Abstract
Although colchicine has been a focus of research, debate and controversy for thousands of years, it was only approved by the United States Food and Drug Administration in 2009. Over the past decade, advances in the knowledge of colchicine pharmacology, drug safety and mechanisms of action have led to changes in colchicine dosing and to potential new uses for this very old drug. In this review, we discuss the pharmacologic properties of colchicine and summarize what is currently known about its mechanisms of action. We then discuss and update the use of colchicine in a variety of illnesses, including rheumatic and, most recently cardiovascular diseases.
Keywords: colchicine, inflammation, gout, cardiovascular disease
Introduction
Although colchicine’s medicinal properties have been recognized for centuries, the drug was first approved in 2009, under the United States Food and Drug Administration (FDA) unapproved drugs initiative.1 FDA approval brought changes in colchicine dosing regimens, and a greater emphasis on safety in the context of co-morbidities and drug-drug interactions. Even before FDA approval, investigations had begun to widen colchicine’s range of clinical use, from gout and familial Mediterranean fever to a variety of rheumatologic and cardiovascular applications (Table 1). Here we review colchicine’s basic biology and classical uses, and provide an update on newer and potential uses in diseases with inflammatory etiologies.
Table 1.
| Conditions | Study Names/Authors and Reference | General Conclusions of studies |
|---|---|---|
| Crystal arthropathies | AGREE - Terkeltaub et al. 201039 Wortmann et al. 201041 Tabatabai et al. 198043 |
Established colchicine efficacy in acute gout and acute calcium pyrophosphate crystal arthritis, as well as prophylaxis of gout flares. |
| Familial Mediterranean fever | S.E. Goldfinger, 197253 Dinarello et al. 197454 Zemer et al. 197455 Ben-Chetrit et al. 199156 |
Colchicine significantly reduced frequency of Familial Mediterranean fever attacks and risk of amyloid renal failure. |
| Behçet’s Disease | Yurdakul et al. 200158 Davatchi et al. 200959 Aktulga et al. 198060 |
Colchicine significantly improved oral aphthosis, genital aphthosis, skin lesions, and joint manifestations in most studies. |
| Chronic cutaneous vasculitis and Sweet’s Syndrome | Callen JP. 198561 Suehisa et al. 198366 Maillard et al. 199967 |
Colchicine use improved skin lesions, as well as (Sweet’s) inflammatory markers, fever and neutrophilia. |
| Initial and Recurrent Pericarditis (I = Initial, R = recurrent) | COPE trial - Imazio et al. 2005 (I)73 Imazio et al. 2013 (I)74 CORE trial - Imazio et al. 2005 (R)69 CORP trial - Imazio et al. 2011 (R)70 |
Colchicine accelerated symptom relief in acute pericarditis compared with aspirin alone or placebo, and reduced recurrence rate. |
| Stable Coronary Artery Disease (CAD), Myocardial Infarction and In-stent restenosis in Diabetes Mellitus | Nidorf et al. 200775 Nidorf et al. 201378 Crittenden et al. 201277 Deftereos et al. 201379 |
Colchicine reduced CRP, cardiac arrest, ACS, or ischemic stroke in stable CAD. Prevalence of myocardial infarction was reduced in gout colchicine users. Decreased in-stent restenosis in colchicine users. |
| Atrial Fibrillation | Imazio et al. 201180 COPPS-2: Imazio et al. 201481 Deftereos et al. 201282 |
Colchicine lowered incidence of post-operative and postablation atrial fibrillation compared with placebo in most studies. |
Pharmacologic properties
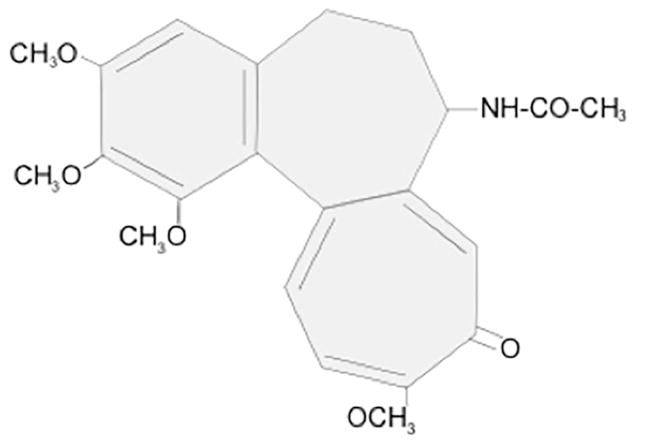
Colchicine is a tricyclic, lipid-soluble alkaloid with a long terminal half-life (20 to 40 hours) and bioavailability ranging from 24% to 88% (Figure 1). Within the bloodstream, ~40% of colchicine binds to albumin.2 Although peak plasma concentrations occur 1 hour after administration, maximal anti-inflammatory effects develop over 24 to 48 hours, based on intra-leukocyte accumulation.3 Colchicine reaches much higher concentrations within leukocytes than in plasma, and persists there for several days after ingestion,2,3 with concentrations ranging from 4 to 64 ng/109.4
Figure 1.
Colchicine preferentially binds three proteins: tubulin, cytochrome P3A4 (CYP3A4) and P-glycoprotein. The dissociation half-life of colchicine from tubulin is 20–40 hours, which primarily defines colchicine’s long clinical half-life.5 Colchicine’s persistence on tubulin prevents the fusion of autophagic vacuoles with lysosomes in neuronal, marrow and muscle cells, resulting in risk of damage to these organ systems,6 particularly for patients with hepatic and/or renal impairment.7,8
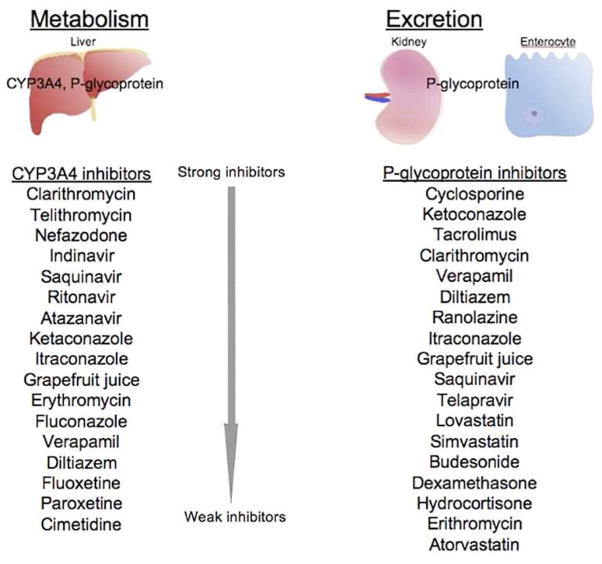
CYP3A4 and P-glycoprotein are largely responsible for colchicine metabolism (Figure 2). CYP3A4 is found in hepatocytes and enterocytes, and metabolizes colchicine to 2- and 3-demethylcolchicine. P-glycoprotein is an ATPase efflux pump found in enterocytes, hepatocytes, renal cells and the blood-brain barrier; it extrudes colchicine from the gastrointestinal tract to limit gastrointestinal absorption. Together with renal excretion, these systems determine overall colchicine serum levels. Individuals vary in their levels of expression of CYP3A4 and P-glycoprotein; lack of adequate response to colchicine in certain patients may relate to over-expression of one or both of these proteins.5
Figure 2.
