefore, we conducted analyses for both definitions, but obtained similar results.
Our study was based on diagnoses made by physicians, avoiding self-reported bias, and we subsequently selected the chronic diagnoses included in Salisbury’s list [32] in accordance to previous studies [11, 33]. However, because we used routine data for administrative purposes, our study shares the limitations of other multimorbidity studies, particularly reliance on the quality of the data recorded [49]. Nevertheless, ICPC-2 data from administrative claims is validated for comparison of groups [50, 51], which was our aim. To reduce potential misclassification of diagnoses by the physicians and to increase the comparability of our study with others, we used the EDCs created by the ACG System [5, 52]. Incomplete register of diagnoses is another potential limitation, as the physicians may choose only one diagnosis in a given consultation despite the presence of several diseases. On the other hand, multimorbidity levels of those patients not attending to primary care cannot be registered. However, including the number of visits for each individual to primary health care during the study period in the analyses did not change the direction of the associations between immigrant status and multimorbidity. The “salmon bias” effect, according to which elderly sick patients would travel back to their countries of origin [53], could also have confounded our results, since these patients would not have visited primary care in the study year and thus not been diagnosed. However, recent analyses for other non-Western immigrants in Norway indicate that a low proportion among the elderly move back to their countries of origin [54]. Nevertheless, the low prevalence of multimorbidity among the oldest groups in our study is most likely explained because the HELFO-database does not include consultations for individuals living in nursing homes. Unfortunately, we have no data on the proportion of immigrants that live in nursing homes, but immigrants might be more reluctant than Norwegians to live away from their own homes [55], which would increase the differences in multimorbidity that we find between the Norwegian-born and the immigrant oldest patients. Our figures of multimorbidity are thus lower than the 42% prevalence of multimorbidity reported in a recent study based on self-reported disease in Norway [56] and should not be used as comprehensive prevalences.
Despite the lower levels of multimorbidity among immigrants compared to Norwegians, immigration is often related to lower socioeconomic status, low health literacy [57] and barriers to health care services use [58], which in turn can additionally complicate the impact of multimorbidity on some immigrant groups. In a recent systematic review, general practitioners identified four difficult areas in caring for patients with multimorbidity: disorganisation and fragmentation of care, inadequacy of current disease specific guidelines, challenges in delivering patient centred care, and barriers to shared decision making [2]. Many of these areas are even further complicated when physicians interact with vulnerable immigrant groups [59–62]. Although access to health services is necessary for health care, more access alone does not necessarily result in equitable health care outcomes [63]. Viewing immigrant patients holistically rather than disease-by-disease [8], and increasing the awareness of the complexity of multimorbidity among elderly immigrants might play a major role in developing effective preventive and treatment strategies in the clinical encounter with immigrants.
Conclusions
Our study confirmed the associations between multimorbidity and immigrant’s area of origin. Immigrants showed a lower prevalence of multimorbidity compared to Norwegian-born, despite the former frequently having lower socio economic and literacy levels. The similarities regarding the type and composition of the multimorbidity patterns found in both groups confirm common physiopathological basis of diseases. The greater complexity of multimorbidity patterns for some immigrant groups requires further investigation. These complexities imply that health care policies and practice will require a more holistic approach for specific population groups in order to meet their health needs and curb and prevent diseases.
Supporting Information
(DOCX)
Acknowledgments
Dr Gimeno-Feliu from the EpiChron Research Group on Chronic Diseases received a World University Network grant from the University of Bergen that allowed us to continue the previously started collaboration between the research groups.
Abbreviations
- ACG®
Adjusted Clinical Groups
- CHF
Congestive Heart Failure
- COPD
Chronic Obstructive Pulmonary Disease
- CVD
Cardio Vascular Disease
- EDC
Expanded Diagnostic Clusters
- ER
Emergency Room
- GP
General Practitioner
- HELFO
Norwegian Health Economics Administration database
- ICPC
International Classification of Primary Care
- KMO
Kaiser-Meyer-Olkin
- NOK
Norwegian Crowns
Data Availability
The dataset contains sensitive information. Although data are pseudonymised, there is still a possibility of recognizing individuals due to the low number of immigrants from some countries to Norway. Thus, the Norwegian Data Inspectorate does not allow this information to be published or shared with other institutions than the University of Bergen.
