Abstract
Osteoclasts are unique bone remodeling cells derived from multinucleated myeloid progenitor cells. They play homeostatic vital roles in skeletal modeling and remodeling but also destroy bone masses in many pathological conditions such as osteoporosis and rheumatoid arthritis. Receptor activation of NF-κB ligand (RANKL) is essential to osteoclastogenesis. In this study, we investigated the effects of bromo-honaucin A (Br-H A)isolated from Leptolyngbya crossbyana (cyanobacterium). To investigate the mechanism of the inhibitory effect of Br-H A on osteoclastogenesis, we employed Br-H A in RANKL-treated murine monocyte/macrophage RAW 264.7 cells for osteoclastic differentiation in-vitro. The inhibitory effects on in-vitro osteoclastogenesis was evaluated by counting the number of Tartarate resistant acid phospatase (TRAP) positive multinucleated cells and by measuring the expression level of osteoclast-specific genes like matrix metalloproteinase 9 (MMP9), cathepsin K (CATH K), GRB2-associated-binding protein 2 (GAB2), c-terminal myc kinase (C-MYC), C-terminal Src kinase (C-SRC) and Microphthalmia-associated transcription factor (MITF). Moreover, Br-H A blocked the resorbing capacity of RAW 264.7 cells on calcium phosphate-coated plates. Finally, Br-H A clearly decreased the expression of Akt and also decreased the activation of ERK. Thus, the study identifies Br-H A as potent inhibitor potentialin the treatment of diseases involving abnormal bone lysis such as osteoporosis, rheumatoid arthritis, and periodontal bone degradation.
Keywords: Br-Honaucin A, RAW 264.7, Osteoclastogenesis, RANKL, p38, ERK, Akt
Graphical Abstract
1. Introduction
Bone remodeling is a physiological process that involves the resorption of bone by osteoclasts and formation of bone matrix by osteoblasts. Osteoclasts are multinucleated cells that are responsible for bone resorption and derive from hematopoetic stem cells (Boyle et al., 2003; Karsenty et al., 2002). An imbalance in bone remodeling that results in more bone resorption than bone formation underlies such pathologic bone disorders such as osteoporosis (Weitzmann and Pacifici., 2006) and rheumatoid arthritis (Durand et al., 2011; Hirayama et al., 2002). Osteoclasts arise from precursor cells of the monocyte/macrophage lineage and are the primary cells responsible for physiological and pathological bone resorption. Therefore, these cells are identified targets for therapies designed to retard or abolish metabolic bone loss, such as in osteoporosis. An inflamed state resulting in abnormal levels of cytokines and growth factors in the bone marrow microenvironment contributes to the pathological resorption of bone by osteoclasts (Mundy et al., 1995). Osteoclasts are known to be formed by the fusion of hematopoietic cells of the monocyte macrophage lineage during the early stage of the differentiation process (Mohamed et al., 2007). The receptor activator of nuclear factor-kappa B ligand (RANKL) produced by osteoblasts plays a key role in osteoclast differentiation and activation (Suda et al., 1999). Binding of RANKL to RANK triggers downstream signaling events that lead to the induction of osteoclastogenesis. The activation of molecular adaptor Grb-2-associated binder 2 (GAB2) subsequently induces the activation of transcription factors, mitogen-activated protein kinases (MAPKs), C-SRC, and AKT (Boyle et al., 2003). Hydrogen ions (H+) was secreted by mature osteoclasts and proteinases such as cathepsin K and matrix metalloproteinase (MMP)-9 from the ruffled border, and these dissolve the inorganic and organic components of bone, respectively. Tartrate-resistant acid phosphatase (TRAP) has also been suggested to be involved in the bone resorptive activity of osteoclasts (Angel et al., 2000). Hydrogen ions are produced via carbonic anhydrase II (CAII) in the cytoplasm and are secreted extracellularly by H+-ATPases (Vaananen and Laitala-Leinonen, 2008). Transcriptional factor involved in osteoclast differentiation and dendritic cell-specific transmembrane protein (DC-STAMP), an essential molecule for cell–cell fusion (Hartgers et al., 2000; Kukita et al., 2004). The c-myc oncogene has been implicated in the control of cell proliferation, differentiation, and programmed cell death as well as in neoplastic transformation (Fuhrmann et al., 1999; Dang et al., 1999; Grandori et al., 2000). C-SRC permits the receptor/kinase complex to organize the osteoclast cytoskeleton by activating the cytoskeleton-organizing molecules (Teitelbaum 2011; Jurdic et al., 2006). RANKL signal in osteoclast precursor cells evokes the activation of mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 (Lee et al., 2003). The phosphoinositide kinase-3/Akt pathway is also stimulated by RANKL. These signaling pathways ultimately lead to induction and activation of the transcription factors involved in expression of genes that characterize osteoclasts differentiation and function.
Honaucin A was isolated from the cyanobacterium Leptolyngbya crossbyana that was found on overgrowing corals in the Hawaiian coast (Choi et al., 2012). Marine cyanobacteria are one of the richest sources of biologically active and structurally unique natural products (Tidgewell et al., 2010) having many biological properties of potential utility in the treatment of cancer, microbial infections, inflammation, or neurological diseases. Chronic inflammatory disorders such as rheumatoid arthritis have pervasive impacts on human health (Grivennikov et al., 2010; Tousoulis et al., 2011). Bone-resorbing osteoclasts are important effector cells in inflammation-induced bone loss such as rheumatoid arthritis or periodontitis (Jimi et al., 2004). We predicted that Br-H A might display a protective effect against bone loss. So, we examined the anti-osteoclastogenic effect and signaling pathways of Br-H A with RANKL stimulated macrophages. We demonstrate here for the first time that Br-H A significantly suppressed RANKL–induced osteoclast differentiation by modulating osteoclast-specific genes, transcription factors and signaling molecules.
2. Materials and methods
2.1. Materials
Cell culture medium, fetal bovine serum (FBS), and horse serum were obtained from Invitrogen (Gaithersburg, MD, USA). RANKL was obtained from PeproTech (Rocky Hill, NJ, USA). A commercially available BONE RESORPTION ASSAY KIT 48 for the osteoclastic bone resorption assay was obtained from PG Research (TOKYO, JAPAN). All other chemicals were purchased from Sigma.
2.2. Preparation of Honaucin A, Hex-Honaucin A, Br-Honaucin A and I-Honaucin A
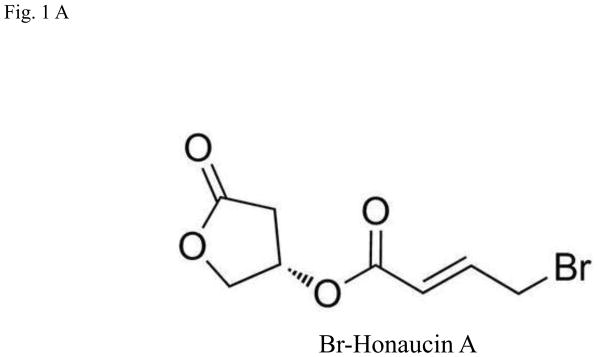
Honaucin A is a natural product originally isolated from a cyanobacterium, Leptolyngbya crossbyana, found overgrowing corals on the Hawaiian coast. Honaucin A and its synthetic derivatives were prepared by previously reported synthetic scheme (Choi et al., 2012). Honaucin A was synthesized by Steglich esterification with N,N′-dicyclohexylcarbodiimide between (S)-3-hydroxy-γ-butyrolactone and 4-chlorocrotonic acid. Hex-honaucin A was also prepared by 1-ethyl-3-(3 A was allaminopropyl) carbodiimide hydrochloride from (S)-3-hydroxy-γ-butyrolactone and (E)-4-chlorohex-2-enoic acid, which was obtained by selenocatalyticallylic chlorination by PhSeCl from (E)-hex-3-enoic acid. Br-H A (Fig. 1A) and I-honaucin A were produced from honaucin A by the treatment at 50°C for 5 days with NaBr and NaI, respectively.