CYP3A4 and P-glycoprotein are also largely responsible for colchicine’s numerous drug-drug interactions, as each interacts with a range of other drugs. Because of CYP3A4 interactions, colchicine metabolism is impaired in patients taking clarithromycin, fluoxetine, paroxetine, nefazodone, indinavir and other protease inhibitors, tolbutamide and azole antifungals, cimetidine, and several non-dihydropyridine calcium channel blockers, each of which is metabolized through this pathway.9 Fatalities have been reported with unadjusted co-administration of colchicine and clarithromycin.10,11 Drugs that are eliminated by the enterocyte P-glycoprotein transporter include macrolides, protease inhibitors, chemotherapeutic agents, glucocorticoids, calcium channel blockers, and certain statins.9 Colchicine particularly interacts with cyclosporine because of P-glycoprotein interaction with both of these medications.12 Dose adjustment of colchicine in the setting of many of the aforementioned agents is mandated and described in detail in the manufacturer’s prescribing information.13
Bioavailability of colchicine in the elderly is comparable to that in younger adults. However, colchicine’s volume of distribution and total body clearance are significantly reduced, leading to a higher plasma concentration and a significantly greater risk of toxicity.14 To counter this effect, some experts recommend reducing the colchicine dose by up to 50% in patients who are older than 70 years of age.15
Mechanisms of action
Colchicine binds to free tubulin dimers which, when incorporated into nascent microtubules, disrupt further microtubule polymerization. Abrogation of microtubule polymerization inhibits vesicle transport, cytokine secretion, phagocytosis, migration and division. At higher concentrations colchicine may also induce some microtubule dissociation.15
In neutrophils, colchicine inhibits intracellular signaling molecules including tyrosine kinases and phospholipases, and inhibits chemotaxis and lysosomal enzyme release during phagocytosis.16 Recent studies suggest that colchicine also modulates neutrophil deformability to suppress neutrophil extravasation.17 In addition, colchicine inhibits neutrophil superoxide anion production and increases leukocyte cAMP, which is known to suppress neutrophil function.18
At low concentrations (e.g., 3 nM), colchicine modulates the distribution of E-selectin adhesion molecules on endothelial cells, inhibiting neutrophil adhesion to, and extravasation from the vasculature. At higher concentrations (e.g., 300 nM), colchicine reduces L-selectin expression on neutrophils, further impeding neutrophil-endothelial cell adhesion. These concentration-dependent effects may partly explain the observation that low doses (e.g., 0.6 mg/day) of colchicine may prevent, but doses higher (e.g., ≥1.8 mg) are needed to suppress acute gouty attacks.19
Colchicine has recently been shown to suppress the activation of caspase-1, the enzymatic component of the nucleotide-binding oligomerization domain receptor (NOD-like receptor) family pyrin 3 (NLRP3) inflammasome. Caspase-1 suppression blocks conversion of pro-IL-1β to active IL-1β, leading to secondary reductions in cytokines such as tumor necrosis factor-α (TNF-α) and IL-6. Colchicine’s effect on this process may be upstream of, rather than directly on, the inflammasome.20 To date, inflammasome inhibition has been assessed at colchicine concentrations 10- to 100-fold higher than those achieved in serum.9 Whether colchicine inhibits caspase-1 at physiologic concentrations, or whether colchicine accumulation in leukocytes is sufficient to block the inflammasome, remains to be determined.
Other potential anti-inflammatory activities include modulation of pyrin expression (see familial Mediterranean fever section), downregulation of lipopolysaccharide-induced TNF-α mRNA production, inhibition of histamine release by mast cells, suppression of pro-collagen synthesis, and promotion of collagenase activity.21–23
Colchicine toxicity
The commonest side effects of colchicine are gastrointestinal, with nausea, vomiting and particularly diarrhea occurring in five to ten percent of patients even at recommended doses. Dose reduction may permit resolution of these symptoms. Colchicine doses of 0.5 to 0.8 mg/kg are highly toxic, and doses of more than 0.8 mg/kg are typically lethal; to reduce the risk of irreversible bolus overdose, the FDA withdrew approval for intravenous colchicine.16,24 Acute overdose usually manifests as gastrointestinal symptoms within 24 hours of ingestion, widespread organ system dysfunction (renal failure, circulatory collapse, marrow failure, muscle weakness, rhabdomyolysis and respiratory failure) within seven days, and finally either resolution of symptoms or worsening organ dysfunction and death. Treatment for acute colchicine overdose is supportive.7,25–27 Chronic colchicine overdose can occur when daily colchicine doses are not adjusted for reduced renal function or interacting medications; colchicine neuromyopathy and cytopenias are classic features of chronic-type overdose.28 Several case reports and one retrospective review suggest that the combination of colchicine plus a statin, typically a statin that interacts with CYP3A4, may occasionally increase the risk of acute myopathy.29–33
Colchicine in rheumatic diseases
Gout and calcium pyrophosphate crystal arthritis
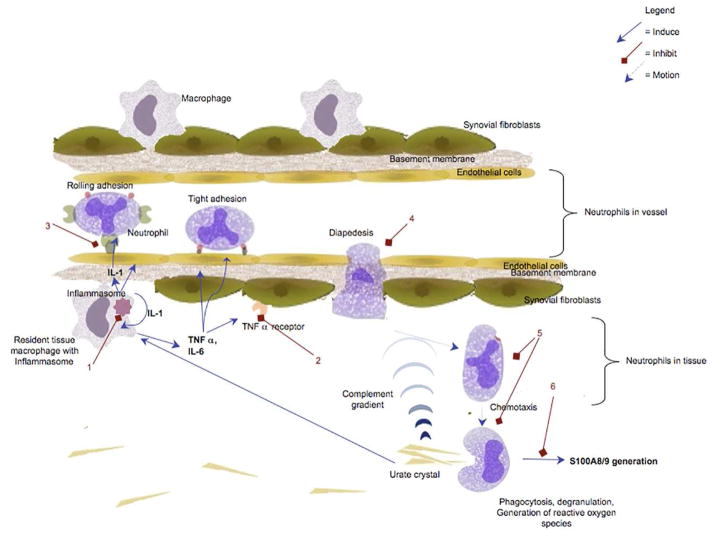
Many of the inflammatory processes discussed above are relevant to gout pathogenesis. Gouty inflammation relies on the activation of macrophages and neutrophils, depends upon the expression of adhesion molecules on leukocytes and vasculature, and is triggered by crystal-induced inflammasome activation and IL-1β generation, all of which can be abrogated by colchicine (Figure 3).34–38
Figure 3.