Funding Statement
Dr Gimeno-Feliu from the EpiChron Research Group on Chronic Diseases received a World University Network grant from the University of Bergen that allowed the authors to continue the previously started collaboration between the research groups. Otherwise, the authors received no specific funding for this work.
References
- 1. Violan C, Foguet-Boreu Q, Flores-Mateo G, Salisbury C, Blom J, Freitag M, et al. Prevalence, determinants and patterns of multimorbidity in primary care: a systematic review of observational studies. PLoS One. 2014;9(7):e102149 10.1371/journal.pone.0102149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176 10.1136/bmj.h176 . [DOI] [PubMed] [Google Scholar]
- 3. Mercer SW, Guthrie B, Furler J, Watt GC, Hart JT. Multimorbidity and the inverse care law in primary care. BMJ. 2012;344:e4152 10.1136/bmj.e4152 . [DOI] [PubMed] [Google Scholar]
- 4. Kuwornu JP, Lix LM, Shooshtari S. Multimorbidity disease clusters in Aboriginal and non-Aboriginal Caucasian populations in Canada. Chronic diseases and injuries in Canada. 2014;34(4):218–25. . [PubMed] [Google Scholar]
- 5. Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–41. 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calderon-Larranaga A, Poblador-Plou B, Gonzalez-Rubio F, Gimeno-Feliu LA, Abad-Diez JM, Prados-Torres A. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well? The British journal of general practice: the journal of the Royal College of General Practitioners. 2012;62(605):e821–6. 10.3399/bjgp12X659295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quinones AR, Liang J, Bennett JM, Xu X, Ye W. How does the trajectory of multimorbidity vary across Black, White, and Mexican Americans in middle and old age? The journals of gerontology Series B, Psychological sciences and social sciences. 2011;66(6):739–49. 10.1093/geronb/gbr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starfield B, Kinder K. Multimorbidity and its measurement. Health policy. 2011;103(1):3–8. 10.1016/j.healthpol.2011.09.004 . [DOI] [PubMed] [Google Scholar]
- 9. Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–66. 10.1016/j.jclinepi.2013.09.021 . [DOI] [PubMed] [Google Scholar]
- 10. Harrison C, Britt H, Miller G, Henderson J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open. 2014;4(7):e004694 10.1136/bmjopen-2013-004694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poblador-Plou B, van den Akker M, Vos R, Calderon-Larranaga A, Metsemakers J, Prados-Torres A. Similar multimorbidity patterns in primary care patients from two European regions: results of a factor analysis. PLoS One. 2014;9(6):e100375 10.1371/journal.pone.0100375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rechel B, Mladovsky P, Ingleby D, Mackenbach JP, McKee M. Migration and health in an increasingly diverse Europe. Lancet. 2013;381(9873):1235–45. 10.1016/S0140-6736(12)62086-8 . [DOI] [PubMed] [Google Scholar]
- 13. Razum O, Zeeb H, Rohrmann S. The 'healthy migrant effect'- not merely a fallacy of inaccurate denominator figures. Int J Epidemiol. 2000;29(1):191–2. . [DOI] [PubMed] [Google Scholar]
- 14. McDonald JT, Kennedy S. Insights into the "healthy immigrant effect": health status and health service use of immigrants to Canada. Soc Sci Med. 2004;59:1613–27. [DOI] [PubMed] [Google Scholar]
- 15. Jatrana S, Pasupuleti SS, Richardson K. Nativity, duration of residence and chronic health conditions in Australia: do trends converge towards the native-born population? Soc Sci Med. 2014;119:53–63. 10.1016/j.socscimed.2014.08.008 . [DOI] [PubMed] [Google Scholar]
