Abstract
FHbp is a major serogroup B meningococcal vaccine antigen. Binding of complement Factor H (FH) to FHbp is specific for human and some non-human primate FH. In previous studies, FH binding to FHbp vaccines impaired protective anti-FHbp antibody responses. In this study we investigated anti-FHbp antibody responses to a third dose of a licensed serogroup B vaccine (MenB-4C) in infant macaques vaccinated in a previous study with MenB-4C. Six macaques with high binding of FH to FHbp (FHhigh), and six with FHlow baseline phenotypes, were immunized three months after dose 2. After dose 2, macaques with the FHlow baseline phenotype had serum anti-FHbp antibodies that enhanced FH binding to FHbp (functionally converting them to a FHhigh phenotype). In this group, activation of the classical complement pathway (C4b deposition) by serum anti-FHbp antibody, and anti-FHbp serum bactericidal titers were lower after dose 3 than after dose 2 (p<0.02). In macaques with the FHhigh baseline phenotype, the respective anti-FHbp C4b deposition and bactericidal titers were similar after doses 2 and 3. Two macaques developed serum anti-FH autoantibodies after dose 2, which were not detected after dose 3. In conclusion, in macaques with the FHlow baseline phenotype whose post-dose 2 serum anti-FHbp antibodies had converted them to FHhigh, the anti-FHbp antibody repertoire to dose 3 was skewed to less protective epitopes than after dose 2. Mutant FHbp vaccines that eliminate FH binding may avoid eliciting anti-FHbp antibodies that enhance FH binding, and confer greater protection with less risk of inducing anti-FH autoantibodies than FHbp vaccines that bind FH.
Keywords: Neisseria meningitidis, vaccine, complement, Factor H, Factor H binding protein, non-human primate, 4CMenB, Bexsero®
1. Introduction
Two protein-based meningococcal serogroup B vaccines were licensed in the U.S. in October 2014 and January 2015. Both contain Factor H binding protein (FHbp) [1], which binds complement Factor H (FH). Binding of FH to meningococci down-regulates the alternative complement pathway and enables the bacteria to evade complement [2, 3].
Binding of FH to FHbp has been reported to be specific for human and some non-human primate FH [4–6]. In human FH transgenic mice, binding of FH to FHbp vaccines decreased protective anti-FHbp antibody responses [7, 8]. In a recent study we investigated the immunogenicity of a licensed four-component meningococcal serogroup B vaccine in infant rhesus macaques in relation to binding of FH to the FHbp vaccine antigen [9]. The vaccine contains FHbp and three other antigens capable of eliciting serum bactericidal activity [10]. In Europe, where currently there is only one licensed serogroup B vaccine, this vaccine, which is manufactured by GSK, is referred to 4CMenB. However, in the U.S. where there is a second licensed serogroup B vaccine that only contains FHbp (manufactured by Pfizer, referred to “MenB-FHbp”), the four component vaccine is referred to as MenB-4C [11], which we will utilize herein.
Because of two amino acid polymorphisms in macaque FH domain 6, some macaques have FH that binds strongly to FHbp, while other macaques have FH that binds only weakly [4, 6]. In macaques immunized with two doses of MenB-4C, those with the low binding FH phenotype (FHlow) had higher anti-FHbp serum bactericidal antibody responses than macaques with high binding (FHhigh) phenotypes [9]. Interestingly, in both groups, the vaccine-induced serum anti-FHbp antibodies after dose 2 enhanced binding of FH to FHbp. Thus, after dose 2 the animals with the baseline FHlow phenotype had functionally converted and had similar FH binding to FHbp as the animals with the FHhigh baseline phenotype. In the present study, we investigated the anti-FHbp antibody responses of these animals to a third dose of MenB-4C vaccine.
2.0 Materials and Methods
2.1. Immunization of rhesus macaques
The animals were housed at the California National Primate Research Center, Davis, California in accordance with American Association for Accreditation of Laboratory Animal Care Standards. We strictly adhered to the “Guide for the Care and Use of Laboratory Animals” [12]. The study was approved by the Institutional Animal Care and Use Committee of the University of California, Davis. We screened 25 macaques for FH binding and identified 8 with the FH high phenotype (32%) and 17 (68%) with the FH low phenotype. Of these, we selected six animals with the highest FH binding to FHbp (FHhigh phenotype), and six with the lowest FH binding (FHlow) for inclusion in our study. At age 3 months, the 12 animals were immunized IM with the MenB-4C vaccine and were given a second injection one month later. We used the dose recommended for human infants. Six additional macaques (two FHhigh and four FHlow) were followed as non-vaccinated, negative controls [9]. In the present study, we administered a third IM dose of MenB-4C three months after the second dose to the 12 animals immunized in our previous study. Blood samples were obtained 4 weeks after dose 3.