Although colchicine has been used to treat gout for centuries, relatively few controlled trials have assessed its efficacy. The most recent major trial, Acute Gout Flare Receiving Colchicine Evaluation (AGREE), randomized 184 patients with acute gout to either a lower-dose colchicine regimen (1.2 mg dose followed by one 0.6 mg dose one hour later), a traditional higher dose regimen (1.2 mg dose followed by 0.6 mg every hour for a maximum of 4.8 mg) or placebo within 12 hours of the onset of attack.39 The lower- and higher-dose regimens demonstrated similar efficacy (37.8% vs 32.7% achieving ≥50% improvement within 24 hours), but adverse events in the lower-dose regimen were similar to placebo. Accordingly, the lower-dose regimen was approved by the FDA. American College of Rheumatology guidelines recommend the lower-dose colchicine regimen as a first-line therapy option for acute gouty attacks.40
In addition to its role in acute gout, colchicine is used prophylactically to reduce gout flare frequency, particularly when patients are initiating urate-lowering therapy. An analysis from three randomized controlled trials found that colchicine use for up to 6 months during initial urate lowering provided greater prophylaxis of flares than its use for only 8 weeks.41 American College of Rheumatology guidelines therefore recommend the use of colchicine or another prophylactic agent for a minimum of 6 months, depending upon clinical scenario.40
Colchicine is also recommended for prophylaxis and acute treatment of calcium pyrophosphate crystal arthritis (i.e., pseudogout). Recent European League Against Rheumatism (EULAR) guidelines recommend colchicine 0.5 mg three times daily with or without a 1 mg loading dose for acute attacks and colchicine 0.5 mg daily for prophylaxis, based largely on expert opinion and a single uncontrolled trial. 42,43
Familial Mediterranean Fever
Familial Mediterranean fever patients suffer recurrent attacks of fever, arthritis and serositis, characterized by sterile neutrophil infiltration. 44 Familial Mediterranean fever is caused by mutations in the gene for pyrin, a myeloid-lineage protein that regulates inflammasome activation.45–48 Pyrin gene mutations lower the threshold for IL-1β production and inflammatory attacks.49
Although colchicine’s mechanism of action in Familial Mediterranean fever is not fully determined, the disease pathogenesis suggests that, similar to gout attacks, colchicine alters adhesion molecules, inhibits chemotaxis through microtubule destabilization, and suppresses NLRP3/caspase-1 activity. In vitro, colchicine has been shown to enhance pyrin expression in response to IFN-α,50 and to disrupt inflammasome-like complexes.51 Mansfield et al observed that pyrin interacts with the cytoskeletons of granulocytes and monocytes, suggesting a possible mechanistic relationship between colchicine, pyrin and microtubules.52
Colchicine use in familial Mediterranean fever was spurred by a 1972 letter by S.E. Goldfinger in the New England Journal of Medicine, describing his experience using 1 to 3 mg daily colchicine in 5 familial Mediterranean fever patients.53 This was followed by two double-blind trials in 1974. In one, 11 subjects with familial Mediterranean fever were randomized to a 28-day course of 0.6 mg colchicine three times daily vs placebo. 54 Subjects receiving colchicine experienced significantly fewer attacks (12% vs 63%). In the other trial, 22 familial Mediterranean fever patients received either two months of twice daily colchicine 0.5 mg or placebo, followed by two months of crossover treatment. 55 Patients taking colchicine experienced significantly fewer attacks.
Long-term follow-up studies of familial Mediterranean fever patients on colchicine, including a 1991 study by Ben-Chetrit and Levy in which 45 patients were followed for at least 15 years, provide evidence for the efficacy and safety of chronic colchicine use.56 Colchicine is now considered first-line therapy for familial Mediterranean fever.57 A daily colchicine dose of 1 to 2 mg induces disease remission or reduces frequency of attacks in 90% to 95% of patients. Importantly, reactive Amyloid A amyloidosis (the major cause of renal failure in familial Mediterranean fever) is almost universally prevented in patients who adhere to colchicine therapy, even those who experience inadequate reduction of attacks.49
Vasculitis
Colchicine is recommended for several types of vasculitis. Colchicine’s activity in vasculitis is likely associated with downregulation of cell surface adhesion molecules and decreased neutrophil adhesion and migration.
In Behçet’s disease, colchicine is recommended for mucocutaneous and joint symptoms. Yurdakul et al randomized 116 patients with active mucocutaneous disease to receive placebo or colchicine 1 to 2 mg daily for two years.58 Among women, colchicine use significantly reduced the incidence of genital ulcers, erythema nodosum and arthritis. Among men, colchicine significantly reduced the incidence of arthritis. Davatchi et al conducted a double-blind, placebo-controlled crossover trial of 169 Behçet’s disease patients without major organ involvement, in which daily colchicine use improved overall disease activity, oral and genital ulcers, skin lesions and joint manifestations.59 In contrast, Aktulga et al found no benefit for colchicine in treating Behçet’s disease-related oral and genital ulcers.60
Colchicine is recommended for treating mild acute or chronic cutaneous vasculitis. Callen treated 13 patients with chronic cutaneous vasculitis with 0.6 mg colchicine twice daily, and reported that 12 of 13 patients responded within 10 days of starting therapy, with 9 achieving complete remission.61 Additionally, 4 of 5 patients with concomitant arthralgia experienced joint symptom resolution. Case reports support these findings and suggest a possible benefit of colchicine in treating the physiologically-similar condition Henoch-Schönlein purpura.62–65
Neutrophilic dermatoses
Colchicine is beneficial in neutrophilic skin conditions. Sweet’s syndrome typically occurs in women aged 30 to 50 years and is characterized by fever, neutrophilia, arthralgias, tender erythematous skin lesions, and neutrophilic infiltrates in the upper dermis. The benefit of colchicine for Sweet’s syndrome was first reported in 1981.66 Later, Maillard et al gave 20 patients with Sweet’s syndrome 0.5 mg colchicine thrice daily for 10 to 21 days, and reported that 18 experienced resolution of fever, skin lesions, arthralgias, and neutrophilia within14 days.67
Colchicine and Cardiovascular Disease
The accumulating understanding of the role of inflammation in cardiovascular diseases has been accompanied by recognition of the possible anti-inflammatory benefit of colchicine in these settings.
