Abstract
The basic Helix-Loop-Helix (bHLH) proteins represent a well-known class of transcriptional regulators. Many bHLH proteins act as heterodimers with members of a class of ubiquitous partners, the E-proteins. A widely-expressed class of inhibitory heterodimer partners- the Inhibitor of DNA-binding (ID) proteins- also exists. Genetic and molecular analyses in humans and in knockout mice implicate E-proteins and ID-proteins in a wide variety of diseases, belying the notion that they are non-specific partner proteins. Here, we explore relationships of E-proteins and ID-proteins to a variety of disease processes and highlight gaps in knowledge of disease mechanisms.
Keywords: Helix-loop-helix protein, E-protein, Id-protein, Differentiation, Cell cycle regulation, Cellular senescence, Neuronal morphogenesis, Development, Disease, Pitt-Hopkins Syndrome, Fuchs corneal dystrophy, Schizophrenia, Rett Syndrome, Atherosclerosis, Diabetes, Osteoporosis, arterial vascular disease, pulmonary arterial hypertension congenital hydronephrosis, Parkinson's disease, Sjogren's syndrome, mammary gland, sertoli cell, trophoblast
E-proteins and Id-proteins are widely-expressed transcriptional regulators with very general functions. They are implicated in diseases by evidence ranging from confirmed Mendelian inheritance, association studies, and mouse models that resemble human disorders. Here we briefly recap the properties of E-proteins and Id-proteins in development and differentiation, cell proliferation and cancer that have been gleaned from decades of study (Massari and Murre, 2000; Murre, 2005; Belle and Zhuang, 2014; Lasorella et al., 2014; Ling et al., 2014; Nair et al., 2014), before describing disease associations where the mechanisms of E-proteins and Id-proteins contributions is incompletely understood.
HLH proteins in differentiation
The helix-loop-helix domain was first recognized in dimeric transcription factors that regulate the IgG enhancer in B cells. Since these bind to Ephrussi-box (E-box) sequences (CANNTG), the first HLH proteins were named E-proteins (Murre et al., 1989). Mammalian E proteins include E12 and E47, which arise through alternative splicing of the E2A (HGNC:11633) gene, E2-2 (HGNC:11634), and HEB (HGNC:11623). Renaming these proteins TCF3, TCF4 and TCF12 respectively has been suggested, but caution must be used to avoid confusion since the acronym TCF had already been assigned to unrelated proteins (Table 1). There is a single E-protein in Drosophila, the Daughterless (Da) protein.
Table 1.
HLH protein nomenclature
| Protein name | Old gene name | New gene name |
|---|---|---|
| E12, E47 | E2A | TCF3 |
| E2-2 | E2-2 | TCF4 |
| HEB | HEB | TCF12 |
E proteins have many functions as heterodimer partners of the tissue-specific bHLH proteins that are sometimes called Class II bHLH proteins. The diverse Class II bHLH proteins include the myogenic transcription factors exemplified by MYOD, a factor sufficient to transform cultured fibroblasts into myoblasts, the conserved proneural proteins such as Achaete and Scute from Drosophila, and hematopoiesis protein T-cell acute lymphocytic leukemia 1 (TAL1) (Massari and Murre, 2000; Powell and Jarman, 2008). The paradigm that was soon established is that tissue-specific Class II bHLH factors whose specific expression defines specification and differentiation programs such as myogenesis or neurogenesis act by conferring DNA binding specificity and transcriptional activation on heterodimers with the ubiquitous E-proteins (Figure 1).
Figure 1. Transcriptional modalities of HLH proteins.
(A) Transcriptional activation of Drosophila proneural genes requires the ubiquitously expressed E protein (Da) forming heterodimers with tissue-specific bHLH proteins (e.g. Ac/Sc) and binds to promoter E boxes. (B) Transcription of myogenic genes is controlled by the binding of E protein-myogenic specific bHLH protein (MyoD) heterodimers to promoter E boxes. (C) E protein (or Da)-ID (or Emc) protein heterodimers fail to bind DNA and can not activate transcription.
Another class of pervasive HLH proteins act in opposition to E-proteins. These Inhibitor of DNA binding (ID) proteins lack a basic DNA binding region, so that heterodimers of ID proteins with E-proteins or with Class II bHLH proteins are unable to bind DNA or activate transcription (Benezra et al., 1990). ID protein levels may be low in differentiated cells, but are abundant in proliferating, multipotent cells including stem cell populations (Ling et al., 2014). Like the E-proteins, ID-proteins are represented by a single homolog in Drosophila, the extra macrochaetae (emc) gene, which was identified in parallel with mammalian ID proteins. emc encodes a negative regulator of proneural bHLH proteins including Achaete and Scute, and is a negative regulator of neurogenesis so that emc mutants exhibit ectopic neural differentiation (Ellis et al., 1990; Garrell and Modolell, 1990). Figure 1 summarizes the basic outline of HLH function in cell type specification and differentiation, in which specific expression of particular class II bHLH proteins must compete with ID proteins to heterodimerize with E proteins and form tissue- and cell-type-specific transcription factors that drive specification and differentiation. bHLH proteins are now known to regulate differentiation in too many additional situations to be listed here. For example, E-proteins and ID-proteins regulate Sertoli cell differentiation (Chaudhary et al., 2005), possibly through the class II bHLH proteins POD1/Epicardin (TCF21) and Scleraxis (Muir et al., 2006; Bhandari et al., 2011).
Expression of the only E protein, Da, in Drosophila seems to be ubiquitous, whereas particular mammalian E-protein and Id-protein genes have various overlapping patterns of expression. Id1 and Id3 in particular are often upregulated by BMP signals, and Id1 by cytokines (Ling et al., 2014). TGFβ,FGF and TCR signaling also regulate Id gene expression (Lasorella et al., 2014; Ling et al., 2014). Both Drosophila and mammals exhibit cross-talk between Id proteins and Notch signaling, consistent with their common regulation of bHLH-mediated differentiation (Baonza et al., 2000; Adam and Montell, 2004; Bhattacharya and Baker, 2009; Lasorella et al., 2014; Ling et al., 2014). It is increasingly recognized that Id genes can be transcriptional targets of E proteins, in Drosophila and in mammals. This makes Id proteins feedback inhibitors of E protein activity, which may be particularly important as a brake on E protein autoregulation (Bhattacharya and Baker, 2011; Schmitz et al., 2012) (Figure 2). Id proteins are also regulated at the level of protein stability (Lingbeck et al., 2005; Lasorella et al., 2006; Kim et al., 2009; Williams et al., 2011b; Lasorella et al., 2014).
Figure 2. Autoregulation and feedback inhibition in normal and disease conditions.
(A) Emc/ID expression requires Da activity. Once Emc/ID level is upregulated, it will in turn block autoregulation of da and Da activity (Bhattacharya and Baker, 2011). This regulation may ensure the steady state of E protein and ID protein levels in normal progenitor cells. (B) ID proteins are transcriptional targets of E proteins in Burkitt lymphoma cells. Both gain of function mutations of E protein (indicated by+symbol) and loss of function mutations of ID protein (indicated by X symbol) found in Burkitt lymphoma interrupt the feedback network between E and ID proteins. Cyclin D3 is also a target gene of E proteins in Burkitt lymphoma cells, and may contribute to the excessive proliferation of tumor cells (Schmitz et al., 2012).
Since mouse knock outs of each of the E-proteins and Id proteins have mutant phenotypes, neither E-proteins nor Id-proteins can be completely redundant. The example of Id1 and Id3 double null (Id1−/−; Id3−/−) mice, which exhibit premature neuronal differentiation, forebrain vascular abnormalities, cardiac defects and embryonic lethality not seen in the single mutants (Lyden et al., 1999; Lasorella et al., 2014), illustrates significant functional overlap that may also occur for other Id-proteins, and similarly between E-proteins (Table 2). Non-overlapping expression accounts for some non-redundancy, although unique protein functions may also exist, especially in the case of E-proteins that differ in basic region sequence and DNA-binding preference. It is thought that any unique functions of individual proteins are superimposed on the overall ratio of total E-proteins to total Id-proteins within a cell that is an important index of competence in differentiation and other processes. For example, elevating Da levels, which are controlled by feedback through Emc, affect the capacity of Ato or Sc to induce neurons in Drosophila (Bhattacharya and Baker, 2011), and elevated E protein levels are thought to act similarly in the postnatal mouse brain (Fischer et al., 2014).
Table 2.
Phenotypes of HLH protein knockout mice
| Genotype | Phenotype | References |
|---|---|---|
| E2A−/− | Disrupted B cell development; lymphopoietic developmental defects with high frequency of T-cell lymphomas | Bain et al., 1994; Bain et al., 1997; Yan et al., 1997 |
| HEB−/− | Reduced pro-B cells; Developmental arrest of thymocyte development; | Zhuang et al., 1996 |
| E2-2−/− | Reduced pro-B cells; Normal mammary gland development; abnormal hindbrain development in the pontine nucleus; | Zhuang et al., 1996; Flora et al., 2007; Itahana et al., 2008 |
| Id1−/− | Altered lipid and glucose metabolism; osteoporotic phenotype; normal mammary gland development; impaired cancer stem cell properties in glioma | de Candia et al., 2004; Chan et al., 2009; Satyanarayana et al., 2012; Lasorella et al., 2014 |
| Id2−/− | Altered lipid and glucose metabolism; hydronephrosis (smooth muscle hypertrophy at the ureteropelvic junction); Parkinson’s disease-like features (impaired dopaminergic system); reduced proliferation in mammary epithelia (lactation defect); reduced body size; absence of lymph nodes and Peyer’s patches; absence of NK cells and Langerhans cells | Aoki et al., 2004; de Candia et al., 2004; Havrda et al., 2008; Park et al., 2008; Hou et al., 2009; Mathew et al., 2013; Tripathi et al., 2012; Havrda et al., 2013; Lasorella et al., 2014 |
| Id3−/− | symptoms of Sjögren’s syndrome (impaired tear and saliva secretion due to defective autoimmune system); normal mammary gland development | de Candia et al., 2004; Li et al., 2004 |
| Id4−/− | premature neuronal differentiation; altered lipid and glucose metabolism; reduce osteoblast differentiation; enhance adipocyte differentiation; reduced proliferation and increased apoptosis in mammary cells | Dong et al., 2011; Murad et al., 2010; Tokuzawa et al., 2010; Lasorella et al., 2014; |
| Id1−/− Id3−/− | premature neuronal differentiation; cardiac developmental defects; brain vascular abnormalities; embryonic lethality | Lyden et al., 1999; Lasorella et al., 2014 |
| Id1−/+ Id3−/− | decreases of proliferation and mineralization in osteoblasts; vascular defects and growth failure in tumor cell xenografts genetic models of cancer | Maeda et al., 2004; Lasorella et al., 2014 |
| Id3−/− ApoE−/− or Id3−/− Ldlr−/− | Enhanced atherosclerosis | Doran et al., 2010; Lipinski et al., 2012 |
HLH Proteins in the cell cycle and senescence
The commitment of progenitor cells to begin cell differentiation is often associated with cell cycle withdrawal. MYOD provided the first example connecting bHLH proteins to cell cycle arrest, inhibiting cell cycling as myoblasts differentiate (Gu et al., 1993; Halevy et al., 1995; Guo et al., 1995; Zhang et al., 1999). Subsequent studies have uncovered a common relationship between E-and ID-proteins and cell cycle progression. In many cells, E12 or E47 homodimers activate transcription of the CDK inhibitors (CDKIs) p15, p16 (also known as INK4a), p21, p27 and p57 (Sloan et al., 1996; Prabhu et al., 1997; Pagliuca et al., 2000)(Figure 3). Through this mechanism, E proteins inhibit cell cycle progression, and are also implicated in cellular senescence (Ling et al., 2014). In fact E47 is necessary for senescence of human fibroblasts, which is prevented by E-protein knockdown (Zheng et al., 2004).