2.2. Serum IgG antibody responses
We used an ELISA to measure serum IgG antibody titers to the four antigens in MenB-4C that elicit serum bactericidal antibodies: FHbp, NadA, NHba, and PorA P1.4 (present in detergent extracted outer membrane vesicles; OMV) [10, 13]. The ELISA was performed as previously described [7], except that bound macaque IgG was detected with alkaline phosphatase-conjugated goat anti-human IgG (Fc specific, Sigma) that cross-reacted with macaque IgG.
2.3. Serum anti-FH autoantibody
Binding of serum IgG antibodies to human FH was measured by ELISA [8] except that macaque IgG anti-FH antibody was detected with goat anti-human IgG (Fc specific) conjugated with alkaline phosphatase (Sigma). In some experiments, we investigated whether the addition of soluble human FH, or previously described recombinant human FH domains 6 and 7 or domains 18 to 20 fused to mouse IgG1 Fc (FH6,7/Fc or FH18-20/Fc, respectively) [14], inhibited serum anti-FH reactivity.
2.4. Serum bactericidal activity
The assay was performed as previously described [9]. The exogenous human complement was serum from a healthy adult that was IgG-depleted using a protein G column (HiTrap Protein G; GE Life Sciences) [7]. In some experiments to inhibit alternative complement pathway activation, we added 100 μg/ml of a mouse anti-Bb mAb (A227; Quidel) [15] to the bactericidal reaction as described [16]. Two mouse mAbs, anti-PorA P1.7 (MN14C11.6; [17]) and anti-capsular (SEAM 12 [18]), which elicit bactericidal activity primarily via the classical complement pathway [16, 19], were used as negative controls.
2.5. C4b deposition on N. meningitidis
We used flow cytometry to measure deposition of human C4b on the surface of live bacteria [9]. Bound human C4b was detected with a 1:100 dilution of fluorescein isothiocyanate-conjugated anti-human C4b (Meridian Life Science).
2.6. Effect of serum anti-FHbp antibodies on binding of macaque FH to meningococci
The assay was performed with live bacteria and a 1:150 dilution of macaque serum as a source of FH and anti-FHbp antibody [9]. Bound macaque FH was detected with a sheep polyclonal antiserum to human FH (Abcam) followed by washing the bacteria and adding donkey anti-sheep IgG antibody (Sigma) conjugated with AlexaFluor 488.
3.0 Results
3.1. Lack of serum antibody booster responses to MenB-4C
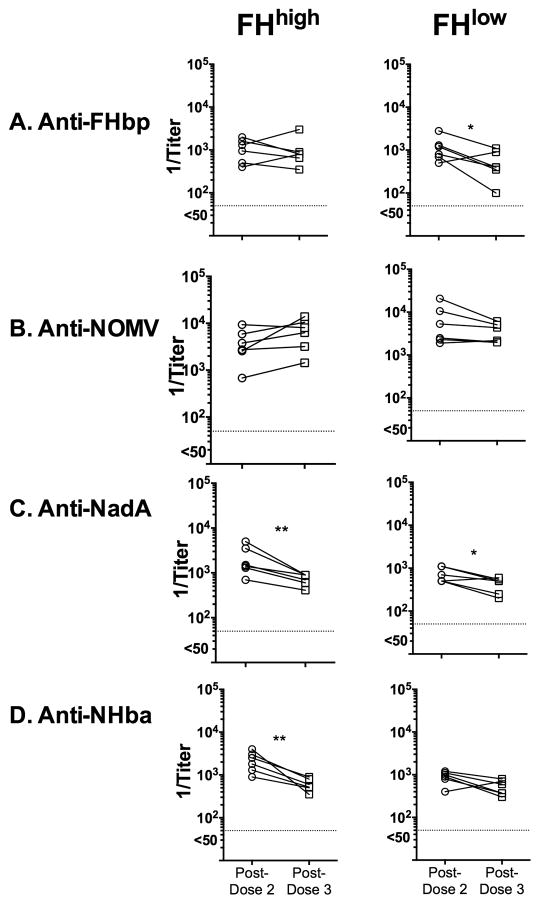
Six macaques with the FHhigh phenotype and six with the FHlow phenotype as determined in sera obtained before dose 1 (“baseline”) were immunized with MenB-4C three months after dose 2. We compared the respective IgG antibody titers against FHbp, OMV, NadA and NHba in sera obtained 4 weeks post-dose 2 and post-dose 3 (Figure 1). For this comparison, we assayed post-dose 2 and 3 sera in parallel. Compared to titers after dose 2, neither group of macaques showed increases in serum IgG titers to any of the antigens after dose 3. Indeed there were small decreases in antibody titers after dose 3 to NadA and NHba in macaques with the FHhigh phenotype, and to NadA and FHbp in macaques with the FHlow baseline phenotype (p<0.05 by paired t-test).
