Abstract
Background
Mitochondrial large-conductance Ca2+-sensitive potassium (mBKCa) channels are involved in myocardial ischemic preconditioning. Their role in sildenafil-induced cardioprotection is unknown. We investigated whether sildenafil-induced acute cardioprotection is mediated by activation of mBKCa channels in the rat heart in vitro.
Methods
Male Wistar rats (n = 8 per group) were randomized and anesthetized with pentobarbital (90 mg/kg). Hearts were isolated, mounted on a Langendorff system and perfused with Krebs-Henseleit buffer at a constant pressure of 80 mmHg. Hearts underwent 30 min of global ischemia followed by 60 min of reperfusion. At the end of the experiments infarct size was determined by TTC staining. In the control group rats were not further treated. Sildenafil (3 μM) was administered over 10 min before the beginning of ischemia. The mBKCa channel inhibitor paxilline (1 μM) was administered with and without sildenafil before the onset of ischemia. The pathway underlying sildenafil-induced cardioprotection was further investigated with the protein kinase G blocker KT5823 (1 μM). Myocardial cGMP concentration was measured by ELISA. Data (mean±SD) were analysed with a one and two-way analysis of variance as appropriate.
Results
In control animals infarct size was 52±8%. Sildenafil increased cGMP concentration and reduced infarct size to 35±6% (P<0.05 vs. control). Paxilline and KT5823 completely blocked sildenafil-induced cardioprotection (paxilline+sildenafil: 50±8%, KT5823+sildenafil: 45±8%; both P<0.05 vs. sildenafil). Functional heart parameters and coronary flow were not different between the study groups.
Conclusion
This study shows that in male rats protein kinase G-dependent opening of mBKCa channels plays a pivotal role in sildenafil-induced cardioprotection.
Introduction
Ischemic heart disease is the leading cause of death worldwide, accounting for about 11% of all deaths globally [1]. After an acute myocardial infarction early reperfusion is the most important therapy for improving morbidity and mortality. However, reperfusion itself induces injury that accounts for up to 50% of the final size of a myocardial infarction and paradoxically reduces the beneficial effects of a restored coronary blood flow [2]. Since the end of the last century much effort has, therefore, been focused on developing cardioprotective strategies that render the heart more resistant to ischemia-reperfusion injury.
Sildenafil is a phosphodiesterase-5-inhibitor that was first approved for the treatment of erectile dysfunction in 1998 [3], and approved for the treatment of pulmonary arterial hypertension in 2005 [4]. The effectiveness of sildenafil under these conditions is attributed to its vasodilating and anti-proliferative properties. The vasodilating effect is mediated by inhibitition of the breakdown of cyclic guanosine monophosphate (cGMP), which relaxes smooth muscle cells [5], whereas the anti-proliferative effect involves protein kinase A and protein kinase G (PKG) activated pathways [6]. Several animal studies suggest that sildenafil also protects against myocardial ischemia-reperfusion injury and thereby reduces infarct size by about 35–75% relative to untreated conditions [5,7–13]. The cardioprotective effects of sildenafil are thought to be mediated by opening of mitochondrial ATP-sensitive potassium (mito-KATP) channels [14], enhanced expression of endothelial nitric oxide synthase (NOS) and inducible NOS [5], and activation of protein kinase C and PKG [15]. However, the precise molecular mechanisms and signalling pathways underlying the cardioprotective effects remain to be determined.
The present study investigated whether sildenafil-induced acute cardioprotection in rats involves large-conductance Ca2+-sensitive potassium (BKCa) channels. In cardiomyocytes BKCa channels are located on the inner membrane of mitochondria [16], so that they constitute mBKCa channels, which have previously been described by us and others to be a critical downstream target in the signalling pathway of several cardioprotective interventions [17–22]. These channels, i.e. mBKCa channels, are in part regulated by PKG and thus share a common signalling pathway with sildenafil [23,24]. Unravelling the signalling pathway underlying the sildenafil-induced cardioprotection may provide a conceptual framework for developing novel, more specific cardioprotective strategies.
Methods
The current investigation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication number 85–23, revised 1996) and was performed after obtaining approval from the Animal Ethics Committee of the University of Düsseldorf, Germany. Seventy-six male Wistar rats (Charles River), weighing 285±23 g were housed on a 12:12 light/dark schedule with free access to standard chow and water. All chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany).
Surgical preparation
Surgical preparation was performed as described previously [18]. In brief, rats were anesthetized by intraperitoneal injection of pentobarbital (90 mg/kg). After thoracotomy hearts were excised and mounted on a Langendorff system. Perfusion of the hearts was performed at constant pressure (80 mmHg) with a Krebs-Henseleit solution, containing (in mM): 116 NaCl, 4.7 KCl, 1.1 MgSO4, 1.17 KH2PO4, 24.9 NaHCO3, 2.52 CaCl2, 8.3 glucose, and 2.2 pyruvate at 37°C. The perfusate was bubbled with a mix of 95% O2 and 5% CO2, resulting in a pO2 of 540–620 mmHg, a pCO2 of 35–38 mmHg, and a pH of 7.38–7.43, respectively.