Figure 3. Mammalian HLH proteins and cell cycle control.
Cell cycle regulation involving HLH proteins in multiple ways. E protein-mediated activation of the CDK inhibitors p16, p21, p27 and p57 is shown. Cell cycle arrest is promoted by these CDK inhibitors in G1-S transition, S and G2-M phases. This regulation is negatively modulated by ID proteins through the association with E proteins. Rb can antagonize ID2 by direct interaction.
As one might expect, the role of E proteins is blocked by ID proteins (Peverali et al., 1994). ID proteins can promote RB inactivation and activate E2F-mediated transcription and cell-cycle progression indirectly because of their inhibition of E proteins and consequently inhibition of CDKIs’ expression, while ID2 can be sequestered by RB directly (Lasorella et al., 2014; Ling et al., 2014) (Figure 3). ID1 and ID3 are degraded during senescence (Kong et al., 2011). ID proteins regulate proliferation in many important tissues, for example during the expansion of mammary epithelia during pregnancy (de Candia et al., 2004). Because of their dual effects on differentiation and proliferation, Id protein expression is associated with stem cell maintenance, and, in tumors, anaplasia (Nam and Benezra, 2009; Anido et al., 2010; Barrett et al., 2012; Niola et al., 2012; Lasorella et al., 2014; Ling et al., 2014; Nair et al., 2014).
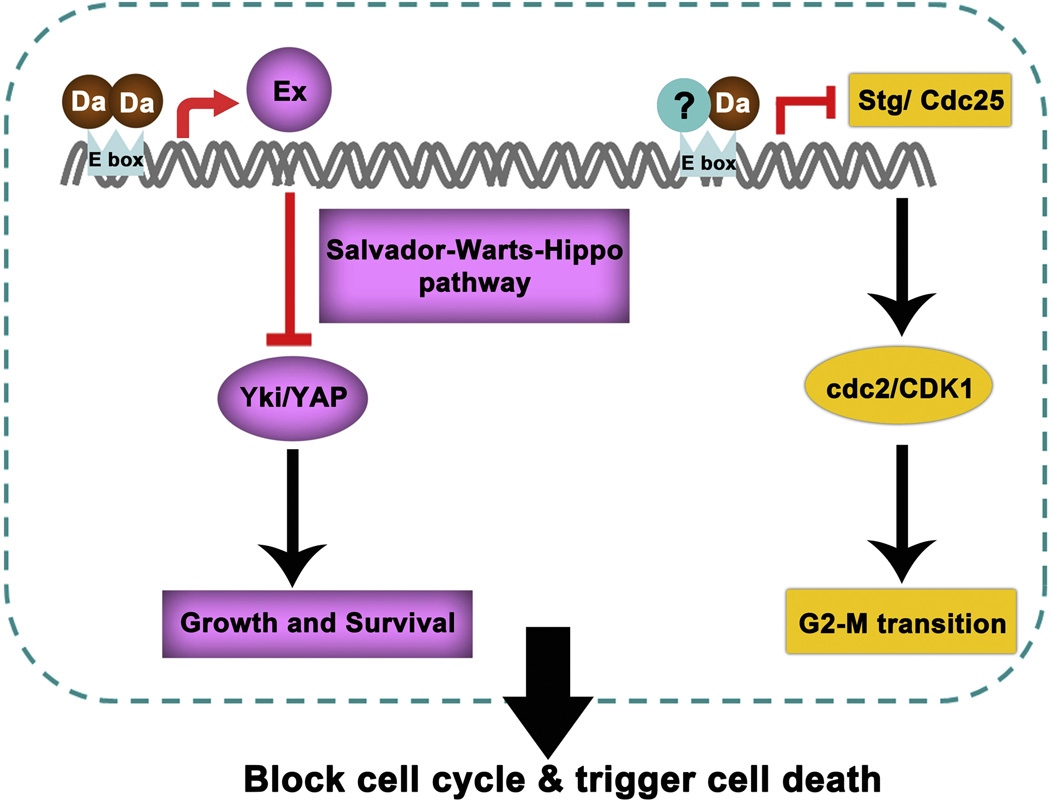
Although this general relationship is seen in many cells there are examples of different effects. Id1 is reported to protect mammary epithelia cells from senescence independently of CDKI expression (Swarbrick et al., 2008), and rapidly-dividing transit amplifying cells surprisingly express less Id1 than their quiescent neural stem cell progenitors (Nam and Benezra, 2009). The poor growth of Drosophila cells lacking emc is also due to Da hyperactivity, not attributed to CDKI expression but to repression of the phosphatase Cdc25/string or to overexpression of the gene expanded within the Salvador-Warts-Hippo pathway of tumor suppressors (Bhattacharya and Baker, 2011; Andrade-Zapata and Baonza, 2014; Wang and Baker, 2015) (Figure 4). It is also envisaged that ID functions independent of dimerization with HLH proteins may exist (Ling et al, 2014).
Figure 4. Drosophila HLH proteins in growth control.
When Drosophila ID protein (emc) is mutated, excess Drosophila E protein (Da) homodimers activate expanded (ex) transcription via binding to E boxes in the cis-regulatory element. The subsequent activation of Salvador-Warts-Hippo signaling pathway inhibits Yki/YAP activity, thereby blocking cell growth and survival. High levels of Da are also involved in blocking cell cycle progression in G2-M transition through inhibiting stg/cdc25 transcription (Wang and Baker, 2015).
E-proteins and ID-proteins in cancer
There is a well-established connection between cancer and E-proteins (Murre, 2005; Belle and Zhuang, 2014). In addition to lymphoma predisposition in E2A mutant mice, E2A mutations have been found in many human lymphomas and leukemias (Murre, 2005; Tijchon et al., 2013; Belle and Zhuang, 2014; Cozen et al., 2014). As would be expected, ID proteins mostly have the opposite properties (Lasorella et al., 2014; Nair et al., 2014). ID levels are elevated in human tumors ranging from melanoma to neuroblastoma (Perk et al., 2005). ID1 and ID3 are clearly implicated in lung cancer initiation, and ID4 is overexpressed by a t(6;14)(p22;q32) chromosomal translocation in leukemia and amplified in ovarian cancer, qualifying it as a proto-oncogene (Perk et al., 2005; Lasorella et al., 2014; Nair et al., 2014).
No doubt many oncogenic and tumor suppressor roles of ID proteins and E proteins reflect their roles in cell proliferation, stemness, and senescence, but there are also contributions to metastasis and angiogenesis(Slattery et al., 2008; Lasorella et al., 2014; Ling et al., 2014; Nair et al., 2014). ID proteins are required to maintain glioma stem cells in the perivascular niche by inhibiting E-protein transcription of the RAP1GAP gene, since RAP1 controls cancer stem cell adhesion to the niche through integrin signaling (Niola et al., 2013). Unexpectedly, ID3 and ID4 sometimes behave as tumor suppressors (Yu et al., 2005; Chen et al., 2011; Love et al., 2012; Richter et al., 2012; Schmitz et al., 2012). For example, recurrent mutations in the E47 (TCF3) and ID3 genes occur in Burkitt’s lymphoma and may allow unrestrained E47 expression to activate both cyclin D3 and PI3K survival signaling (Love et al., 2012; Richter et al., 2012; Schmitz et al., 2012) (Figure 2B).
Post-mitotic ID functions: Neuronal morphogenesis
In addition to roles in cell fate specification and in the regulation of the cell cycle and senescence, in postmitotic neurons ID1, ID2 and ID4 are ubiquitinated by the Anaphase Promoting Complex/Cyclosome (APC/C) and their subsequent turnover affects axon and dendrite development (Lasorella et al., 2006; Kim et al., 2009). Mutations of the APC/C interaction motif of ID2 enhance axonal growth in mouse cerebellar granule neurons both in vitro and in the cerebellar cortex, suggesting that ID2 degradation maintains axon morphology by restraining E protein-dependent axon growth (Lasorella et al., 2006). On the other hand, knockdown of ID1 in the cerebellar cortex or primary hippocampal neurons stimulates dendrite morphogenesis, indicating an inhibitory role of ID1 in dendrite development (Kim et al., 2009). APC/C is important in cell cycle exit (Peters, 2006), and therefore might couple cell cycle exit to dendrogenesis in differentiating neurons. It remains unknown at present whether ID-protein downregulation contributes to terminal cell cycle withdrawal in neurons, and how E proteins affect dendrites. These questions are of considerable interest, as neurological disorders are associated with ID- and E-proteins (see below).
