Abstract
Background
Intestinal parasitic infections are significant cause of morbidity and mortality in endemic countries. In Ethiopia, helminthiasis was the third leading cause of outpatient visits. Despite the health extension program was launched to address this problem, there is limited information on the burden of intestinal parasites after implementation of the program in our setting. Therefore, the aim of this study was to assess the intestinal helminthic infections among clients attending at Anbesame health center, South Gondar, Ethiopia.
Methods
A cross sectional study was conducted at Anbesame health center from March to June 2015. A structured questionnaire was used to collect data from 464 study participants selected consecutively. Stool specimen collection, processing through formol-ether concentration technique and microscopic examination for presence of parasites were carried out. Data were entered, cleaned and analyzed using SPSS Version 20.
Results
Among the total 464 study participants with median (±IQR) age of 25.0 (±21.75) years, 262 (56.5%) were females. Helminthic infection was found in 97 (20.9%) participants. Hookworm (68 [14.7%]) was the predominant parasite followed by S. mansoni (11 [2.4%]), E. vermicularis (9 [1.9%]) and S. stercoralis (5 [1.1%]). Patients with age group ≥15 years (AOR: 5.26; 95% CI: 2.05–13.46; P: 0.001) and walking barefoot (AOR: 2.20; 95% CI: 1.08–4.48; P: 0.031) were more vulnerable from the hookworm infections.
Conclusions
There was a high burden of hookworm infections in our setting. Hence, regular shoes wearing, considering all age groups in the albendazole deworming as mass treatment and environmental hygiene are important interventions to reduce the burden of such neglected tropical disease.
Introduction
Intestinal parasitic infections caused by intestinal helminths and protozoan are among the significant cause of morbidity and mortality in endemic countries. According to WHO estimate, 3.5 billion people were affected, and that 450 million were ill as a result of these infections [1, 2].
In sub-Saharan African countries where attributed largely to poor socioeconomic status, poor sanitation, inadequate medical care and absence of safe drinking water supplies; up to 250 million people are estimated to be infected with at least one or more species of intestinal nematodes [3]. In Ethiopia, helminthiasis was the third leading cause of outpatient visits [4]. Moreover, different studies showed higher prevalence of intestinal helminthiasis [5–7].
As a prevention strategy, Ethiopia launched the health extension program that promotes four areas of care: Disease prevention and control, family health, hygiene and environmental sanitation, and health education and communication [8]. This program is believed to reduce intestinal parasites burden if the environmental sanitation, and disease prevention and control is done as targeted. However, there is limited information on intestinal parasites burden after the program implementation. Therefore, the aim of this study was to assess the intestinal helminthic infections among clients attending at Anbesame health center, South Gondar, Ethiopia.
Methods
A cross sectional study was conducted from March to June 2015 at Anbesame health center, Dera district, South Gondar. It is located at 11° 43' 0'' North and 37° 38' 0'' East. The climate of the area is Woinadega with 2077 meters above sea level. The area has 1300mm3 mean annual rain fall and 26°C mean annual temperature. The main activities of the people in this area are agriculture [9]. The health center serves for 45820 populations in the catchment area. Under this health center, five rural and one urban health posts are assigned to implement the health extension program. Health Extension Workers (HEWs) spend 75% of their time visiting families in their homes and performing outreach activities in the community. To address strong community demands for basic curative care, HEWs are trained to provide first aid; treat malaria, dysentery, intestinal parasites, and other ailments; and to refer cases to the nearest health center when more complicated care is needed [10]. 400 mg albendazole deworming has been given to <5 years children and to all pregnant women at 2nd and 3rd trimesters in the five health posts. The deworming coverage was 85.9% in these health posts [11].
A total of 464 clients following their health outcome at Anbesame health center were selected consecutively as study subjects. Clients treated for intestinal helminthiasis within one month were excluded from this study. Two trained interviewers collected the data from clients using a structured questionnaire containing socio-demographic variables, and prevention and control measures on intestinal parasites.
The laboratory investigation was performed by three laboratory technologists who have more than 5 years of experience in stool examination at health facilities in West Amhara region. About 2 grams of fresh stool specimen was collected from each study participant and placed in a labeled clean plastic stool container. Quality of reagents and instruments were checked before starting the laboratory investigation. A portion of stool was examined by direct wet mount with saline to observe motile intestinal parasites. The remaining stool specimen was processed by formol-ether concentration technique and examined under light microscope at 100X and 400X magnifications for the presence ova of parasites [12].
The data were entered, cleaned and analyzed using SPSS version 20. Frequencies of parasite burden were calculated. Multiple logistic regression was used to check associations with parasite burden. Variables having P value < 0.05 was taken as significant association.
