Abstract
Steroid hormones are crucial for many biological events in multicellular organisms. In insects, the principal steroid hormones are ecdysteroids, which play essential roles in regulating molting and metamorphosis. During larval and pupal development, ecdysteroids are synthesized in the prothoracic gland (PG) from dietary cholesterol via a series of hydroxylation and oxidation steps. The expression of all but one of the known ecdysteroid biosynthetic enzymes is restricted to the PG, but the transcriptional regulatory networks responsible for generating such exquisite tissue-specific regulation is only beginning to be elucidated. Here, we report identification and characterization of the C2H2-type zinc finger transcription factor Ouija board (Ouib) necessary for ecdysteroid production in the PG in the fruit fly Drosophila melanogaster. Expression of ouib is predominantly limited to the PG, and genetic null mutants of ouib result in larval developmental arrest that can be rescued by administrating an active ecdysteroid. Interestingly, ouib mutant animals exhibit a strong reduction in the expression of one ecdysteroid biosynthetic enzyme, spookier. Using a cell culture-based luciferase reporter assay, Ouib protein stimulates transcription of spok by binding to a specific ~15 bp response element in the spok PG enhancer element. Most remarkable, the developmental arrest phenotype of ouib mutants is rescued by over-expression of a functionally-equivalent paralog of spookier. These observations imply that the main biological function of Ouib is to specifically regulate spookier transcription during Drosophila development.
Author Summary
Steroid hormones are crucial for development and reproduction in multicellular organisms. The spatially-restricted expression of almost all steroid biosynthesis genes is key to the specialization of steroid producing cells. In the last decade, insects have become the focus for research on the biosynthesis of the principal steroid hormones, ecdysteroids. However, the transcriptional regulatory mechanisms controlling the ecdysteroid biosynthesis genes are largely unknown. Here we show that a novel zinc finger transcription factor Ouija board (Ouib) is essential for activating the expression of one ecdysteroid biosynthesis gene, spookier, in the ecdysteroid producing cells. Ouib is the first invertebrate transcription factor that is predominantly expressed in the steroidogenic organs and essential for development via inducing expression of the steroidogenic gene. In addition, this is the first report showing the catalytic step-specific control of steroid hormone biosynthesis through transcriptional regulation.
Introduction
Steroid hormones are responsible for the coordination and regulation of many biological events during development of multicellular organisms. In all species, steroid hormones are synthesized from cholesterol and/or other phytosterols by multiple steroidogenic enzymes, epitomized by the members of the steroidogenic cytochrome P450 monooxygenases. High-level steroid hormone biosynthesis generally occurs in specialized steroidogenic tissues. Thus, an important condition for achieving tissue-specificity of steroid biosynthesis is providing a regulatory mechanism that ensures tissue-specific expression of the steroidogenic enzyme genes.
In vertebrates, major sites of steroid hormone biosynthesis are the adrenal cortex, gonads and placenta, that express steroidogenic enzyme genes such as Cyp11a1, P450c17a, 3β-HSD and 17β-HSD [1]. Key transcriptional regulators for these genes are the orphan nuclear receptors NR5A1 and NR5A2, also known as Ad4BP/Steroidogenic Factor 1 (SF-1) and Liver receptor homolog-1 (LRH-1), respectively [2–5]. Ad4BP/SF-1 and LRH-1 are predominantly expressed in the steroidogenic cells. A collective body of previous studies has established that Ad4BP/SF-1 controls steroid hormone biosynthesis through the transcriptional regulation of all steroidogenic genes [3,5]. Moreover, forced expression of this gene is sufficient to differentiate embryonic stem cells and human induced pluripotent stem cells into the steroidogenic cells [6,7] and to induce ectopic adrenal formation [8], indicating that Ad4BP/SF-1 acts as a master regulator for steroid hormone biosynthesis in vertebrates.
In insects, the principal steroid hormones are ecdysteroids, including ecdysone and its active derivative 20-hydroxyecdysone (20E), which plays pivotal roles in controlling a number of developmental and physiological events, especially in guiding transition from one developmental stage to the next via molting and metamorphosis [9–13]. During larval and pupal development, ecdysone is synthesized from dietary cholesterol in a specialized endocrine organ called the prothoracic gland (PG). After release from the PG, ecdysone is converted to 20E in the peripheral tissues through the action of Shade, the terminal P450 monoxygenase in the biosynthetic pathway [14]. In the last 15 years, a number of genes encoding essential ecdysteroidogenic enzymes acting in the PG have been identified and characterized, including noppera-bo [15–17], neverland (nvd) [18,19], Cyp307a1/spook (spo) [20,21], Cyp307a2/spookier (spok) [21], non-molting glossy/shroud (sro) [22], Cyp306a1/phantom (phm) [23,24], Cyp302a1/disembodied (dib) [25,26] and Cyp315a1/shadow (sad) [26]. All of these enzymes (except nvd and spok) are collectively referred to as the Halloween genes [13,27].
Previous studies have identified multiple transcription factors essential for ecdysteroidogenic functions in the PG. For example, the Ecdysone receptor-Ultraspiracle complex and several other ecdysteroid-regulated transcription factors such as βFTZ-F1, Broad, E75A and DHR4 are involved in both forward and feedback regulation of cyclic ecdysteroid production [28–35]. Ecdysteroid biosynthesis is also transcriptionally regulated by other factors including Without children [36], Molting defective (Mld) [37], the CncC-dKeap1 complex [38], Ventral veins lacking (Vvl) [39,40], Knirps [39] and FOXO [35]. Importantly, it has been reported that Broad, CncC, dKeap1, Vvl and Knirps directly bind to enhancer regions of some ecdysteroidogenic enzyme genes [31,33,38,39].
However, it should be noted that, unlike vertebrate Ad4BP/SF-1 and LHR-1, all of the identified steroidogenic transcription factors in insects are highly expressed not only in the PG, but also in many other non-ecdysteroidogenic tissues. Furthermore, some of these transcription factors have important functions other than ecdysteroid biosynthesis. For example, FOXO is well characterized as the primary transcriptional mediator of the insulin/insulin-like peptide signaling pathway in almost all cells [41]. Similarly, the CncC-dKeap1 complex is known to regulate xenobiotic responses [42] while Vvl and Knirps play key roles in cellular differentiation and morphogenesis of several tissues during embryogenesis including the PG (i.e. [43,44]). More notably, βFTZ-F1, the insect homolog of vertebrate Ad4BP/SF-1, plays a crucial role in ecdysteroid-dependent transcriptional cascades in not only the PG but also many other tissues [4,9].
In contrast to the broad roles that all these steroidogenic factors play in other tissues during development, we describe here a much more specific role for the transcription factor coded by the gene ouija board (ouib). Ouib is a C2H2-type zinc finger transcription factor, that is specifically expressed in the Drosophila PG and our genetic analysis clearly demonstrates that ouib is only essential for the expression of Spookier (Spok), a potential rate-limiting enzyme in the ecdysone biosynthetic pathway. Most remarkable, however, is that spok appears to be the essential target of ouib since resupply of a Spok paralog in PG tissue rescues ouib mutants to viability. Since orthologs of ouib and spok are found only in Drosophiladae genomes, this study also suggests a presence of insect clade-specific transcriptional regulatory mechanisms of ecdysone biosynthesis.
Results
CG11762/ouija board is predominantly expressed in the prothoracic gland during embryonic and larval development
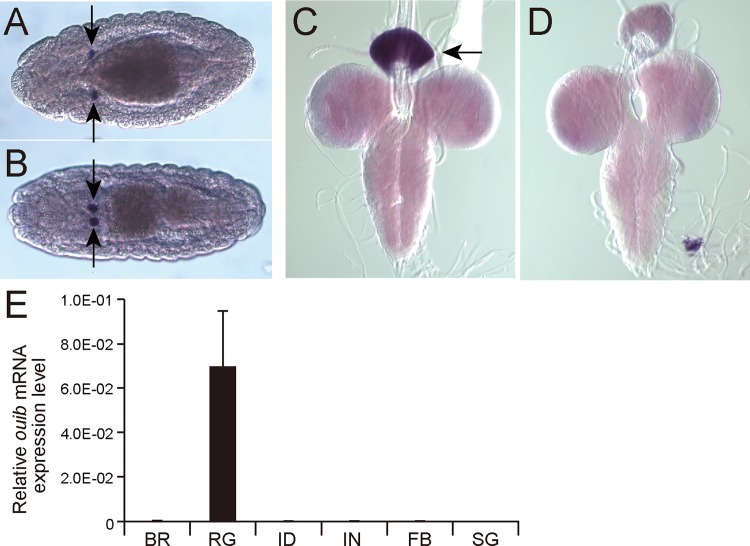
We identified CG11762, designated ouija board (ouib), as a gene predominantly expressed in the PG primordia in the embryonic in situ gene expression pattern database of the Berkeley Drosophila Genome Project Experiment ID RT01107 [45]. We confirmed the PG restricted expression of ouib in embryos using RNA in situ hybridization (Figs 1A, 1B and S1). Additional RNA in situ hybridization and quantitative reverse-transcription (qRT)-PCR experiments revealed that ouib is also predominantly expressed in the ring gland including the PG cells during larval development (Fig 1C, 1D and 1E). These results suggest that ouib may be involved in ecdysteroid biosynthesis.
Fig 1. Expression analysis of ouib in Drosophila larva and embryo.
(A, B) RNA in situ hybridization of stage 14 (A) and stage 16 (B) embryos with the ouib antisense RNA probe. Dorsal views are shown. ouib signal was detected in the primordia of PG cells (arrows). An image with sense RNA probe is shown in S1 Fig (C, D) in situ hybridization of third instar larval brain-ring gland complexes with the ouib antisense (C) and sense (D) RNA probes. ouib signal is detected in the ring gland including the PG cells (arrow). (E) The expression levels of ouib in several tissues quantified by qRT-PCR (N = 3). Total RNA was prepared from wandering third instar larvae. BR, brain; RG, ring gland; ID, imaginal disc; IN, intestine; FB, fat body; SG, salivary gland. Error bars indicate the s.e.m.
ouija board encodes a ZAD-C2H2 zinc-finger protein and is conserved only in Drosophilidae species
The predicted open reading frame of ouib encodes a protein that belongs to the family of the zinc-finger associated domain (ZAD) containing C2H2 zinc-finger proteins (ZFPs) [46,47]. The ZAD-ZFP family constitutes the largest subgroup of C2H2 ZFPs especially in insect species, and are characterized by an N-terminal ZAD consisting of ∼75 amino acid residues that are thought to serve as a protein-protein interaction domain [48]. In D. melanogaster, there are 98 independent loci encoding ZAD-ZFPs [47]. At least some of ZAD-ZFPs are thought to act as transcription factors, since several of them have been reported to bind DNA [49,50]. Notably, 5 paralogs of ouib are duplicated at the 85A9 cytological position of the third chromosome in D. melanogaster genome (S2 Fig), and one of the paralogs designated M1BP codes for a general transcription factor [51], raising the possibility that Ouib acts as a transcription factor in the PG.