Beyond developmental defects and cancer: Connections to disease
E-proteins and ID-proteins have been implicated in a number of human diseases, which may be related to the roles of E- and ID-proteins in differentiation and cycle regulation, or perhaps reflect other mechanisms (Table 3). It is interesting to catalogue these potential disease relationships because their wide range argues persuasively that E- and ID-proteins represent central regulators active in most or all cells, rather than ubiquitous heterodimer partners of little intrinsic interest, and also so that gaps in knowledge can be identified. The following summaries are ordered according to the strength of the evidence linking to human disease, beginning with unambiguous Mendelian genetic disease in humans and progressing through other human data to mouse models that are suggestive but for which direct evidence in human disease is wanting.
Table 3.
HLH protein diseases and mechanisms
| Disease | Affected organ or tissue |
HLH protein | evidence | Process affected |
|---|---|---|---|---|
| Pitt-Hopkins syndrome | Brain, face | E2-2 | Human mutation | Differentiation? Cell cycle? Neuronal morphogenesis? |
| Fuchs corneal dystrophy | eye | E2-2 | Human gene association | senescence? |
| schizophrenia | brain | E2-2 | Human gene association | Differentiation? Cell cycle? Neuronal morphogenesis? |
| Rett syndrome | brain | E2A, ID1-4 | Gene expression | Differentiation? Cell cycle? Neuronal morphogenesis? |
| atherosclerosis | arteries | E2A, ID3 | Human, mouse gene association | Differentiation? |
| Diamond Blackfan anemia | Bone marrow | E2A, E2-2, ID2 | Gene expression | Differentiation? |
| Diabetes, glucose and lipid metabolism | multiple | ID1–2,4 | Mouse mutant models | Differentiation? |
| Osteoporosis | bone | ID1, ID4 | Mouse mutant models | Differentiation? |
| Arterial vascular disease | arteries | ID1, ID3 | Mouse mutant models | Cell cycle? |
| Congenital hydronephrosis | kidney | ID2 | Mouse mutant models | Differentiation? |
| Polycystic kidney disease | Kidney | E2A, ID2 | Gene expression | Cell cycle? |
| Parkinson’s disease | Brain, nerves | ID2 | Mouse mutant models | Differentiation? |
| Sjogren’s syndrome | Lachrymal, salivary glands, skin | ID3 | Mouse mutant models | Differentiation? |
Pitt-Hopkins Syndrome
Haploinsufficiency for the E protein transcription factor E2-2 (TCF4) due to heterozygous mutations or deletions is responsible for Pitt-Hopkins Syndrome (PTHS), an autosomal dominant disorder characterized by severe intellectual disability, global developmental delay, recurrent seizure and hyperventilation (Zweier et al., 2007; Amiel et al., 2007; Giurgea et al., 2008; de Pontual L. et al., 2009). Most of the mutations found in PTHS patients disrupt E2-2 transcriptional activity (Forrest et al., 2012; Sepp et al., 2012). In the developing and mature central nervous system, E2-2 is a heterodimer partner for Class II bHLH proneural and neuronal precursor proteins. Hence, heterozygous loss of function affects a large number of target genes, including the neuronal adhesion receptor genes contactin associated protein-like 2 (CNTNAP2) and neurexin 1 (NRXN1) (Forrest et al., 2012). Both CNTNAP2 and NRXN1 mutations have been associated with PTHS-like disorder, schizophrenia and autism (Alarcon et al., 2008; Zweier et al., 2009; Kirov et al., 2009). PTHS could reflect defective expression of such neuronal differentiation genes that depend on E2-2/proneural gene heterodimers, defective maintenance of cell cycle withdrawal (since unscheduled cell cycle re-entry can be associated with defective neuronal differentiation (Ruggiero et al., 2012)) or be related to the roles of ID1 and ID2 in dendrite and axon morphogenesis, or multiple of these mechanisms. It is also noteworthy that a particular E2-2 mutation has been found in Rett Syndrome (see below), suggesting that related molecular mechanisms lead to PTHS and Rett Syndrome.
Fuchs corneal dystrophy
Multiple SNPs in E2-2 are associated with Fuchs corneal dystrophy (Baratz et al., 2010; Li et al., 2011; Thalamuthu et al., 2011; Eghrari et al., 2012; Stamler et al., 2013; Lau et al., 2014), a common, dominant, progressive, late onset disease in which endothelial cells are gradually lost from the internal surface of the cornea. The hallmark of this disease is the increasing density of tiny bumps, termed guttae, that form on the cornea, loss of the fluid-pumping function of the endothelium cells, decrease in corneal transparency and resultant loss of vision. The lack of Fuchs corneal dystrophy in PTHS patients that have heterozygous loss-of-function E2-2 mutations, suggests that Fuchs corneal dystrophy might involve E2-2 gain-of-function. Interestingly, endothelial samples from Fuchs corneal dystrophy patients exhibit reduced ID1 expression, which could be another route to elevate E2-2 activity (Matthaei et al., 2014)(Figure 1). Since corneal endothelium is normally non-proliferative, cell cycle arrest is unlikely to cause disease, but altered expression of senescence-related genes has been reported (Matthaei et al., 2014). In Drosophila, elevated Da expression can be toxic and activates the Salvador-Warts-Hippo pathway of tumor suppressors (Wang and Baker, 2015), but it is not known whether this occurs in Fuchs corneal dystrophy. A different view is suggested, however, by studies of SNP rs613872 associated with an intronic trinucleotide repeat expansion (Breschel et al., 1997; Wieben et al., 2012). This expansion causes mis-splicing and RNA toxicity, similar to other trinucleotide expansion disorders such as myotonic dystrophy type 1 and 2, fragile X syndrome, and frontotemporal dementia (Du et al., 2015). RNA toxicity affects expression of many other genes, and the effects of this SNP might have little to do with E2-2 function.
Schizophrenia
In addition to linkage to PTHS and Fuchs corneal dystrophy, several SNPs in E2- 2 are significantly associated with schizophrenia (Stefansson et al., 2009; Forrest et al., 2014). E2-2 is also a target of micro-RNA 137, which has been mapped as a schizophrenia susceptibility locus (Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium, 2011; Wright et al., 2013), and predicted to bind other miRNAs associated with schizophrenia, autism and other CNS disorders (Perkins et al., 2007; Talebizadeh et al., 2008). However, none of the variants affect the E2-2 protein, so how E2-2 activity is affected in schizophrenia remains uncertain (Williams et al., 2011a). This has not been resolved by mouse studies. E2-2 homozygous null (E2-2−/−) mice displayed abnormal hindbrain development in the pontine nucleus (Flora et al., 2007) (Table 2). Pontine nucleus development requires heterodimers of E2-2 with Atoh-1 (Flora et al., 2007). Thus, schizophrenia could have a developmental origin caused by reduction of E2-2 function. On the other hand, E2-2 overexpressing transgenic mice have defects in fear conditioning and sensorimotor gating (Brzozka et al., 2010). Sensorimotor gating has been used for a long time in animal model studies because it resembles the oversensitivity to sensory stimulation seen in schizophrenic patients (Braff and Geyer, 1990). Clearly, it would be very informative to resolve how schizophrenia-associated SNPs affect E2-2 function, and how they differ from those associated with Fuchs Corneal Dystrophy.
Rett Syndrome
Rett syndrome is an X-linked dominant disorder primarily caused by loss-of-function mutations in the methyl-CpG-binding protein 2 (MeCP2) gene, which lead to intellectual disability including language deficits, epileptic seizures and respiratory dysfunction (Bedogni et al., 2014). Microarray and chromatin immunoprecipitation analyses identified ID1, ID2, ID3 and ID4 genes as primary targets of MeCP2 (Peddada et al., 2006). MeCP2 is a repressor, and upregulation of all four IDs has been found in MeCP2 null mouse brain (MeCP2-/Y hemizygotes) as well as brain tissue from Rett Syndrome patients (Peddada et al., 2006). The MeCP2 null mutant mouse also has reduced expression of the neural precursor gene NeuroD1 (Peddada et al., 2006). Since E47 stimulates NeuroD1 transcription (Sharma et al., 1999), high ID-protein levels could reduce NeuroD1 expression. Interestingly, a frameshift mutation of E2-2 was recently discovered in a variant Rett syndrome patient who did not have mutations in MeCP2 (Armani et al., 2012). E2-2 loss of function is expected to mimic effects of ID-protein overexpression. Other E2-2 loss of function mutations cause Pitt-Hopkins syndrome (see above), which shares symptoms with Rett syndrome. Although the cellular basis of Rett Syndrome is not yet clear, altered neural differentiation as a consequence of reduced NeuroD expression, defects in neuronal cell cycle withdrawal, or altered axon or dendrite morphogenesis are all plausible candidates.
A further connection between HLH proteins and autism spectrum disorders is that ID3 has been reported as a target of miR-29b, a brain-specific miRNA that is expressed more highly in autism spectrum disorders. ID3 expression is significantly downregulated in miR-29b-transfected cells (Sarachana et al., 2010). Whether ID3 is really involved in autism, whether its function in this regard is related to that of E2-2, and if differentiation, cell cycle, neuronal morphology or other defects are involved remains to be elucidated. Intriguingly, one trait that is strongly associated with autism is circadian rhythm dysfunction (Hu et al., 2009). ID proteins play essential roles in controlling circadian rhythm by sequestering the bHLH transcription factors CLOCK and BMAL that are central components of the circadian pacemaker (Duffield et al., 2009).
Atherosclerosis
ID3 maps to atherosclerosis susceptibility loci in both humans and mice (Lusis et al., 2004). A single-nucleotide polymorphism (SNP rs11574) substitutes Thr for Ala105 in the human ID3 protein and is associated with increased carotid intima-media thickness (IMT) (Doran et al., 2010). This mutation decreases ID3 function as measured by binding to E12 in NIH 3T3 cells (Doran et al., 2010).
Atherosclerosis is strongly enhanced when Id3 is deleted in ApoE or Ldlr knockout mice (ie in Id3−/− ApoE−/− or Id3−/− Ldlr−/− genotypes) (Doran et al., 2010; Lipinski et al., 2012) (Table 2). Bone marrow transplantation from Id3+/+ ApoE−/− donors is atheroprotective, pointing to a role in bone marrow-derived cell types (Doran et al., 2012). These mice have reduced B-cell numbers but increased macrophage numbers in the aorta (Doran et al., 2012; Lipinski et al., 2012). Chemokines, and vascular cell adhesion molecule 1 (VCAM-1) are elevated, and may promote these changes in leukocyte adhesion and recruitment into the arterial wall. The VCAM-1 promoter is regulated by E12, and could be activated by E12 following a reduction in ID3 (Lipinski et al., 2012). One model, therefore, is that ID3 protects against atherosclerosis by altering leukocyte recruitment to artery walls (Doran et al., 2010; Lipinski et al., 2012; Doran et al., 2012).