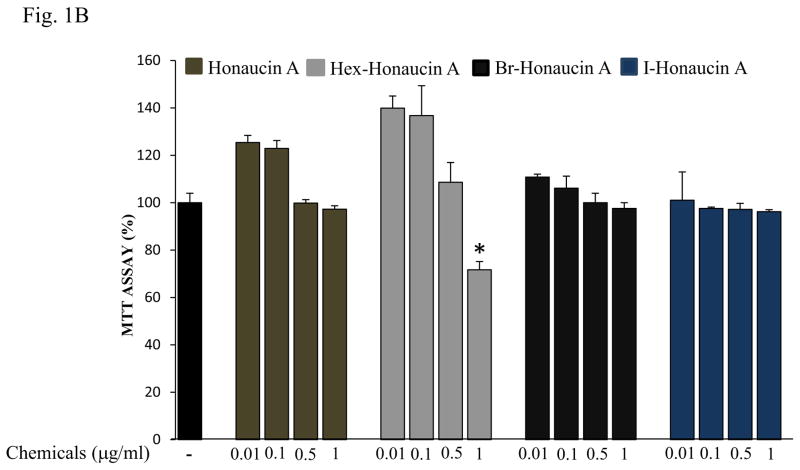
Fig. 1.
Effects of Honaucin derivatives on cell viability in RAW264.7 cells. (A) Chemical structure of bromo-honaucin A (Br-H A). (B) The effect of Honaucin A and it’s derivatives on cell viability was measured using the MTT assay. Values are expressed as means ± S.D. of triplicate experiments.*P < 0.05 indicates significant differences compared to the control.
2.3. Cell viability
Cell viability was measured by conventional MTT assay. RAW264.7 cells (3 × 104) were seeded on 96-well plates and incubated for 24 h in media that was supplemented with 10% FBS. Various concentration (0.01, 0.1, 0.5, 1) μg/ml of Honaucin A and Hex-Honaucin A, Br-Honaucin A and I-Honaucin A were added to the cell. The cells were incubated for additional 24 h at 37°C in 95% air and 5% CO2 atmosphere incubator and then washed with phosphate buffered-saline (PBS) and a medium containing 100 μg/ml of MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] for 2 h at 37°C. The cells were washed with PBS and dissolved in 200 μl of Dimethyl sulfoxide (DMSO). The resulting intracellular purple formazan was quantified by measuring the absorbance at a wavelength of 540 nm using spectrophotometer.
2.4. Cell culture and treatment
The murine monocyte/macrophage cell line RAW264.7 was purchased from American Type Culture Collection (Manasas, VA, USA) and grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). All cells were grown in a humidified atmosphere containing 5% CO2 at 37°C. For osteoclastic differentiation, RAW264.7 cells were suspended in a-MEM containing 10% FBS, 2 mM-glutamate, 100 μg/ml penicillin, and 100 μg/ml streptomycin and then seeded at 2×103 cells/well in 96-well culture plates and cultured with 50 ng/ml soluble RANKL for 4 days in osteoclastogenic medium. Cells were cytochemically stained for tartrate-resistant acid phosphatase (TRAP), an osteoclast marker protein.
2.5. Tartrate-resistant acid phosphatase (TRAP) staining
For TRAP staining, cells were washed with PBS and fixed with 3.7% formaldehyde for 10 min. After washing with PBS, cells were incubated with 0.1% (v/v) Triton X-100 for 1 min and then incubated for 40 min at 37 °C in dark with a mixture of solutions of Fast Garnet GBC, sodium nitrite, naphthol AS-BI phosphoric acid, acetate, and tartrate on the Leukocyte Acid Phosphatase Assay kit (Sigma) according to the manufacturer’s instructions. Cells were washed with distilled water and TRAP-positive multinucleated cells containing three or more nuclei were counted using a light microscope. Furthermore, IC50 was calculated. To calculate IC50, a scatter graph was made in MS EXCEL (where X axis is concentration and Y axis is % activity) then a slope equation “Y=mx+c” is used (where Y=50, and value of m and c is present in equation itself). Now, the equation is solved and the value of “X” obtained will be IC50 value for that graph.
2.6. RT-PCR analysis
Total RNA was isolated from cultured cells using TRIzol (Invitrogen) and cDNA synthesis was performed with Super Script II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol and stored at −80°C. All PCR primers were purchased from Bioneer (Daejeon, Korea). Primer sequences and PCR conditions used in this study are listed in Table 1. After initial denaturation at 94°C for 1 min, PCR was performed for various cycles (30 s at 94°C, 1 min at annealing temperature and 2 min at 72°C) using Taq polymerase (Promega, Madison, WI, USA). Reaction products were separated on a 1% agarose gel, stained with ethidium bromide, and analyzed by densitometry using a Phosphoimager and Quantity One software (Version 4.3.1) (Bio-Rad, Hercules, CA, USA). Levels of each gene expression in all experimental groups were compared to the expression levels of the control group.
Table 1.
Primer sequences and conditions for RT-PCR
| Target genes (Accession number) | Primer (Forward, Reverse) | Anneling Tm(°C) | PCR cycles |
|---|---|---|---|
| TRAP (NM_007388) | 5′-ctgctgggcctacaaatcat-3′ 5′-ggtagtaagggctggggaag-3′ |
54 | 30 |
| RANK (NM_009399) | 5′-aaaccttggaccaactgcac-3′ 5′-accatcttctcctcccgagt-3′ |
53.7 | 30 |
| MMP9 (NM_013599) | 5′-cgtcgtgatccccacttact-3′ 5′-agagtactgcttgcccagga-3′ |
57.5 | 36 |
| Cathepsin K NM_007802) | 5′-aggcggctatatgaccactg-3′ 5′-ccgagccaagagagcatatc-3′ |
57.5 | 26 |
| MMP2 (NM_008610) | 5′-ggctggaacactaggac-3′ 5′-cgatgccatcaaagacaatg-3′ |
60 | 36 |
| CAII (K00811) | 5′ ttggctgttttgggctatttt-3′ 5′- cgctttgatctttctattctt -3′ |
52 | 35 |
| DC-STAMP (AY517483) | 5′- ctaaggagaagaaacccttg-3′ 5′- cagcatagaagacaacaatcc -3′ |
54 | 35 |
| PU.1 (M_32370) | 5′- cttcccttatcaaaccttgtc-3′ 5′- aggtgagcttcttcttgactt -3′ |
54 | 35 |
| MITF (NM_008601) | 5′- agacctgacatgtacgacaac-3′ 5′- tatgcagggctactgataag -3′ |
56 | 35 |
| c-src (U01149) | 5′- tccaggctgaggagtggtactttgg-3′ 5′- atacggtagtgaggcggtgacacag -3′ |
64 | 35 |
| c-myc (AB040746) | 5′- caccagcagcgactctgaagaagag-3′ 5′- agaggtgagcttgtgctcgtctgc -3′ |
64 | 35 |
| GAB2 (AB018414) | 5′- ctggacaagaaccacaatgc-3′ 5′- agtctttcctggaggttcag -3′ |
56 | 30 |
| β-actin (NM_007393) | 5′-ttctacaatgagctgcgtgt-3′ 5′-ctcatagctcttctccaggg-3′ |
50 | 26 |
2.7. Western blotting
Cells were harvested and lysed in lysis buffer [20 mM Tris–HCl (pH 7.5), 137 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM Na3VO4, 1 mM phenyl methyl sulfonyl fluoride (PMSF), and 1 X protease inhibitor cocktail]. After centrifugation at 13,000 × g for 15 min, supernatants were used as cell extracts and supernatants were stored at −70°C until use. Cell extracts were separated by SDS-PAGE on 8–10% gels and then transferred to polyvinylidenedifluoride (PVDF) membranes (Bio-Rad) with a glycine transfer buffer (192 mM glycine, 25 mM Tris–HCl [pH 8.8], 20% MeOH [v/v]). Membranes were blocked with 5% nonfat skim milk in Tris-buffered saline (TBS) containing 0.25% Tween-20 (TTBS) at room temperature for 1 h and then incubated for 16 h at 4°C with rabbit anti-phospho-ERK1/2 (Cell Signaling Technology Inc., Beverly, MA, USA), anti-phospho-JNK (Cell Signaling Technology), anti-phospho-AKT(ser 473) (Cell Signaling Technology), or anti-β-actin (Santa Cruz Biotechnology) antibodies diluted 1:1000 in 5% nonfat skim milk in TTBS. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse antibodies (Santa Cruz Biotechnology) were used as secondary antibodies (1:5000–1:10,000 dilution in 5% nonfat skim milk in TTBS, 1 h incubation at room temperature) and the antigen–antibody complexes were visualized with an ECL Plus kit (Amersham Biosciences, Piscataway, NJ, USA). Protein expression of osteoclast markers P-Akt, P-JNK and P-ERK are divided by Akt, JNK and ERK respectively.