A fluid filled balloon was inserted into the left ventricle and end-diastolic pressure was set at 1–4 mmHg. All hearts underwent an equilibration period of 20 minutes. Thereafter, heart rate, the rate pressure product (RPP, calculated as heart rate x (maximal left ventricular pressure—minimal left ventricular pressure)), left ventricular end-diastolic pressure (LVEDP), and coronary flow were measured continuously and digitized using an analogue to digital converter (PowerLab/8SP, ADInstruments Pty Ltd, Castle Hill, Australia) at a sampling rate of 500 Hz. The data were continuously recorded on a personal computer using Chart for Windows v5.0 (ADInstruments Pty Ltd, Castle Hill, Australia). Maximal contracture and time to maximal contracture were detected by checking the course of contracture development during index ischemia and selecting the time point when contracture reached its highest level in each experiment. Arrhythmic intervals were not used for data analysis.
Experimental protocol
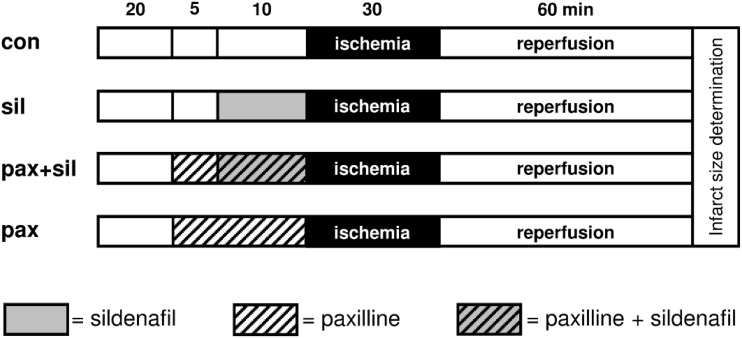
Figs 1 and 2 summarize the study design. In the first part of the study (Fig 1) hearts were randomly assigned to one of four experimental groups (n = 8 in each group). In group 1 (control) hearts were kept under baseline conditions for 35 minutes before they underwent 30 minutes of ischemia followed by 60 minutes of reperfusion. Group 2 (sildenafil) was designed to investigate the cardioprotective effect of sildenafil. For this purpose, 3 μM sildenafil were given over 10 minutes prior to ischemia. Group 3 (paxilline+sildenafil) was designed in order to characterize the role of mBKCa channels in the signalling cascade underlying sildenafil-induced cardioprotection. In this group the potent and well established [18,22,25,26] BKCa channel inhibitor paxilline (1 μM) was given 5 minutes before sildenafil was administered. To rule out an effect on myocardial infarction size by paxilline itself, paxilline (1 μM) was also administered without sildenafil (group 4, paxilline). Sildenafil and paxilline were each separately infused into a mixing chamber placed in the perfusion system. After 60 minutes of reperfusion, the hearts were cut into transverse slices, which were then stained with 0.75% triphenyltetrazoliumchloride (TTC) solution. The infarcted area was determined by planimetry using SigmaScan Pro 5® computer software (SPSS Science Software, Chicago, IL).
Fig 1. Experimental design of the 1st part of the study: con = control, sil = sildenafil, pax = paxilline.
After a 20 minutes equilibration period paxilline (1 μM) was administered to group 3 and group 4. In group 2 and 3 sildenafil (3 μM) was administered 10 minutes before global myocardial ischemia was induced.
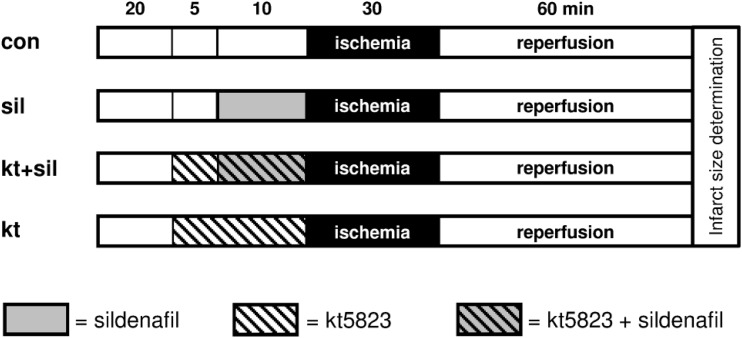
Fig 2. Experimental design of the 2nd part of the study to investigate the role of protein kinase G (PKG) in sildenafil-induced cardioprotection.: con = control, sil = sildenafil, kt = KT5823 (PKG blocker).