The effect of Id proteins on the vasculature is complicated, however (Yang et al., 2014). ID3 inhibits adiponectin expression by preventing E47 from binding to E-boxes in the adiponectin promoter (Doran et al., 2008), Adiponectin is an adipocyte-derived cytokine that attenuates plaque formation: low adiponectin levels are associated with coronary artery disease, whereas adiponectin over-expression reduced atherosclerosis in ApoE-deficient mice. Atherosclerosis might also be impacted by the roles of Id proteins in vascular smooth muscle cells and endothelial cells (see below). For example, alternative splicing of Id3 in vascular lesions may produce a protein with a uniquely atheroprotective role (Forrest et al., 2004).
Arterial Vascular Disease
Mutations in the bmpr2 gene that encodes the BMP type II receptor are found in most cases of heritable pulmonary arterial hypertension (PAH), in which smooth muscle cells proliferate to occlude the vessel (Lane et al., 2000; Machado et al., 2006). ID1 and ID3 are targets of BMP signaling in pulmonary artery smooth muscle cells, as in other cell types, and a recent study suggests that Id proteins mediate anti-proliferative effect of BMP signaling (Yang et al., 2013). The mechanism is unclear, however, because both Id2 and Id3 are also reported to inhibit p21 expression in proliferating vascular smooth muscle cells, as in many other cell types (Matsumura et al., 2002; Forrest et al., 2004; Taylor et al., 2006)(Figure 3). Id proteins also have roles in endothelial cells (Ling et al., 2014; Yang et al., 2014). Id1−/−;Id3−/− mice exhibit cardiac developmental defects, brain vascular abnormalities, and embryonic lethality (Lyden et al., 1999) (Table 2).
Bone Marrow Failure
A connection between HLH proteins and bone marrow failure has been suggested based only on ID2 upregulation and E47 and HEB downregulation in Diamond Blackfan Anemia (DBA) patients (Zhang et al., 1997). E47, TCF4, and a tissue-restricted expressed bHLH protein, SCL, regulate early erythroid differentiation and are reduced during maturation when ID2 is upregulated, so that elevated ID2 would be expected to decrease the expression of erythroid specific genes and erythroid differentiation. This could be re-evaluated now that it is known that approximately 65% of patients harbor mutations in ribosomal protein genes (Farrar et al., 2011), and that many aspects of DBA have been attributed to p53 hyperactivity caused by nucleolar stress (Ellis, 2014). Although p53 can regulate ID1 and ID2 transcription in neural stem cells, repression is observed, contrary to the notion that ID2 is upregulated in DBA patients (Paolella et al., 2011). It is not impossible that differentiation defects can contribute to DBA, however, as suggested by mutations in the hematopoietic transcription factor GATA1 that were recently found in some DBA cases (Sankaran et al., 2012).
Diabetes, glucose and lipid metabolism
Insulin affects glucose and lipid metabolism and both are disrupted in diabetes. β-cells in the islets of Langerhans of diabetic mice, or isolated β-cells exposed to hyperglycemia or lipid, have elevated ID1/ID3 expression (Wice et al., 2001; Busch et al., 2002; Kjorholt et al., 2005; Billestrup, 2011). Consistent with the notion that ID proteins impair glucose and lipid metabolism, islets from Id1−/− mice are protected against high-fat diet-induced repression of β-cell genes, such as pancreatic duodenal homeobox-1, NeuroD1 (also called Beta2), Glut2, pyruvate carboxylase, and Gpr40 (Akerfeldt and Laybutt, 2011). Id1−/− mice show less insulin resistance in skeletal muscle, liver and white adipose tissue, and increased Uncoupling Protein 1 and Peroxisome proliferator-activated receptor gamma coactivator 1 in brown adipose tissue (corresponding to increased energy expenditure) (Satyanarayana et al., 2012). Mice over-expressing E-proteins revealed similar phenotypes (Zhao et al., 2014).
Although Id1 expression is down-regulated and the protein apparently degraded during adipocyte differentiation, adipogenesis appears normal in Id1−/− mice, suggesting that it is effects on oxygen consumption and thermogenesis that protect them against a high-fat diet (Satyanarayana et al., 2012). E2A and E2-2 proteins can also directly increase insulin expression in β-cells (Vierra and Nelson, 1995).
Other ID proteins also regulate metabolism. Mice with homozygous deletions of Id2 or Id4 have less body fat and gain much less weight on a high-fat diet (Murad et al., 2010; Mathew et al., 2013). Id2−/− mice have altered lipid metabolism, impaired adipogenesis, and reduced gonadal white adipose deposits and liver lipid content (Park et al., 2008; Hou et al., 2009; Mathew et al., 2013) (Table 2). Male Id2−/− mice also reveal increased insulin sensitivity, increased glucose uptake by skeletal muscle and brown adipose tissue, and reduced intramuscular triacylglycerol and diacylglycerol levels (Mathew et al., 2013). Apparently, therefore, ID1, 2, and 4 play a variety of roles in insulin secretion and energy metabolism.
Osteopenia and osteoporosis
Id1−/− mice have an osteoporotic phenotype ie reduced bone density and increased bone fragility (Chan et al., 2009) (Table 2). Healthy bone volume is controlled by the balance between bone deposition by osteoblasts and bone resorption by osteoclasts. Excess of bone resorption over bone formation results in osteoporosis and its milder potential precursor, osteopenia. Osteoclasts and osteoblasts are derived from distinct lineages: osteoclasts differentiate from hematopoietic stem cell (HSC) precursors (Suda et al., 1992), while osteoblasts arise from mesenchymal stem cells (MSCs). The bone phenotype of Id1−/− is due to excess osteoclastogenesis (Chan et al., 2009). Genes required for osteoclastogenesis are up-regulated including Trap, Oscar and Ctsk; over-expressing ID1 downregulates these genes (Chan et al., 2009). It would be reasonable to anticipate a positive role of one or more E-proteins in osteoclast differentiation and maturation, although none seems to have been reported.
Osteoporosis and age-related osteopenia also result from differentiation of MSCs into bone marrow adipocytes rather than osteoblasts. Adipocytes additionally suppress osteogenesis because secreted adipogenic signals influence osteoclast differentiation by HSCs. Id4−/− mice reduce osteoblast differentiation and enhance adipocyte differentiation (Tokuzawa et al., 2010) (Table 2). ID4 competes with the bHLH factor Hes1 to dimerize with Hey2, allowing Hes1 to activate osteoblast-specific gene transcription and to promote osteogenesis through the key osteoblast differentiation factor Runx2 (Tokuzawa et al., 2010). It is not known how Hes1 regulates Runx2 levels (Ikawa et al., 2006). Other ID proteins also affect osteoblasts, which show reduced proliferation and mineralization in Id1+/−; Id3−/− mice (Maeda et al., 2004). Taken together, these findings make both ID- and perhaps E-proteins potential targets for the prevention and treatment of osteoporosis, because of their roles in osteoclast and osteoblast specification.
Renal disease
Id2+/− or Id2−/− mice develop congenital hydronephrosis, which is common in humans and may result in early-onset renal failure (Aoki et al., 2004; Tripathi et al., 2012). The cause of hydronephrosis is frequently obstruction at the ureteropelvic junction. Id2 mutant mice display irregular, hypertophic muscle layers at the ureteropelvic junction, suggesting that smooth muscle hypertrophy at the ureteropelvic junction could be the cause of hydronephrosis (Aoki et al., 2004). E protein-dependent myogenesis could be stimulated when ID2 levels are decreased, but the usual role of E-proteins is to inhibit proliferation, not promote it (Figure 1 and 3).
A role for ID proteins was also suggested in autosomal dominant polycystic kidney disease (PKD), which is mostly caused by mutations in the PKD1 and PKD2 genes (Peters and Sandkuijl, 1992). In this disease bilateral renal cysts replace the normal renal parenchyma, often resulting in end-stage renal disease. Cysts are lined by a single layer of epithelium that is characterized by increased cellular proliferation and decreased differentiation (Nadasdy et al., 1995). It was reported that Polycystin-2, the product of the PKD2 gene, sequestered ID2 in the cytoplasm in a PKD1-dependent manner, suppressing inappropriate proliferation through E47-dependent p21 transcription (Li et al., 2005; Zhou, 2009). More recent publications focus on defects in Ca2+ regulation at the cilium as a cause of PKD, however, and the relationship to cell cycle regulation by ID- and E-proteins is unclear (Fliegauf et al., 2007; Fedeles et al., 2011).
Parkinsonism
Id2 homozygous null mutant mice display features of Parkinson’s disease, such as fewer dopaminergic neurons in the olfactory bulb and reduced olfactory discrimination (Havrda et al., 2008). Id2−/− mice also have reduced dopamine transporter expression, activated caspase-3, as well as glial infiltration in the substantia nigra pars compacta of brain (Havrda et al., 2013) (Table 2). These observations suggest that ID2 is required for the development of midbrain dopaminergic neurons and raise the question of how ID2 might be involved in Parkinson’s disease and other disorders of the dopaminergic system such as attention deficit hyperactivity disorder, schizophrenia, and drug abuse. Intriguingly, significant inductions of ID1, ID2 and ID3 expression by decreased dopamine levels have been detected in rodents under continuous stress conditions (Konishi et al., 2010). At present, it is not known whether E-proteins play specific roles in dopaminergic neurons, other than their general role in neuronal differentiation (Figure 1).