Pericarditis
In 2004, the European Society of Cardiology recommended colchicine for pericarditis, based on small, non-randomized trials and expert consensus.68 These recommendations have subsequently been supported by randomized trials. The COlchicine for REcurrent pericarditis (CORE trial) was the first randomized trial to assess colchicine’s efficacy in recurrent pericarditis. 69 Eighty-four consecutive patients with a first episode of recurrent pericarditis were randomized to 1 month of aspirin or prednisone with or without 6 months of colchicine. Patients receiving colchicine experienced a significant reduction in symptoms at 72 hours after onset of pericarditis compared with those not treated (10% vs 31%, p=0.03). After 18 months, the colchicine group experienced a 51% reduction in the rate of recurrent pericarditis versus 24% in the no colchicine group (p=0.02). The subsequent COlchicine for Recurrent Pericarditis (CORP) trial found similar risk reductions in a randomized, double-blind, placebo-controlled multicenter trial of 120 patients.70 Most recently, the CORP-2 trial evaluated 240 patients with two or more recurrences in a randomized, double-blind, placebo-controlled multicenter trial. Colchicine was as effective at reducing multiple recurrences of pericarditis as it was for first recurrences. 71,72
Similar benefits were observed for treatment of an initial attack of acute pericarditis. The open-label COlchicine for acute PEricarditis (COPE) trial randomized 120 patients to receive colchicine plus aspirin or aspirin alone. Symptom persistence at 72 hours was reduced (11.7% vs 36.7%; p=0.003), and the rate of recurrence over 18 months was 11% in the aspirin/colchicine group versus 32% in the aspirin-only group (p=0.004).73 Similar findings were reported in a multicenter, double-blind, placebo-controlled trial by Imazio et al.74
Current European guidelines for pericarditis treatment recommend colchicine, 2 mg daily for 1 to 2 days, followed by a maintenance dose of 1 mg daily. However, recently-studied weight-adjusted doses appear to improve gastrointestinal tolerance.74
Coronary Artery Disease
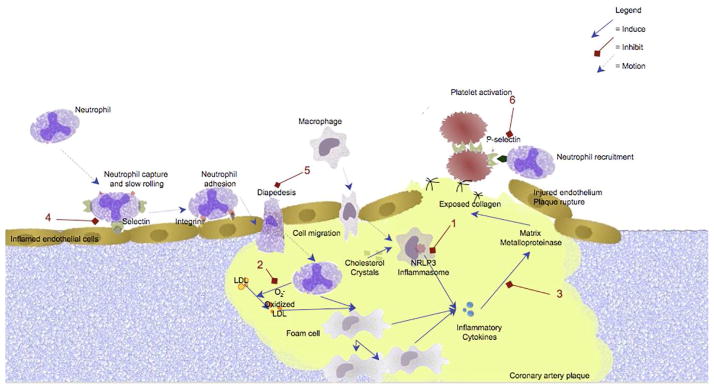
Based upon the paradigm of atherosclerosis as an inflammatory process, colchicine has recently been investigated as a possible therapy for the prevention and/or treatment of stable coronary artery disease and acute coronary syndromes. Hypothetically, colchicine’s effects on macrophages, adhesion molecules and neutrophils, as well as its ability to reduce cytokine and matrix metalloproteinase production, could all play roles in reducing adverse cardiovascular outcomes (Figure 4).
Figure 4.
In an open-label trial of 60 patients, Nidorf et al demonstrated a 60% reduction in the acute phase reactant and cardiovascular risk marker C-reactive protein (CRP) with colchicine 0.5 mg twice daily, in patients with stable coronary artery disease already on an aspirin and statin regimen.75 However, in a separate, randomized study of 80 patients with acute coronary syndrome or stroke, Nidorf et al found no significant association between colchicine use (1 mg daily) and CRP reduction,76 suggesting either that the dose studied was insufficient in the acute setting, or that colchicine may be more effective when administered as a prophylactic rather than treatment agent.
Given that gout patients are at high risk for cardiovascular disease, and that they often take colchicine on a chronic basis, Crittenden et al systematically evaluated the prevalence of myocardial infarction among 1288 gout patients at a VA hospital.77 This cross-sectional study found a reduced prevalence of myocardial infarction among gout patients who used colchicine (1.2%) versus those who did not (2.6%; p=0.03). Patients taking colchicine also demonstrated trends towards reduced mortality and lower CRP levels.
More recently, Nidorf et al performed a randomized, observer-blinded endpoint study to evaluate the effect of colchicine 0.5 mg daily on secondary cardiovascular events in 532 patients with stable coronary artery disease already on aspirin and/or clopidogrel and statin therapy.78 Patients were followed for a median of three years. The primary outcome--the composite incidence of acute syndromes, out-of-hospital cardiac arrest, and non-cardioembolic ischemic stroke--occurred in 5.3% of colchicine patients versus 16% of placebo patients, warranting further study of colchicine’s potential benefit in the prevention of acute coronary syndrome.
Colchicine has shown promise in preventing in-stent restenosis among patients with diabetes mellitus. With growing evidence that inflammation plays a role in the restenosis process, one study randomized 196 patients with diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention with bare-metal stent placement to receive either colchicine 0.5 mg or placebo twice daily for six months. 79 Subsequent angiography indicated that the rate of angiographic in-stent restenosis was 16% in the colchicine group versus 33% in the placebo group (p=0.007).