This study was ethically reviewed and approved by the Amhara Regional Health Bureau Ethical Review Committee. Official permission was obtained from the Anbesame health center. Written informed consent, which was approved by the ethics committee, was obtained from each study participate. For participants under 18 years old, we obtained the written informed consent from their parents or guardians. Results were kept confidential and study participants found positive for intestinal parasites were linked to the health center's clinician.
Results
Socio-demographic characteristics
From the total of 464 study participants enrolled in this study, 262 (56.5%) were females. The median (±IQR) age of participants was 25.0 (±21.75) years. More than half of the participants were illiterate (284 [61.2%]) and married (257 [55.4%]). One hundred forty one (30.4%), 137 (29.5%) and 109 (23.5%) were farmers, house wives and students in occupation, respectively (Table 1).
Table 1. Sociodemographic characteristics of study participants at Anbesame HC, 2015.
| Characteristics | Number | Percent | |
|---|---|---|---|
| Age (years) | <15 | 132 | 28.4 |
| 15–24 | 93 | 20.0 | |
| 25–49 | 184 | 39.7 | |
| 50+ | 55 | 11.9 | |
| Sex | Male | 202 | 43.5 |
| Female | 262 | 56.5 | |
| Marital status | Married | 257 | 55.4 |
| Single | 177 | 38.1 | |
| Divorced | 18 | 3.9 | |
| Widowed | 12 | 2.6 | |
| Educational status | College &above | 13 | 2.8 |
| High school | 32 | 6.9 | |
| Elementary | 135 | 29.1 | |
| Illiterate | 284 | 61.2 | |
| Occupation | Farmer | 141 | 30.4 |
| Student | 109 | 23.5 | |
| Gov employee | 13 | 2.8 | |
| NA (under aged) | 55 | 11.9 | |
| Merchant | 9 | 1.9 | |
| House wife | 137 | 29.5 | |
Intestinal parasites prevention and control practice
The participants got drinking water from river (289 [62.3%]), well (53 [11.4%]) and pipe (122 [26.3%]). Only 141 (30.4%) worn shoes regularly and 55 (11.9%) took 400 mg albendazole deworming. More than half (301 [64.9%]) of the participants had latrine. Of which, 242 (80.4%) did not use the latrine regularly. Most of the participants, 405 (87.3%), had open field defecation practice. Hand washing after toilet was not practiced in 374 (80.6%) clients.
Helminthic infections
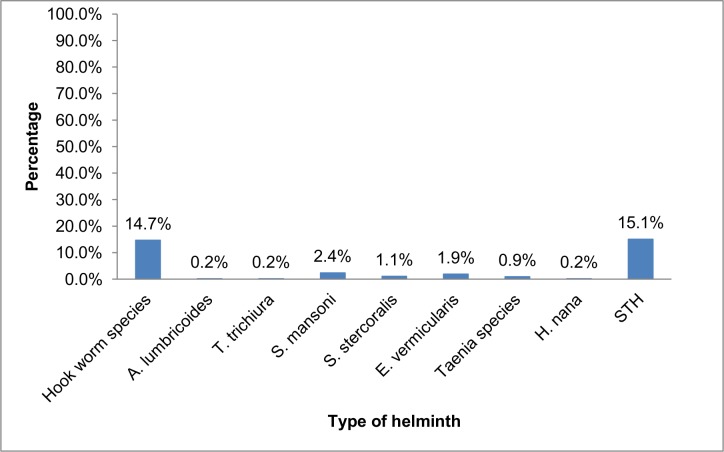
Of the total 464 stool specimens examined for intestinal parasites, the overall prevalence of helminthiasis was 20.9% (97/464). Soil transmitted helminths (hookworm, A. lumbricoides and T. trichiura) were found in 70 (15.1%) patients. Hookworm (68 [14.7%]) was the predominant parasite followed by S. mansoni (11 [2.4%]), E. vermicularis (9 [1.9%]) and S. stercoralis (5 [1.1%]) (Fig 1). Among the patients with hookworm infections, three patients were co-infected with S. mansoni, E. vermicularis and S. stercoralis.
Fig 1. Proportion of helminthic infections among clients attending Anbesame health center, South Gondar, 2015.