2.8. Pit formation assay
RAW 264.7 cells were suspended in phenol α-MEM containing 10% FBS and plated at a concentration of 5×103 cells/well on calcium phosphate (CaP)-coated 48 well plate. Binding of Fluoresceinamine –labeled chondroitin sulfate (FACS) to CaP-coated plate and cell culture was performed in aseptic conditions. 0.25 ml of bone resorption assay facs was added on BONE RESORPTION ASSAY PLATE 48 (Code#: CSR-BRA-48KIT), and the plate was covered with lid and incubated at 37°C for 1–2 h in light-shielded conditions. After, incubation the plate was washed twice with 0.5 ml of 1x PBS, and 0.5 ml of culture medium (without phenol-red) was added. FACS-labeled CaP-coated plate was kept in light-shielded conditions during cell proliferation. The media was removed from the plate and RAW264.7 cells were inoculated into each well in the culture medium (phenol red-free αMEM containing 10% FBS), in the presence or absence of 100 ng/ml RANKL and Br-H A for 6 days without changing medium. Multinuclear osteoclast cells are observed in 6 days. On day 6, 100 μl of the conditioned medium was transferred from each well into the wells of a 96-well plate (black plate for fluorescence measurement). Then, 50 μl of BONE RESORPTION ASSAY BUFFER was added to each well and mixed using a plate shaker. Fluorescence intensity was measured using an excitation wavelength of 485 nm and an emission wavelength of 535 nm. To measure the pit area, the plate was then treated with 5% sodium hypochlorite for 5 min to remove the cells. Then the plate was washed with water and dried. Finally, the regions in each well are photographed using a microscope, and the pit area was measured with image J software.
2.9. Statistics analysis
All of the experimental data shown are expressed as means ± S.D. and all experiments were repeated at least three times, unless otherwise indicated. Statistical analyses were performed by Dunnett’s multiple comparison test using SPSS ver. 12.0 software and P-values less than 0.05 were considered significant.
3. Results
3.1 Effects of Honaucin derivatives on cell viability
We evaluated four honaucin-type compounds effects on cell growth. RAW264.7 cells were treated with honaucin A or its derivatives at different concentrations (0.01 μg/ml, 0.1 μg/ml, 0.5 μg/ml and 1 μg/ml) for 24 h and cell growth was measured by MTT assay. Honaucin A and its derivatives at 0.01 μg/ml, 0.1 μg/ml concentrations exhibited no cytotoxic effects in cells. Furthermore, of all the derivatives at 0.5 μg/ml, and 1 μg/ml concentrations, Hex- H A at 1 μg/ml showed high toxicity, only 71.6% cell viability was observed. While Honaucin A, Br-H A and I-H A did not showed any toxicity compared to the control cells that had received no treatment (Fig. 1B).
3.2 Effects of Honaucin derivatives on cell viability, TRAP activity and IC50 in RANKL-induced Osteoclastogenesis
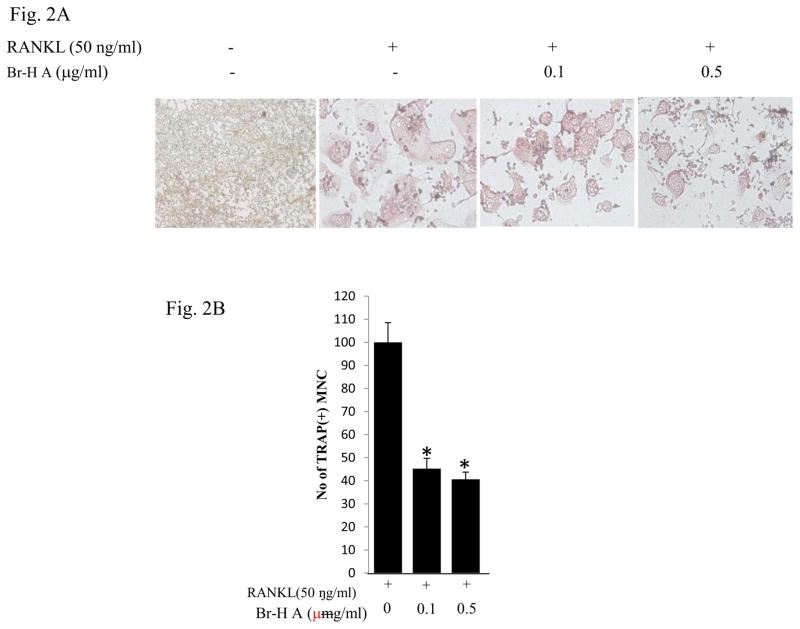
Next, we evaluated the inhibitory activity of 0.1 μg/ml and 0.5μg/ml honaucin A and its derivatives to 50 ng/ml RANKL-induced osteoclastogenesis in RAW 264.7 cells. We found TRAP activity was decreased by Br-honaucin A > I-honaucin A >hex-honaucin A >honaucin A, respectively (Table 2). 0.1 μg/ml Br-H A caused 50% more inhibition of RANKL-induced osteoclastogenesis than did I-honaucinA, hex-honaucin A and honaucin A, as determined by measuring the cellular TRAP activity (Table 2). Their anti-osteoclastogenic effects were not attributed to cellular toxicity as confirmed by MTT cell viability assay of cells treated with TRAP and honaucin A or its derivatives at 0.5 μg/ml. Br-H A did not affect the cell growth rate of RAW264.7 cells at the 1 and 3 day time-points but did reduce the number of TRAP-positive multinucleated cells to 40.58 ± 1.8% compared to control. Of the four honaucin derivatives, Br-H A was the most potent inhibitor of RANKL-induced osteoclastogenesis with an IC50 value of approximately 0.54 ± 0.06 μg/ml compared to RANKL treatment as 100% activity (Table 2). Next, we examined whether Br-H A has an effect on multinucleated osteoclast-like cell formation in RANKL-stimulated RAW264.7 cells. Br- Honaucin A markedly inhibited the RANKL stimulated multinucleated osteoclast-like cell formation in RAW264.7 cells in a dose-dependent manner (Fig. 2A and 2B).