Orthologs of ouib are found in genomes of 11 other Drosphilidae species (S1 Table) [52]. FlyBase (http://flybase.org/reports/FBgn0037618.html) also indicates the presence of potential orthologs of ouib in the mosquito species Aedes aegypti, Anopheles gambiae and Culex quinquefasciatus. However, a reciprocal BLAST search does not support the idea that the mosquito genomes have true ouib orthologs. In addition, a standard BLAST search did not detect any orthologous counterparts of ouib in any organisms other than Drosophilidae species. This is consistent with the previous report [47], that no orthologs of ouib are found in genomes of the silkworm Bombyx mori and the beetle Tribolium castatenum. Taken together, these results suggest that ouib is a Drosophilidae-specific ecdysteroidogenic component.
ouija board is essential for larval development
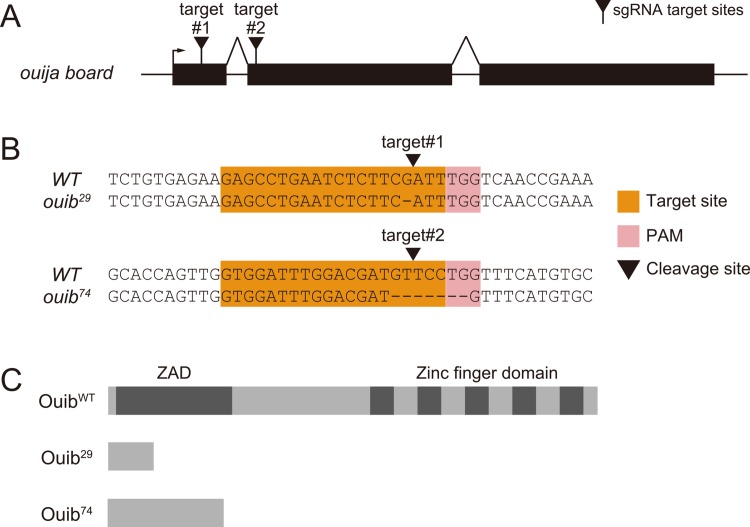
To assess the in vivo functional importance of ouib, we generated ouib loss-of-function alleles by a CRISPR/Cas9-dependent genome editing technology [53]. We succeeded in isolating two independent mutant alleles, ouib 29 and ouib 74, each of which had a small deletion induced by different CRISPR single guide RNAs (sgRNAs; Fig 2A and 2B). Both ouib 29 and ouib 74 alleles led to premature stop codons in the putative coding sequence of ouib, eliminating all 5 zinc-finger domains in the C-terminal region of Ouib (Fig 2C).
Fig 2. Generation of ouib mutant alleles by CRISPR/Cas9 system.
(A) A schema of the ouib gene showing the sgRNA target sites. Exons are shown as black boxes, the transcriptional start site as arrow, and sgRNA target sites as black triangles. (B) Sequences of sgRNA target sites. The 20 bp target sequence corresponding to each target site is indicated in orange, along with the neighboring NGG protospacer adjacent motif (PAM) in pink and the cleavage site of Cas9 is shown as black triangles. (C) Predicted protein structures of ouib alleles. Ouib29 and Ouib74 are composed of 29 and 74 amino acids, respectively.
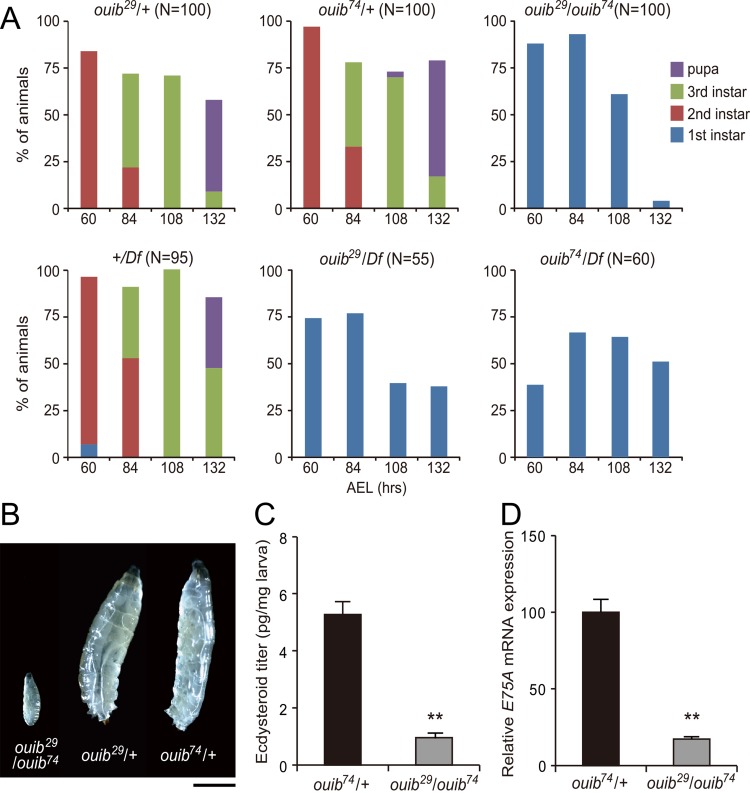
Embryos transheterozygous for ouib 29/ouib 74 completed embryogenesis, hatched normally, and showed no apparent morphological defects after hatching. However, ouib 29/ouib 74 transheterozygotes arrested development in the first instar larval stage and, even 108 hours after egg laying (AEL) or later, never molted into second instars and (Fig 3A and 3B). Eventually all ouib 29/ouib 74 transheterozygous animals died by 144 hours AEL retaining the first instar larva-type morphology. In contrast, the majority of control ouib 29/+ or ouib 74/+ heterozygous animals became pupae (Fig 3A and 3B) by this time. To rule out the possibility that the observed phenotype was due to off-target mutations by CRISPR/Cas9 system, we combined ouib 29 or ouib 74 allele with a deficiency (Df) line that deletes a genomic region containing ouib locus. Similar to ouib 29/ouib 74 transheterozygotes, ouib 29 /Df or ouib 74 /Df animals died in the first instar stage, while +/Df animals were fully viable. This result provides evidence that ouib locus is responsible for the lethal phenotype. These results demonstrate that Ouib is essential for larval development.
Fig 3. Larval lethality and developmental arrest phenotype of ouib mutant larvae.
(A) The survival rate and developmental progression of control and ouib mutant animals (N = 50~100). (B) Comparison of body size and developmental stage between control (right and middle) and ouib mutant (left) at 108 hours AEL. Control animals became third instar larvae, while ouib mutant animals were first instar larvae. Scale bar: 1 mm. (C) Ecdysteroid levels in control and ouib mutant first instar larvae at 12 hours AH measured by ELISA (N = 4). (D) Ecdysteroid levels, as measured by the ecdysone inducible gene E75A, in control and ouib mutant first instar larvae at 36 hours AEL measured by qRT-PCR (N = 3). Error bars indicate s.e.m. **; P<0.01 with Student’s t-test.
The ouija board loss-of-function phenotype is due to ecdysteroid deficiency
We next examined whether the larval arrest and lethality phenotype of ouib mutant animals was due to the loss of ecdysteroids. An ELISA assay revealed that the ecdysteroid titer in ouib 29/ouib 74 transheterozygotes was significantly reduced compared to control animals (Fig 3C). Consistent with this observation, the expression of E75A, which is an early ecdysteroid-inducible gene, was greatly reduced in ouib 29/ouib 74 transheterozygotes (Fig 3D). Moreover, when ouib 29/ouib 74 animals or ouib 74 /Df animals were fed yeast paste containing 20E after hatching, they molted to the second instar larval stage or later, as judged by the anterior spiracular morphologies (Table 1). These results suggest that loss of ouib mutant phenotype is due to ecdysteroid deficiency and that ouib regulates ecdysteroid production in the PG during normal development.
Table 1. Rescue of ouib null mutant animals by oral administration of sterols and ecdysteroids.
| Ecdy-steroid | Number of dead animals at each stage | Total number of animals | ||||
|---|---|---|---|---|---|---|
| 1st instar | 2nd instar and later | |||||
| ouib 29/ouib 74 | ouib 74/Df | ouib 29/ouib 74 | ouib 74/Df | ouib 29/ouib 74 | ouib 74/Df | |
| None | 47 | 36 | 0 | 0 | 47 | 36 |
| C | 48 | - | 0 | - | 48 | - |
| 7dC | 46 | - | 0 | - | 46 | - |
| 5βkd | 1 | - | 43 | - | 44 | - |
| 20E | 0 | 39 | 47 | 24 | 47 | 63 |
The first instar larvae of ouib 29/ouib 74 or ouib 74 /Df animals were collected 36 hours AEL, and then fed yeast pastes containing 0.5% (w/w) each steroid. The number of dead animals at each stage was counted. C, cholesterol; 7dC, 7-dehydrocholesterol; 5βkd, 5β-ketodiol; 20E, 20-hydroxyecdysone; -, not determined.
Loss of ouija board strongly reduces the expression of one ecdysone biosynthetic enzyme gene spookier
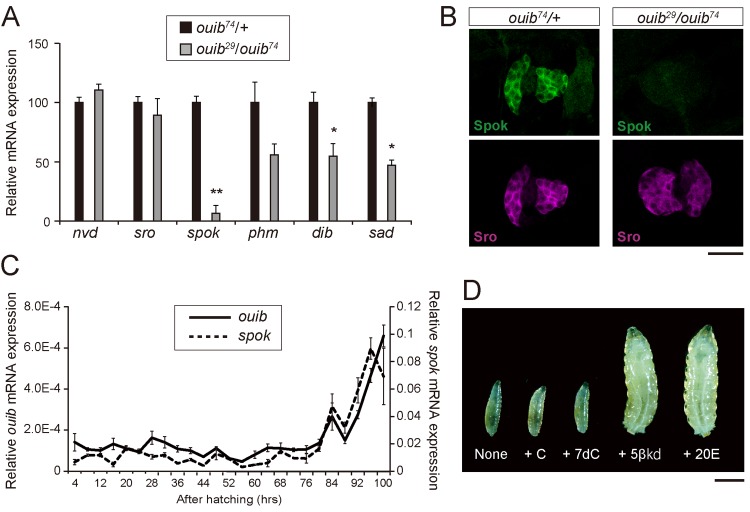
As described above, we expect that Ouib acts as a transcription factor. Considering the spatial expression pattern and the loss-of-function phenotype of ouib, we wondered whether loss of ouib resulted in changes in the expression levels of any ecdysteroidogenic genes in the PG. To address this issue, we conducted qRT-PCR experiment to examine expression levels of 6 ecdysteroidogenic genes in the first instar larvae of control and ouib 29/ouib 74 transheterozygotes. Among the 6 genes, the expression of one gene Cyp307a2/spok was drastically reduced in ouib 29/ouib 74 transheterozygotes as compared to control animals (Fig 4A). An immunohistological analysis using anti-Spok antibody also revealed a strong decrease of Spok protein level in ouib 29/ouib 74 larvae compared to control animals, but not that of the Sro protein, another ecdysone biosynthetic enzyme expressed in the PG. (Fig 4B). We also found that expression of Cyp302a1/dib and Cyp315a1/sad, two other ecdysone biosynthetic P450 genes, were also lower than in ouib 29/ouib 74 animals compared to control animals, but their reduction was just on the threshold of significance (Fig 4A). On the basis of the observation that the mutants cannot induce the expression of “spookier,” we named CG11762 “ouija board” since this is an instrument for calling ghosts in western countries.