STH: Soil Transmitted Helminths
Determinant factors
In multivariate analysis, age group ≥15 years and walking barefoot were significantly associated with hookworm infections. Those clients with ≥15 years old had 5.26 (AOR: 5.26; 95% CI: 2.05–13.46; P: 0.001) times more to have hookworm infections (19.0%) compared to clients below 15 years (3.8%). The 17.6% of hookworm infections found among patients walking in barefoot was 2.21 (AOR: 2.21; 95% CI: 1.11–4.41; P: 0.024) times higher compared to those who worn shoes regularly (7.9%) (Table 2).
Table 2. Determinant factors for hookworm infection among clients attending at Anbesame health center, 2015.
| Variables | Hookworm infection | COR (95% CI) | AOR (95% CI) | P | ||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Ate unwashed fruits | No | 113 | 14 | 1 | ||
| Yes | 283 | 54 | 1.54 (0.82–2.88) | - | - | |
| Open field defecation | No | 55 | 4 | 1 | ||
| Yes | 341 | 64 | 2.58 (0.90–7.37) | - | - | |
| Walking barefoot | No | 129 | 11 | 1 | 1 | |
| Yes | 267 | 57 | 2.50 (1.27–4.94) | 2.21 (1.11–4.41) | 0.024 | |
| Took deworming/year | No | 344 | 65 | 3.28 (0.99–10.80) | 2.80 (0.83–9.40) | 0.096 |
| Yes | 52 | 3 | 1 | 1 | ||
| Age in year | <15 | 127 | 5 | 1 | 1 | |
| ≥15 | 269 | 63 | 5.95 (2.34–15.15) | 5.26 (2.05–13.46) | 0.001 | |
AOR: Adjusted Odds Ratio; CI: Confidence Interval; P: P value
Discussion
Neglected disease including the three soil-transmitted helminths (ascariasis, hookworm infection, and trichuriasis) have been given relatively little attention by national governments and are considered to be low priority international public health issues [13]. However, these neglected diseases constitute major public health problems. For instance, hook worm infection can cause gastrointestinal blood loss, iron and energy deficiencies, protein and zinc deficiencies and thereby causing malnutrition and anemia [1, 14, 15]. In Ethiopia, helminthiasis was the third leading cause of outpatient visits [4].
Hygiene and environmental sanitation are the prevention strategies used in the health extension program that have been implemented through the seven packages (Proper and safe excreta disposal system; proper and safe solid and liquid waste management; water supply safety measures; food hygiene and safety measures; healthy home environment; arthropods and rodent control; personal hygiene) since 2005. HEWs spend 75% of their time visiting families in their homes and perform such activities in the community [8, 10]. However, this study has found that 20.9% prevalence of helminthic infections. Such high prevalence of helminthiasis is indicator of poor performance in the implementation of the prevention strategy.
Hookworm (14.7%) was the predominant parasite followed by S.mansoni (2.4%), E. vermicularis (1.9%) and S. stercoralis (1.1%). The prevalence may be much more especially for S.mansoni and S. stercoralis because of the less sensitive method (formal ether concentration technique) used to diagnose chronic strongyloidiasis in the stool of patient [16], and the only one day fecal examination used for S. mansoni diagnosis is not very sensitive.
In this study, 14.7% of the study participants were infected with hookworm. Similar prevalence of hookworm infections were reported: 11.5% in Delgi primary school [17], and 14.3% in Jimma [18]. However, the finding is higher compared to the 6.6% of hookworm prevalence reported at Teda health center [19], 4.9% in Northern Ethiopia [20] and 6.7% in Babile town, Eastern Ethiopia [21]. The possible reason might be due to improper latrine utilization, unavailability of safe drinking water, lower deworming coverage in the adolescents and adults and no hand washing practice after toilet since our finding has shown such problems.
The 17.6% of hookworm infections found among patients walking in barefoot was 2.21 (AOR: 2.21; 95% CI: 1.11–4.41; P: 0.024) times higher compared to those who worn shoes (7.9%). Similarly, studies conducted at Teda health Center, North Gondar [19] and in Zarima town, Northwest Ethiopia [7] reported significant association between walking barefoot and hookworm infections. This event happens when the infective stages of immature larva (filariform), which hatch in the soil, can penetrate human skin, and people usually become infected by walking barefoot on contaminated soil [22].
Moreover, clients with ≥15 years of age had significantly higher hookworm infections (19.0%) compared to those below 15 years (3.8%). This finding is supported by another study conducted in Southern Ethiopia [23]. This might be due to the difference in albendazole deworming that has been given to all <5 years children every 6 months and to all pregnant women at 2nd and 3rd trimester as a mass treatment in the HEP [11]. Our finding has also shown that significantly lower number of study participants above 15 years old took 400 mg albendazole deworming (9.6%) compared to the 17.4% of deworming utilization in under 15 years (P: 0.025).