Table 2.
The effects of Honaucin A derivatives on cell viability, TRAP activity and IC50 in RANKL-induced Osteoclastogenesis.
| Compound | Cell viability (0.5 μg/ml)
|
TRAP (%) | TRAP (%) | IC50 (μg/ml) | |
|---|---|---|---|---|---|
| Day 1 | Day 3 | 0.1 μg/ml | 0.5μg/ml | ||
| Honaucin A | 106.20 | 93.40 | 69.03 ± 4 | 59.63 ± 1.1 | 0.63 ± 0.09 |
| Hex-Honaucin A | 102.41 | 91.81 | 64.01 ± 1.9 | 47.28 ± 1.8 | 0.68 ± 0.09 |
| Br-Honaucin A | 103.27 | 96.55 | 45.18 ± 2.6 | 40.58 ± 1.8 | 0.54 ± 0.06 |
| I-Honaucin A | 98.79 | 86.90 | 59.83 ± 3.4 | 46.02 ± 2.9 | 0.61± 0.09 |
Cell viability was measured by MTT assay in RAW 264.7 cells upon treatment with individual compounds at 0.5 μg/ml final concentrations for 1 day and 3 days. Cell viability represents the percentage of the control. Relative TRAP activity of individual compounds at 0.1 μg/ml and 0.5 μg/ml in the presence of 50 μg/ml RANKL. IC50 was calculated for TRAP activity. Values are expressed as means ± S.D. of triplicate experiments
Fig. 2.
Inhibitory effects on osteoclast differentiation in RANKL-stimulated RAW264.7 cells. (A) RAW264.7 cells were cultured with 0.1 μg/ml and 0.5 μg/ml concentration of Br-H A in the presence of 50 αg/ml RANKL. (B) The histogram represents the number of TRAP (+) multinucleated cells compared with RANKL treated as a control. Values are expressed as means ± S.D. of triplicate experiments. *P < 0.05 indicates significant differences from the RANKL-stimulated group.
3.3 Effect of Br-H A on mRNA expression of osteoclastic marker genes in RANKL stimulated RAW 264.7 cells
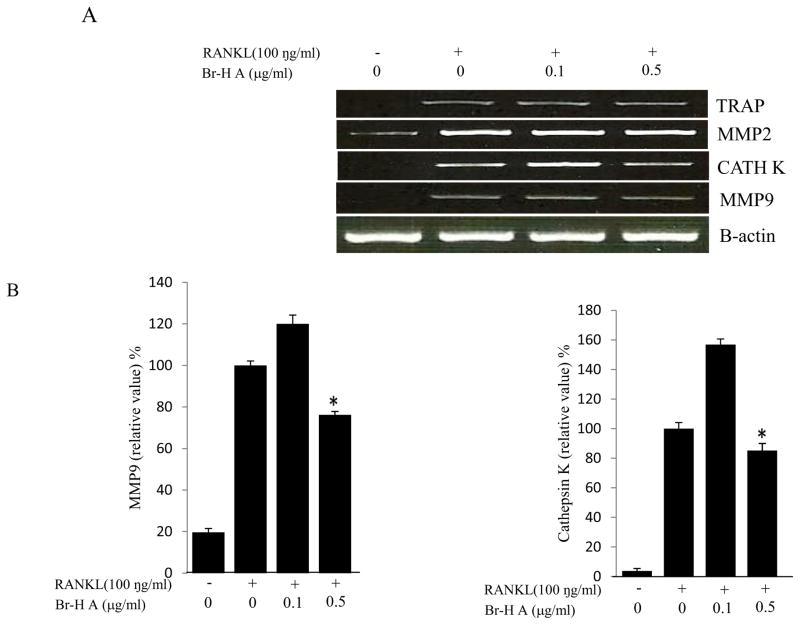
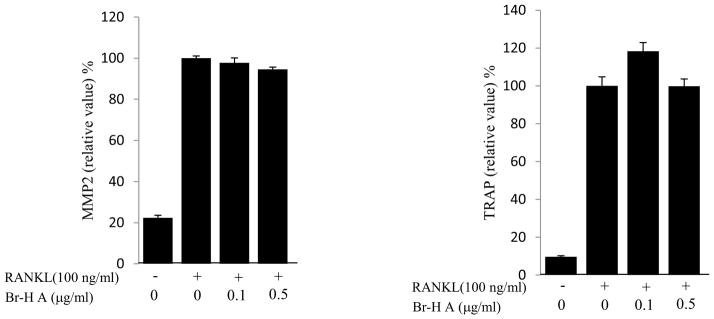
We then monitored the expression levels of the biomarkers and specific transcription factors involved in osteoclastogenesis. Osteoclast differentiation is associated with up-regulation of specific genes in response to RANKL (Miyauchi et al., 2011). To determine if the inhibitory effect of Br-H A corresponded with the expression of osteoclast specific genes, total RNA was prepared from treated RAW 264.7 cells and analyzed by RT-PCR. While RANKL (100 ng/ml) significantly induced the expression of cathepsin K, MMP 9, matrix metalloproteinase 2 (MMP 2) and TRAP in RAW264.7 cells. The expression of cathepsin K and MMP9 increased in a low concentration of Br- H A and significantly reduced in a high concentration of Br-H A. Whereas Br-H A did not show much effect on TRAP and MMP 2 gene (Fig. 3A and 3B). Thus, results show that Br-H A has a specific effect on the regulation of some genes induced during osteoclast differentiation.
Fig. 3.