Fig 4. Expression analysis of ecdysteroidogenic genes and feeding rescue experiment in ouib mutant larvae.
(A) The expression levels of ecdysteroidogenic genes in control and ouib mutant first instar larvae at 38 hours AEL measured by qRT-PCR (N = 3). (B) Immunostaining of the PG cells from control and ouib mutant first instar larvae at 36 hours AEL with antibodies against Spok (green) and Sro (magenta). Scale bar: 25 μm. (C) The transcriptional expression profiles of ouib and spok in w 1118 during larval development measured by qRT-PCR (N = 3). The solid line and dashed lines indicate the expression levels of ouib and spok, respectively. (D) Feeding rescue experiments for ouib mutant (ouib 29 /ouib 74) larvae. Mutant animals fed 5β-ketodiol (5βkd) and 20-hydroxyecdysone (20E) became third instar larvae, while animals fed cholesterol (C), 7-dehydrocholesterol (7dC) and none remained first instar larvae. The lethal stages in each experimental condition were scored and shown in Table 2. Scale bar: 1 mm. Error bars indicate s.e.m. *; P<0.05, **; P<0.01 with Student’s t-test.
We also examined whether there was a correlation between expression of ouib and spok during larval development. Overall the expression of both genes was relatively low during early stages and gradually became higher in late stages. We also found that the temporal expression profile of ouib closely correlates to that of spok in the late third instar stage (Fig 4C). Curiously, the temporal expression profile did not always correlates to the dynamics of ecdysteroid titer during the third instar stage (S3 Fig). For example, ouib expression did not increase prior to white prepupal stage, when the level of ecdysteroid titer was high. This result suggests that the ouib-spok coordinated transcriptional relationship does not fully account for the temporal dynamics of ecdysteroid biosynthesis during development.
ouija board plays an essential role in the “Black Box” where spookier has a crucial function
A previous study reported that Spok plays a crucial role in the “Black Box”, which consists of the conversion steps from 7-dehydrocholesterol (7dC) to 5β-ketodiol (5βkd) in the ecdysteroid biosynthetic pathway [21]. The participation of Spok in the “Black box” reactions was inferred by the observation that the larval arrest phenotype of spok RNAi animals was rescued by oral administration of 5βkd, but not 7dC or the most upstream precursor cholesterol [21]. Indeed, the same tendency as observed in spok RNAi animals was found in ouib loss-of-function animals. When ouib 29/ouib 74 transheterozygotes were fed yeast paste supplemented with cholesterol or 7dC, the larvae still arrested at the first instar larval stage (Fig 4D and Table 1). In contrast, we found that the first instar larval arrest phenotype of ouib 29/ouib 74 transheterozygotes was rescued when the animals were fed yeast paste supplemented with 5βkd (Fig 4D and Table 1). These results suggest that loss of ouib function specifically impairs the catalytic conversion that takes place during the “Black Box” reactions. These results also imply that the moderate reduction seen in dib and sad expression does not contribute in a major way to the ouib mutant phenotype.
The ouija board mutant phenotype is due to loss of spookier expression in the prothoracic gland
In addition to the feeding rescue experiment, we examined whether the ouib mutant phenotype was rescued by forced expression of spok using GAL4-UAS binary gene expression system. We first established UAS-spok transgenic strains to drive spok expression in the PG cells under control of phm-GAL4#22 driver. However, for an unknown reason, none of our UAS-spok transgenes was expressed in the PG of first and second instar larvae with the phm-GAL4#22 driver and thus these constructs were not suitable for our experimental purpose. Therefore, we decided to examine whether the ouib mutant phenotype could be rescued by forced expression of Cyp307a1/spo, a paralog of spok that appears to provide the same enzymatic activity but only in embryos and in the follicular cells of the ovary [20,21]. We confirmed that spo was functionally equivalent to spok in vivo, as spo overexpression rescued the first instar larval arrest phenotype of spok RNAi animals (S2 Table). Indeed, spo overexpression in the PG rescued the larval arrest phenotype of ouib 29/ouib 74 transheterozygotes, and some of the animals grew up to the adult stage (Table 2). These results strongly suggest that the developmental arrest phenotype of ouib mutant is due solely to loss of spok expression in the PG. Our data therefore support the idea that Ouib is a special transcription factor primarily required for inducing expression of one biosynthetic gene spok, and no other essential gene during development.
Table 2. Rescue of ouib mutant animals by spo overexpression in the PG.
| Transgenes | Number of ouib 29 /ouib 74 adults | |||
|---|---|---|---|---|
| Tb | 3rd instar larvae | Pupae | Adults | |
| phm-GAL4#22, UAS-spo | + | 169 (100%) | 124 (73.4%) | 29 (17.2%) |
| - | 246 | n.d. | n.d. | |
| phm-GAL4#22 | + | 0 | 0 | 0 |
| - | 85 | n.d. | n.d. | |
| UAS-spo | + | 0 | 0 | 0 |
| - | 84 | n.d. | n.d. | |
The number of ouib 29 /ouib 74 animals that grew up to the third instar larval, pupal and adult stages was scored. Tb+ indicates ouib 29 /ouib 74 animals, while Tb- indicates ouib 29 /TM6 or ouib 74 /TM6 animals from the parental strains in the same experimental batches. Tb- animals can be used as internal controls. Values in parentheses indicate the percentage of animals that survive to the pupal and adult stages. The animals were fed standard cornmeal food without any steroidal supplements. Detailed genetic crosses for this experiment are described in Materials and Methods. n.d., not determined.
Identification of Ouija board-response element in the spookier enhancer region
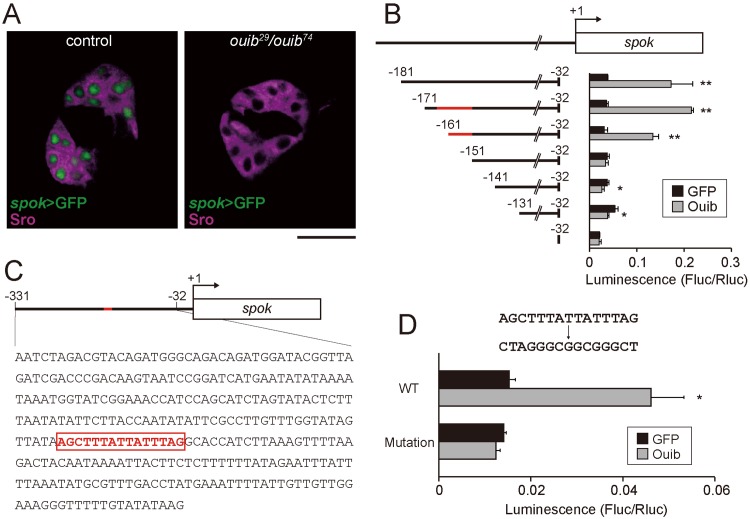
To address whether Ouib protein acts directly on the spok enhancer region to induce spok expression, we initially searched for an Ouib-response element in the enhancer/promoter region of spok. We first identified a ~1.4 kb genomic region upstream of the spok coding sequence that was sufficient to mimic the expression of spok in the PG when fused to a GFP reporter (Figs 5A and S4). The GFP expression driven by the ~1.4 kb spok enhancer region was almost completely abolished in ouib 29/ouib 74 transheterozygotes (Fig 5A), suggesting that the ~1.4 kb element contains a Ouib-response element.
Fig 5. Transcriptional activity of Ouib for the upstream element of spok.
(A) Fluorescence images of the PG cells from control and ouib mutant larvae with spok enhancer/promoter-driven nuclear localized-GFP construct (spok>GFP) at 36 hours AEL. PG cells are immunostained with antibody against Sro (magenta). Scale bar: 20 μm. (B) Luciferase reporter assay with plasmids containing the series of upstream elements of spok. Numbers indicate the distance from the translation initiation site (+1) of spok. The red bar indicates the Ouib-response element (-166 to -152). The white box represents the coding regions. Reporter activities of progressive deletion constructs are shown right (N = 3). The GFP expression plasmid was used as a negative control. (C) Schematic representation of the location of Ouib-response element (-166 to -152) in the spok enhancer/promoter region. The nucleotide sequence is shown below the cartoon of spok gene structure. The bar and box with red color indicate the 15 bp Ouib-response element. The black bar and the box indicate the enhancer/promoter and coding region, respectively. (D) Luciferase reporter assay with plasmids containing the 15 bp transversion mutation in the -166 to -152 region of the 300 bp upstream element of spok (N = 3). The GFP expression plasmid was used as a negative control. Error bars indicate s.e.m. *; P<0.05, **; P<0.01 with Student’s t-test.
In order to identify the cis-regulatory element(s) responsible for the Ouib-mediated control of spok expression, we conducted a promoter/enhancer characterization analysis in a heterologous cell culture system. We generated DNA constructs carrying the upstream region of spok fused with a luciferase (luc) gene cassette and then transfected Drosophila Schneider 2 (S2) cells using these DNA constructs with or without a plasmid for overexpressing FLAG-ouib. We identified a 300 bp genomic region corresponding to the region from -331 bp to -32 bp upstream of the ATG start codon of spok that drives expression of the luc reporter in S2 cells in an Ouib-dependent manner (S5 Fig). The 300 bp region was also sufficient to drive expression of a GFP reporter in the PG cells (S4 Fig). To narrow down the element(s) responsible for the Ouib-dependent expression of spok, we tested several constructs carrying the upstream region of spok with a range of deletions within the 300 bp region (S5 Fig). We first generated the deletion constructs in 50 bp increments from 5´ terminus of the 300 bp region and found that the region from -181 to -131 bp was crucial for the Ouib-dependent luc reporter activity (S5 Fig). We then generated the deletion constructs in 10 bp increments from 5´ terminus of the -181 to -32 region. The construct carrying the -151 to -32 region did not show any induction in luc reporter activity even in the presence of Ouib (Fig 5B). The construct carrying the longer 10 bp 5´ extension (-161 to -32) still retained statistical significant Ouib-dependent luc reporter activity. However, the fold induction of luc reporter activity with the -161 to -32 region was slightly reduced as compared to the -171 to -32 region or longer (Fig 5B). From these results, we hypothesized that the Ouib-response element lay between -166 to -152 bps (Fig 5C). To clarify the importance of this 15 bp region for Ouib-dependent control of gene expression, we introduced transversion mutations of the entire 15 bp sequence. This mutated construct exhibited no luc reporter induction in the presence of Ouib upon transfection into S2 cells (Fig 5D). We also conducted subsequent reporter assays using constructs carrying various mutations in the 15 bp sequence. None of the constructs carrying any of several 3 bp substitutions within the 15bp sequence eliminated the responsive to Ouib (S6 Fig). Therefore, we conclude that Ouib binding tolerates degeneracy throughout the 15 bp sequence (5´-AGCTTTATTATTTAG-3´).
We also examined the evolutionary conservation of the Ouib-response elements in putative spok enhancer regions in 12 Drosophilide species whose genome sequences have been determined [52]. EMBOSS Matcher, an algorithm to identify local similarities between two sequences [54], found sequence motifs similar to the D. melanogster Ouib-response element in almost all of the Drosophilidae species (S7 Fig). In particular, the D. yakuba putative spok enhancer contains exactly the same 15 bp sequence motif. In addition, in the species belonging to the subgenus Sophophora, which includes D. melanogaster, the Ouib-response element-like motifs are found in proximity (within 500 bp) to the spok coding region (S7 Fig). These data suggest that Ouib-like response elements are also evolutionarily conserved to some degree.