Secondary atrial fibrillation
Post-operative atrial fibrillation is a common occurrence after cardiac surgery and is presumed driven by surgery-related inflammation. The COlchicine for the Prevention of the Post-pericardiotomy Syndrome (COPPS) atrial fibrillation substudy demonstrated that post-operative administration of colchicine was associated with a 45% reduction in the incidence of post-operative atrial fibrillation.80 However, a second multicenter, randomized, double-blind, placebo-controlled trial (COPPS-2) recently found no reduction in post-operative atrial fibrillation among patients receiving colchicine. 81
Colchicine may also reduce the risk of recurrence of atrial fibrillation after ablation therapy. In a randomized, double-blind, placebo-controlled study by Deftereos et al, 3 months of colchicine monotherapy was associated with a 16% reduction in the incidence of atrial fibrillation after pulmonary vein isolation with radiofrequency ablation.82 The colchicine group also experienced significant decreases in IL-6 and CRP levels.
Conclusion
Colchicine is one of the oldest therapeutic substances known to mankind, yet the scope of its benefits has only recently been the subject of active study. Because of its wide range of activities among inflammatory cells, its systemic effect and its relatively mild side effect profile at recommended doses, colchicine has proved to be beneficial in some common and frustrating conditions, including cardiovascular disease. Ongoing research will further elucidate the mechanisms of action and therapeutic utility of this historic medicine.
Clinical Significance.
Colchicine is considered first-line therapy for treatment of acute gout, prophylaxis of gout, and familial Mediterranean fever. It is also commonly used in other diseases including pseudogout, pericarditis, Behçet’s disease and neutrophilic dermatoses.
Colchicine’s anti-inflammatory properties may be beneficial in treating or preventing cardiovascular disease, including pericarditis, post-surgical atrial fibrillation and acute cardiovascular syndromes.
Acknowledgments
Funding: None
Support: MHP is supported in part by CTSA grant UL1TR000038 from the National Center for the Advancement of Translational Science, National Institutes of Health. SK is supported in part by a New York State Empire Clinical Research Investigator Program Award.
Footnotes
All authors participated in all aspects of this manuscript, including conception, research, writing and editing.
Conflict of interest: AS: None. SK: None. MHP is the recipient of an investigator-initiated grant and also serves as a study site investigator for a trial supported by Takeda, Inc; is the recipient of an investigator-initiated grant from Savient, Inc; is the recipient of an investigator-initiated grant and has served as a consultant for Crealta, Inc; and has served as a consultant for Astra-Zeneca, Inc. BS is the recipient of an investigator-initiated grant (drug and placebo only) from Takeda, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Research C for DE. [Accessed May 28, 2014];Drug Safety Information for Healthcare Professionals - Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys) Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm174315.htm.
- 2.Sabouraud A, Chappey O, Dupin T, Scherrmann JM. Binding of colchicine and thiocolchicoside to human serum proteins and blood cells. [Accessed May 28, 2014];Int J Clin Pharmacol Ther. 1994 32(8):429–32. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7981928. [PubMed] [Google Scholar]
- 3.Chappey ON, Niel E, Wautier JL, et al. Colchicine disposition in human leukocytes after single and multiple oral administration. [Accessed May 28, 2014];Clin Pharmacol Ther. 1993 54(4):360–7. doi: 10.1038/clpt.1993.161. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8222477. [DOI] [PubMed] [Google Scholar]
- 4.Chappey O, Niel E, Dervichian M, Wautier JL, Scherrmann JM, Cattan D. Colchicine concentration in leukocytes of patients with familial Mediterranean fever. [Accessed January 13, 2014];Br J Clin Pharmacol. 1994 38(1):87–9. doi: 10.1111/j.1365-2125.1994.tb04328.x. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1364844&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niel E, Scherrmann J-M. Colchicine today. [Accessed January 13, 2014];Joint Bone Spine. 2006 73(6):672–8. doi: 10.1016/j.jbspin.2006.03.006. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17067838. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez C, Figarella-Branger D, Alla P, Harlé J-R, Pellissier J-F. Colchicine myopathy: a vacuolar myopathy with selective type I muscle fiber involvement. An immunohistochemical and electron microscopic study of two cases. [Accessed January 13, 2014];Acta Neuropathol. 2002 103(2):100–6. doi: 10.1007/s004010100434. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11810174. [DOI] [PubMed] [Google Scholar]
- 7.Kuncl RW, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. [Accessed May 28, 2014];N Engl J Med. 1987 316(25):1562–8. doi: 10.1056/NEJM198706183162502. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3035372. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Chetrit E, Scherrmann JM, Zylber-Katz E, Levy M. Colchicine disposition in patients with familial Mediterranean fever with renal impairment. [Accessed May 28, 2014];J Rheumatol. 1994 21(4):710–3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8035398. [PubMed] [Google Scholar]
- 9.Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. [Accessed January 13, 2014];J Clin Rheumatol. 2013 19(1):19–29. doi: 10.1097/RHU.0b013e31827d8790. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3551244&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng VCC, Ho PL, Yuen KY. Two probable cases of serious drug interaction between clarithromycin and colchicine. [Accessed January 13, 2014];South Med J. 2005 98(8):811–3. doi: 10.1097/01.SMJ.0000163315.02563.B2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16144178. [DOI] [PubMed] [Google Scholar]
- 11.Dogukan A, Oymak FS, Taskapan H, Güven M, Tokgoz B, Utas C. Acute fatal colchicine intoxication in a patient on continuous ambulatory peritoneal dialysis (CAPD). Possible role of clarithromycin administration. [Accessed January 13, 2014];Clin Nephrol. 2001 55(2):181–2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11269688. [PubMed] [Google Scholar]
- 12.Wason S, Digiacinto JL, Davis MW. Effect of cyclosporine on the pharmacokinetics of colchicine in healthy subjects. [Accessed January 13, 2014];Postgrad Med. 2012 124(4):189–96. doi: 10.3810/pgm.2012.07.2579. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22913907. [DOI] [PubMed] [Google Scholar]