Figure 1. Serum IgG antibody responses to the MenB-4C vaccine.
Each symbol represents a titer of an individual animal as measured by ELISA. Pre-dose 1 titers were negative (<1:100) as were post-dose 2 and post-dose 3 titers of six negative control unvaccinated animals (data not shown). Left. Macaques with FHhigh phenotype. Right. Macaques with FHlow phenotype. A. Anti-FHbp; B. Anti-NOMV; C. Anti-NadA; and D. Anti-NHba. Compared to post-dose 2, the respective titers after dose 3 did not increase and in some groups the titers decreased to specific antigens. For example, after dose 3 the anti-NadA titers were lower than after dose 2 in both groups of macaques. After dose 3, the anti-NHba titers were lower in the FHhigh group (left), and the anti-FHbp titers were lower in the FHlow group. *, p≤0.05; **, p≤0.01 by paired t-test.
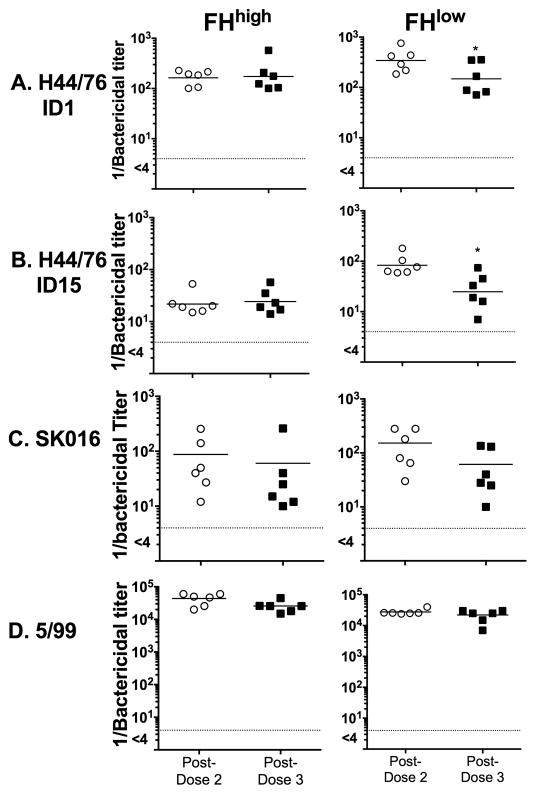
There also were no significant increases in serum bactericidal titers after the third dose of vaccine (Figure 2). For this analysis we tested strains mismatched for three of the four antigens in MenB-4C vaccine that elicit bactericidal antibody [8]. Thus, the bactericidal titers against strains SK016 and 5/99 were specific for antibody to PorA P1.4 and NadA, respectively, and the bactericidal titers to H44/76 were specific for anti-FHbp antibody. FHbp is divided into two sub-families, A and B, based on amino acid sequence similarity [20, 21]. For anti-FHbp bactericidal responses we tested wild-type strain H44/76 that expressed sub-family B FHbp ID 1 (that matched the ID 1 antigen in MenB-4C), and a mutant of H44/76 with a different sub-family B FHbp antigen (ID 15) [9]. In some strains, PorB2 or NspA can bind human FH and increase resistance to anti-FHbp bactericidal antibody [22, 23]. However, strain H44/76 has PorB3, which shows minimal or no binding with human FH [22], and is a low NspA expresser. Thus, its principal FH ligand is FHbp and, therefore, these alternative FH ligands did not influence anti-FHbp bactericidal activity. For the macaques with the FHlow baseline phenotype, the respective anti-FHbp bactericidal titers were lower after dose 3 than after dose 2 against both H44/76 strains (p≤0.02 by paired t- test). As a result, the anti-FHbp bactericidal titers of the macaques with the FHlow baseline phenotype, which had been higher after dose 2 than in macaques with the FHhigh phenotype (p=0.02), were no longer different from each other after dose 3 (p≥0.65).
Figure 2. Serum bactericidal antibody responses.