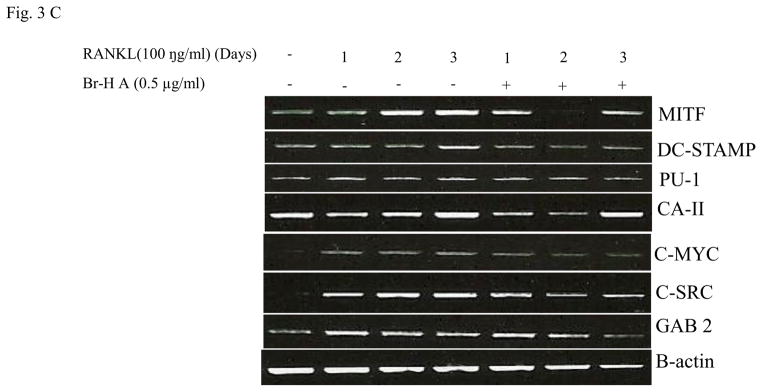
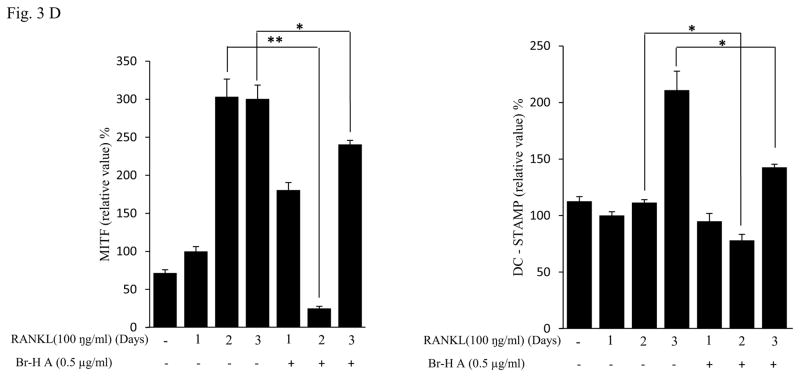
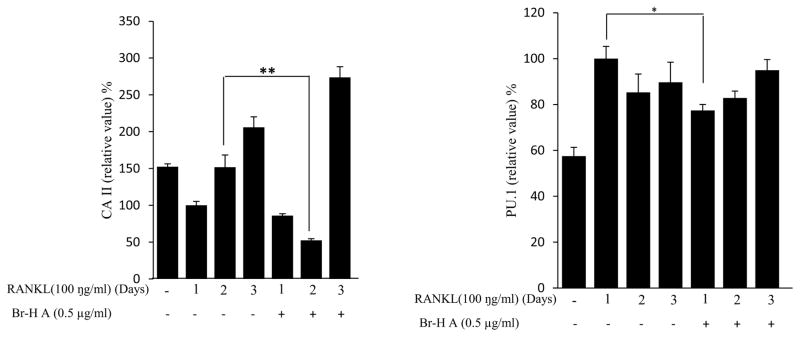
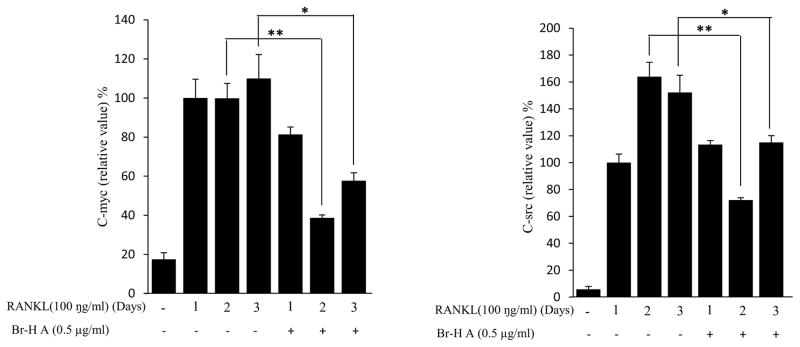
Effect of Br-H A on mRNA expression of osteoclastic marker genes in RANKL stimulated RAW264.7 cells. (A) RAW 264.7 cells (3.0 × 105 cells/mL) were pre-incubated for 16 h, and the cells were stimulated with RANKL (100 ng/mL) in the presence of Br-H A (0.1 and 0.5 μg/ml) for 4 days. The expressions of mRNA osteoclastogenic marker genes were determined using RT-PCR. (B) The histogram represents the levels of the mRNA expression (%) compared with that of the control. Values are expressed as means ± S.D. of triplicate experiments. *P < 0.05 indicates significant differences from the RANKL-stimulated group. (C) RAW264.7 cells (3.0 × 105 cells/ml) were pre-incubated for 16 h, and the cells were stimulated with RANKL (100 ng/ml) in the presence of Br-Honaucin A 0.5 μg/ml for 1, 2, 3 days. mRNA expressions of osteoclastogenic marker genes were determined using RT-PCR. (D) The histogram represents the levels of the mRNA expression (%) compared with that of the control. Values are expressed as means ± S.D. of triplicate experiments. *P < 0.05 and **P < 0.001 indicate significant differences from the RANKL-stimulated group.
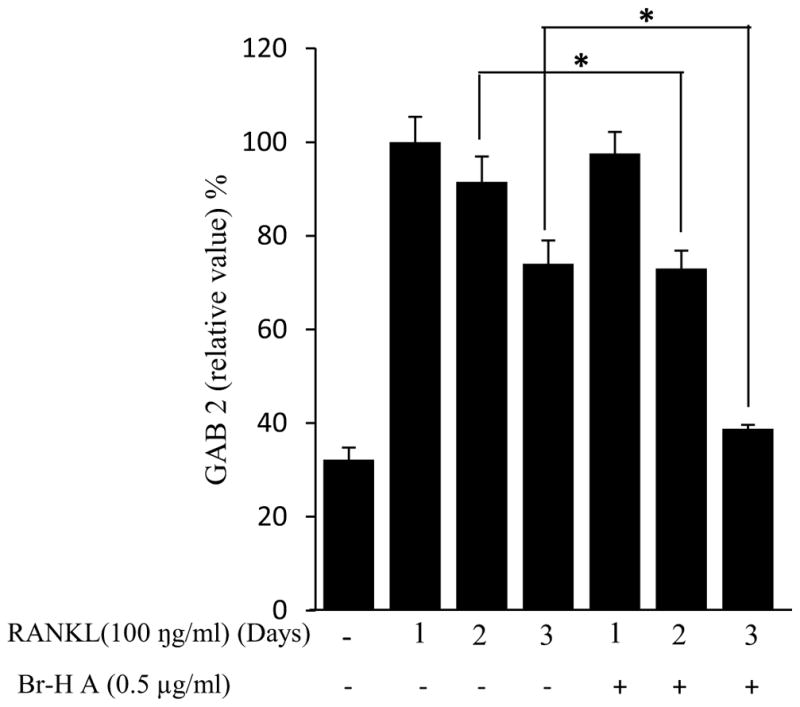
Similarly, RANKL (100 ng/ml) significantly induced the expression of purine rich 1 (PU 1), Microphthalmia-associated transcription factor (MITF), C-SRC, C-MYC, dendritic cell-specific transmembrane protein (DC-STAMP), GAB2 and carbonic anhydrase II (CAII). Br-H A reduced RANKL-induced expression of PU 1 in day 1 and didn’t effect on day 2 and 3. In case of MITF, C-SRC, C-MYC, GAB2 and DC-stamp the RANKL induced expression is significantly reduced in day 2 and day 3 rather than in day 1. Finally, the RANKL-induced expression of CA II is reduced significantly only in day 2 and it didn’t effect in day 1 and 3 (Fig. 3C and 3D) and also did not affect the expression of the housekeeping gene β-actin. These results show that Br-H A has specific effect on the regulation of specific genes in different time intervals during osteoclast differentiation.
3.4 Suppression of RANKL- stimulated pit formation by Br-H A
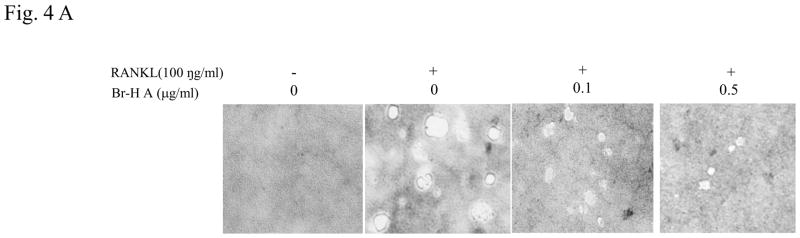
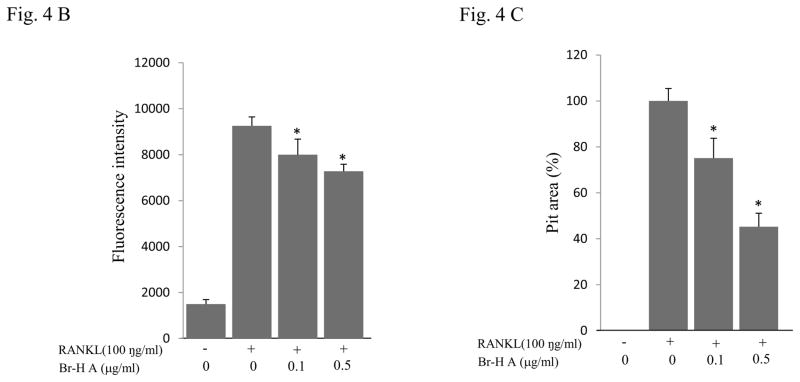
We further examined whether Br-H A had an effect on the ability of mature osteoclasts to resorb bone. RAW264.7 cells were stimulated with (100 ng/ml) RANKL to induce the differentiation of mature osteoclasts with bone-resorbing capacity and plated on calcium phosphate-coated culture plates. RANKL-stimulated cells formed a number of pits on the bottom of the plate, suggesting that the bone resorption activity of RANKL-treated RAW264.7 cells made them functionally active as osteoclasts (Fig. 4A). Furthermore, treatment with Br-H A significantly reduced the fluorescence intensity in a dose dependent manner and also the number and overall area of resorption pits in a concentration-dependent manner as compared to treatment with RANKL alone (Fig. 4B and 4C).