- 16. Pfortmueller CA, Stotz M, Lindner G, Muller T, Rodondi N, Exadaktylos AK. Multimorbidity in adult asylum seekers: a first overview. PLoS One. 2013;8(12):e82671 10.1371/journal.pone.0082671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klinthall M, Lindstrom M. Migration and health: a study of effects of early life experiences and current socio-economic situation on mortality of immigrants in Sweden. Ethnicity & health. 2011;16(6):601–23. 10.1080/13557858.2011.602392 . [DOI] [PubMed] [Google Scholar]
- 18. Viruell-Fuentes EA, Miranda PY, Abdulrahim S. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012;75(12):2099–106. 10.1016/j.socscimed.2011.12.037 . [DOI] [PubMed] [Google Scholar]
- 19. Doamekpor LA, Dinwiddie GY. Allostatic Load in Foreign-Born and US-Born bBacks: Evidence From the 2001–2010 National Health and Nutrition Examination Survey. American journal of Public Health. 2015;105:591–7. 10.2105/AJPH.2014.302285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sole-Auro A, Crimmins EM. Health of Immigrants in European countries. The International migration review. 2008;42(4):861–76. 10.1111/j.1747-7379.2008.00150.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diaz E, Kumar BN, Gimeno-Feliu L-A, Calderón-Larrañaga A, Poblador-Pou B, Prados-Torres A. Multimorbidity among registered immigrants in Norway: the role of reason for migration and length of stay. Tropical Medicine & International Health. 2015;On line 15 Oct 2015. 10.1111/tmi.12615 [DOI] [PubMed] [Google Scholar]
- 22. Diaz E, Gimeno-Feliu LA, Calderón-Larrañaga A, Prados-Torres A. Frequent attenders in general practice and immigrant status in Norway: A nationwide cross-sectional study. Scandinavian Journal of Primary Health Care. 2014;32(4):232–40. 10.3109/02813432.2014.982368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diaz E, Calderón-Larrañaga A, Prado-Torres A, Poblador-Plou B, Gimeno-Feliu L-A. How do immigrants use primary healthcare services? A register-based study in Norway. European Journal Public Health. 2015;25(1):72–8. Epub 31. July [DOI] [PubMed] [Google Scholar]
- 24. Straiton M, Reneflot A, Diaz E. Immigrants’ use of primary health care services for mental health problems. BMC Health Services Research. 2014;14:341 10.1186/1472-6963-14-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grabovschi C, Loignon C, Fortin M. Mapping the concept of vulnerability related to health care disparities: a scoping review. BMC Health Serv Res. 2013;13:94 10.1186/1472-6963-13-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caballero AE. Cultural Competence in Diabetes Mellitus Care: An Urgent Need. Insuline. 2007;2(2):80–91. [Google Scholar]
- 27. Tran AT, Straand J, Diep LM, Meyer HE, Birkeland KI, Jenum AK. Cardiovascular disease by diabetes status in five ethnic minority groups compared to ethnic Norwegians. BMC Public Health. 2011;11:554 10.1186/1471-2458-11-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lynch CP, Gebregziabher M, Axon RN, Hunt KE, Payne E, Egede LE. Geographic and racial/ethnic variations in patterns of multimorbidity burden in patients with type 2 diabetes. J Gen Intern Med. 2015;30(1):25–32. 10.1007/s11606-014-2990-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabanal KS, Lindman AS, Selmer RM, Aamodt G. Ethnic differences in risk factors and total risk of cardiovascular disease based on the Norwegian CONOR study. European journal of preventive cardiology. 2012;20(6):1013–21. 10.1177/2047487312450539 [DOI] [PubMed] [Google Scholar]
- 30. Diaz E, Kumar BN. Differential utilization of Primary Health Care services among older immigrants and Norwegians. A register-based comparative study in Norway. BMC Health Services Research. 2014;14:623 10.1186/s12913-014-0623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SSB. Country and citizenship in social statistics. 2008.