Sjogren’s Syndrome
ID3 homozygous null (Id3−/−) mice develop many symptoms similar to those found in Sjögren’s Syndrome, an autoimmune disease in which immune cells chronically attack the lachrymal and salivary glands, resulting in impaired tear and saliva secretion (Li et al., 2004) (Table 2). The effect of ID3 gene inactivation may be on lymphocyte differentiation because adoptive transfer of Id3−/− bone marrow cells is sufficient to induce symptoms of Sjögren’s syndrome (Li et al., 2004; Guo et al., 2011), and because CD20 antibody treatment, which depletes B cells, relieves the Sjögren’s symptoms of Id3−/− mice (Hayakawa et al., 2007). Since ID3 is required to downregulate E proteins during T cell development, defective T cells may also contribute to the disease (Engel and Murre, 2002; Kim et al., 2002a). Although no changes in ID3 mRNA levels have been seen in T cells from human patients, it remains an interesting possibility that defects in E-protein-dependent lymphocyte differentiation contribute to Sjögren’s Syndrome (Ling et al., 2014)
Conclusions and Prospects
Although genetic evidence in humans directly implicates E- and ID-proteins in Pitt-Hopkins Syndrome, schizophrenia, Rett Syndrome, atherosclerosis, and perhaps Fuchs Corneal Dystrophy and pulmonary arterial hypertension, how these diseases are related to the well-studied roles of E- and ID-proteins in normal development is not yet clear. More information would be useful to substantiate the contributions of E- and ID-proteins to Diamond Blackfan Anemia and Polycystic Kidney Disease. At the moment changes in E-proteins or ID-proteins are not implicated as direct causes of defects in metabolism or diabetes, bone fragility, hydronephrosis, Parkinsonism, or Sjogrens’ Syndrome in humans, but their mouse mutations provide models of these disorders and suggest they may be involved. This variety of diseases and models reinforce the notion that E-and ID-proteins, and their relative expression levels, constitute an important aspect of the cellular regulatory environment that remains important throughout life, not only during early development. The mechanisms established for HLH protein regulation in normal development provide a useful starting point for disease mechanisms (Figures 1–2). Do, for example, ID-proteins regulate dendrite growth through E-proteins and transcription, and is this relevant to neurological conditions? More still remains to be learned concerning normal E-and ID-protein expression and function, especially at the post-transcriptional level. Post-translational modification and changes in subcellular localization have all been reported (Lingbeck et al., 2005; Kurooka and Yokota, 2005; Sun et al., 2005; Rollin et al., 2009; Nio-Kobayashi et al., 2013; Lasorella et al., 2014). Protein turnover and stability may be a regulatory factor and therapeutic target. Recent work shows that the Drosophila E-protein Da is destabilized by Notch signaling, and this may be one of the mechanisms by which Notch regulates so many differentiation processes (Kiparaki et al., 2015). These studies exemplify the broad effects, and potential therapeutic opportunities, expected to accrue from modulating these very widely-expressed and important HLH proteins.
Acknowledgements
We thank A. Bhattacharya and K. Li for contributions to work on HLH proteins in our laboratory, and J. Hebert, H. Lachman, B. Morrow, N. Sibinga and the anonymous reviewers for comments on the manuscript. Our work on HLH proteins is supported by the NIH (GM047892) and by an Unrestricted Grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adam JC, Montell DJ. A role for extra macrochaetae downstream of Notch in follicle cell differentiation. Development. 2004;131:5971–5980. doi: 10.1242/dev.01442. [DOI] [PubMed] [Google Scholar]
- Akerfeldt MC, Laybutt DR. Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance. Diabetes. 2011;60:2506–2514. doi: 10.2337/db11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, Nelson SF, Cantor RM, Geschwind DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am. J. Hum. Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Rio M, de PL, Redon R, Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A, Colleaux L. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am. J. Hum. Genet. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Zapata I, Baonza A. The bHLH factors extramacrochaetae and daughterless control cell cycle in Drosophila imaginal discs through the transcriptional regulation of the Cdc25 phosphatase string. PLoS. Genet. 2014;10:e1004233. doi: 10.1371/journal.pgen.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sanchez RM, Barba I, Martinez-Saez E, Prudkin L, Cuartas I, Raventos C, Martinez-Ricarte F, Poca MA, Garcia-Dorado D, Lahn MM, Yingling JM, Rodon J, Sahuquillo J, Baselga J, Seoane J. TGF-beta Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Mori S, Kitajima K, Yokoyama O, Kanamaru H, Okada K, Yokota Y. Id2 haploinsufficiency in mice leads to congenital hydronephrosis resembling that in humans. Genes Cells. 2004;9:1287–1296. doi: 10.1111/j.1365-2443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Armani R, Archer H, Clarke A, Vasudevan P, Zweier C, Ho G, Williamson S, Cloosterman D, Yang N, Christodoulou J. Transcription factor 4 and myocyte enhancer factor 2C mutations are not common causes of Rett syndrome. Am. J. Med. Genet. A. 2012;158A:713–719. doi: 10.1002/ajmg.a.34206. [DOI] [PubMed] [Google Scholar]
- Baonza A, de Celis JF, Garcia-Bellido A. Relationships between extramacrochaetae and Notch signalling in Drosophila wing development. Development. 2000;127:2383–2393. doi: 10.1242/dev.127.11.2383. [DOI] [PubMed] [Google Scholar]
- Baratz KH, Tosakulwong N, Ryu E, Brown WL, Branham K, Chen W, Tran KD, Schmid-Kubista KE, Heckenlively JR, Swaroop A, Abecasis G, Bailey KR, Edwards AO. E2-2 protein and Fuchs’s corneal dystrophy. N. Engl. J. Med. 2010;363:1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- Barrett LE, Granot Z, Coker C, Iavarone A, Hambardzumyan D, Holland EC, Nam HS, Benezra R. Self-renewal does not predict tumor growth potential in mouse models of high-grade glioma. Cancer Cell. 2012;21:11–24. doi: 10.1016/j.ccr.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Bedogni F, Rossi RL, Galli F, Cobolli GC, Gandaglia A, Kilstrup-Nielsen C, Landsberger N. Rett syndrome and the urge of novel approaches to study MeCP2 functions and mechanisms of action. Neurosci. Biobehav. Rev. 2014;46(Pt 2):187–201. doi: 10.1016/j.neubiorev.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Belle I, Zhuang Y. E proteins in lymphocyte development and lymphoid diseases. Curr. Top. Dev. Biol. 2014;110:153–187. doi: 10.1016/B978-0-12-405943-6.00004-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Best SA, Hutt KJ, Fu NY, Vaillant F, Liew SH, Hartley L, Scott CL, Lindeman GJ, Visvader JE. Dual roles for Id4 in the regulation of estrogen signaling in the mammary gland and ovary. Development. 2014;141:3159–3164. doi: 10.1242/dev.108498. [DOI] [PubMed] [Google Scholar]
- Bhandari RK, Sadler-Riggleman I, Clement TM, Skinner MK. Basic helix-loop-helix transcription factor TCF21 is a downstream target of the male sex determining gene SRY. PLoS. One. 2011;6:e19935. doi: 10.1371/journal.pone.0019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Baker NE. The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye. Dev. Biol. 2009;327:288–300. doi: 10.1016/j.ydbio.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Baker NE. A network of broadly expressed HLH genes regulates tissue-specific cell fates. Cell. 2011;147:881–892. doi: 10.1016/j.cell.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billestrup N. ID’ing a novel inhibitor of beta-cell function, Id1. Diabetes. 2011;60:2455–2456. doi: 10.2337/db11-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch. Gen. Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- Breschel TS, McInnis MG, Margolis RL, Sirugo G, Corneliussen B, Simpson SG, McMahon FJ, MacKinnon DF, Xu JF, Pleasant N, Huo Y, Ashworth RG, Grundstrom C, Grundstrom T, Kidd KK, DePaulo JR, Ross CA. A novel, heritable, expanding CTG repeat in an intron of the SEF2-1 gene on chromosome 18q21.1. Hum. Mol. Genet. 1997;6:1855–1863. doi: 10.1093/hmg/6.11.1855. [DOI] [PubMed] [Google Scholar]
- Brzozka MM, Radyushkin K, Wichert SP, Ehrenreich H, Rossner MJ. Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the brain. Biol. Psychiatry. 2010;68:33–40. doi: 10.1016/j.biopsych.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ., Jr Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat. Genet. 2004;36:343–350. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic beta-cell function. Diabetes. 2002;51:977–987. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- Casanovas O, Miro F, Estanyol JM, Itarte E, Agell N, Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 2000;275:35091–35097. doi: 10.1074/jbc.M006324200. [DOI] [PubMed] [Google Scholar]
- Chan AS, Jensen KK, Skokos D, Doty S, Lederman HK, Kaplan RN, Rafii S, Rivella S, Lyden D. Id1 represses osteoclast-dependent transcription and affects bone formation and hematopoiesis. PLoS. One. 2009;4:e7955. doi: 10.1371/journal.pone.0007955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK. The helix-loop-helix inhibitor of differentiation (ID) proteins induce post-mitotic terminally differentiated Sertoli cells to re-enter the cell cycle and proliferate. Biol. Reprod. 2005;72:1205–1217. doi: 10.1095/biolreprod.104.035717. [DOI] [PubMed] [Google Scholar]