In the second part of the study (Fig 2) the effect of the selective PKG inhibitor KT5823 on sildenafil-induced cardioprotection was investigated. For this purpose hearts were randomized to four experimental groups (n = 8 in each group). Group 1 (control) and group 2 (sildenafil) underwent the same protocol as described for these groups above. In group 3 KT5823 (1 μM) was given 5 minutes before sildenafil was administered. To rule out an effect of KT5823 on myocardial infarction size, KT5823 (1 μM) was also administered without sildenafil (group 4). After 60 minutes of reperfusion the infarct size was determined as described above.
In a subset of experiments myocardial cGMP concentration was measured in both control animals (n = 6) and in animals exposed to sildenafil (n = 6). These measurements were performed after administration of sildenafil was terminated, i.e. directly before ischemia was induced. cGMP concentration was measured by ELISA as described previously [27].
Statistical analysis
Sample size was calculated using GraphPad StatMate™ Version 1.01 (GraphPad Software, San Diego, CA, USA), and yielded a group size of n = 8 as necessary to detect a difference in infarct size of 25% with a power of 80% and an α < 0.05 (two-tailed). The estimations of the mean difference of 25% and the standard deviation of 15% were based on previous own data [18]. Hemodynamic parameters were measured during baseline, and during the ischemic and reperfusion period. Comparisons of hemodynamics between groups or between different time points within a group were performed with a two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test (SPSS Science Software, version 12.0.1). Infarct sizes were determined by a researcher blinded to the experimental groups and analyzed by One-way ANOVA followed by Student Newman-Keuls post hoc test. Data are expressed as mean±SD. Changes within and among groups were considered statistically significant if P<0.05.
Results
Infarct size
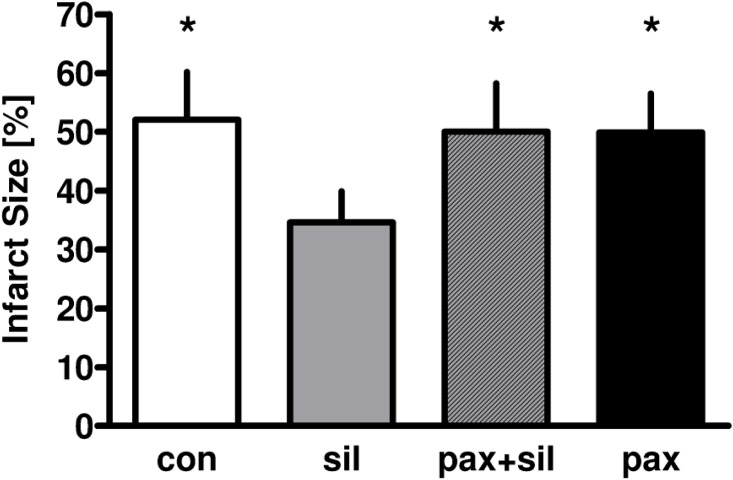
In the first part of the study infarct size of the control group was 52±8% of the whole heart (Fig 3). Sildenafil reduced infarct size to 35±6% (P<0.05 versus control). When paxilline was administered prior to sildenafil, the infarct size was about the same as in untreated controls (50±8%, P<0.05 versus sildenafil alone), indicating that the BKCa-channel inhibitor paxilline completely blocked the cardioprotective effect of sildenafil. Paxilline alone hat no effect on infarct size (50±7%, P<0.05 versus sildenafil alone).
Fig 3. Infarct size of untreated control hearts (con), and of hearts exposed to sildenafil (sil, 3 μM) with or without the mBKCa-channel inhibitor paxilline (pax, 1 μM).
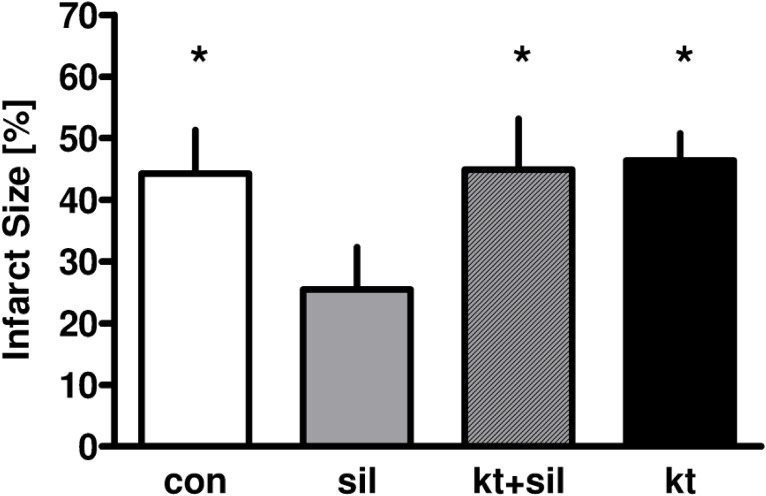
In the second part of the study, which was designed to investigate the effect of the PKG inhibitor KT5823, the infarct size of the control group was 44±7% (Fig 4). Sildenafil decreased infarct size to 26±7% (P<0.05 versus control), and this effect was completely blocked by KT5823 (infract size 45±8%, P<0.05 versus sildenafil alone). KT5823 alone had no effect on infarct size (46±4%, P<0.05 versus sildenafil alone).