- 32. Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. British Journal of General Practice. 2011. 10.3399/bjgp11X548929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prados-Torres A, Poblador-Plou B, Calderon-Larrañaga A, Gimeno-Feliu LA, Gonzalez-Rubio F, Poncel-falco A, et al. Multimorbidity Patterns in pimary Care: Interactions among Chronic Diseases Using factor Analysis. PLOS ONE. 2012;7(2):e32190 10.1371/journal.pone.0032190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberts KC, Rao DP, Bennett TL, Loukine L, Jayaraman GC. Prevalence and patterns of chronic disease multimorbidity and associated determinants in Canada. Health Promot Chronic Dis Prev Can. 2015;35(6):87–94. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cooper B. Schizophrenia, social class and immigrant status: the epidemiological evidence. Epidemiologia e psichiatria sociale. 2005;14(3):137–44. . [DOI] [PubMed] [Google Scholar]
- 36. Diaz E, Kumar BN, Engedal K. Immigrant Patients with Dementia and Memory Impairment in Primary Health Care in Norway: A National Registry Study. Dementia and geriatric cognitive disorders. 2015;39:321–31. 10.1159/000375526 [DOI] [PubMed] [Google Scholar]
- 37. Shiroma PR, Alarcon RD. Time for healing: somatization among chronically mentally ill immigrants. Journal of cultural diversity. 2011;18(1):3–7. . [PubMed] [Google Scholar]
- 38. O'Mahoney JM, Donnelly TT. The influence of culture on immigrant women's mental health care experiences from the perspectives of health care providers. Issues in mental health nursing. 2007;28(5):453–71. [DOI] [PubMed] [Google Scholar]
- 39. Gatta G, Mallone S, van der Zwan JM, Trama A, Siesling S, Capocaccia R, et al. Cancer prevalence estimates in Europe at the beginning of 2000. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(6):1660–6. 10.1093/annonc/mdt030 . [DOI] [PubMed] [Google Scholar]
- 40. Lyratzopoulus G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in the number of general practitioner consultations before hospital referral for cancer: findings from a the 2010 National Cancer Patient Experience Survey in England. The Lancet Oncology. 2012;13(4):353–65. 10.1016/S1470-2045(12)70041-4 [DOI] [PubMed] [Google Scholar]
- 41. Morrone A, Nosotti L, Piombo L, Scardella P, Spada R, Pitidis A. Iron deficiency anaemia prevalence in a population of immigrated women in Italy. Eur J Public Health. 2012;22(2):256–62. 10.1093/eurpub/ckq144 . [DOI] [PubMed] [Google Scholar]
- 42. de-la-Iglesia-Inigo S, Carranza-Rodriguez C, Ropero-Gradilla P, Gonzalez-Fernandez FA, Molero-Labarta T, Hemmersbach-Miller M, et al. Red blood cell disorders in recently arrived African immigrants to Gran Canaria, Spain. Trans R Soc Trop Med Hyg. 2013;107(2):91–7. 10.1093/trstmh/trs017 . [DOI] [PubMed] [Google Scholar]
- 43. Rassjo EB, Byrskog U, Samir R, Klingberg-Allvin M. Somali women's use of maternity health services and the outcome of their pregnancies: a descriptive study comparing Somali immigrants with native-born Swedish women. Sexual & reproductive healthcare: official journal of the Swedish Association of Midwives. 2013;4(3):99–106. 10.1016/j.srhc.2013.06.001 . [DOI] [PubMed] [Google Scholar]
- 44. McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. 10.1196/annals.1314.001 . [DOI] [PubMed] [Google Scholar]
- 45. Offidani E, Ruini C. Psychobiological correlates of allostatic overload in a healthy population. Brain, behavior, and immunity. 2012;26(2):284–91. 10.1016/j.bbi.2011.09.009 . [DOI] [PubMed] [Google Scholar]
- 46. Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139(1):165–73. 10.1378/chest.10-1252 . [DOI] [PubMed] [Google Scholar]
- 47. Le Reste JY, Nabbe P, Lygidakis C, Doerr C, Lingner H, Czachowski S, et al. A research group from the European General Practice Research Network (EGPRN) explores the concept of multimorbidity for further research into long term care. Journal of the American Medical Directors Association. 2013;14(2):132–3. 10.1016/j.jamda.2012.07.017 . [DOI] [PubMed] [Google Scholar]
- 48. Schäfer I, Kaduszkiewicz H, Wagner H-O, Schön G, Scherer M, Bussche Hvd. Reducing complexity: a visualisation of multimorbidity by combining disease clusters and triads. BMC Public Health. 2014;14:1285 10.1186/1471-2458-14-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. 10.1016/S0140-6736(12)60240-2 . [DOI] [PubMed] [Google Scholar]
- 50.Information CfH. International Classification of Primary Care Second Edition (ICPC-2): Literature review. 2006.