Fig. 4.
RAW264.7 cells were plated on to Bone Resorption Assay Kit 48 plates and cultured in differentiation medium without (control) or with Br-H A (0.1 and 0.5 μg/ml) for 6 days. (A) A microscopic photograph of a CaP coated plate (on day 6), without RANKL, with RANKL only (100 ng/ml), and with RANKL and Br-H A (0.1 and 0.5 μg/ml) respectively. (B) The inhibitory effects of Br-H A on the resorption of CaP induced by RANKL (100 ng/ml) were evaluated by fluorescence intensity. (C) The histogram represents the relative pit area (%) compared with that of the control (#). The results are expressed as mean ± S.D. of triplicate experiments. *P<0.05 indicates significant differences from the RANKL stimulated group.
3.5 Effect on MAPK and Akt in RANKL stimulated RAW 264.7 cells
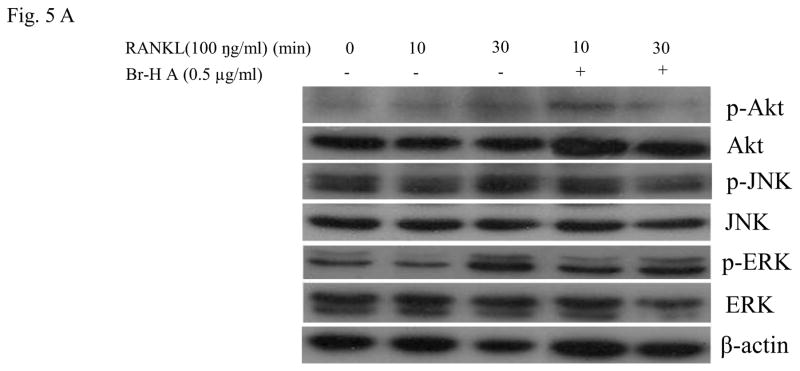
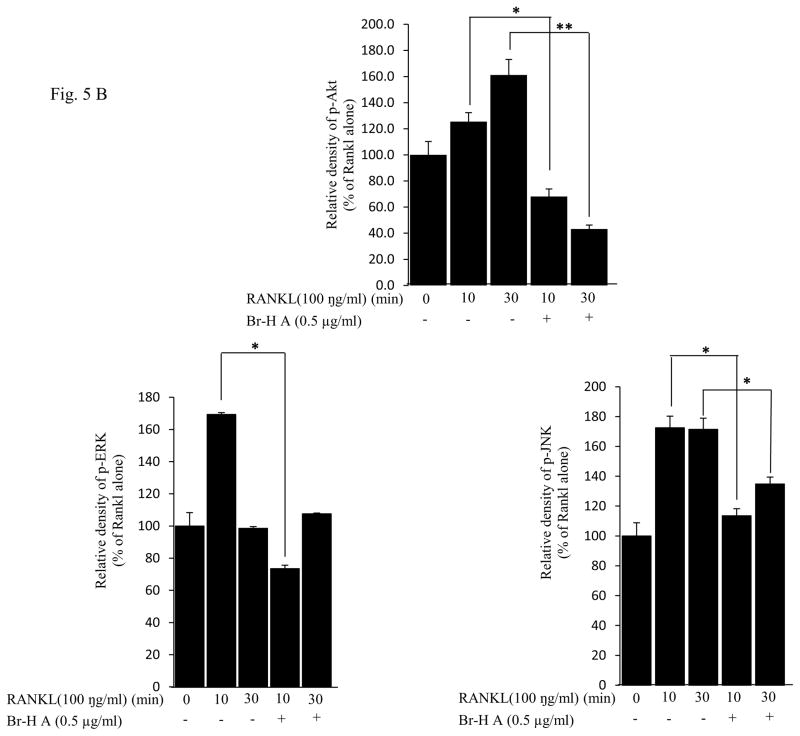
Families of (MAPKs) JNK, ERK, have been shown to be activated in response to RANKL in osteoclast precursor cells (Lee et al., 2002) and RAW 264.7 cells (Mozar et al., 2008). To determine the intracellular mechanism of Br-H A, we investigated the activation of MAPKs and Akt involved in the RANKL-signaling pathway using the RAW264.7 cells. The JNK, ERK1/2 and Akt activation states were determined by Western blot analysis using antibodies specifically directed against the phosphorylated forms of these kinases, and compared to data obtained with antibodies directed against their un-phosphorylated states. RANKL (100 ng/ml) markedly induced the activation of MAPKs within 10 min of treatment in RAW 264.7 cells. However, the phosphorylation of ERK1/2 appeared to be inhibited by the treatment with Br-H A at 10 and 30 min, again the activation of JNK was not inhibited by the treatment with Br-H A at 10 and 30 min. Likewise RANKL (100 ng/ml) also induced the activation of Akt (ser 473) within 10 min of treatment. This RANKL-mediated activation of Akt appeared to be inhibited by treatment with Br-H A at 10 and 30 min (Fig. 5A and 5B).
Fig. 5.
Effect on MAPK and Akt. (A) MAPK protein level in RANKL-stimulated RAW264.7 cells. RAW264.7 cells (1.0 × 106 cells/mL) were cultured for 16 h, pre-incubated with 0.5 μg/ml of Br-H A for 30 min, and stimulated with RANKL (100 ng/ml) for the indicated times. Cell extracts were analyzed by Western blot using antibodies specifically directed against the phosphorylated forms of the enzymes, compared to data obtained with antibodies directed against the unphosphorylated states of the kinases. Equal amounts of protein were loaded in each lane as calibrated by the level of β-actin. (B) The histogram represents the percent relative density of the MAPK compared with that of the control. Values are expressed as mean ± S.D. of triplicate experiments. *P<0.05 and **P<0.001 indicate significant differences from the RANKL
4. Discussion
Honaucin A is a marine natural product found originally in a Hawaiian marine cyanobacterium. As a synthetically tractable compound, various, halogen atoms (Br, I) could be attached to the primary site of the crotonic acid residue. In this study, we tried to screen Honaucin A and its derivatives and select the most potent drug. So, we performed MTT assay and TRAP activity. Hex- H A didn’t showed toxic effect on day1 but showed toxic effect on day 3 in MTT assay so we didn’t choose Hex-H A. Honaucin A didn’t showed toxic effect in MTT assay and has good effect in TRAP activity. But concentration of Honaucin A was doubled than Br-H A so we choose Br-H A. While I-honaucin A was a very active compound, it was found to be quite labile. Therefore, Br-honaucin A was chosen as the most effective drug for further study (Choi et al., 2012).