Fig 4. Infarct size in the 2nd part of the study that investigated the effect of the PKG-blocker KT5823 on infarct size.
Data are mean±SD. *P<0.05 versus sildenafil.
cGMP formation
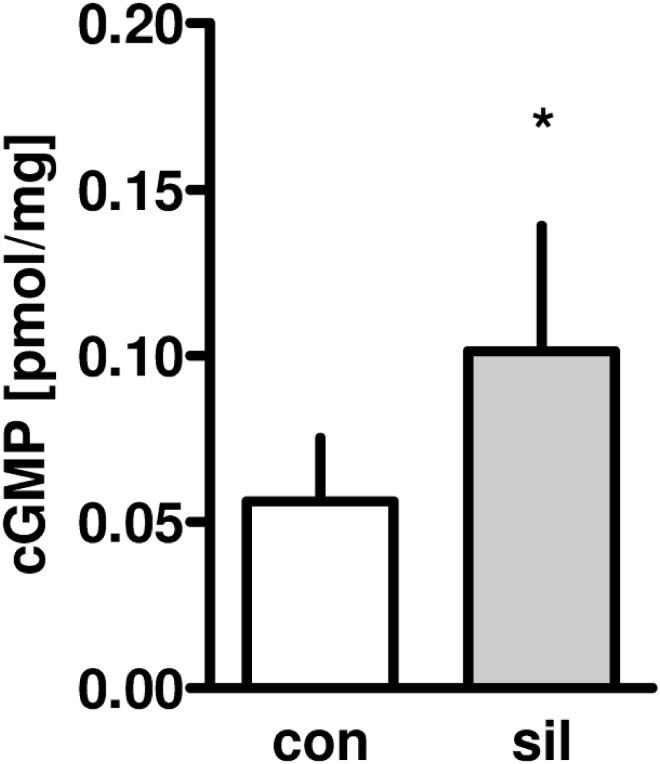
As shown in Fig 5, sildenafil lead to a significant increase in myocardial cGMP formation compared to untreated controls (P<0.05).
Fig 5. Effect of sildenafil on myocardial cGMP concentration (n = 6 per group).
Cardiac function
As summarized in Table 1 (first part of the study) and Table 2 (second part of the study), at none of the different time points was there a significant difference in the heart rate, RPP, LVEDP, and coronary blood flow between the different experimental groups. Over the duration of the experiments heart rate remained stable in all groups, whereas RPP and LVEDP increased during the reperfusion period (all P<0.05 versus baseline). In contrast, coronary flow decreased during the reperfusion period (P<0.05 versus baseline).
Table 1. Hemodynamic variables.
| baseline | drug perfusion | ischemia | reperfusion | ||
|---|---|---|---|---|---|
| 30 | 30 | 60 | |||
| Heart Rate (bpm) | |||||
| con | 326 ± 29 | 330 ± 43 | 293 ± 36 | 275 ± 24 | |
| sil | 305 ± 28 | 336 ± 20 | 330 ± 39 | 278 ± 26 | |
| pax+sil | 308 ± 58 | 308 ± 57 | 264 ± 50 | 277 ± 41 | |
| pax | 338 ± 50 | 334 ± 53 | 296 ± 50 | 275 ± 37 | |
| RPP (mmHg * bpm) | |||||
| con | 42704 ± 6340 | 42704 ± 6340 | 7940 ± 4844* | 9544 ± 3368* | |
| sil | 43200 ± 9234 | 46046 ± 6831 | 8662 ± 5223* | 9972 ± 2840* | |
| pax+sil | 45168 ± 9736 | 43034 ± 8034 | 7817 ± 4875* | 10135 ± 2852* | |
| pax | 44487 ± 6362 | 40855 ± 7904 | 6873 ± 2347* | 8018 ± 2185* | |
| LVEDP (mmHg) | |||||
| con | 7 ± 3 | 8 ± 3 | 39 ± 9* | 85 ± 15* | 78 ± 13* |
| sil | 8 ± 1 | 10 ± 1 | 33 ± 6* | 87 ± 13* | 80 ± 11* |
| pax+sil | 9 ± 4 | 11 ± 3 | 36 ± 4* | 84 ± 8* | 77 ± 10* |
| pax | 10 ± 5 | 14 ± 6 | 40 ± 12* | 89 ± 15* | 82 ± 15* |
| Coronary flow (ml * min -1 /g dry heart weight) | |||||
| con | 105 ± 15 | 113 ± 17 | 66 ± 15* | 57 ± 12* | |
| sil | 107 ± 10 | 117 ± 9 | 60 ± 15* | 55 ± 17* | |
| pax+sil | 114 ± 16 | 114 ± 19 | 51 ± 22* | 51 ± 5* | |
| pax | 112 ± 13 | 103 ± 13 | 61 ± 11* | 53 ± 8* | |
Data are mean±SD. con = control; sil = sildenafil; pax = paxilline. RPP = rate pressure product; LVEDP = left ventricular end-diastolic pressure.