- 51. Frese T, Herrmann K, Bungert-Kahl P, Sandholzer H. Inter-rater reliability of the ICPC-2 in a German general practice setting. Swiss medical weekly. 2012;142:w13621 10.4414/smw.2012.13621 . [DOI] [PubMed] [Google Scholar]
- 52. Carlsson L, Borjesson U, Edgren L. Patient based 'burden-of-illness' in Swedish primary health care. Applying the Johns Hopkins ACG case-mix system in a retrospective study of electronic patient records. The International journal of health planning and management. 2002;17(3):269–82. 10.1002/hpm.674 . [DOI] [PubMed] [Google Scholar]
- 53. Razum O. Commentary: of salmon and time travellers- musing on the mystery of migrant mortality. Int J Epidemiol. 2006;35(4):919–21. 10.1093/ije/dyl143 . [DOI] [PubMed] [Google Scholar]
- 54.Mørk E. Seniorer i Norge 2010 (Elderly in Norway 2010). Oslo: 2011 Contract No.: ISBN 978-82-537-8048-1.
- 55.Nergård TB. Eldre innvandreres bruk av pleie- og omsorgstjenester. Rapport fra fem norske storbykommuner (The elderlies' use of Social Care Services. Report fron five Norwegian communities). 2008.
- 56. Tomasdottir MO, Getz L, Sigurdsson JA, Petursson H, Kirkengen AL, Krokstad S, et al. Co- and multi-morbidity patterns in an unselected Norwegian population: cross-sectional analysis based on the HUNT Study and theoretical reflections concerning basic medical models. European Journal for Person Centered Healthcare. 2013;2(3):335–45. [Google Scholar]
- 57. Kreps GL, Sparks L. Meeting the health literacy needs of immigrant populations. Patient Educ Couns. 2008;71(3):328–32. 10.1016/j.pec.2008.03.001 . [DOI] [PubMed] [Google Scholar]
- 58. Nørredam M, Nielsen SS, Krasnik A. Migrants' utilization of somatic healthcare services in Europe—a systematic review. Eur J Public Health. 2010;20(5):555–63. 10.1093/eurpub/ckp195 . [DOI] [PubMed] [Google Scholar]
- 59. Varvin S, Aasland OG. Legers forhold til flyktningpasienten (Physicians ' relation to refugees). Tidsskrift for den Norske Legeforening. 2009;129(15):1488–90. [DOI] [PubMed] [Google Scholar]
- 60. Bathia R, Wallace P. Experiences of refugees and asylum seekers in general practice: a qualitative study. BMC Family Practice. 2007;8(48). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jensen NK, Nørredam M, Priebe S, Krasnik A. How do general practitioners experience providing care to refugees with mental health problems? A qualitative study from Denmark. BMC Fam Pract. 2013;14:17 10.1186/1471-2296-14-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rousseau C, Kuile St, Muñoz M, Nadeau L, Ouimet M-J, Kirmayer L, et al. Health care Access for Refugees and Immigrants with precarious Status. Public Health and Human Right Challenges. Canadian Journal of Public Health. 2008;99(4):290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Adepoju O. Capsule commentary on Lynch et al. , Geographic and racial/ethnic variations in patterns of multimorbidity burden in patients with type 2 diabetes. J Gen Intern Med. 2015;30(1):93 10.1007/s11606-014-3005-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The dataset contains sensitive information. Although data are pseudonymised, there is still a possibility of recognizing individuals due to the low number of immigrants from some countries to Norway. Thus, the Norwegian Data Inspectorate does not allow this information to be published or shared with other institutions than the University of Bergen.