Ouija board physically associates with the Ouija board-response element
We sought to further establish if Ouib binds directly to the Ouib-response element by performing a DNA/protein binding assay. We first examined the physical interaction between the Ouib-response element sequence and Ouib protein by an ABCD assay, which uses biotin conjugated, double-stranded oligonucleotides containing the Ouib-response element sequences. Nuclear extracts obtained from S2 cells expressing FLAG-ouib were mixed with the biotin-labeled oligonucleotide, and then the protein-oligonucleotide complexes were pulled down using streptavidin beads. We found that FLAG-Ouib protein bound strongly to the wild type Ouib-response element probe, but not to the mutated probe (Fig 6A). In the control experiments, a biotinylated probe corresponding to M1BP (another ZAD-ZFP homolog of Ouib) binding sequence in the enhancer of smoothened locus [51] did not efficiently precipitate FLAG-Ouib. Conversely, FLAG-M1BP protein did not bind to the Ouib-response element, while it bound to M1BP binding element (Fig 6A).
Fig 6. DNA-Binding analysis of Ouib for the upstream element of spok.
(A) ABCD assay with nuclear extracts from S2 cells and avidin-biotin-conjugated double-stranded DNA probes. After pull-down, proteins were detected by western blotting using anti-FLAG antibody. (B) EMSA using recombinant proteins of GST alone or GST-fusion zinc finger domains of Ouib (amino acids 150–313) with 32P-labeled double-stranded oligonucleotide probes containing the wild type 15 bp Ouib-response element (ORE). The x100 and x200 amounts of the non-labeled probes of the wild type ORE (ORE WT), the mutated ORE (ORE Mut) and the wild type M1BP-response element (MRE WT) were used as cold competitors. (C) Densitometric analysis of the EMSA band radioactive intensities in the same experimental condition as B with 3 independent replicates. Average radioactivity of the ORE/GST-Ouib-Zf complex is set as 100%. Note that the complexes with the ORE were outcompeted by the unlabeled ORE WT probe, but not by the unlabeled MRE WT probe. *P<0.05 and **P<0.01 by Tukey's multiple comparisons test. n.s., not significant. (D) EMSA using recombinant proteins of GST-fusion zinc finger domains of Ouib (amino acids 150–313) with 32P-labeled double-stranded oligonucleotide probes of ORE WT, ORE Mut and MRE WT.
To exclude the possibility that FLAG-Ouib protein isolated from cultured cells, indirectly associated with the probe through a complex containing some other endogenous transcription factor unrelated to Ouib, we prepared an E. coli produced recombinant protein containing the C-terminal 5 zinc finger domains (Ouib-Zf), and performed electrophoretic mobility shift assays (EMSAs) between the recombinant protein and the 15 bp Ouib-response element. We utilized 45 bp radiolabeled DNA probes, whose sequences corresponded to the spok enhancer region containing the 15 bp Ouib-response element. We found that the wild type oligonucleotide probes formed DNA/protein complexes with GST-Ouib-Zf, but not with GST alone (Fig 6B and 6C). In contrast, such DNA/protein complexes were not detected when radiolabeled mutated Ouib-response element sequences or sequences corresponding to the M1BP site [51] (Fig 6D) were used as probes, thereby confirming the specificity of the binding. Moreover, the complexes with the wild type 45 bp probes were outcompeted by unlabeled 45 bp DNA probes with the wild type Ouib-response element sequences, but not by the unlabeled mutated DNA probes or by the unlabeled M1BP probes (Fig 6B and 6C). Taken together, these findings strongly support the idea that Ouib specifically regulates spok transcription by direct binding to the Ouib-response element in the spok enhancer.
ouija board transcript level is not affected by PTTH signaling
Previous studies found that the increase of spok expression in the late third instar larvae is positively controlled by prothoracicotropic hormone (PTTH) [55,56]. We therefore examined whether ouib expression changed in response to down regulation of PTTH signaling. However, when the levels of the PTTH receptor gene torso were knocked down in the PG by RNAi, we observed no change in ouib expression, suggesting that PTTH regulation of spok is not mediated through ouib (S8 Fig).
Discussion
In this study, we have demonstrated that the ZAD-ZFP Ouib is required for ecdysteroid biosynthesis in the PG during D. melanogaster development. The following points summarize our finding. First, ouib is predominantly expressed in the PG during embryonic and larval stages. Second, ouib null mutants exhibit early (first instar) larval developmental arrest due to a low ecdysteroid titer. Third, the larval arrest phenotype is caused by a failure of spok expression in the PG, and is rescued by sole overexpression of a spok paralog. Finally, a specific Ouib-response element that binds Ouib was identified in the enhancer region of spok. Our study reports on the discovery of the first invertebrate tissue-specific, steroidogenic transcription factor.
ouib mutants exhibit a drastic reduction of spok expression. However, we point out that ouib mutants also show a mild statistically-significant reduction of dib and sad (Fig 4A). In fact, while no DNA sequences exactly matching the spok Ouib-response element (5´-AGCTTTATTATTTAG-3´) are found elsewhere in D. melanogaster genome, a number of degenerate sequences do exist in the genome, including the regions upstream of ecdysteroidogenic gene coding regions (S9 Fig). Considering the fact that the luciferase constructs carrying any of several 3 bp substitutions within the 15bp sequence are still responsive to Ouib (S6 Fig), we cannot completely rule out the possibility that Ouib is also involved in direct transcriptional regulation of genes other than spok, particularly dib and sad. Nevertheless, our results indicate that the impairment of expression of dib and sad seems not to contribute to the ouib phenotype in a major way. First, the arrest during the first instar larval stage of ouib mutants is rescued by oral administration of 5βkd. Since Dib and Sad play roles in the terminal hydroxylation steps downstream of the conversion of 5βkd to ecdysone [23,24,26], this finding suggests that the enzymatic levels of Dib and Sad are still sufficient to make functional levels of ecdysone. Second and more importantly, the first instar larval arrest phenotype of ouib mutants is rescued by a sole overexpression of spo, which is functionally equivalent to spok. Therefore, in addition to the PG specificity, we would argue that the key additional feature of Ouib is its specific role in spok expression. To further clarify the extent to which Ouib regulates other genes, and the functional importance of these genes, will require additional studies including transcriptome analysis/ChIP-seq analysis together with eventual mutational analysis of any identified targets.
Curiously, the presence of ouib only in the Drosophilidae genomes is concordant with the Drosophilidae-specific duplication of Cyp307a P450 subfamily. While members of the Cyp307 P450 subfamily, which includes spok, are found in all arthropod species examined so far [20,21,57,58], Drosophilidae Cyp307 genes have been duplicated within the Drosophila radiation [21,59]. In the case of D. melanogaster, the duplicated Cyp307 genes are Cyp307a1/spo and Cyp307a2/spok, which are sub-functionally divergent in terms of gene expression pattern; spo is expressed in early embryogenesis and oogenesis, while spok is expressed in the PG cells in late embryogenesis as well as the larval and pupal stages [20,21]. Our data demonstrate that the spatiotemporal expression pattern of ouib closely matches that of spok but not spo. Notably, neither ouib nor spok transcripts are detected in embryonic stages 5–9 when the embryonic ecdysteroid titer is maximal [21,45], indicating that these genes do not contribute to producing embryonic ecdysteroids. Therefore, an acquisition of ouib might be a critical event for the sub-functionalization of two Cyp307 genes by changing the regulation of their expression during the Drosophilidae evolution. In terms of evolution, it is worth mentioning that there is a case where evolution changed the activity of a single ecdysteroidogenic enzyme (Nvd) dramatically limiting the food source of Drosophila pachea to a single species of cactus [60]. Further assessment of the biological and evolutionary roles of ouib and spok will require determining which transcription factors are involved in the transcriptional regulation of D. melanogaster spo and Cyp307a genes in other insects. Since there are many divergent ZAD-ZFP genes in each insect genome and they are expanded in insect lineage-specific manner [47], it is possible that a different ZAD-ZFP gene whose primary structure is not orthologous to ouib could be a transcription factor for other Cyp307a genes.
Regarding the evolutionarily aspect of ouib, it is important to recognize that spok expression is regulated by another ZAD-ZFP called Molting defective (Mld) [21,37,39]. Interestingly, just like ouib, mld genes are also found only in genomes of Drosophilidae but not other insects [21,37]. In contrast to ouib, Mld does not appear to be specific for the regulation of spok expression. First, Mld, unlike ouib, is expressed in several other tissues during development besides the PG [37]. Second, Mld is essential for regulating expression Nvd as well as spok and perhaps other genes [39]. Third and most important, the mld loss–of-function phenotype is not rescued by overexpressing either spo or spok [21]. Therefore, Ouib and Mld overlap in their regulation of spok expression, but also have distinct functions during development. While it is still unclear whether Mld is a transcription factor, it would be intriguing to examine a functional relationship between Ouib and Mld for induction of spok expression in the PG. According to our qRT-PCR data, it is less likely that Mld controls ouib expression in the PG (S10 Fig).
Another question to be answered is how ouib expression is regulated during larval development. As shown above, it does not seem to be by PTTH. However, recent work as shown that spok and other ecdysteroidogenic enzyme genes are also influenced by humoral factors such as TGFβ/Activin [61] and monoaminergic tropic factors [62,63]. It will be interesting to determine whether these factors affect spok expression in the PG through modulation of ouib levels.
An additional significant aspect of this work is to provide the first evidence for the existence of a catalytic step-specific transcriptional regulation of steroid hormone biosynthesis in organisms. Whereas the substrate of Spok and its product have not yet been identified, Spok appears to play a crucial role in the “Black Box” step of ecdysteroid biosynthetic pathway, and it is a strong candidate for acting as a rate-limiting enzyme in the pathway [10,21,64]. Interestingly, a recent study has reported that pre-mRNA splicing of spok, but not any other ecdysteroidogenic genes expressed in the PG, seems to specifically depend on a protein encoded by ecdysoneless (ecd), whose mutant phenotype includes ecdysteroid deficiency [65]. Thus, a rate-limiting step of ecdysteroid biosynthesis catalyzed by Spok could be under tight control by both specific transcriptional and post-transcriptional mechanisms. Currently, it is unknown whether such catalytic-specific transcriptional and/or posttranscriptional mechanisms also exist in other organisms including vertebrates.
Similar to ecdysteroids, vertebrate steroid hormones are synthesized via several intermediates by multiple steroidogenic enzymes. Among them, the rate-limiting step in vertebrate steroid hormone productions is the delivery of substrate cholesterol from the outer mitochondrial membrane to the inner one and the subsequent conversion of cholesterol to pregnenolone by CYP11A1. It is attractive to hypothesize that the rate-limiting step in vertebrate steroid hormone biosynthesis is also specifically regulated by unidentified transcriptional and/or splicing regulator(s). Whereas no apparent orthologs of ouib are found in vertebrates, their genomes possess a ZAD-ZFP gene called ZFP276, which is a tumor suppressor gene [66]. Interestingly a ecd ortholog is also found in humans and may also contribute to the malignancy of certain tumor types [65]. It would be worth examining roles of these genes in steroid hormone biosynthesis in vertebrates.