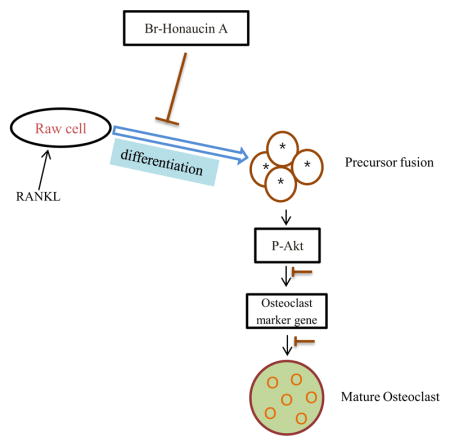
Osteoclasts are tissue-specific multinucleated cells generated from the differentiation of monocyte/macrophage precursors (Boyle et al., 2003). Excessive bone resorption plays a vital role in the formation of age-related osteoporosis (Cummings et al., 2002). In this study, we determined that Br-H A is a potent inhibitor of osteoclastogenesis in RANKL-stimulated RAW 264.7 cells. Br-H A inhibited RANKL-induced formation of osteoclasts from precursor cells without cytotoxicity. Br-H A also led to the decrease of osteoclast-specific genes like cathepsin K, MMP9, PU-1, MITF, C-SRC, C-MYC, GAB2, DC-stamp and CA II. Moreover, Br-H A inhibited the resorbing capacity of RAW 264.7 cells on calcium phosphate-coated plates. Br-H A has inhibitory effects on the activation of ERK, JNK and Akt.
Over expression of cathepsin K causes excessive bone loss (Siddiqi et al., 2015; Zhuoet al., 2014). In this study, we found that Br-H A reduced the RANKL-induced expression of cathepsin K. TRAP is an iron-binding protein that is highly expressed in osteoclasts and also induced in differentiation of osteoclasts (Reddy et al., 1995). In our study, Br-H A did not reduce the RANKL-induced expression of TRAP. Over expression of MMP9 increased osteoclast differentiation (Park et al., 2014). In this study we found that Br-H A reduced the RANKL-induced expression of MMP9. Bone matrix degradation is also associated with the expression of MMP2 (Kang et al., 2003). But our study shows that Br-H A did not reduce the RANKL-induced expression of MMP2. MITF regulates the development and function of several cell lineages, including osteoclasts (Lu et al., 2010). The genetic and physical interactions between MITF and PU 1 are necessary for osteoclast gene expression and differentiation (Lucin et al., 2001). In this study, we examined whether MITF and PU 1 regulate osteoclast. We found Br-H A reduced the RANKL-induced expression of MITF and PU 1. Furthermore, c-myc is a downstream target of RANKL and its expression is required for RANKL-induced osteoclastogenesis (Takeshita et al., 2002). In our study, we found Br-H A dramatically reduced the RANKL-induced expression of c-myc. Likewise, c-src play important roles in regulating bone resorption of osteoclasts by mediating their migration (Zou et al., 2007). In our study, Br-H A reduced the RANKL-induced expression of c-src. GAB2 is the target of several receptor and non-receptor tyrosine kinases as well as to be modified by other receptors known to be expressed on the osteoclast or its precursors including Fc (Sarmay et al., 2006). Our study showed that Br-H A reduced the RANKL-induced expression of GAB 2. In addition, DC-STAMP is not only involved in cell-cell fusion during osteoclastogenesis but also participates in osteoclast differentiation (Ya-Hui et al., 2012). In this study, Br-H A reduced the RANKL-induced expression of DC-STAMP. CA II is essential in bone resorption and also in osteoclast differentiation (Lehenkaril et al., 1998) and appears in osteoclasts at an early stage of differentiation (Laitala et al., 1993). In our study, Br-H A reduced the RANKL-induced expression of CA II.
Br-H A also had inhibitory effects on the activation of MAPKs and Akt. Osteoclastogenesis is stimulated by inflammatory cytokines, leading to the resorption of bone typical of osteoporosis (Yoon et al., 2013). RANKL binds to RANK in osteoclast precursor and differentiating osteoclasts cells, resulting in activation of various intracellular signaling pathways involving p38, JNK, ERK and Akt (Boyle et al., 2003; Lee et al., 2003). RANKL-mediated activation of Akt is dependent on TRAF6-Src-PI 3-kinase interaction (Wong et al., 1999). Akt primarily plays a role in promoting osteoclast survival (Lee et al., 2001). Br-H A displayed a potent inhibitory effect on osteoclast differentiation due to Akt inhibition. Similarly, JNK and ERK play a functional role in osteoclast differentiation (Lee et al., 2002; Yu et al., 2014). In our study, Br-H A suppressed the activation of JNK and ERK in response to RANKL in a dose dependent manner. An intricate interplay of these pathways determines the specific changes in gene expression and cytoskeletal reorganization for differentiation, function, and survival of osteoclasts.
In summary, the present study demonstrated that Br-H A inhibits osteoclastogenesis from macrophage cell line in vitro. Br-H A reduced the RANKL-induced expression of osteoclast-associated genes MMP9, Cath K, GAB2, C-MYC, C-SRC, MITF, PU 1 and DC-STAMP. In addition, Br-H A attenuated the RANKL-induced expression of ERK and Akt activation. Although additional experiments are needed to confirm the efficacy of Br-H A in vivo, our results indicate that it may have potential as a therapeutic drug for disorders associated with bone loss.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Science, ICT & Future Planning(NRF-2014R1A1A2A16055076), Research Base Construction Fund Support Program funded by Chonbuk National University in 2015 (YS) and the National Institutes of Health under grant CA100851 (WHG).