- 13.FDA prescribing information for colchicine. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 14.Rochdi M, Sabouraud A, Girre C, Venet R, Scherrmann JM. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. [Accessed May 27, 2014];Eur J Clin Pharmacol. 1994 46(4):351–4. doi: 10.1007/BF00194404. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7957521. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EW. The Mechanism of Colchicine Inhibition of Mitosis. I. Kinetics of Inhibition and the Binding of H3-Colchicine. [Accessed May 28, 2014];J Cell Biol. 1965 25(SUPPL):145–60. doi: 10.1083/jcb.25.1.145. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2106604&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terkeltaub RA. Colchicine update: 2008. [Accessed January 13, 2014];Semin Arthritis Rheum. 2009 38(6):411–9. doi: 10.1016/j.semarthrit.2008.08.006. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18973929. [DOI] [PubMed] [Google Scholar]
- 17.Paschke S, Weidner AF, Paust T, Marti O, Beil M, Ben-Chetrit E. Technical advance: Inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments. [Accessed January 15, 2014];J Leukoc Biol. 2013 94(5):1091–6. doi: 10.1189/jlb.1012510. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23901122. [DOI] [PubMed] [Google Scholar]
- 18.Rudolph SA, Greengard P, Malawista SE. Effects of colchicine on cyclic AMP levels in human leukocytes. [Accessed January 13, 2014];Proc Natl Acad Sci U S A. 1977 74(8):3404–8. doi: 10.1073/pnas.74.8.3404. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=431582&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. [Accessed January 13, 2014];J Clin Invest. 1995 96(2):994–1002. doi: 10.1172/JCI118147. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=185287&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. [Accessed January 9, 2014];Nature. 2006 440(7081):237–41. doi: 10.1038/nature04516. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16407889. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Davis GS, Mohr C, Nain M, Gemsa D. Suppression of LPS-induced tumor necrosis factor-alpha gene expression by microtubule disrupting agents. [Accessed January 13, 2014];Immunobiology. 1996 195(4–5):640–54. doi: 10.1016/s0171-2985(96)80028-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8933163. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie E, Levine RJ, Malawista SE. Histamine release from rat peritoneal mast cells: inhibition by colchicine and potentiation by deuterium oxide. [Accessed January 13, 2014];J Pharmacol Exp Ther. 1968 164(1):158–65. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4177465. [PubMed] [Google Scholar]
- 23.Fell HB, Lawrence CE, Bagga MR, Hembry RM, Reynolds JJ. The degradation of collagen in pig synovium in vitro and the effect of colchicine. [Accessed January 13, 2014];Matrix. 1989 9(2):116–26. doi: 10.1016/s0934-8832(89)80029-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2542741. [DOI] [PubMed] [Google Scholar]
- 24.Finkelstein Y, Aks SE, Hutson JR, et al. Colchicine poisoning: the dark side of an ancient drug. [Accessed October 16, 2014];Clin Toxicol (Phila) 2010 48(5):407–14. doi: 10.3109/15563650.2010.495348. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20586571. [DOI] [PubMed] [Google Scholar]
- 25.Mullins ME, Carrico EA, Horowitz BZ. Fatal cardiovascular collapse following acute colchicine ingestion. [Accessed October 21, 2014];J Toxicol Clin Toxicol. 2000 38(1):51–4. doi: 10.1081/clt-100100916. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10696925. [DOI] [PubMed] [Google Scholar]
- 26.Aghabiklooei A, Zamani N, Hassanian-Moghaddam H, Nasouhi S, Mashayekhian M. Acute colchicine overdose: report of three cases. [Accessed October 21, 2014];Reumatismo. 2013 65(6):307–11. doi: 10.4081/reumatismo.2013.720. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24705036. [DOI] [PubMed] [Google Scholar]
- 27.Yousuf Bhat Z, Reddy S, Pillai U, Doshi M, Wilpula E. Colchicine-Induced Myopathy in a Tacrolimus-Treated Renal Transplant Recipient: Case Report and Literature Review. [Accessed October 21, 2014];Am J Ther. 2014 doi: 10.1097/MJT.0000000000000044. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24732905. [DOI] [PubMed]
- 28.Kuncl RW, Duncan G. Chronic human colchicine myopathy and neuropathy. [Accessed October 23, 2014];Arch Neurol. 1988 45(3):245–6. doi: 10.1001/archneur.1988.00520270015009. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3341942. [DOI] [PubMed] [Google Scholar]
- 29.Boonmuang P, Nathisuwan S, Chaiyakunapruk N, et al. Characterization of Statin-Associated Myopathy Case Reports in Thailand Using the Health Product Vigilance Center Database. [Accessed October 26, 2014];Drug Saf. 2013 doi: 10.1007/s40264-013-0055-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23615756. [DOI] [PubMed]
- 30.Oh DHJ, Chan SQ, Wilson AM. Myopathy and possible intestinal dysfunction in a patient treated with colchicine and simvastatin. [Accessed October 26, 2014];Med J Aust. 2012 197(6):332–3. doi: 10.5694/mja12.10333. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22994827. [DOI] [PubMed] [Google Scholar]
- 31.Justiniano M, Dold S, Espinoza LR. Rapid onset of muscle weakness (rhabdomyolysis) associated with the combined use of simvastatin and colchicine. [Accessed October 26, 2014];J Clin Rheumatol. 2007 13(5):266–8. doi: 10.1097/RHU.0b013e318156d977. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17921794. [DOI] [PubMed] [Google Scholar]
- 32.Tufan A, Dede DS, Cavus S, Altintas ND, Iskit AB, Topeli A. Rhabdomyolysis in a patient treated with colchicine and atorvastatin. [Accessed October 26, 2014];Ann Pharmacother. 40(7–8):1466–9. doi: 10.1345/aph.1H064. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16772404. [DOI] [PubMed] [Google Scholar]
- 33.Atasoyu EM, Evrenkaya TR, Solmazgul E. Possible colchicine rhabdomyolysis in a fluvastatin-treated patient. [Accessed October 26, 2014];Ann Pharmacother. 39(7–8):1368–9. doi: 10.1345/aph.1E653. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15956236. [DOI] [PubMed] [Google Scholar]
- 34.Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. Monosodium urate monohydrate crystal-recruited noninflammatory monocytes differentiate into M1-like proinflammatory macrophages in a peritoneal murine model of gout. [Accessed January 15, 2014];Arthritis Rheum. 2011 63(5):1322–32. doi: 10.1002/art.30249. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21538316. [DOI] [PubMed] [Google Scholar]
- 35.Terkeltaub RA, Sklar LA, Mueller H. Neutrophil activation by inflammatory microcrystals of monosodium urate monohydrate utilizes pertussis toxin-insensitive and -sensitive pathways. [Accessed January 15, 2014];J Immunol. 1990 144(7):2719–24. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2108211. [PubMed] [Google Scholar]