Materials and Methods
Drosophila strains
Drosophila melanogaster flies were reared on standard agar-cornmeal medium at 25°C under a 12:12 h light/dark cycle. w 1118, yw and Oregon R were used as the wild type strain. phm–GAL4#22 [55] and w; UAS-dicer2; phm-GAL4#22/TM6 Ubi-GFP was used as the strain to drive forced gene expression in the PG. UAS-spo [20] and UAS-spok-IR [21] transgenic flies were obtained from Hiroshi Kataoka (The University of Tokyo) and Hajime Ono (Kyoto University), respectively. y 1 v 1 nos-phiC31; attP40, v 1 and y 2 cho 2 v 1 ; attP40{nos-Cas9}/CyO [53] were obtained from National Institute of Genetics, Japan. The w; sna Sco /CyO; P{w+mC = tubP-GAL80 ts }7 (stock number #130453) and w 1118 ; Df(3R)ED5330/TM6C Sb 1, a deficiency strain that deletes a genomic region including the ouib locus (stock umber #150241) [67], were obtained from Drosophila Genetic Resource Center. UAS-torso-IR (stock number #101154) and UAS-mld-IR (stock number #17329) were obtained from the Vienna Drosophila RNAi center.
in situ RNA hybridization
Digoxygenin (DIG)-labeled antisense RNA probes were synthesized using DIG RNA labeling mix (Roche) and T3 and T7 RNA polymerase (Fermentas). To generate the ouib probe, the ouib ORF was amplified by PCR with cDNA derived from whole bodies of Oregon R larvae and the primers described in S3 Table. PCR product was inserted into SmaI-digested pBluescript II SK (-), and then used as the templates for synthesizing RNA probes. Fixation, hybridization and detection were performed as [23,68].
Quantitative reverse transcription (qRT)-PCR
RNA was isolated using the RNAiso Plus reagent (TaKaRa). Genomic DNA digestion and cDNA synthesis were performed using the ReverTra Ace qPCR RT Kit (TOYOBO). qRT-PCR was performed using the THUNDERBIRD SYBR qPCR Mix (TOYOBO) or Universal SYBR Select Master Mix (Applied Biosystems) with a Thermal Cycler Dice TP800 or TP870 system (TaKaRa). Serial dilutions of a plasmid containing the ORF of each gene were used as a standard. The expression levels of the target genes were normalized to an endogenous control ribosomal protein 49 (rp49) in the same sample. The primers for quantifying D. melanogaster ouib and E75A are described in S3 Table. Primers amplifying nvd, sro, spok, phm, dib, sad and rp49 were previously described [22,55].
Immunostaining
Tissue dissections were performed in PBS followed by fixation in 4% PFA for 20 minutes at room temperature. For this study, the following primary antibodies were: mouse anti-FLAG M5 (1:1,000) (Sigma); rabbit anti-Phm (1:200) [30], guinea pig anti-Spok (1:200) [61]; guinea pig anti-Sro (1:1,000) [62]. Tissues were incubated over night with primary antibodies at 4°C. Fluorescent conjugated secondary antibodies used in this study, goat anti-mouse Alexa Fluor 488, goat anti-guinea pig Alexa Fluor 488, goat anti-rabbit Alexa Fluor 555 and goat anti-guinea pig Alexa Fluor 555, were purchased from Life Technologies. Secondary antibodies were diluted 1:500 and incubated for 1 hour at room temperature. Confocal images were captured using Carl Zeiss LSM 700 laser scanning microscope.
UAS vectors, overexpression of genes and generation of transgenic strains
The GAL4-UAS system [69] was used to overexpress genes in D. melanogaster. To generate pUAST vector to overexpress ouib, specific primers including a sequence coding FLAG tag at N terminal were used for PCR to add EcoRI and XbaI sites to the 5´ and 3´ ends, respectively (S3 Table). Template cDNAs were reverse transcribed using total RNA of the ring gland from D. melanogaster using ReverTra Ace qPCR RT Kit (TOYOBO). PCR was performed using KOD Plus Neo (TOYOBO). The amplified CDS region of ouib was digested with EcoRI and XbaI, and then ligated into a pWALIUM10-moe vector [70]. Transformants were established by BestGene, Inc.
Generation of the ouib alleles
Generation of the ouib allele was carried out by CRISPR/Cas9 system using the pBFv-U6.2 vector [53] provided by the National Institute of Genetics, Japan. We selected 2 independent target sites (target#1 and target#2 as shown in Fig 2). To minimize off-target effects of CRISPR/Cas9 system, we confirmed by BLAST search that no 15 nucleotide stretches within the selected target sequence (23 nucleotides including PAM motif) matched any other sequence on the 3rd chromosome. Sense and antisense oligonucleotides corresponding to sgRNA target sequences (S3 Table) were annealed and inserted into BbsI-digested pBFv-U6.2 vector. The ouib sgRNA vectors were injected into the embryos of the y 1 v 1 nos-phiC31; attP40 strain. The nos-Cas9-based gene targeting was carried out as previously described [53]. Males carrying nos-Cas9 and a sgRNA transgene were crossed to wild-type flies by mass mating. From their progeny, 10 and 50 single males for the target#1 and target#2 sites, respectively, were isolated. Each male was crossed with w; TM3 Sb/TM6 Tb females and then the independent isogenized strains were established. Among them, we surveyed the strains showing homozygous lethality and eventually 1 target#1 and 29 target#2 lethal strains were selected. To confirm indel mutations at ouib locus in each strain, we performed the T7EI assay as previously described [53]. In this assay, genome DNA from the heterozygous adults of each strain was extracted as previously described [53]. To amplify the DNA fragment including Cas9 target sites, PCR was conducted with KOD FX Neo (TOYOBO), the extracted genome DNA, and the primers listed in S3 Table [53]. The PCR products were treated with T7 endonuclease (NEB). The reacted samples were analyzed by agarose gel electrophoresis. Out of 30 total candidate strains, 1 target#1 and 8 target#2 strains were selected as candidate flies possessing indel mutations in ouib region. The PCR products from the 9 strains were subcloned into a SmaI-digested pBluescript II (Promega) and then sequenced with T3 and T7 primers. We detected small deletions in 8 out of the 9 strains. The minimal and maximal deletion sizes were 1 bp and 13 bp, respectively. We chose 1 strain for each target sites for further analyses and renamed them ouib 29 and ouib 74, both of which caused frameshift mutations for ouib locus (Fig 2).
Scoring of developmental progression of ouib mutants
ouib 29 /TM3 Act-GFP flies, ouib 74 /TM3 Act-GFP flies and w 1118 flies were crossed each other. Eggs were laid on grape plates with yeast pastes at 25°C for 8 hours. 36 hours AEL, 100 hatched GFP negative (ouib 29 /+, ouib 74 /+ and ouib 29 /ouib 74) first instar larvae were transferred into vials with standard cornmeal food (25 animals per vial). Every 24 hours, developmental stages were scored by tracheal morphology as previously described [22].
Feeding rescue experiments with ecdysteroids and intermediates
For the rescue experiments, 20 mg of dry yeast was mixed with 38 μl H2O and 2 μl ethanol or supplemented with 2 μl of the following sterols dissolved in ethanol: cholesterol (Wako; 150 mg/ml), 7-dehydrocholesterol (Sigma; 150 mg/ml), 5β-ketodiol (kindly gifted from Yoshinori Fujimoto, Tokyo Institute of Technology; 150 mg/ml) and 20-hydroxyecdysone (Sigma; 50 mg/ml). We crossed ouib 29 /TM3 Ser 1 GMR2 Act-GFP flies with ouib 74 /TM3 Ser 1 GMR2 Act-GFP flies. Eggs were laid on grape plates with yeast pastes at 25°C for 12 hours. At 36 hours AEL, 50 hatched GFP negative (ouib 29 /ouib 74) first instar larvae were transferred to the yeast paste on grape plates and kept at 25°C. Every 24 hours, developmental stages were scored by tracheal morphology as previously described [22].
Genetic rescue experiments with ouib and spo
For the rescue experiments of ouib mutant by ouib overexpression, ouib 29 phm-GAL4#22/TM3 Act-GFP was established by chromosomal recombination. The flies of UAS-FLAG-ouib-1M; ouib 74 /TM6 Ubi-GFP were crossed with the flies of ouib 29 phm-GAL4#22/TM3 Act-GFP, the flies of ouib 74 /TM3 Act-GFP were crossed with the flies of ouib 29 phm-GAL4#22/TM3 Act-GFP, and the flies of UAS-FLAG-ouib-1M; ouib 74 /TM6 Ubi-GFP were crossed with the flies of ouib 29 /TM3 Act-GFP. Eggs were laid on grape plates with yeast pastes at 25°C for 12 hours. At 36 hours AEL, 50 hatched GFP negative (UAS-FLAG-ouib-1M/+; ouib 29 phm-GAL4#22/ouib 74, ouib 29 phm-GAL4#22/ouib 74 and UAS-FLAG-ouib-1M/+; ouib 29 /ouib 74) first instar larvae were transferred to the standard agar-cornmeal medium. Developmental stages were scored 108 hours AEL by tracheal morphology as previously described [22].
For the rescue experiments of spok RNAi by spo overexpression, UAS-spok-IR UAS-spo was established by chromosomal recombination on third chromosome. The flies of UAS-spok-IR UAS-spo strain was crossed with w; UAS-dicer2; phm-GAL4#22/TM6 Ubi-GFP flies. Eggs were laid on standard agar-cornmeal medium at 25°C for 24 hours. After 7 days, developmental stages of the animals on the wall were scored by presence of TM6 balancer.
For the rescue experiments of ouib mutant by spo overexpression, Roi/CyO; ouib 29 phm-GAL4#22/TM6, Roi/CyO; ouib 29 UAS-spo/TM6 and Roi/CyO; ouib 74 UAS-spo/TM6 were established by chromosomal recombination on third chromosome. The flies of Roi/CyO; ouib 29 phm-GAL4#22/TM6 were crossed with Roi/CyO; ouib 74 UAS-spo/TM6, the flies of Roi/CyO; ouib 29 phm-GAL4#22/TM6 were crossed with Roi/CyO; ouib 74 /TM6 and Roi/CyO; ouib 74 /TM6 were crossed with Roi/CyO; ouib 29 UAS-spo/TM6. Eggs were laid on standard agar-cornmeal medium at 25°C for 24 hours. After 7 days, developmental stages of the animals on the wall were scored by presence of TM6 balancer.
Ecdysteroid measurement
ouib 29 /TM3 Ser 1 GMR2 Act-GFP flies and w 1118 flies were crossed with ouib 74 /TM3 Ser 1 GMR2 Act-GFP flies. Eggs were laid on grape plates with yeast pastes at 25°C and the hatched larvae were cleared. After 8 hours, GFP negative (ouib 74 /+ and ouib 29 /ouib 74) first instar larvae were transferred into vials with standard cornmeal food. At 12 hours AH, whole larvae were rinsed in water and homogenized in 50 μl methanol and supernatant was collected following centrifugation at 14,000 rpm at 4°C. The remaining tissue was re-extracted in 50 μl methanol over night at 4°C. The supernatants were evaporated using a EYELA CVE-2000 (Tokyo Rikakikai) and redissolved in 50 μl EIA buffer [0.1 M PBS/0.1% BSA, 0.4 M NaCl, 1 mM EDTA and 0.01% NaN3]. ELISA was performed according to manufacturer’s instructions using 20-Hydroxyecdysone EIA Antiserum, 20-Hydroxyecdysone AChE Tracer and Ellman’s Reagent (Cayman Chemical) that detects 20-hydroxyecdysone with the same affinity. Standard curves were generated using 20E (Sigma). Absorbance was measured at 415 nm on a plate reader, Multiskan GO (Thermo Scientific) using the SkanIt Software 3.2 (Thermo Scientific).