*P<0.05 versus baseline.
Table 2. Hemodynamic variables.
| baseline | drug perfusion | ischemia | reperfusion | ||
|---|---|---|---|---|---|
| 30 | 30 | 60 | |||
| Heart Rate (bpm) | |||||
| con | 334 ± 37 | 316 ± 36 | 238 ± 43* | 249 ± 41* | |
| sil | 295 ± 40 | 305 ± 43 | 226 ± 117 | 244 ± 39 | |
| kt+sil | 313 ± 57 | 306 ± 54 | 198 ± 109* | 252 ± 56 | |
| kt | 317 ± 32 | 298 ± 36 | 287 ± 48 | 256 ± 34 | |
| RPP (mmHg * bpm) | |||||
| con | 38004 ± 6029 | 35803 ± 6717 | 6021 ± 3845* | 7840 ± 3220* | |
| sil | 34296 ± 4887 | 35525 ± 4167 | 4036 ± 2255* | 5964 ± 2297* | |
| kt+sil | 36083 ± 6829 | 32697 ± 4745 | 6403 ± 5231* | 6076 ± 2809* | |
| kt | 37895 ± 6834 | 33894 ± 3521 | 6300 ± 4834* | 6736 ± 3338* | |
| LVEDP (mmHg) | |||||
| con | 4 ± 2 | 5 ± 4 | 32 ± 13* | 72 ± 12* | 66 ± 9* |
| sil | 5 ± 2 | 7 ± 3 | 30 ± 6* | 70 ± 9* | 64 ± 8* |
| kt+sil | 4 ± 2 | 6 ± 3 | 31 ± 8* | 64 ± 12* | 60 ± 9* |
| kt | 4 ± 2 | 6 ± 3 | 31 ± 9* | 77 ± 10* | 70 ± 8* |
| Coronary flow (ml * min -1 /g dry heart weight) | |||||
| con | 101 ± 15 | 100 ± 14 | 59 ± 5* | 56 ± 3* | |
| sil | 95 ± 22 | 100 ± 21 | 66 ± 15* | 59 ± 13* | |
| kt+sil | 100 ± 21 | 100 ± 21 | 61 ± 11* | 57 ± 14* | |
| kt | 92 ± 20 | 89 ± 23 | 62 ± 6* | 59 ± 7* | |
Data are mean±SD. con = control; sil = sildenafil; kt = kt5823. RPP = rate pressure product; LVEDP = left ventricular end-diastolic pressure.
*P<0.05 versus baseline.
Animal characteristics
There were no differences in body weight or heart weight, respectively, between the different experimental groups (Tables 3 and 4).
Table 3. Weights and ischemic contracture.
| Body weight (g) | Heart weight dry (g) | Time of max. ischemic contracture (min) | Level of max. ischemic contracture (mmHg) | |
|---|---|---|---|---|
| con | 279 ± 22 | 0.14 ± 0.02 | 15 ± 1 | 66 ± 12 |
| sil | 284 ± 23 | 0.15 ± 0.02 | 15 ± 1 | 59 ± 9 |
| pax+sil | 285 ± 19 | 0.15 ± 0.01 | 16 ± 1 | 60 ± 11 |
| pax | 287 ± 29 | 0.15 ± 0.01 | 15 ± 1 | 66 ± 18 |
Data are mean±SD. con = control; sil = sildenafil; pax = paxilline.
Table 4. Weights and ischemic contracture.
| Body weight (g) | Heart weight dry (g) | Time of max. ischemic contracture (min) | Level of max. ischemic contracture (mmHg) | |
|---|---|---|---|---|
| con | 270 ± 16 | 0.16 ± 0.01 | 15 ± 1 | 48 ± 14 |
| sil | 291 ± 21 | 0.16 ± 0.02 | 15 ± 2 | 50 ± 6 |
| kt+sil | 295 ± 32 | 0.16 ± 0.02 | 15 ± 2 | 46 ± 12 |
| kt | 264 ± 19 | 0.16 ± 0.01 | 16 ± 1 | 47 ± 9 |
Data are mean±SD. con = control; sil = sildenafil; kt = kt5823.
Discussion
Over the past 30 years enormous progress has been made in understanding the mechanisms underlying myocardial ischemia-reperfusion injury, and has led to the development of several experimental cardioprotective strategies. Despite their unquestionable efficacy in these settings, however, only few of them have been integrated into clinical pathways yet. This may be in part due to the lack of safe and effective drugs with an acceptable side effect profile that exert powerful cardioprotection.