- Chen SS, Claus R, Lucas DM, Yu L, Qian J, Ruppert AS, West DA, Williams KE, Johnson AJ, Sablitzky F, Plass C, Byrd JC. Silencing of the inhibitor of DNA binding protein 4 (ID4) contributes to the pathogenesis of mouse and human CLL. Blood. 2011;117:862–871. doi: 10.1182/blood-2010-05-284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozen W, Timofeeva MN, Li D, Diepstra A, Hazelett D, Delahaye-Sourdeix M, Edlund CK, Franke L, Rostgaard K, Van Den Berg DJ, Cortessis VK, Smedby KE, Glaser SL, Westra HJ, Robison LL, Mack TM, Ghesquieres H, Hwang AE, Nieters A, de SS, Lightfoot T, Becker N, Maynadie M, Foretova L, Roman E, Benavente Y, Rand KA, Nathwani BN, Glimelius B, Staines A, Boffetta P, Link BK, Kiemeney L, Ansell SM, Bhatia S, Strong LC, Galan P, Vatten L, Habermann TM, Duell EJ, Lake A, Veenstra RN, Visser L, Liu Y, Urayama KY, Montgomery D, Gaborieau V, Weiss LM, Byrnes G, Lathrop M, Cocco P, Best T, Skol AD, Adami HO, Melbye M, Cerhan JR, Gallagher A, Taylor GM, Slager SL, Brennan P, Coetzee GA, Conti DV, Onel K, Jarrett RF, Hjalgrim H, van den Berg A, McKay JD. A meta-analysis of Hodgkin lymphoma reveals 19p13.3 TCF3 as a novel susceptibility locus. Nat. Commun. 2014;5:3856. doi: 10.1038/ncomms4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Candia P, Akram M, Benezra R, Brogi E. Id4 messenger RNA and estrogen receptor expression: inverse correlation in human normal breast epithelium and carcinoma. Hum. Pathol. 2006;37:1032–1041. doi: 10.1016/j.humpath.2006.03.004. [DOI] [PubMed] [Google Scholar]
- de Candia P, Benera R, Solit DB. A role for Id proteins in mammary gland physiology and tumorigenesis. Adv. Cancer Res. 2004;92:81–94. doi: 10.1016/S0065-230X(04)92004-0. [DOI] [PubMed] [Google Scholar]
- de Pontual L, Mathieu Y, Golzio C, Rio M, Malan V, Boddaert N, Soufflet C, Picard C, Durandy A, Dobbie A, Heron D, Isidor B, Motte J, Newburry-Ecob R, Pasquier L, Tardieu M, Viot G, Jaubert F, Munnich A, Colleaux L, Vekemans M, Etchevers H, Lyonnet S, Amiel J. Mutational, functional, and expression studies of the TCF4 gene in Pitt-Hopkins syndrome. Hum. Mutat. 2009;30:669–676. doi: 10.1002/humu.20935. [DOI] [PubMed] [Google Scholar]
- Dong J, Huang S, Caikovski M, Ji S, McGrath A, Custorio MG, Creighton CJ, Maliakkal P, Bogoslovskaia E, Du Z, Zhang X, Lewis MT, Sablitzky F, Brisken C, Li Y. ID4 regulates mammary gland development by suppressing p38MAPK activity. Development. 2011;138:5247–5256. doi: 10.1242/dev.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Lehtinen AB, Meller N, Lipinski MJ, Slayton RP, Oldham SN, Skaflen MD, Yeboah J, Rich SS, Bowden DW, McNamara CA. Id3 is a novel atheroprotective factor containing a functionally significant single-nucleotide polymorphism associated with intima-media thickness in humans. Circ. Res. 2010;106:1303–1311. doi: 10.1161/CIRCRESAHA.109.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, Cutchins A, Lee DJ, Glover DK, Kelly KA, Galkina EV, Ley K, Witztum JL, Tsimikas S, Bender TP, McNamara CA. B-cell aortic homing and atheroprotection depend on Id3. Circ. Res. 2012;110:e1–e12. doi: 10.1161/CIRCRESAHA.111.256438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Meller N, Cutchins A, Deliri H, Slayton RP, Oldham SN, Kim JB, Keller SR, McNamara CA. The helix-loop-helix factors Id3 and E47 are novel regulators of adiponectin. Circ. Res. 2008;103:624–634. doi: 10.1161/CIRCRESAHA.108.175893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Aleff RA, Soragni E, Kalari K, Nie J, Tang X, Davila J, Kocher JP, Patel SV, Gottesfeld JM, Baratz KH, Wieben ED. RNA Toxicity and Missplicing in the Common Eye Disease Fuchs Endothelial Corneal Dystrophy. J. Biol. Chem. 2015 doi: 10.1074/jbc.M114.621607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield GE, Watson NP, Mantani A, Peirson SN, Robles-Murguia M, Loros JJ, Israel MA, Dunlap JC. A role for Id2 in regulating photic entrainment of the mammalian circadian system. Curr. Biol. 2009;19:297–304. doi: 10.1016/j.cub.2008.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghrari AO, McGlumphy EJ, Iliff BW, Wang J, Emmert D, Riazuddin SA, Katsanis N, Gottsch JD. Prevalence and severity of fuchs corneal dystrophy in Tangier Island. Am. J. Ophthalmol. 2012;153:1067–1072. doi: 10.1016/j.ajo.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HM, Spann DR, Posakony JW. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990;61:27–38. doi: 10.1016/0092-8674(90)90212-w. [DOI] [PubMed] [Google Scholar]
- Ellis SR. Nucleolar stress in Diamond Blackfan anemia pathophysiology. Biochim. Biophys. Acta. 2014;1842:765–768. doi: 10.1016/j.bbadis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Engel I, Murre C. Disruption of pre-TCR expression accelerates lymphomagenesis in E2A–deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11322–11327. doi: 10.1073/pnas.162373999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JE, Vlachos A, Atsidaftos E, Carlson-Donohoe H, Markello TC, Arceci RJ, Ellis SR, Lipton JM, Bodine DM. Ribosomal protein gene deletions in Diamond-Blackfan anemia. Blood. 2011;118:6943–6951. doi: 10.1182/blood-2011-08-375170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedeles SV, Tian X, Gallagher AR, Mitobe M, Nishio S, Lee SH, Cai Y, Geng L, Crews CM, Somlo S. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 2011;43:639–647. doi: 10.1038/ng.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Creighton CJ, Buser AC, DeMayo FJ, Edwards DP, Lydon JP. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology. 2008;149:6236–6250. doi: 10.1210/en.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Azim K, Hurtado-Chong A, Ramelli S, Fernandez M, Raineteau O. E-proteins orchestrate the progression of neural stem cell differentiation in the postnatal forebrain. Neural Dev. 2014;9:23. doi: 10.1186/1749-8104-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M, Chapman RM, Doyle AM, Tinsley CL, Waite A, Blake DJ. Functional analysis of TCF4 missense mutations that cause Pitt-Hopkins syndrome. Hum. Mutat. 2012;33:1676–1686. doi: 10.1002/humu.22160. [DOI] [PubMed] [Google Scholar]
- Forrest MP, Hill MJ, Quantock AJ, Martin-Rendon E, Blake DJ. The emerging roles of TCF4 in disease and development. Trends Mol. Med. 2014;20:322–331. doi: 10.1016/j.molmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Forrest ST, Barringhaus KG, Perlegas D, Hammarskjold ML, McNamara CA. Intron retention generates a novel Id3 isoform that inhibits vascular lesion formation. J. Biol. Chem. 2004;279:32897–32903. doi: 10.1074/jbc.M404882200. [DOI] [PubMed] [Google Scholar]
- Garrell J, Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990;61:39–48. doi: 10.1016/0092-8674(90)90213-x. [DOI] [PubMed] [Google Scholar]
- Giurgea I, Missirian C, Cacciagli P, Whalen S, Fredriksen T, Gaillon T, Rankin J, Mathieu-Dramard M, Morin G, Martin-Coignard D, Dubourg C, Chabrol B, Arfi J, Giuliano F, Claude LJ, Philip N, Sarda P, Villard L, Goossens M, Moncla A. TCF4 deletions in Pitt-Hopkins Syndrome. Hum. Mutat. 2008;29:E242–E251. doi: 10.1002/humu.20859. [DOI] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Guo K, Wang J, Andres V, Smith RC, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol. Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Li H, Han M, Xu T, Wu X, Zhuang Y. Modeling Sjogren’s syndrome with Id3 conditional knockout mice. Immunol. Lett. 2011;135:34–42. doi: 10.1016/j.imlet.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Havrda MC, Harris BT, Mantani A, Ward NM, Paolella BR, Cuzon VC, Yeh HH, Israel MA. Id2 is required for specification of dopaminergic neurons during adult olfactory neurogenesis. J. Neurosci. 2008;28:14074–14086. doi: 10.1523/JNEUROSCI.3188-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havrda MC, Paolella BR, Ward NM, Holroyd KB. Behavioral abnormalities and Parkinson’s-like histological changes resulting from Id2 inactivation in mice. Dis. Model. Mech. 2013;6:819–827. doi: 10.1242/dmm.010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I, Tedder TF, Zhuang Y. B-lymphocyte depletion ameliorates Sjogren’s syndrome in Id3 knockout mice. Immunology. 2007;122:73–79. doi: 10.1111/j.1365-2567.2007.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou TY, Ward SM, Murad JM, Watson NP, Israel MA, Duffield GE. ID2 (inhibitor of DNA binding 2) is a rhythmically expressed transcriptional repressor required for circadian clock output in mouse liver. J. Biol. Chem. 2009;284:31735–31745. doi: 10.1074/jbc.M109.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, Luu T, Lai Y, Lee NH. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res. 2009;2:78–97. doi: 10.1002/aur.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J. Exp. Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana Y, Piens M, Fong S, Singh J, Sumida T, Desprez PY. Expression of Id and ITF-2 genes in the mammary gland during pregnancy. Biochem. Biophys. Res. Commun. 2008;372:826–830. doi: 10.1016/j.bbrc.2008.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Puram SV, Bilimoria PM, Ikeuchi Y, Keough S, Wong M, Rowitch D, Bonni A. A centrosomal Cdc20-APC pathway controls dendrite morphogenesis in postmitotic neurons. Cell. 2009;136:322–336. doi: 10.1016/j.cell.2008.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Xu M, Nie L, Peng XC, Jimi E, Voll RE, Nguyen T, Ghosh S, Sun XH. Helix-loop-helix proteins regulate pre-TCR and TCR signaling through modulation of Rel/NF-kappaB activities. Immunity. 2002a;16:9–21. doi: 10.1016/s1074-7613(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Kim GY, Mercer SE, Ewton DZ, Yan Z, Jin K, Friedman E. The stress-activated protein kinases p38 alpha and JNK1 stabilize p21(Cip1) by phosphorylation. J. Biol. Chem. 2002b;277:29792–29802. doi: 10.1074/jbc.M201299200. [DOI] [PubMed] [Google Scholar]
- Kiparaki M, Zarifi I, Delidakis C. bHLH proteins involved in Drosophila neurogenesis are mutually regulated at the level of stability. Nucleic Acids Res. 2015;43:2543–2559. doi: 10.1093/nar/gkv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Rujescu D, Ingason A, Collier DA, O’Donovan MC, Owen MJ. Neurexin 1 (NRXN1) deletions in schizophrenia. Schizophr. Bull. 2009;35:851–854. doi: 10.1093/schbul/sbp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005;54:2755–2763. doi: 10.2337/diabetes.54.9.2755. [DOI] [PubMed] [Google Scholar]