- 36.Popa-Nita O, Naccache PH. Crystal-induced neutrophil activation. [Accessed January 15, 2014];Immunol Cell Biol. 2010 88(1):32–40. doi: 10.1038/icb.2009.98. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19949421. [DOI] [PubMed] [Google Scholar]
- 37.Mitroulis I, Kambas K, Ritis K. Neutrophils, IL-1β, and gout: is there a link? [Accessed January 15, 2014];Semin Immunopathol. 2013 35(4):501–12. doi: 10.1007/s00281-013-0361-0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23344781. [DOI] [PubMed] [Google Scholar]
- 38.Haskard DO, Landis RC. Interactions between leukocytes and endothelial cells in gout: lessons from a self-limiting inflammatory response. [Accessed January 15, 2014];Arthritis Res. 2002 4(Suppl 3):S91–7. doi: 10.1186/ar562. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3240138&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. [Accessed January 13, 2014];Arthritis Rheum. 2010 62(4):1060–8. doi: 10.1002/art.27327. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20131255. [DOI] [PubMed] [Google Scholar]
- 40.Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. [Accessed January 14, 2014];Arthritis Care Res (Hoboken) 2012 64(10):1447–61. doi: 10.1002/acr.21773. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3662546&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wortmann RL, Macdonald PA, Hunt B, Jackson RL. Effect of prophylaxis on gout flares after the initiation of urate-lowering therapy: analysis of data from three phase III trials. [Accessed April 6, 2014];Clin Ther. 2010 32(14):2386–97. doi: 10.1016/j.clinthera.2011.01.008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21353107. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Doherty M, Pascual E, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. [Accessed January 15, 2014];Ann Rheum Dis. 2011 70(4):571–5. doi: 10.1136/ard.2010.139360. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21257614. [DOI] [PubMed] [Google Scholar]
- 43.Tabatabai MR, Cummings NA. Intravenous colchicine in the treatment of acute pseudogout. [Accessed November 2, 2014];Arthritis Rheum. 1980 23(3):370–4. doi: 10.1002/art.1780230320. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7362691. [DOI] [PubMed] [Google Scholar]
- 44.Portincasa P, Scaccianoce G, Palasciano G. Familial mediterranean fever: a fascinating model of inherited autoinflammatory disorder. [Accessed January 13, 2014];Eur J Clin Invest. 2013 43(12):1314–27. doi: 10.1111/eci.12170. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24117178. [DOI] [PubMed] [Google Scholar]
- 45.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. [Accessed January 22, 2014];Cell. 1997 90(4):797–807. doi: 10.1016/s0092-8674(00)80539-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9288758. [DOI] [PubMed] [Google Scholar]
- 46.A candidate gene for familial Mediterranean fever. [Accessed January 22, 2014];Nat Genet. 1997 17(1):25–31. doi: 10.1038/ng0997-25. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9288094. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasula SM, Poyet J-L, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. [Accessed January 22, 2014];J Biol Chem. 2002 277(24):21119–22. doi: 10.1074/jbc.C200179200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11967258. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Manji GA, Grenier JM, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. [Accessed January 22, 2014];J Biol Chem. 2002 277(33):29874–80. doi: 10.1074/jbc.M203915200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12019269. [DOI] [PubMed] [Google Scholar]
- 49.Lidar M, Livneh A. Familial Mediterranean fever: clinical, molecular and management advancements. [Accessed January 13, 2014];Neth J Med. 2007 65(9):318–24. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17954950. [PubMed] [Google Scholar]
- 50.Centola M, Wood G, Frucht DM, et al. The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. [Accessed May 28, 2014];Blood. 2000 95(10):3223–31. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10807793. [PubMed] [Google Scholar]
- 51.Taskiran EZ, Cetinkaya A, Balci-Peynircioglu B, Akkaya YZ, Yilmaz E. The effect of colchicine on pyrin and pyrin interacting proteins. [Accessed January 13, 2014];J Cell Biochem. 2012 113(11):3536–46. doi: 10.1002/jcb.24231. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22730186. [DOI] [PubMed] [Google Scholar]
- 52.Mansfield E, Chae JJ, Komarow HD, et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. [Accessed January 13, 2014];Blood. 2001 98(3):851–9. doi: 10.1182/blood.v98.3.851. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11468188. [DOI] [PubMed] [Google Scholar]
- 53.Goldfinger SE. Colchicine for familial Mediterranean fever. [Accessed January 13, 2014];N Engl J Med. 1972 287(25):1302. doi: 10.1056/NEJM197212212872514. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4636899. [DOI] [PubMed] [Google Scholar]
- 54.Dinarello CA, Wolff SM, Goldfinger SE, Dale DC, Alling DW. Colchicine therapy for familial mediterranean fever. A double-blind trial. [Accessed January 13, 2014];N Engl J Med. 1974 291(18):934–7. doi: 10.1056/NEJM197410312911804. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4606353. [DOI] [PubMed] [Google Scholar]
- 55.Zemer D, Revach M, Pras M, et al. A controlled trial of colchicine in preventing attacks of familial mediterranean fever. [Accessed January 13, 2014];N Engl J Med. 1974 291(18):932–4. doi: 10.1056/NEJM197410312911803. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4606109. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Chetrit E, Levy M. Colchicine prophylaxis in familial Mediterranean fever: reappraisal after 15 years. [Accessed January 13, 2014];Semin Arthritis Rheum. 1991 20(4):241–6. doi: 10.1016/0049-0172(91)90019-v. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2042056. [DOI] [PubMed] [Google Scholar]
- 57.Grattagliano I, Bonfrate L, Ruggiero V, Scaccianoce G, Palasciano G, Portincasa P. Novel therapeutics for the treatment of familial mediterranean Fever: from colchicine to biologics. [Accessed January 15, 2014];Clin Pharmacol Ther. 2013 95(1):89–97. doi: 10.1038/clpt.2013.148. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23867542. [DOI] [PubMed] [Google Scholar]
- 58.Yurdakul S, Mat C, Tüzün Y, et al. A double-blind trial of colchicine in Behçet’s syndrome. [Accessed January 13, 2014];Arthritis Rheum. 2001 44(11):2686–92. doi: 10.1002/1529-0131(200111)44:11<2686::aid-art448>3.0.co;2-h. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11710724. [DOI] [PubMed] [Google Scholar]