This study was not without limitation. Due to budget constraint: Kato-Katz smear technique was not used, and only one day fecal examination was carried out to check the presence of helminths that could affect diagnostic sensitivity of parasites such us S. mansoni.
Conclusions
There was a high burden of hookworm infections in our setting. Participants walking bare foot (AOR: 2.21; 95% CI: 1.11–4.41; P: 0.024) and age group ≥15 years (AOR: 5.26; 95% CI: 2.05–13.46; P: 0.001) were vulnerable from the hookworm infections. Hence, regular shoes wearing, considering all age groups in the albendazole deworming as mass treatment and environmental hygiene are important interventions to reduce the burden of such neglected tropical disease.
Acknowledgments
The authors would like to thank the study participants for their participation and the data collectors for their commitment. We also thank the Anbesame health center's management and laboratory staffs for their cooperation to conduct this study.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Haque R. Human intestinal parasites. J Health Popul Nutr 2007; 25 (4): 387–91. [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). Control of tropical diseases Geneva: WHO; 1998. [Google Scholar]
- 3.World Health Organization. Prevention and control of schistosomiasis and soiltaransmitted helminthiasis. Geneva: 2002.
- 4. Ministry of Health (MOH). Health and Health Related Indicators. Addis Ababa, Ethiopia: Planning and Programming Department; 2011. [Google Scholar]
- 5. Demissie F, Petros B, Kebede A. Hookworm species distribution among school children in Asendabo town, Jimma Zone, South West Ethiopia. Ethiop J Health Sci 2008; 18 (2). [Google Scholar]
- 6. Erosie L, Merid Y, Ashiko A, Ayine M, Balihu A, Muzeyin S, et al. Prevalence of Hookworm infection and hemoglobin status among rural elementary school children in Southern Ethiopia. Ethiop J Health Dev 2002; 16 (1): 113–5. [Google Scholar]
- 7. Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, Mathewos B, et al. Soil transmitted helminths and schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis 2011;9(11):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebhatu A. The implementation of Ethiopia's Health Extension Program: An overview. Addis Ababa, Ethiopia; 2008.
- 9.Dera Woreda Office of agriculture Annual Report, Anbesame, Ethiopia, 2014.
- 10.USAID/ Pathfinder International. The Ethiopian Health Extension Program, 2008.
- 11.Anbesame health center semi-annual report January-June, 2015.
- 12. Cheesbrough M. District Laboratory practice in tropical countries, Part 1. second ed; New York: Cambridge University Press; 2006. [Google Scholar]
- 13. Hotez P, Molyneux D, Fenwick A, Kumaresan J, SE S, Sachs J, et al. Control of Neglected Tropical Diseases. The New Engl J Med 2007; 357:1018–27. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt H. Do intestinal nematodes affect productivity in adulthood? Parasitol Today 2000; 16:153–8. [DOI] [PubMed] [Google Scholar]
- 15.Skolnik R, Ahmed A. Ending the Neglect of Neglected Tropical Diseases, 2010.
- 16. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis Infection. Clinical Infectious Diseases 2001; 33:1040–7. [DOI] [PubMed] [Google Scholar]
- 17. Ayalew A, Debebe T, Worku A. Prevalence and risk factor of intestinal parasites among Delgi School children, North Gondar, Ethiopia. Journal of Parasitology and Vector Biology 2011; 3 (5):75–81. [Google Scholar]
- 18. Debalke S, Worku A, Jahur N, Mekonnen Z. Soil transmitted helminths and associated factors among school children in Jimma Town, South west Ethiopia. Ethiop J Health Sci 2013; 23 (3):237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abate A, Kibret B, Bekalu E, Abera S, Teklu T, Yalew A, et al. Cross-Sectional Study on the prevalence of intestinal parasites and associated risk factors in Teda health centre, Northwest Ethiopia. ISRN Parasitology 2013:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mathewos B, Alemu A, Woldeyohannes D, Alemu A, Addis Z, Tiruneh M, et al. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Research Notes 2014; 7(88):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tadesse G. The prevalence of intestinal helminthic infections and associated risk factors among schoolchildren in Babile town Eastern Ethiopia. The Ethiopian Journal of Health Development 2005;19 (2):140–7. [Google Scholar]
- 22. Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of Sanitation on Soil-Transmitted Helminth Infection: Systematic Review and Meta-Analysis. PLoS Med 2012; 9: e1001162 doi: 10.1371/journal.pmed.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wegayehu T, Tsalla T, Seifu B, Teklu T. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health 2013;13:151 doi: 10.1186/1471-2458-13-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.