- Kong Y, Cui H, Zhang H. Smurf2-mediated ubiquitination and degradation of Id1 regulates p16 expression during senescence. Aging Cell. 2011;10:1038–1046. doi: 10.1111/j.1474-9726.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Ogawa T, Nakagomi S, Inoue K, Tohyama M, Kiyama H. Id1, Id2 and Id3 are induced in rat melanotrophs of the pituitary gland by dopamine suppression under continuous stress. NeuroScience. 2010;169:1527–1534. doi: 10.1016/j.neuroscience.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Kurooka H, Yokota Y. Nucleo-cytoplasmic shuttling of Id2, a negative regulator of basic helix-loop-helix transcription factors. J. Biol. Chem. 2005;280:4313–4320. doi: 10.1074/jbc.M412614200. [DOI] [PubMed] [Google Scholar]
- Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Benezra R, Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat. Rev. Cancer. 2014;14:77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, Torre-Ubieta L, Pagano M, Bonni A, Iavarone A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature. 2006;442:471–474. doi: 10.1038/nature04895. [DOI] [PubMed] [Google Scholar]
- Lau LC, Ma L, Young AL, Rong SS, Jhanji V, Brelen ME, Pang CP, Chen LJ. Association of common variants in TCF4 and PTPRG with Fuchs’ corneal dystrophy: a systematic review and meta-analysis. PLoS. One. 2014;9:e109142. doi: 10.1371/journal.pone.0109142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dai M, Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity. 2004;21:551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 2005;7:1202–1212. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- Li YJ, Minear MA, Rimmler J, Zhao B, Balajonda E, Hauser MA, Allingham RR, Eghrari AO, Riazuddin SA, Katsanis N, Gottsch JD, Gregory SG, Klintworth GK, Afshari NA. Replication of TCF4 through association and linkage studies in late-onset Fuchs endothelial corneal dystrophy. PLoS. One. 2011;6:e18044. doi: 10.1371/journal.pone.0018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Kang B, Sun XH. Id proteins: small molecules, mighty regulators. Curr. Top. Dev. Biol. 2014;110:189–216. doi: 10.1016/B978-0-12-405943-6.00005-1. [DOI] [PubMed] [Google Scholar]
- Lingbeck JM, Trausch-Azar JS, Ciechanover A, Schwartz AL. E12 and E47 modulate cellular localization and proteasome-mediated degradation of MyoD and Id1. Oncogene. 2005;24:6376–6384. doi: 10.1038/sj.onc.1208789. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ, Campbell KA, Duong SQ, Welch TJ, Garmey JC, Doran AC, Skaflen MD, Oldham SN, Kelly KA, McNamara CA. Loss of Id3 increases VCAM-1 expression, macrophage accumulation, and atherogenesis in Ldlr-/mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:2855–2861. doi: 10.1161/ATVBAHA.112.300352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, Lugar PL, Rizzieri DA, Lagoo AS, Bernal-Mizrachi L, Mann KP, Flowers CR, Naresh KN, Evens AM, Chadburn A, Gordon LI, Czader MB, Gill JI, Hsi ED, Greenough A, Moffitt AB, McKinney M, Banerjee A, Grubor V, Levy S, Dunson DB, Dave SS. The genetic landscape of mutations in Burkitt lymphoma. Nat. Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis AJ, Mar R, Pajukanta P. Genetics of atherosclerosis. Annu. Rev. Genomics Hum. Genet. 2004;5:189–218. doi: 10.1146/annurev.genom.5.061903.175930. [DOI] [PubMed] [Google Scholar]
- Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O’Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, Eickelberg O, Olschewski H, Elliott CG, Glissmeyer E, Carlquist J, Kim M, Torbicki A, Fijalkowska A, Szewczyk G, Parma J, Abramowicz MJ, Galie N, Morisaki H, Kyotani S, Nakanishi N, Morisaki T, Humbert M, Simonneau G, Sitbon O, Soubrier F, Coulet F, Morrell NW, Trembath RC. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum. Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tsuji K, Nifuji A, Noda M. Inhibitory helix-loop-helix transcription factors Id1/Id3 promote bone formation in vivo. J. Cell Biochem. 2004;93:337–344. doi: 10.1002/jcb.20154. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Zhou P, Pywell CM, van der Veen DR, Shao J, Xi Y, Bonar NA, Hummel AD, Chapman S, Leevy WM, Duffield GE. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS. One. 2013;8:e73064. doi: 10.1371/journal.pone.0073064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura ME, Lobe DR, McNamara CA. Contribution of the helix-loop-helix factor Id2 to regulation of vascular smooth muscle cell proliferation. J. Biol. Chem. 2002;277:7293–7297. doi: 10.1074/jbc.M108986200. [DOI] [PubMed] [Google Scholar]
- Matthaei M, Zhu AY, Kallay L, Eberhart CG, Cursiefen C, Jun AS. Transcript profile of cellular senescence-related genes in Fuchs endothelial corneal dystrophy. Exp. Eye Res. 2014;129:13–17. doi: 10.1016/j.exer.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir T, Sadler-Riggleman I, Stevens JD, Skinner MK. Role of the basic helix-loop-helix protein ITF2 in the hormonal regulation of Sertoli cell differentiation. Mol. Reprod. Dev. 2006;73:491–500. doi: 10.1002/mrd.20397. [DOI] [PubMed] [Google Scholar]
- Murad JM, Place CS, Ran C, Hekmatyar SK, Watson NP, Kauppinen RA, Israel MA. Inhibitor of DNA binding 4 (ID4) regulation of adipocyte differentiation and adipose tissue formation in mice. J. Biol. Chem. 2010;285:24164–24173. doi: 10.1074/jbc.M110.128744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C. Helix-loop-helix proteins and lymphocyte development. Nat. Immunol. 2005;6:1079–1086. doi: 10.1038/ni1260. [DOI] [PubMed] [Google Scholar]
- Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nadasdy T, Laszik Z, Lajoie G, Blick KE, Wheeler DE, Silva FG. Proliferative activity of cyst epithelium in human renal cystic diseases. J. Am. Soc. Nephrol. 1995;5:1462–1468. doi: 10.1681/ASN.V571462. [DOI] [PubMed] [Google Scholar]
- Nair R, Teo WS, Mittal V, Swarbrick A. ID proteins regulate diverse aspects of cancer progression and provide novel therapeutic opportunities. Mol. Ther. 2014;22:1407–1415. doi: 10.1038/mt.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5:515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Narayanan R, Giakoumelou S, Boswell L, Hogg K, Duncan WC. Expression and localization of inhibitor of differentiation (ID) proteins during tissue and vascular remodelling in the human corpus luteum. Mol. Hum. Reprod. 2013;19:82–92. doi: 10.1093/molehr/gas052. [DOI] [PubMed] [Google Scholar]
- Niola F, Zhao X, Singh D, Castano A, Sullivan R, Lauria M, Nam HS, Zhuang Y, Benezra R, Di BD, Iavarone A, Lasorella A. Id proteins synchronize stemness and anchorage to the niche of neural stem cells. Nat. Cell Biol. 2012;14:477–487. doi: 10.1038/ncb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niola F, Zhao X, Singh D, Sullivan R, Castano A, Verrico A, Zoppoli P, Friedmann-Morvinski D, Sulman E, Barrett L, Zhuang Y, Verma I, Benezra R, Aldape K, Iavarone A, Lasorella A. Mesenchymal high-grade glioma is maintained by the ID-RAP1 axis. J. Clin. Invest. 2013;123:405–417. doi: 10.1172/JCI63811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca A, Gallo P, De LP, Lania L. Class A helix-loop-helix proteins are positive regulators of several cyclin-dependent kinase inhibitors’ promoter activity and negatively affect cell growth. Cancer Res. 2000;60:1376–1382. [PubMed] [Google Scholar]
- Paolella BR, Havrda MC, Mantani A, Wray CM, Zhang Z, Israel MA. p53 directly represses Id2 to inhibit the proliferation of neural progenitor cells. Stem Cells. 2011;29:1090–1101. doi: 10.1002/stem.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Waki H, Villanueva CJ, Monticelli LA, Hong C, Kang S, MacDougald OA, Goldrath AW, Tontonoz P. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-gamma expression and adipocyte differentiation. Mol. Endocrinol. 2008;22:2038–2048. doi: 10.1210/me.2007-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddada S, Yasui DH, LaSalle JM. Inhibitors of differentiation (ID1, ID2, ID3 and ID4) genes are neuronal targets of MeCP2 that are elevated in Rett syndrome. Hum. Mol. Genet. 2006;15:2003–2014. doi: 10.1093/hmg/ddl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DJ, Sandkuijl LA. Genetic heterogeneity of polycystic kidney disease in Europe. Contrib. Nephrol. 1992;97:128–139. doi: 10.1159/000421651. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Peverali FA, Ramqvist T, Saffrich R, Pepperkok R, Barone MV, Philipson L. Regulation of G1 progression by E2A and Id helix-loop-helix proteins. EMBO J. 1994;13:4291–4301. doi: 10.1002/j.1460-2075.1994.tb06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Jarman AP. Context dependence of proneural bHLH proteins. Curr. Opin. Genet. Dev. 2008;18:411–417. doi: 10.1016/j.gde.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu S, Ignatova A, Park ST, Sun XH. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell Biol. 1997;17:5888–5896. doi: 10.1128/mcb.17.10.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Schlesner M, Hoffmann S, Kreuz M, Leich E, Burkhardt B, Rosolowski M, Ammerpohl O, Wagener R, Bernhart SH, Lenze D, Szczepanowski M, Paulsen M, Lipinski S, Russell RB, Adam-Klages S, Apic G, Claviez A, Hasenclever D, Hovestadt V, Hornig N, Korbel JO, Kube D, Langenberger D, Lawerenz C, Lisfeld J, Meyer K, Picelli S, Pischimarov J, Radlwimmer B, Rausch T, Rohde M, Schilhabel M, Scholtysik R, Spang R, Trautmann H, Zenz T, Borkhardt A, Drexler HG, Moller P, MacLeod RA, Pott C, Schreiber S, Trumper L, Loeffler M, Stadler PF, Lichter P, Eils R, Kuppers R, Hummel M, Klapper W, Rosenstiel P, Rosenwald A, Brors B, Siebert R. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat. Genet. 2012;44:1316–1320. doi: 10.1038/ng.2469. [DOI] [PubMed] [Google Scholar]