Sildenafil-induced acute cardioprotection
The present study shows that the phoshphodiesterase-5-inhibitor sildenafil has a powerful acute cardioprotective effect by reducing ischemia-reperfusion injury of the isolated perfused rat heart subjected to 30 minutes of global ischemia and 60 minutes of reperfusion in the Langendorff model, which is in line with previous studies, e.g. [7,12,13]. With regard to the clinical situation this finding might be of particular interest: first, sildenafil is a drug with a desirable side effect profile that is widely used for the treatment of erectile dysfunction and pulmonary arterial hypertension [14]. Its pharmacokinetic and pharmacodynamic profile is well described and the drug is widely available. Second, measures that acutely reduce the extent of an ischemia-reperfusion injury can be administered just before a planned coronary occlusion or before coronary reperfusion occurs, e.g. in coronary bypass surgery, what usually is a realizable approach in the clinical setting. Of note, and in addition to its infarct size reducing effect, administration of sildenafil before coronary occlusion has previously been shown to reduce cardiac hypertrophy and apoptosis [28], to improve coronary vascular resistance and hemodynamics [29], and to improve survival [28] in several experimental animal models. The sildenafil dose (3 μM) used in the present study is in the same range as in previous studies investigating the beneficial effect of sildenafil on erectile dysfunction and on lower urinary tract symptoms [30–32].
The use of sildenafil for acute cardioprotection in the setting of ischemia-reperfusion appears promising, and unravelling the underlying mechanisms is an attractive strategy for developing more specific clinical interventions capable of protecting an ischemic myocardium against an ischemia-reperfusion injury. However, since the present study was performed on male rats only, the findings are not necessarily applicable to females.
Signalling pathway in sildenafil-induced acute cardioprotection
The results of the present study show that sildenafil-induced acute cardioprotection was completely abolished when paxilline, a potent and selective BKCa channel blocker [18,22,25,26], was administered before sildenafil was injected, while paxilline itself had no effect on infarct size. Because in cardiomyocytes BKCa channels are located on the inner mitochondrial membrane and not in other subcellular structures [19], this finding suggests that opening of mitochondrial BKCa (mBKCa) channels is essential in the early cardioprotective effect of sildenafil. As described previously, mitochondria play a pivotal role in controlling cell life and death by ATP synthesis and Ca2+ homeostasis, and thus in ischemia-reperfusion injury [7]. We and others previously found mBKCa channels to be a critical downstream target in the signalling pathway of several cardioprotective interventions [17–22]. These channels are members of the voltage-gated K+ channel superfamily and are critical in maintaining Ca2+ homeostasis, mainly via their ability to sense transmembrane voltage and intracellular Ca2+ concentration [33]. mBKCa channels are in part regulated by PKG and thus share a common signalling pathway with sildenafil [15,23,24,34]. Therefore it appeared possible that sildenafil-induced cardioprotection is PKG-dependent. Indeed, our results show that administration of the selective PKG inhibitor KT5823 completely blocked the cardioprotective effect of sildenafil, suggesting that PKG plays a crucial role in the signalling cascade underlying sildenafil-induced cardioprotection as illustrated in Fig 6. This finding is supported by the observation that the selective knockdown of protein kinase G in cardiomyocytes has been shown to block sildenafil-induced cardioprotection [33]. Because sildenafil also increased cGMP formation the results of the present study indicate that sildenafil-induced cardioprotection is mediated, at least in part, by enhanced cGMP synthesis and PKG activation, which, in turn, opens myocardial mBKCa channels.
Fig 6. Schematic model of the cardioprotective pathway of sildenafil-induced acute cardioprotection as reflected by the present study.
PDE = phosphodiesterase, PKG = protein kinase G, KT5823 = protein kinase G inhibitor, Paxilline = mBKCa-channel inhibitor.
Effects of sildenafil on cardiac function
Intravenous administration of sildenafil over 10 minutes prior to ischemia did not alter cardiac function. While heart rate remained stable throughout the whole duration of the experiments, RPP decreased and LVEDP increased upon reperfusion without differences between the treatment groups. These findings indicate that sildenafil did not affect cardiac performance, e. g. cardiac contractility, which is in line with previous observations, e.g. [13]. In addition, coronary flow was significantly reduced after ischemia and was not affected by sildenafil, suggesting that the acute cardioprotective effect of sildenafil occurred independent of potential vasodilating effects in the coronary vasculature. Why coronary flow was not increased in response to sildenafil remains, however, unclear.
Limitations
In the present study the BKCa channel blocker paxilline was used to characterize the signaling pathway underlying sildenafil-induced cardioprotection. Paxilline is a potent and well established blocker of various BKCa channels [18,22,25,26]. Therefore, its exclusive use as a pharmacological indicator of the function of mitochondrial BKCa channels needs to be taken with caution. Several studies suggest that BKCa channels in cardiomyocytes are exclusively located on the inner mitochondrial membrane, and not in other subcellular structures [16,35–38], indicating that the paxilline results of the present study reflect the role of mBKCa channels in sildenafil-induced cardioprotection. This interpretation is in line with the observation that pharmacological activation of mitochondrial BKCa channels has previously been shown to protect isolated cardiomyocytes against reperfusion injury [35]. However, a recent study by Lai et al. [39] found BKCa channels to be located in plasma membranes of sinoatrial node cells, so that in the heart the expression of BKCa channels outside of mitochondria cannot conlusively be ruled out.