- 59.Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, et al. Colchicine versus placebo in Behçet’s disease: randomized, double-blind, controlled crossover trial. [Accessed January 13, 2014];Mod Rheumatol. 2009 19(5):542–9. doi: 10.1007/s10165-009-0200-2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19597921. [DOI] [PubMed] [Google Scholar]
- 60.Aktulga E, Altaç M, Müftüoglu A, et al. A double blind study of colchicine in Behçet’s disease. [Accessed January 13, 2014];Haematologica. 1980 65(3):399–402. Available at: http://www.ncbi.nlm.nih.gov/pubmed/6778795. [PubMed] [Google Scholar]
- 61.Callen JP. Colchicine is effective in controlling chronic cutaneous leukocytoclastic vasculitis. [Accessed January 13, 2014];J Am Acad Dermatol. 1985 13(2 Pt 1):193–200. doi: 10.1016/s0190-9622(85)70158-2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4044947. [DOI] [PubMed] [Google Scholar]
- 62.Ashida A, Murata H, Ohashi A, Ogawa E, Uhara H, Okuyama R. A case of hypocomplementaemic urticarial vasculitis with a high serum level of rheumatoid factor. [Accessed January 13, 2014];Australas J Dermatol. 2013 54(3):e62–3. doi: 10.1111/j.1440-0960.2011.00873.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23905981. [DOI] [PubMed] [Google Scholar]
- 63.Werni R, Schwarz T, Gschnait F. Colchicine treatment of urticarial vasculitis. [Accessed January 13, 2014];Dermatologica. 1986 172(1):36–40. doi: 10.1159/000249290. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3956819. [DOI] [PubMed] [Google Scholar]
- 64.Saulsbury FT. Successful treatment of prolonged Henoch-Schönlein purpura with colchicine. [Accessed January 13, 2014];Clin Pediatr (Phila) 2009 48(8):866–8. doi: 10.1177/0009922809337532. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19460892. [DOI] [PubMed] [Google Scholar]
- 65.Pyne D, Mootoo R, Bhanji A. Colchicine for the treatment of recurrent Henoch-Schönlein purpura in an adult. [Accessed January 13, 2014];Rheumatology (Oxford) 2001 40(12):1430–1. doi: 10.1093/rheumatology/40.12.1430. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11752528. [DOI] [PubMed] [Google Scholar]
- 66.Suehisa S, Tagami H. Treatment of acute febrile neutrophilic dermatosis (Sweet’s syndrome) with colchicine. [Accessed January 13, 2014];Br J Dermatol. 1981 105(4):483. doi: 10.1111/j.1365-2133.1981.tb00785.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7295564. [DOI] [PubMed] [Google Scholar]
- 67.Maillard H, Leclech C, Peria P, Avenel-Audran M, Verret JL. Colchicine for Sweet’s syndrome. A study of 20 cases. [Accessed January 13, 2014];Br J Dermatol. 1999 140(3):565–6. doi: 10.1046/j.1365-2133.1999.02747.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10233302. [DOI] [PubMed] [Google Scholar]
- 68.Maisch B, Seferoviæ PM, Ristiæ AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. [Accessed January 13, 2014];Eur Heart J. 2004 25(7):587–610. doi: 10.1016/j.ehj.2004.02.002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15120056. [DOI] [PubMed] [Google Scholar]
- 69.Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. [Accessed January 13, 2014];Arch Intern Med. 2005 165(17):1987–91. doi: 10.1001/archinte.165.17.1987. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16186468. [DOI] [PubMed] [Google Scholar]
- 70.Imazio M, Brucato A, Cemin R, et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. [Accessed January 13, 2014];Ann Intern Med. 2011 155(7):409–14. doi: 10.7326/0003-4819-155-7-201110040-00359. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21873705. [DOI] [PubMed] [Google Scholar]
- 71.Imazio M, Belli R, Brucato A, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. [April 1, 2014];Lancet. 2014 doi: 10.1016/S0140-6736(13)62709-9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24694983. [DOI] [PubMed]
- 72.Cacoub PP. Colchicine for treatment of acute or recurrent pericarditis. [Accessed October 23, 2014];Lancet. 2014 383(9936):2193–4. doi: 10.1016/S0140-6736(14)60113-6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24694984. [DOI] [PubMed] [Google Scholar]
- 73.Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. [Accessed January 10, 2014];Circulation. 2005 112(13):2012–6. doi: 10.1161/CIRCULATIONAHA.105.542738. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16186437. [DOI] [PubMed] [Google Scholar]
- 74.Imazio M, Brucato A, Cemin R, et al. A randomized trial of colchicine for acute pericarditis. [Accessed January 10, 2014];N Engl J Med. 2013 369(16):1522–8. doi: 10.1056/NEJMoa1208536. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23992557. [DOI] [PubMed] [Google Scholar]
- 75.Nidorf M, Thompson PL. Effect of colchicine (0.5 mg twice daily) on high-sensitivity C-reactive protein independent of aspirin and atorvastatin in patients with stable coronary artery disease. [Accessed January 13, 2014];Am J Cardiol. 2007 99(6):805–7. doi: 10.1016/j.amjcard.2006.10.039. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17350370. [DOI] [PubMed] [Google Scholar]
- 76.Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. [Accessed January 13, 2014];J Thromb Thrombolysis. 2012 33(1):88–94. doi: 10.1007/s11239-011-0637-y. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21918905. [DOI] [PubMed] [Google Scholar]
- 77.Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. [Accessed January 13, 2014];J Rheumatol. 2012 39(7):1458–64. doi: 10.3899/jrheum.111533. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3733459&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. [Accessed January 13, 2014];J Am Coll Cardiol. 2013 61(4):404–10. doi: 10.1016/j.jacc.2012.10.027. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23265346. [DOI] [PubMed] [Google Scholar]
- 79.Deftereos S, Giannopoulos G, Raisakis K, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. [Accessed January 13, 2014];J Am Coll Cardiol. 2013 61(16):1679–85. doi: 10.1016/j.jacc.2013.01.055. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23500260. [DOI] [PubMed] [Google Scholar]
- 80.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. [Accessed January 13, 2014];Circulation. 2011 124(21):2290–5. doi: 10.1161/CIRCULATIONAHA.111.026153. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22090167. [DOI] [PubMed] [Google Scholar]
- 81.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. [Accessed October 19, 2014];JAMA. 2014 312(10):1016–23. doi: 10.1001/jama.2014.11026. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25172965. [DOI] [PubMed] [Google Scholar]
- 82.Deftereos S, Giannopoulos G, Kossyvakis C, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. [Accessed January 13, 2014];J Am Coll Cardiol. 2012 60(18):1790–6. doi: 10.1016/j.jacc.2012.07.031. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23040570. [DOI] [PubMed] [Google Scholar]