- Rollin J, Blechet C, Regina S, Tenenhaus A, Guyetant S, Gidrol X. The intracellular localization of ID2 expression has a predictive value in non small cell lung cancer. PLoS. One. 2009;4:e4158. doi: 10.1371/journal.pone.0004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero R, Kale A, Thomas B, Baker NE. Mitosis in neurons: Roughex and APC/C maintain cell cycle exit to prevent cytokinetic and axonal defects in Drosophila photoreceptor neurons. PLoS. Genet. 2012;8:e1003049. doi: 10.1371/journal.pgen.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran VG, Ghazvinian R, Do R, Thiru P, Vergilio JA, Beggs AH, Sieff CA, Orkin SH, Nathan DG, Lander ES, Gazda HT. Exome sequencing identifies GATA1 mutations resulting in Diamond-Blackfan anemia. J. Clin. Invest. 2012;122:2439–2443. doi: 10.1172/JCI63597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarachana T, Zhou R, Chen G, Manji HK, Hu VW. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2:23. doi: 10.1186/gm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana A, Klarmann KD, Gavrilova O, Keller JR. Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis. FASEB J. 2012;26:309–323. doi: 10.1096/fj.11-190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, Liu X, Powell J, Yang Y, Xu W, Zhao H, Kohlhammer H, Rosenwald A, Kluin P, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Ogwang MD, Reynolds SJ, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pittaluga S, Wilson W, Waldmann TA, Rowe M, Mbulaiteye SM, Rickinson AB, Staudt LM. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp M, Pruunsild P, Timmusk T. Pitt-Hopkins syndrome-associated mutations in TCF4 lead to variable impairment of the transcription factor function ranging from hypomorphic to dominant-negative effects. Hum. Mol. Genet. 2012;21:2873–2888. doi: 10.1093/hmg/dds112. [DOI] [PubMed] [Google Scholar]
- Sharma A, Moore M, Marcora E, Lee JE, Qiu Y, Samaras S, Stein R. The NeuroD1/BETA2 sequences essential for insulin gene transcription colocalize with those necessary for neurogenesis and p300/CREB binding protein binding. Mol. Cell Biol. 1999;19:704–713. doi: 10.1128/mcb.19.1.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery C, Ryan MP, McMorrow T. E2A proteins: regulators of cell phenotype in normal physiology and disease. Int. J. Biochem. Cell Biol. 2008;40:1431–1436. doi: 10.1016/j.biocel.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Sloan SR, Shen CP, McCarrick-Walmsley R, Kadesch T. Phosphorylation of E47 as a potential determinant of B-cell-specific activity. Mol. Cell Biol. 1996;16:6900–6908. doi: 10.1128/mcb.16.12.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JF, Roos BR, Wagoner MD, Goins KM, Kitzmann AS, Riley JB, Stone EM, Fingert JH. Confirmation of the association between the TCF4 risk allele and Fuchs endothelial corneal dystrophy in patients from the Midwestern United States. Ophthalmic Genet. 2013;34:32–34. doi: 10.3109/13816810.2012.726396. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de FR, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St CD, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr. Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Sun L, Trausch-Azar JS, Ciechanover A, Schwartz AL. Ubiquitin-proteasome-mediated degradation, intracellular localization, and protein synthesis of MyoD and Id1 during muscle differentiation. J. Biol. Chem. 2005;280:26448–26456. doi: 10.1074/jbc.M500373200. [DOI] [PubMed] [Google Scholar]
- Swarbrick A, Roy E, Allen T, Bishop JM. Id1 cooperates with oncogenic Ras to induce metastatic mammary carcinoma by subversion of the cellular senescence response. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5402–5407. doi: 10.1073/pnas.0801505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebizadeh Z, Butler MG, Theodoro MF. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res. 2008;1:240–250. doi: 10.1002/aur.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Li F, Thimmalapura P, Gerrity RG, Sarembock IJ, Forrest S, Rutherford S, McNamara CA. Hyperlipemia and oxidation of LDL induce vascular smooth muscle cell growth: an effect mediated by the HLH factor Id3. J. Vasc. Res. 2006;43:123–130. doi: 10.1159/000090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalamuthu A, Khor CC, Venkataraman D, Koh LW, Tan DT, Aung T, Mehta JS, Vithana EN. Association of TCF4 gene polymorphisms with Fuchs’ corneal dystrophy in the Chinese. Invest Ophthalmol. Vis. Sci. 2011;52:5573–5578. doi: 10.1167/iovs.11-7568. [DOI] [PubMed] [Google Scholar]
- Tijchon E, Havinga J, van Leeuwen FN, Scheijen B. B-lineage transcription factors and cooperating gene lesions required for leukemia development. Leukemia. 2013;27:541–552. doi: 10.1038/leu.2012.293. [DOI] [PubMed] [Google Scholar]
- Tokuzawa Y, Yagi K, Yamashita Y, Nakachi Y, Nikaido I, Bono H, Ninomiya Y, Kanesaki-Yatsuka Y, Akita M, Motegi H, Wakana S, Noda T, Sablitzky F, Arai S, Kurokawa R, Fukuda T, Katagiri T, Schonbach C, Suda T, Mizuno Y, Okazaki Y. Id4, a new candidate gene for senile osteoporosis, acts as a molecular switch promoting osteoblast differentiation. PLoS. Genet. 2010;6:e1001019. doi: 10.1371/journal.pgen.1001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Wang Y, Casey AM, Chen F. Absence of canonical Smad signaling in ureteral and bladder mesenchyme causes ureteropelvic junction obstruction. J. Am. Soc. Nephrol. 2012;23:618–628. doi: 10.1681/ASN.2011060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierra CA, Nelson C. The Pan basic helix-loop-helix proteins are required for insulin gene expression. Mol. Endocrinol. 1995;9:64–71. doi: 10.1210/mend.9.1.7760851. [DOI] [PubMed] [Google Scholar]
- Wang LH, Baker NE. Salvador-warts-hippo pathway in a developmental checkpoint monitoring helix-loop-helix proteins. Dev. Cell. 2015;32:191–202. doi: 10.1016/j.devcel.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wice BM, Bernal-Mizrachi E, Permutt MA. Glucose and other insulin secretagogues induce, rather than inhibit, expression of Id-1 and Id-3 in pancreatic islet beta cells. Diabetologia. 2001;44:453–463. doi: 10.1007/s001250051643. [DOI] [PubMed] [Google Scholar]
- Wieben ED, Aleff RA, Tosakulwong N, Butz ML, Highsmith WE, Edwards AO, Baratz KH. A common trinucleotide repeat expansion within the transcription factor 4 (TCF4, E2-2) gene predicts Fuchs corneal dystrophy. PLoS. One. 2012;7:e49083. doi: 10.1371/journal.pone.0049083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HJ, Moskvina V, Smith RL, Dwyer S, Russo G, Owen MJ, O’Donovan MC. Association between TCF4 and schizophrenia does not exert its effect by common nonsynonymous variation or by influencing cis-acting regulation of mRNA expression in adult human brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011a;156B:781–784. doi: 10.1002/ajmg.b.31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, Cao TC, Carano RA, Dixit VM. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell. 2011b;146:918–930. doi: 10.1016/j.cell.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Wright C, Turner JA, Calhoun VD, Perrone-Bizzozero N. Potential Impact of miR-137 and Its Targets in Schizophrenia. Front Genet. 2013;4:58. doi: 10.3389/fgene.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li X, Li Y, Southwood M, Ye L, Long L, Al-Lamki RS, Morrell NW. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am. J. Physiol Lung Cell Mol. Physiol. 2013;305:L312–L321. doi: 10.1152/ajplung.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li X, Morrell NW. Id proteins in the vasculature: from molecular biology to cardiopulmonary medicine. Cardiovasc. Res. 2014;104:388–398. doi: 10.1093/cvr/cvu215. [DOI] [PubMed] [Google Scholar]
- Yu L, Liu C, Vandeusen J, Becknell B, Dai Z, Wu YZ, Raval A, Liu TH, Ding W, Mao C, Liu S, Smith LT, Lee S, Rassenti L, Marcucci G, Byrd J, Caligiuri MA, Plass C. Global assessment of promoter methylation in a mouse model of cancer identifies ID4 as a putative tumor-suppressor gene in human leukemia. Nat. Genet. 2005;37:265–274. doi: 10.1038/ng1521. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Zhao X, Wei Q, Paterson BM. Direct inhibition of G(1) cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J. 1999;18:6983–6993. doi: 10.1093/emboj/18.24.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MY, Clawson GA, Olivieri NF, Bell LL, Begley CG, Miller BA. Expression of SCL is normal in transfusion-dependent Diamond-Blackfan anemia but other bHLH proteins are deficient. Blood. 1997;90:2068–2074. [PubMed] [Google Scholar]
- Zhao Y, Johansson C, Tran T, Bettencourt R, Itahana Y, Desprez PY, Konieczny SF. Identification of a basic helix-loop-helix transcription factor expressed in mammary gland alveolar cells and required for maintenance of the differentiated state. Mol. Endocrinol. 2006;20:2187–2198. doi: 10.1210/me.2005-0214. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ling F, Griffin TM, He T, Towner R, Ruan H, Sun XH. Up-regulation of the Sirtuin 1 (Sirt1) and peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) genes in white adipose tissue of Id1 protein-deficient mice: implications in the protection against diet and age-induced glucose intolerance. J. Biol. Chem. 2014;289:29112–29122. doi: 10.1074/jbc.M114.571679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Wang H, Xue L, Zhang Z, Tong T. Regulation of cellular senescence and p16(INK4a) expression by Id1 and E47 proteins in human diploid fibroblast. J. Biol. Chem. 2004;279:31524–31532. doi: 10.1074/jbc.M400365200. [DOI] [PubMed] [Google Scholar]
- Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu. Rev. Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, Schenck A, Rauch A. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am. J. Hum. Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, Reardon W, Saraiva J, Cabral A, Gohring I, Devriendt K, de RT, Bijlsma EK, Hennekam RC, Orrico A, Cohen M, Dreweke A, Reis A, Nurnberg P, Rauch A. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Am. J. Hum. Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]