GFP reporter assay
To generate the spok>GFP reporter construct, a ~1.4 kb fragment immediately upstream of the spok transcription unit was amplified from yw genomic DNA using the primers 1.45spok-p_F and 1.45spok-p_R (S3 Table). This fragment was first subcloned into the pCR2.1-TOPO vector (Life Technologies) and then removed as an EcoRI fragment and cloned into the Drosophila transformation vector pH-Stinger [71]. To refine the location of the PG enhancer, seven 250–300 bp overlapping fragments that covered the entire 1.4 kb fragment were derived through PCR and each cloned into hH- Stinger. The only fragment that gave expression in the PG of transgenic animals was the ~300 bp fragment immediately upstream of the transcriptional start site. This fragment was generated using the primers 300spok-p_F and 300spok-p_R (S3 Table). Transgenic lines were generated through standard means using a w 1118 host background. The GFP reporter strains of spok>GFP; ouib 29 /TM6 Ubi-GFP and spok>GFP; ouib 74 /TM6 Ubi-GFP were established and crossed each other. Eggs were laid on grape plates with yeast pastes at 25°C for 4 hours. The first instar larvae were dissected 36 hours AEL and immunostained.
Construction of luciferase reporter plasmids
The upstream regions of spok were amplified from Oregon R genomic DNA by specific primers to add SacI and BglII sites to the 5´ and 3´ ends, respectively. PCR was performed using KOD Plus Neo (TOYOBO). The amplified upstream regions of spok were digested with SacI and BglII, and then ligated into a pGL3-Basic vector luciferase reporter plasmid (Promega). Reporter plasmids carrying mutated regions were constructed from the pGL3-Basic plasmid containing WT upstream 300 bp region by inverse PCR. The primers for PCR are listed in S3 Table.
Transfection and luciferase reporter assays
S2 cells were seeded in 1 ml Schneider’s Drosophila Medium (GIBCO) in a 24-well plate (greiner bio-one) 1 day before transfection. Transfection of S2 cells was performed using the Effectene Transfection Reagent (Qiagen). GFP-pUAST [23] and FLAG-ouib-pWALIUM10-moe plasmids were transfected, respectively, along with the Actin5C-GAL4 construct (a gift from Yasushi Hiromi, National Institute of Genetics) and the luciferase reporter plasmids. The Copia Renilla Control plasmid (addgene; #38093) [72] was used as the reference. The cells were incubated for 2 days after transfection. Then they were processed by using the Dual-Luciferase Reporter Assay System (Promega) in accordance with the manufacturer’s instructions and were analyzed with Flash’n glow LB 955 (Berthold Technologies).
Preparation of S2 cell nuclear extracts
S2 cells overexpressing FLAG-ouib or FLAG-M1BP were collected and washed with TBS. Cells were then centrifuged at 4000 g at 4°C for 5 min. The pellet was suspended and vortexed with 400 μl Buffer A [10 mM Hepes pH 7.9, 10 mM KCl, 1 mM DTT and 1 unit Complete Mini (Roche)] and 25 μl 10% NP-40. Then sample was centrifuged at 1500 g at 4°C for 5 min. The pellet was suspended and vortexed with 50 μl Buffer C [20 mM Hepes pH7.9, 400 mM NaCl, 2 mM MgSO4, 1mM DTT and Complete Mini (Roche)], then shaked at 4°C for 30 min. After shaking, sample was centrifuged at 14,000 rpm at 4°C for 5 min and supernatant was collected.
Western blotting
Samples were boiled with SDS sample buffer [150 mM Tris-HCl pH 6.8, 0.6% SDS, 15% glycerol, 0.009 mg/μl Bromophenol blue, 5% 2-mercaptoethanol and 1 unit Complete Mini (Roche)] for 5 min, and loaded on 12% polyacrylamide gel followed by transfer onto PVDF membrane (GE Healthcare). Anti-FLAG M5 monoclonal antibody (1:1,000; Sigma) was used for primary antibody and ECL Peroxidase labeled anti-mouse antibody (1:10,000; GE Healthcare) was used for secondary antibody. The band was detected by ECL Ultra Lumigen TMA-6 (GE Healthcare) and Ez-Capture MG (ATTO).
Avidin-Biotin-Conjugated DNA-Binding (ABCD) assay
Preparation of S2 cell nuclear extracts is described in the Supplemental Materials. ABCD assay was conducted essentially as previously described [73]. Biotin-labeled DNA probes were purchased from Life Technologies. The probes were incubated with Dynabeads M-280 Streptavidin (Life Technologies) at room temperature for 15 min. DNA-beads complexes were mixed with nuclear extracts and ABCD Binding Buffer [50 mM Hepes pH 7.9, 150 mM NaCl, 0.5% Triton X-100, 20 ng/μl poly(dI/dC)], and incubated at 4°C for 1 hour. After incubation, the beads were washed with ABCD Binding Buffer. The biotin-labeled oligonucleotides are listed in S3 Table.
Preparation of recombinant proteins in E coli
GST proteins fused with or without 150–313 amino acid residues of Ouib (GST-Ouib-Zf) containing 5 zinc finger domains were expressed using pGEX-4T-3 vector system (GE Healthcare) in Escherichia coli BL-21 strain. E. coli cells were harvested and crashed with sonication. GST alone and GST-Ouib-Zf were purified from the supernatant with AKTA start equipped with GSTrap affinity column (GE Healthcare).
Electrophoretic mobility shift assay (EMSA)
Electrophoretic mobility shift assay was conducted as previously described [74,75]. 45 bp double-stranded oligonucleotide probes containing wild type M1BP binding site, wild type and mutated (transversion) Ouib response element were prepared by annealing single-strand oligonucleotides listed in S3 Table. The wild type M1BP binding site was derived from the smoothened promoter [51]. Double-stranded DNA fragment was end-labeled by using T4 polynucleotide kinase (TOYOBO) and [γ-32P]ATP. GST or GST-Ouib fusion proteins (400 ng) were incubated for 30 min at 4°C in the reaction mixture [12 mM Hepes, pH 7.9, 1 mM dithiothreitol, 1 mM EDTA, 60 mM KCl, 4 mM MgCl2, 2 mM ZnSO4, 50 ng/ul poly(dI-dC), 1 mg/ml BSA and 12% Glycerol] in the presence or absence of 100–200-fold molar excess of specific double-stranded competitor DNA. A radiolabeled DNA probe (0.3 ng, 40,000 cpm) was added, and the incubation was continued for 20 min at 4°C. The incubation mixture was directly loaded on a 5% non-denaturing polyacrylamide gel in 1 × TBE buffer [89 mM Tris-HCl, pH 8.0, 89 mM boric acid, and 2 mM EDTA], and electrophoresed at 4°C with buffer circulation. The gels were dried and analyzed with a bio-imaging analyzer Typhoon 8600 (Amersham Pharmacia Biotech Inc). The competitor oligonucleotides used are listed in the S3 Table.
Supporting Information
The orthologs of ouija board in 12 Drosophilidae species are described in the FlyBase website (http://flybase.org/reports/FBgn0209782.html). We confirmed by the BLAST search that the amino acid sequence of any of these putative proteins as a query is most similar to that of D. melanogaster CG11762.
(PDF)
The number of spok RNAi animals that grew up to the 3rd instar larval stage or later stage was scored. Detailed genetic crosses for this experiment are described in Materials and Methods. The animals were fed standard cornmeal food without any steroidal supplements. Values in parentheses indicate the number of control non-RNAi progeny from the parental strains in the same experimental batches.
(PDF)
Small letters indicate the restriction enzyme recognition sequences. Under lines indicate the transversion mutation sequences. Asterisks indicate 5´ biotinylation.
(PDF)
Dorsal views are shown. (A) Signals with antisense probe. (B) Signals with sense probe. Arrows indicate positions of the PG primordia. Scale bar: 100 μm.
(PDF)
The data are derived from the FlyBase GBrowse website (http://flybase.org/cgi-bin/gbrowse2/dmel/?Search=1;name=FBgn0037618). Numbers indicate the nucleotide positions at the 85A9 cytological position of the chromosome 3R scaffold. Boxed arrows represent gene spans and their directions. The 5 ZAD-ZNF genes are colored by magenta.
(PDF)
ouib expression and ecdysteroid levels in w 1118 during the 3rd instar stage measured by qRT-PCR (N = 3) and ELISA (N = 4). The blue line indicates the relative expression level of ouib, normalized to the level of 0–6 hours after L2-L3 molting (0–6 hr A3L). Error bars indicate the s. e. m.
(PDF)
(A, B) Phase-contrast (left) and fluorescence (right) images of the 108 hours AEL 3rd instar larval brain-ring gland complexes with spok>GFP construct. The PG cells were immunostained with anti-Sro antibody (magenta). The spok>GFP constructs contain 1.45 kbp (A) and 300 bp (B) enhancer regiosn of spok, respectively. Scale bar: 100 μm.
(PDF)
Numbers indicate the distance from the translation initiation site (+1) of spok, and white box represents the coding region of spok. Luc reporter activities of progressive deletion constructs are shown in right. Bars and error bars represent the average and the s. e. m., respectively, of three independent experiments. **; P<0.01 by Student’s t-test.
(PDF)
The introduced transversion mutations in the 1’, 2’, 3’, 4’ and 5’ constructs are shown in the top. The GFP expression plasmid was used as a negative control. Bars and error bars represent the average and the s. e. m., respectively, of three independent experiments. **; P<0.01 by Student’s t-test.
(PDF)
EMBOSS Matcher [54] was used to search for sequences similar to the D. melanogaster Ouib response element (15 bp) within the 1 kb regions upstream of the translation initiation site of the spok loci from 12 Drosophilidae species. Numbers before and after nucleotide sequences indicate the distance from the translation initiation site of spok. Parentheses indicate numbers of identical matches to D. melanogaster Ouib response element. “S” and “D” indicate the subgenera Sophophora and Drosophila, respectively. spok genes are DG27210 (D. simulans #1), GD27133 (D. simulans #2), GD28291 (D. simulans #3), GM22791 (D. sechellia), GG16659 (D. erecta), GE19452 (D. yakuba), GF20000 (D. ananassae), GA31537 (D. pseudoobscura), GL21970 (D. persimilis), GK19177 (D. willistoni), GI23968 (D. mojavensis), GH21174 (D. grimshawi) and GJ26360 (D. virilis). Note that a BLAST search using D. melanogaster Spok protein sequence as a query hit 3 D. simulans spok candidate genes. Also note that the BLAST search hit only one spo/spok family gene in D. grimshawi genome and thus it is not faithfully judged if GH21174 is orthologous to spo or spok.