The present study did not investigate gender-dependent differences and instead focused on male animals as most of the studies investigating sildenafil-induced cardioprotection did. It thus remains unclear whether the results are applicable to female rats. It also remains unclear whether the results are transferrable to multi-morbid patients. Clinical studies investigating patient populations with an according diseases profile are needed to address this issue.
Conclusion
In recent years, several studies investigated the role and mechanisms of sildenafil in protecting against ischemia-reperfusion injury. These studies identified the expression of nitric oxide synthases, accumulation of cGMP, activation of kinases (such as PKC and ERK), and opening of mitochondrial KATP channels as part of the signalling cascade. The present study investigated the acute cardioprotective effect of sildenafil and found that sildenafil-induced cardioprotection is mediated, at least in part, by enhanced cGMP synthesis and PKG activation, which, in turn, opens myocardial mBKCa channels (Fig 6).
Acknowledgments
This study was supported by institutional and departmental sources. The authors have no conflicts of interest.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1.WHO (2013) The top 10 causes of death. [Internet] (Accessed 30 March 2014. Available: http://www.hoint/mediacentre/factsheets/fs310/en/).
- 2. Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA (1998) Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 338: 1397–1404. [DOI] [PubMed] [Google Scholar]
- 4. Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. (2005) Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 5. Salloum F, Yin C, Xi L, Kukreja RC (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–597. [DOI] [PubMed] [Google Scholar]
- 6. Tantini B, Manes A, Fiumana E, Pignatti C, Guarnieri C, Zannoli R, et al. (2005) Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol 100: 131–138. [DOI] [PubMed] [Google Scholar]
- 7. Lee KH, Kwon SJ, Woo JS, Lee GJ, Lee SR, Jang HH, et al. (2014) Effects of sildenafil on nanostructural and nanomechanical changes in mitochondria in an ischaemia-reperfusion rat model. Clin Exp Pharmacol Physiol 41: 763–768. 10.1111/1440-1681.12290 [DOI] [PubMed] [Google Scholar]
- 8. Milano G, Bianciardi P, Rochemont V, Vassalli G, Segesser LK, Corno AF, et al. (2011) Phosphodiesterase-5 inhibition mimics intermittent reoxygenation and improves cardioprotection in the hypoxic myocardium. PLoS One 6: e27910 10.1371/journal.pone.0027910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Madhani M, Hall AR, Cuello F, Charles RL, Burgoyne JR, Fuller W, et al. (2010) Phospholemman Ser69 phosphorylation contributes to sildenafil-induced cardioprotection against reperfusion injury. Am J Physiol Heart Circ Physiol 299: H827–836. 10.1152/ajpheart.00129.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC (2009) ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296: H1236–1243. 10.1152/ajpheart.00100.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koneru S, Varma Penumathsa S, Thirunavukkarasu M, Vidavalur R, Zhan L, Singal PK, et al. (2008) Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med 12: 2651–2664. 10.1111/j.1582-4934.2008.00319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das S, Maulik N, Das DK, Kadowitz PJ, Bivalacqua TJ (2002) Cardioprotection with sildenafil, a selective inhibitor of cyclic 3',5'-monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res 28: 213–219. [PubMed] [Google Scholar]
- 13. Ockaili R, Salloum F, Hawkins J, Kukreja RC (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283: H1263–1269. [DOI] [PubMed] [Google Scholar]
- 14. Das A, Durrant D, Salloum FN, Xi L, Kukreja RC (2014) PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Das A, Xi L, Kukreja RC (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283: 29572–29585. 10.1074/jbc.M801547200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bentzen BH, Osadchii O, Jespersen T, Hansen RS, Olesen SP, Grunnet M (2009) Activation of big conductance Ca(2+)-activated K (+) channels (BK) protects the heart against ischemia-reperfusion injury. Pflugers Arch 457: 979–988. 10.1007/s00424-008-0583-5 [DOI] [PubMed] [Google Scholar]
- 17. Heinen A, Strothoff M, Schmidt A, Stracke N, Behmenburg F, Bauer I, et al. (2014) Pharmacological options to protect the aged heart from ischemia and reperfusion injury by targeting the PKA-BK(Ca) signaling pathway. Exp Gerontol 56: 99–105. 10.1016/j.exger.2014.03.029 [DOI] [PubMed] [Google Scholar]