Footnotes
CONFLICT OF INTEREST
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angel NZ, Walsh N, Forwood MR, Ostrowski MC, Cassady AI, Hume DA. Transgenic mice overexpressing tartrate-resistant acid phosphatase exhibit an increased rate of bone turnover. J Bone Miner Res. 2000;15:103–110. doi: 10.1359/jbmr.2000.15.1.103. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Mensah KA, Schwarz EM, Ju Y, Takahata M, Feng C, McMahon LA, Hicks DG, Panepento B, Keng PC, Ritchlin CT. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP) J Bone Miner Res. 2012;27:79–92. doi: 10.1002/jbmr.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Mascuch SJ, Villa FA, Byrum T, Teasdale ME, Smith JE, Preskitt LB, Rowley DC, Gerwick L, Gerwick WH. Honaucins A–C, Potent Inhibitors of Inflammation and Bacterial Quorum Sensing: Synthetic Derivatives and Structure-Activity Relationships. Chem Biol. 2012;19:589–598. doi: 10.1016/j.chembiol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- Dang CV, Resar LM, Emison E, Kim S, Li Q, Prescott JE, Wonsey D, Zeller K. Function of the c-Myc oncogenic transcription factor. Exp Cell Res. 1999;253:63–77. doi: 10.1006/excr.1999.4686. [DOI] [PubMed] [Google Scholar]
- Durand M, Boire G, Komarova SV, Dixon SJ, Sims SM, Harrison RE. The increased in vitro osteoclastogenesis in patients with rheumatoid arthritis is due to increased percentage of precursors and decreased apoptosis – The in Vitro osteoclast differentiation in arthritis (IODA) study. Bone. 2011;48:588–596. doi: 10.1016/j.bone.2010.10.167. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G, Rosenberger G, Grusch M, Klein N, Hofmann J, Krupitza G. The MYC dualism in growth and death. Mutat Res. 1999;437:205–217. doi: 10.1016/s1383-5742(99)00084-8. [DOI] [PubMed] [Google Scholar]
- Grandori C, Cowley SM, James LP, Eisenman RN. The myc/MAX/MAD network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Yang D, Chang EJ, Lee Y, Huang H, Sung SH, Lee ZH, Kim YC, Kim HH. Inhibition of osteoclast differentiation and bone resorption by sauchinone. Biochem Pharmacol. 2007;74:911–923. doi: 10.1016/j.bcp.2007.06.044. [DOI] [PubMed] [Google Scholar]
- Hartgers FC, Vissers JL, Looman MW, van Zoelen C, Huffine C, Figdor CG, Adema GJ. DC-STAMP, a novel multi membrane spanning molecule preferentially expressed by dendritic cells. Eur J Immunol. 2000;30:3585–3590. doi: 10.1002/1521-4141(200012)30:12<3585::AID-IMMU3585>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatol (Oxford) 2002;41:1232–1239. doi: 10.1093/rheumatology/41.11.1232. [DOI] [PubMed] [Google Scholar]
- Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Kang BS, Park YG, Cho JY, Kim JK, Lee TK, Kim DW, Gu YH, Suzuki I, Chang YC, Kim CH. Interleukin-1 and tumor necrosis factor-alpha induce collagenolysis and bone resorption by regulation of matrix metalloproteinase-2 in mouse calvarial bone cells. Immunopharmacol Immunotoxicol. 2003;25:347–364. doi: 10.1081/iph-120024503. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, Nagata K, Iijima T, Horiuchi M, Matsusaki H, Hieshima K, Yoshie O, Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitala T, Vaananen K. Proton channel part of vacuolar H (+)-ATPase and carbonic anhydrase II expression is stimulated in resorbing osteoclasts. J Bone Miner Res. 1993;8:119– 126. doi: 10.1002/jbmr.5650080115. [DOI] [PubMed] [Google Scholar]
- Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem. 2001;276:49343–49349. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidyl inositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30:71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214. doi: 10.1016/s0006-291x(03)00695-8. [DOI] [PubMed] [Google Scholar]
- Lehenkari P, Hentunen TA, Laitala-Leinonen T, Tuukkanen J, Väänänen HK. Carbonic Anhydrase II Plays a Major Role in Osteoclast Differentiation and Bone Resorption by Effecting the Steady State Intracellular pH and Ca21. Exp Cell Res. 1998;242:128–137. doi: 10.1006/excr.1998.4071. [DOI] [PubMed] [Google Scholar]
- Lu SY, Li M, Lin YL. Mitf induction by RANKL is critical for osteoclastogenesis. Mol Biol Cell. 2010;21:1763–1771. doi: 10.1091/mbc.E09-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchin A, Suchting S, Merson T, Rosol TJ, Hume DA, Cassad AI, Ostrowski C. Genetic and physical interactions between Microphthalmia transcription factor and PU. 1 are necessary for osteoclast gene expression and differentiation. J Biol Chem. 2001;276:36703–36710. doi: 10.1074/jbc.M106418200. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Miyamoto T. Suppressive effects for osteoclastogenesis regulated by RANKL signal. Clin Calcium. 2011;21(8):1141–1147. [PubMed] [Google Scholar]
- Mohamed SG, Sugiyama E, Shinoda K, Taki H, Hounoki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Ogawa H, Miyahara T. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW 264.7 cells and mouse bone marrow cells. Bone. 2007;41:592–602. doi: 10.1016/j.bone.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Mozar A, Haren N, Chasseraud M, Louvet L, Maziere C, Wattel A, Mentaverri R, Morliere P, Kamel S, Brazier M, Maziere JC, Massy ZA. High extracellular inorganic phosphate concentration inhibits RANK–RANKL signaling in osteoclast like cells. J Cell Physiol. 2008;215:47–54. doi: 10.1002/jcp.21283. [DOI] [PubMed] [Google Scholar]
- Mundy GR, Boyce B, Hughes D, Wright K, Bonewald L, Dallas S, Harris S, Ghosh-Choudhury N, Chen D, Dunstan C. The effects of cytokines and growth factors on osteoblastic cells. Bone. 1995;17:71S–75S. doi: 10.1016/8756-3282(95)00182-d. [DOI] [PubMed] [Google Scholar]
- Park K, Ju WC, Yeo JH, Kim JY, Seo HS, Uchida Y, Cho Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int J Mol Med. 2014;33:178–184. doi: 10.3892/ijmm.2013.1557. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Hundley JE, Windle JJ, Alcantara O, Linn R, Leach RJ, Boldt DH, Roodman GD. Characterization of the mouse tartrate-resistant acid phosphatase (TRAP) gene promoter. J Bone Miner Res. 1995;10:601–606. doi: 10.1002/jbmr.5650100413. [DOI] [PubMed] [Google Scholar]
- Sarmay G, Angyal A, Kertesz A, Maus M, Medgyesi D. The multiple function of Grb2 associated binder (Gab) adaptor/scaffolding protein in immune cell signaling. Immunol Lett. 2006;104:76–82. doi: 10.1016/j.imlet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Siddiqi MH, Siddiqi MZ, Kang S, Noh HY, Ahn S, Simu SY, Aziz MA, Sathishkumar N, Jiménez Pérez ZE, Yang DC. Inhibition of Osteoclast Differentiation by Ginsenoside Rg3 in RAW 264.7 Cells via RANKL, JNK and p38 MAPK Pathways through a Modulation of Cathepsin K: An In Silico and In Vitro Study. Phytother Res. 2015;9:1286–1294. doi: 10.1002/ptr.5374. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15:1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. The osteoclast and its unique cytoskeleton. Ann N Y Acad Sci. 2011;1240:14–17. doi: 10.1111/j.1749-6632.2011.06283.x. [DOI] [PubMed] [Google Scholar]
- Tidgewell K, Clark BT, Gerwick WH. The natural products chemistry of cyanobacteria. In: Moore B, Crews P, editors. Comprehensive Natural Products Chemistry. 2. Oxford, UK: Elsevier; 2010. pp. 141–188. [Google Scholar]
- Tousoulis D, Kampoli AM, Papageorgiou N, Androulakis E, Antoniades C, Toutouzas K, Stefanadis C. Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des. 2011;17:4089–4110. doi: 10.2174/138161211798764843. [DOI] [PubMed] [Google Scholar]
- Väänänen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–138. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Weitzmann MN, Pacifici R. Estrogen regulation of immune cell bone interactions. Ann N Y Acad Sci. 2006;1068:256–274. doi: 10.1196/annals.1346.030. [DOI] [PubMed] [Google Scholar]
- Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, Choi Y. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- Yoon WJ, Kim KN, Heo SJ, Han SC, Kim J, Ko YJ, Kang HK, Yoo ES. Sargachromanol G inhibits osteoclastogenesis by suppressing the activation NF-κB and MAPKs in RANKL-induced RAW 264.7 cells. Biochem Biophys Res Commun. 2013;434:892–897. doi: 10.1016/j.bbrc.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Yu M, Chen X, Lv C, Yi X, Zhang Y, Xue M, He S, Zhu G, Wang H. Curcumol suppresses RANKL-induced osteoclast formation by attenuating the JNK signaling pathway. Biochem Biophys Res Commun. 2014;447:364–70. doi: 10.1016/j.bbrc.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Gauthier JY, Black WC, Percival MD, Duong LT. Inhibition of bone resorption by the cathepsin k inhibitor odanacatib is fully reversible. Bone. 2014;67:269–280. doi: 10.1016/j.bone.2014.07.013. [DOI] [PubMed] [Google Scholar]