(PDF)
Amounts of ouib mRNAs were measured by qRT-PCR. phm>+ and phm>torso-IR indicate w 1118 ; +/+; phm-GAL4#22/+ and w 1118 ; UAS-torso-IR/+; phm-GAL4#22/+, respectively. RNA samples were collected 140 hours after egg laying. Bars and error bars represent the average and the s. e. m., respectively, of three biological replicates. n.s. means P>0.05 by Student’s t-test.
(PDF)
EMBOSS Matcher [54] was used to search for sequences similar to D. melanogaster Ouib response element (15 bp) within the putative enhancer/promoter regions of D. melanogaster ecdysteroidogenic enzyme genes. Numbers before and after nucleotide sequences indicate the distance from the translation initiation site of each gene. Parentheses indicate numbers of identical matches to D. melanogaster Ouib response element. Except for phm, a enhancer/promoter region was defined as a genomic region between the translation initiation site of each ecdysteroidogenic enzyme gene and the 3´ end of a gene next to the enzymatic gene. A phm enhancer/promoter is a -500 to -1 region as previously characterized [31].
(PDF)
Amounts of spok and ouib mRNAs were measured by qRT-PCR. phm>dicer2 and phm>dicer2+mld-IR indicate w 1118 ; UAS-dicer2/+; phm-GAL4#22/+ and w 1118 ; UAS-dicer2/UAS-mld-IR; phm-GAL4#22/+, respectively. RNA samples were collected 36 hours after egg laying. Bars and error bars represent the average and the s. e. m., respectively, of three biological replicates. ** and n.s. mean P<0.01 and P>0.05 by Student’s t-test, respectively.
(PDF)
Acknowledgments
We thank Yosuke Umei for his technical assistance; Tomoki Chiba and Fuminori Tsuruta for use of their luminometer; Yoshinori Fujimoto, Hiroshi Kataoka, Yasushi Hiromi, Shu Kondo, Hajime Ono, the Drosophila Genetic Resource Center in Kyoto, the National Institute of Genetics, the Vienna Drosophila RNAi Center and the Developmental Studies Hybridoma Bank for stocks and reagents; and Aya Fukuda, Barry M. Honda, Marek Jindra, Takao Naganuma, Kazushige Nagaosa, Koji Nakamura, Yoshinobu Nakanishi, Kim F. Rewitz, and Yuichi Shima for helpful discussion and critical reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Precursory Research for Embryonic Science and Technology, Japan Science and Technology Agency http://www.jst.go.jp/kisoken/presto/en/index.html; The Naito Foundation; https://www.naito-f.or.jp/en/ and by the National Institutes of Health, USA; grant number R01 GM093301; http://grants.nih.gov/grants/funding/r01.htm. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med. 2009;234: 880–907. [DOI] [PubMed] [Google Scholar]
- 2. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18: 361–377. [DOI] [PubMed] [Google Scholar]
- 3. Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57: 19–36. [DOI] [PubMed] [Google Scholar]
- 4. Fayard E, Auwerx J, Schoonjans K. LRH-1: An orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14: 250–260. [DOI] [PubMed] [Google Scholar]
- 5. Morohashi K, Baba T, Tanaka M. Steroid hormones and the development of reproductive organs. Sex Dev. 2013;7: 61–79. 10.1159/000342272 [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto K, Yazawa T, Mizutani T, Imamichi Y, Kawabe S, Kanno M, et al. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol Cell Endocrinol. 2011;336: 123–126. 10.1016/j.mce.2010.11.031 [DOI] [PubMed] [Google Scholar]
- 7. Sonoyama T, Sone M, Honda K, Taura D, Kojima K, Inuzuka M, et al. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153: 4336–4345. 10.1210/en.2012-1060 [DOI] [PubMed] [Google Scholar]
- 8. Zubair M, Oka S, Parker K, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23: 1657–1667. 10.1210/me.2009-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thummel CS. Molecular Mechanisms of Developmental Timing in C. elegans and Drosophila . Dev Cell. 2001;1: 453–465. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47: 883–916. [DOI] [PubMed] [Google Scholar]
- 11. Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol. 2013;58: 497–516. 10.1146/annurev-ento-120811-153608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niwa YS, Niwa R. Neural control of steroid hormone biosynthesis during development in the fruit fly Drosophila melanogaster . Genes Genet Syst. 2014;89: 27–34. [DOI] [PubMed] [Google Scholar]
- 13. Niwa R, Niwa YS. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci Biotechnol Biochem. 2014;78: 1283–1292. 10.1080/09168451.2014.942250 [DOI] [PubMed] [Google Scholar]
- 14. Petryk A, Warren JT, Marqués G, Jarcho MP, Gilbert LI, Kahler J, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc Natl Acad Sci U S A. 2003;100: 13773–13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Enya S, Ameku T, Igarashi F, Iga M, Kataoka H, Shinoda T, et al. A Halloween gene noppera-bo encodes a glutathione S-transferase essential for ecdysteroid biosynthesis via regulating the behaviour of cholesterol in Drosophila . Sci Rep. 2014;4: 6586 10.1038/srep06586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chanut-delalande H, Hashimoto Y, Pelissier-monier A, Spokony R, Dib A, Kondo T, et al. Pri peptides are mediators of ecdysone for the temporal control of development. Nat Cell Biol. 2014;16: 1035–1044. 10.1038/ncb3052 [DOI] [PubMed] [Google Scholar]
- 17. Enya S, Daimon T, Igarashi F, Kataoka H, Uchibori M, Sezutsu H, et al. The silkworm glutathione S-transferase gene noppera-bo is required for ecdysteroid biosynthesis and larval development. Insect Biochem Mol Biol. 2015;61: 1–7. 10.1016/j.ibmb.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 18. Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development. 2006;133: 2565–2574. [DOI] [PubMed] [Google Scholar]
- 19. Yoshiyama-Yanagawa T, Enya S, Shimada-Niwa Y, Yaguchi S, Haramoto Y, Matsuya T, et al. The conserved rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J Biol Chem. 2011;286: 25756–25762. 10.1074/jbc.M111.244384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Namiki T, Niwa R, Sakudoh T, Shirai K-I, Takeuchi H, Kataoka H. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem Biophys Res Commun. 2005;337: 367–374. [DOI] [PubMed] [Google Scholar]
- 21. Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol. 2006;298: 555–570. [DOI] [PubMed] [Google Scholar]
- 22. Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, Kawaoka S, et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the “Black Box” of the ecdysteroid biosynthesis pathway. Development. 2010;137: 1991–1999. 10.1242/dev.045641 [DOI] [PubMed] [Google Scholar]
- 23. Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, et al. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila . J Biol Chem. 2004;279: 35942–35949. [DOI] [PubMed] [Google Scholar]
- 24. Warren JT, Petryk A, Marqués G, Parvy J-P, Shinoda T, Itoyama K, et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: a P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34: 991–1010. [DOI] [PubMed] [Google Scholar]
- 25. Chávez VM, Marqués G, Delbecque JP, Kobayashi K, Hollingsworth M, Burr J, et al. The Drosophila disembodied gene controls late embryonic morphogenesis and codes for a cytochrome P450 enzyme that regulates embryonic ecdysone levels. Development. 2000;127: 4115–4126. [DOI] [PubMed] [Google Scholar]
- 26. Warren JT, Petryk A, Marque G, Jarcho M, Parvy J-P, Dauphin-villemant C, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster . Proc Natl Acad Sci U S A. 2002;99: 11043–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans. 2006;34: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 28. Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila . Dev Cell. 2002;3: 209–220. [DOI] [PubMed] [Google Scholar]
- 29. Ou Q, Magico A, King-Jones K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 2011;9: e1001160 10.1371/journal.pbio.1001160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parvy J-P, Blais C, Bernard F, Warren JT, Petryk A, Gilbert LI, et al. A role for βFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster . Dev Biol. 2005;282: 84–94. [DOI] [PubMed] [Google Scholar]
- 31. Moeller ME, Danielsen ET, Herder R, O’Connor MB, Rewitz KF. Dynamic feedback circuits function as a switch for shaping a maturation-inducing steroid pulse in Drosophila . Development. 2013;140: 4730–4739. 10.1242/dev.099739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parvy J-P, Wang P, Garrido D, Maria A, Blais C, Poidevin M, et al. Forward and feedback regulation of cyclic steroid production in Drosophila melanogaster . Development. 2014;141: 3955–3965. 10.1242/dev.102020 [DOI] [PubMed] [Google Scholar]
- 33. Xiang Y, Liu Z, Huang X. Br regulates the expression of the ecdysone biosynthesis gene npc1 . Dev Biol. 2010;344: 800–808. 10.1016/j.ydbio.2010.05.510 [DOI] [PubMed] [Google Scholar]
- 34. Talamillo A, Herboso L, Pirone L, Pérez C, González M, Sánchez J, et al. Scavenger receptors mediate the role of SUMO and Ftz-f1 in Drosophila steroidogenesis. PLoS Genet. 2013;9: e1003473 10.1371/journal.pgen.1003473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koyama T, Rodrigues MA, Athanasiadis A, Shingleton AW, Mirth CK. Nutritional control of body size through FoxO-Ultraspiracle mediated ecdysone biosynthesis. Elife. 2014;3: e03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wismar J, Habtemichael N, Warren JT, Dai JD, Gilbert LI, Gateff E. The mutation without children rgl causes ecdysteroid deficiency in third-instar larvae of Drosophila melanogaster . Dev Biol. 2000;226: 1–17. [DOI] [PubMed] [Google Scholar]
- 37. Neubueser D, Warren JT, Gilbert LI, Cohen SM. molting defective is required for ecdysone biosynthesis. Dev Biol. 2005;280: 362–372. [DOI] [PubMed] [Google Scholar]
- 38. Deng H, Kerppola TK. Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet. 2013;9: e1003263 10.1371/journal.pgen.1003263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Danielsen ET, Moeller ME, Dorry E, Komura-Kawa T, Fujimoto Y, Troelsen JT, et al. Transcriptional control of steroid biosynthesis genes in the Drosophila prothoracic gland by Ventral veins lacking and Knirps. PLoS Genet. 2014;10: e1004343 10.1371/journal.pgen.1004343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng C, Ko A, Chaieb L, Koyama T, Sarwar P, Mirth CK, et al. The POU Factor Ventral veins lacking/Drifter directs the timing of metamorphosis through ecdysteroid and juvenile hormone signaling. PLoS Genet. 2014;10: e1004425 10.1371/journal.pgen.1004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puig O, Mattila J. Understanding Forkhead box class O function: lessons from Drosophila melanogaster . Antioxid Redox Signal. 2011;14: 635–647. 10.1089/ars.2010.3407 [DOI] [PubMed] [Google Scholar]
- 42. Misra JR, Horner MA, Lam G, Thummel CS. Transcriptional regulation of xenobiotic detoxification in Drosophila . Genes Dev. 2011;25: 1796–1806. 10.1101/gad.17280911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung S, Chavez C, Andrew DJ. Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis. Dev Biol. 2011;360: 160–172. 10.1016/j.ydbio.2011.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sánchez-Higueras C, Sotillos S, Castelli-Gair Hombría J. Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr Biol. 2014;24: 76–81. 10.1016/j.cub.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 45.Fisher B, Weiszmann R, Frise E, Hammonds A, Tomancak P, Beaton A, et al. BDGP insitu homepage. 2012;http://insitu.fruitfly.org/cgi-bin/ex/insitu.pl