- 18. Frassdorf J, Huhn R, Niersmann C, Weber NC, Schlack W, Preckel B, et al. (2010) Morphine induces preconditioning via activation of mitochondrial K(Ca) channels. Can J Anaesth 57: 767–773. 10.1007/s12630-010-9325-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, et al. (2002) Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033. [DOI] [PubMed] [Google Scholar]
- 20. Huhn R, Weber NC, Preckel B, Schlack W, Bauer I, Hollmann MW, et al. (2012) Age-related loss of cardiac preconditioning: impact of protein kinase A. Exp Gerontol 47: 116–121. 10.1016/j.exger.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 21. Shintani Y, Node K, Asanuma H, Sanada S, Takashima S, Asano Y, et al. (2004) Opening of Ca2+- activated K+ channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol 37: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 22. Huhn R, Heinen A, Weber NC, Schlack W, Preckel B, Hollmann MW (2010) Ischaemic and morphine-induced post-conditioning: impact of mK(Ca) channels. Br J Anaesth 105: 589–595. 10.1093/bja/aeq213 [DOI] [PubMed] [Google Scholar]
- 23. Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE (2012) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302: H115–123. 10.1152/ajpheart.00046.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, et al. (2004) The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J 18: 1382–1391. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez M, McManus OB (1996) Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968. [DOI] [PubMed] [Google Scholar]
- 26. Wang X, Fisher PW, Xi L, Kukreja RC (2008) Essential role of mitochondrial Ca2+- activated and ATP-sensitive K+ channels in sildenafil-induced late cardioprotection. J Mol Cell Cardiol 44: 105–113. [DOI] [PubMed] [Google Scholar]
- 27. Cattaneo MG, Cappellini E, Benfante R, Ragni M, Omodeo-Sale F, Nisoli E, et al. (2011) Chronic deficiency of nitric oxide affects hypoxia inducible factor-1alpha (HIF-1alpha) stability and migration in human endothelial cells. PLoS One 6: e29680 10.1371/journal.pone.0029680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, et al. (2008) Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–1406. 10.1152/ajpheart.91438.2007 [DOI] [PubMed] [Google Scholar]
- 29. Reffelmann T, Kloner RA (2003) Effects of sildenafil on myocardial infarct size, microvascular function, and acute ischemic left ventricular dilation. Cardiovasc Res 59: 441–449. [DOI] [PubMed] [Google Scholar]
- 30. Fusco F, di Villa Bianca R, Mitidieri E, Cirino G, Sorrentino R, Mirone V (2012) Sildenafil effect on the human bladder involves the L-cysteine/hydrogen sulfide pathway: a novel mechanism of action of phosphodiesterase type 5 inhibitors. Eur Urol 62: 1174–1180. 10.1016/j.eururo.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 31. Oger S, Behr-Roussel D, Gorny D, Lebret T, Validire P, Cathelineau X, et al. (2010) Signalling pathways involved in sildenafil-induced relaxation of human bladder dome smooth muscle. Br J Pharmacol 160: 1135–1143. 10.1111/j.1476-5381.2010.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dikmen A, di Villa Bianca R, Mitidieri E, Donnarumma E, Sevin G, Cirino G (2013) New mechanism for the beneficial effect of sildenafil on erectile dsyfunction: H2S. Nitric Oxide 31: S38. [Google Scholar]
- 33. Kukreja RC, Salloum FN, Das A, Koka S, Ockaili RA, Xi L (2011) Emerging new uses of phosphodiesterase-5 inhibitors in cardiovascular diseases. Exp Clin Cardiol 16: e30–35. [PMC free article] [PubMed] [Google Scholar]
- 34. Thebaud B, Michelakis E, Wu XC, Harry G, Hashimoto K, Archer SL (2002) Sildenafil reverses O2 constriction of the rabbit ductus arteriosus by inhibiting type 5 phosphodiesterase and activating BK(Ca) channels. Pediatr Res 52: 19–24. [DOI] [PubMed] [Google Scholar]
- 35. Borchert GH, Hlavackova M, Kolar F (2013) Pharmacological activation of mitochondrial BK(Ca) channels protects isolated cardiomyocytes against simulated reperfusion-induced injury. Exp Biol Med (Maywood) 238: 233–241. [DOI] [PubMed] [Google Scholar]
- 36. Balderas E, Zhang J, Stefani E, Toro L (2015) Mitochondrial BKCa channel. Front Physiol 6: 104 10.3389/fphys.2015.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitt N, Grunnet M, Olesen SP (2014) Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev 94: 609–653. 10.1152/physrev.00022.2013 [DOI] [PubMed] [Google Scholar]
- 38. Clements RT, Terentyev D, Sellke FW (2015) Ca(2+)-activated K(+) channels as therapeutic targets for myocardial and vascular protection. Circ J 79: 455–462. 10.1253/circj.CJ-15-0015 [DOI] [PubMed] [Google Scholar]
- 39. Lai MH, Wu Y, Gao Z, Anderson ME, Dalziel JE, Meredith AL (2014) BK channels regulate sinoatrial node firing rate and cardiac pacing in vivo. Am J Physiol Heart Circ Physiol 307: H1327–1338. 10.1152/ajpheart.00354.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.