- 46. Chung HR, Schäfer U, Jäckle H, Böhm S. Genomic expansion and clustering of ZAD-containing C2H2 zinc-finger genes in Drosophila . EMBO Rep. 2002;3: 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chung H-R, Löhr U, Jäckle H. Lineage-specific expansion of the zinc finger associated domain ZAD. Mol Biol Evol. 2007;24: 1934–1943. [DOI] [PubMed] [Google Scholar]
- 48. Jauch R, Bourenkov GP, Chung HR, Urlaub H, Reidt U, Jäckle H, et al. The zinc finger-associated domain of the Drosophila transcription factor Grauzone is a novel zinc-coordinating protein-protein interaction module. Structure. 2003;11: 1393–1402. [DOI] [PubMed] [Google Scholar]
- 49. Payre F, Vincent A. Genomic targets of the serendipity β and δ zinc finger proteins and their respective DNA recognition sites. EMBO J. 1991;10: 2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krystel J, Ayyanathan K. Global analysis of target genes of 21 members of the ZAD transcription factor family in Drosophila melanogaster . Gene. 2013;512: 373–382. 10.1016/j.gene.2012.09.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Gilmour DS. Distinct mechanisms of transcriptional pausing orchestrated by GAGA factor and M1BP, a novel transcription factor. EMBO J. 2013;32: 1829–1841. 10.1038/emboj.2013.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450: 203–218. [DOI] [PubMed] [Google Scholar]
- 53. Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila . Genetics. 2013;195: 715–721. 10.1534/genetics.113.156737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McBrayer Z, Ono H, Shimell M, Parvy J-P, Beckstead RB, Warren JT, et al. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila . Dev Cell. 2007;13: 857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rewitz KF, Yamanaka N, Gilbert LI, Connor MBO. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science. 2009;326: 1403–1406. 10.1126/science.1176450 [DOI] [PubMed] [Google Scholar]
- 57. Rewitz KF, O’Connor MB, Gilbert LI. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem Mol Biol. 2007;37: 741–753. [DOI] [PubMed] [Google Scholar]
- 58. Rewitz KF, Gilbert LI. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol. 2008;8: 60 10.1186/1471-2148-8-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sztal T, Chung H, Gramzow L, Daborn PJ, Batterham P, Robin C. Two independent duplications forming the Cyp307a genes in Drosophila . Insect Biochem Mol Biol. 2007;37: 1044–1053. [DOI] [PubMed] [Google Scholar]
- 60. Lang M, Murat S, Clark AG, Gouppil G, Blais C, Matzkin LM, et al. Mutations in the neverland gene turned Drosophila pachea into an obligate specialist species. Science. 2012;337: 1658–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development. 2011;138: 2693–2703. 10.1242/dev.063412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shimada-Niwa Y, Niwa R. Serotonergic neurons respond to nutrients and regulate the timing of steroid hormone biosynthesis in Drosophila . Nat Commun. 2014;5: 5778 10.1038/ncomms6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ohhara Y, Shimada-Niwa Y, Niwa R, Kayashima, Hayashi Y, Akagi K, et al. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc Natl Acad Sci U S A. 2015;112: 1452–1457. 10.1073/pnas.1414966112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rewitz KF, Larsen MR, Lobner-Olesen A, Rybczynski R, O’Connor MB, Gilbert LI. A phosphoproteomics approach to elucidate neuropeptide signal transduction controlling insect metamorphosis. Insect Biochem Mol Biol. 2009;39: 475–483. 10.1016/j.ibmb.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 65. Claudius A-K, Romani P, Lamkemeyer T, Jindra M, Uhlirova M. Unexpected role of the steroid-deficiency protein ecdysoneless in pre-mRNA splicing. PLoS Genet. 2014;10: e1004287 10.1371/journal.pgen.1004287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wong JCY, Gokgoz N, Alon N, Andrulis IL, Buchwald M. Cloning and mutation analysis of ZFP276 as a candidate tumor suppressor in breast cancer. J Hum Genet. 2003;48: 668–671. [DOI] [PubMed] [Google Scholar]
- 67. Ryder E, Blows F, Ashburner M, Bautista-Llacer R, Coulson D, Drummond J, et al. The DrosDel collection: A set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster . Genetics. 2004;167: 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lehmann R, Tautz D. Chapter 30 In situ hybridization to RNA. Methods Cell Biol. 1994;44: 575–598. [DOI] [PubMed] [Google Scholar]
- 69. Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;415: 401–415. [DOI] [PubMed] [Google Scholar]
- 70. Ni J-Q, Zhou R, Czech B, Liu L-P, Holderbaum L, Yang-Zhou D, et al. A genome-scale shRNA resource for transgenic RNAi in Drosophila . Nat Methods. 2011;8: 405–407. 10.1038/nmeth.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barolo S, Carver LA, Posakony JW. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila . Biotechniques. 2000;29: 726–732. [DOI] [PubMed] [Google Scholar]
- 72. Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, et al. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299: 2039–2045. [DOI] [PubMed] [Google Scholar]
- 73. Matsuzaki H, Daitoku H, Hatta M, Aoyama H, Yoshimochi K, Fukamizu A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc Natl Acad Sci U S A. 2005;102: 11278–11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yanai K, Hirota K, Taniguchi-Yanai K, Shigematsu Y, Shimamoto Y, Saito T, et al. Regulated expression of human angiotensinogen gene by hepatocyte nuclear factor 4 and chicken ovalbumin upstream promoter-transcription factor. J Biol Chem. 1999;274: 34605–34612. [DOI] [PubMed] [Google Scholar]
- 75. Shimotsuma M, Okamura E, Matsuzaki H, Fukamizu A, Tanimoto K. DNase I hypersensitivity and ε-globin transcriptional enhancement are separable in locus control region (LCR) HS1 mutant human β-globin YAC transgenic mice. J Biol Chem. 2010;285: 14495–14503. 10.1074/jbc.M110.116525 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The orthologs of ouija board in 12 Drosophilidae species are described in the FlyBase website (http://flybase.org/reports/FBgn0209782.html). We confirmed by the BLAST search that the amino acid sequence of any of these putative proteins as a query is most similar to that of D. melanogaster CG11762.
(PDF)
The number of spok RNAi animals that grew up to the 3rd instar larval stage or later stage was scored. Detailed genetic crosses for this experiment are described in Materials and Methods. The animals were fed standard cornmeal food without any steroidal supplements. Values in parentheses indicate the number of control non-RNAi progeny from the parental strains in the same experimental batches.
(PDF)
Small letters indicate the restriction enzyme recognition sequences. Under lines indicate the transversion mutation sequences. Asterisks indicate 5´ biotinylation.
(PDF)
Dorsal views are shown. (A) Signals with antisense probe. (B) Signals with sense probe. Arrows indicate positions of the PG primordia. Scale bar: 100 μm.
(PDF)
The data are derived from the FlyBase GBrowse website (http://flybase.org/cgi-bin/gbrowse2/dmel/?Search=1;name=FBgn0037618). Numbers indicate the nucleotide positions at the 85A9 cytological position of the chromosome 3R scaffold. Boxed arrows represent gene spans and their directions. The 5 ZAD-ZNF genes are colored by magenta.
(PDF)
ouib expression and ecdysteroid levels in w 1118 during the 3rd instar stage measured by qRT-PCR (N = 3) and ELISA (N = 4). The blue line indicates the relative expression level of ouib, normalized to the level of 0–6 hours after L2-L3 molting (0–6 hr A3L). Error bars indicate the s. e. m.
(PDF)
(A, B) Phase-contrast (left) and fluorescence (right) images of the 108 hours AEL 3rd instar larval brain-ring gland complexes with spok>GFP construct. The PG cells were immunostained with anti-Sro antibody (magenta). The spok>GFP constructs contain 1.45 kbp (A) and 300 bp (B) enhancer regiosn of spok, respectively. Scale bar: 100 μm.
(PDF)
Numbers indicate the distance from the translation initiation site (+1) of spok, and white box represents the coding region of spok. Luc reporter activities of progressive deletion constructs are shown in right. Bars and error bars represent the average and the s. e. m., respectively, of three independent experiments. **; P<0.01 by Student’s t-test.
(PDF)
The introduced transversion mutations in the 1’, 2’, 3’, 4’ and 5’ constructs are shown in the top. The GFP expression plasmid was used as a negative control. Bars and error bars represent the average and the s. e. m., respectively, of three independent experiments. **; P<0.01 by Student’s t-test.
(PDF)
EMBOSS Matcher [54] was used to search for sequences similar to the D. melanogaster Ouib response element (15 bp) within the 1 kb regions upstream of the translation initiation site of the spok loci from 12 Drosophilidae species. Numbers before and after nucleotide sequences indicate the distance from the translation initiation site of spok. Parentheses indicate numbers of identical matches to D. melanogaster Ouib response element. “S” and “D” indicate the subgenera Sophophora and Drosophila, respectively. spok genes are DG27210 (D. simulans #1), GD27133 (D. simulans #2), GD28291 (D. simulans #3), GM22791 (D. sechellia), GG16659 (D. erecta), GE19452 (D. yakuba), GF20000 (D. ananassae), GA31537 (D. pseudoobscura), GL21970 (D. persimilis), GK19177 (D. willistoni), GI23968 (D. mojavensis), GH21174 (D. grimshawi) and GJ26360 (D. virilis). Note that a BLAST search using D. melanogaster Spok protein sequence as a query hit 3 D. simulans spok candidate genes. Also note that the BLAST search hit only one spo/spok family gene in D. grimshawi genome and thus it is not faithfully judged if GH21174 is orthologous to spo or spok.
(PDF)
Amounts of ouib mRNAs were measured by qRT-PCR. phm>+ and phm>torso-IR indicate w 1118 ; +/+; phm-GAL4#22/+ and w 1118 ; UAS-torso-IR/+; phm-GAL4#22/+, respectively. RNA samples were collected 140 hours after egg laying. Bars and error bars represent the average and the s. e. m., respectively, of three biological replicates. n.s. means P>0.05 by Student’s t-test.
(PDF)
EMBOSS Matcher [54] was used to search for sequences similar to D. melanogaster Ouib response element (15 bp) within the putative enhancer/promoter regions of D. melanogaster ecdysteroidogenic enzyme genes. Numbers before and after nucleotide sequences indicate the distance from the translation initiation site of each gene. Parentheses indicate numbers of identical matches to D. melanogaster Ouib response element. Except for phm, a enhancer/promoter region was defined as a genomic region between the translation initiation site of each ecdysteroidogenic enzyme gene and the 3´ end of a gene next to the enzymatic gene. A phm enhancer/promoter is a -500 to -1 region as previously characterized [31].
(PDF)
Amounts of spok and ouib mRNAs were measured by qRT-PCR. phm>dicer2 and phm>dicer2+mld-IR indicate w 1118 ; UAS-dicer2/+; phm-GAL4#22/+ and w 1118 ; UAS-dicer2/UAS-mld-IR; phm-GAL4#22/+, respectively. RNA samples were collected 36 hours after egg laying. Bars and error bars represent the average and the s. e. m., respectively, of three biological replicates. ** and n.s. mean P<0.01 and P>0.05 by Student’s t-test, respectively.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.