Each symbol represents the titer of an individual animal. Not shown, pre-dose 1 titers, which were negative (<1:4) as were titers of six negative control unvaccinated animals. Each test strain was mismatched for three of the four antigens in MenB-4C known to elicit bactericidal antibody (see methods). A and B. Anti-FHbp bactericidal activity (WT strain H44/76 with FHbp ID 1, which is identical to FHbp variant in vaccine) or a mutant of H44/76 with FHbp ID 15 (87% amino acid identity to vaccine antigen). C. Anti-OMV bactericidal activity (WT strain SK106). D. Anti-NadA bactericidal activity (WT strain 5/99). Note that Y-axis in Panel D is on a larger scale (105) than in panels A, B or C (103). Compared to post-dose 2, the respective titers after dose 3 did not increase. For anti-FHbp bactericidal activity (A and B), the titers after dose 3 in the animals with the FHlow phenotype were lower than after dose 2 (p≤0.02 by paired t-test).
3.2. Activation of the classical complement pathway by anti-FHbp antibodies
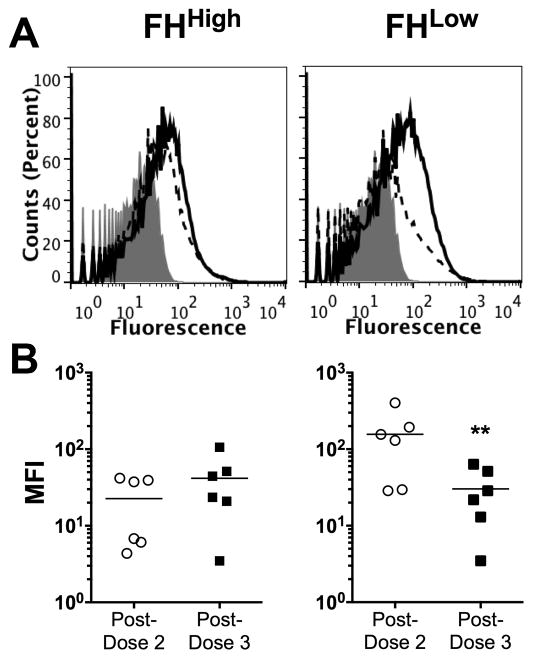
Deposition of human complement C4b on the surface of live N. meningitidis cells is a marker of lectin and/or classical complement pathway activation. In our previous study, the anti-FHbp antibodies in post-dose 2 sera from the macaques with the FHhigh phenotype activated less C4b deposition than the animals with the FHlow phenotype [9]. In the present study we measured C4b deposition by anti-FHbp antibodies in post-dose 3 sera and compared the respective results to post-dose 2 sera (Figure 3). We assayed the post-dose 2 and 3 sera in parallel using the mutant H44/76 strain with FHbp ID 15. Representative data for an individual macaque with the baseline FHhigh phenotype and a macaque with a baseline FHlow phenotype are shown in Figure 3, Panel A. In the macaque with the baseline FHhigh phenotype (left panel), there was similar C4b deposition elicited by serum anti-FHbp antibodies after dose 2 (solid line) and dose 3 (dashed line). In the macaque with the baseline FHlow phenotype (right panel) there was less C4b deposition elicited by post-dose 3 serum anti-FHbp antibodies than post-dose 2. The C4b deposition results for all 12 animals stratified by baseline FHhigh or FHlow phenotypes are summarized in Figure 3, Panel B. Among the macaques with baseline FHhigh phenotypes (left panel), there was no significant change in the fluorescence intensities after dose 3, compared to after dose 2. Among macaques with the FHlow baseline phenotype, there was a significant decline in C4b deposition after dose 3 compared to after dose 2 (p<0.02, paired t-test). These data suggested that the lower anti-FHbp bactericidal titers after dose 3 than dose 2 in macaques with the FHlow baseline phenotype were a result of less activation of the classical complement pathway.
Figure 3. Deposition of human C4b on live meningococci by vaccine-induced macaque anti-FHbp antibodies.
The macaque sera were heat-inactivated and tested at a 1:40 dilution in the presence of 5% human serum that had been depleted of IgG as a source of complement. The test strain was the H44/76 mutant with FHbp ID 15. A. C4b deposition elicited by representative post-immunization sera after dose 2 of vaccine (solid line) or dose 3 (dashed line). The gray region denotes bacteria with 5% human complement but no macaque serum. B. Median fluorescence intensities (MFIs) for C4b deposition elicited by macaque sera (N=6 per group). The horizontal error bars represent geometric means. The difference between the vaccinated post-dose 2 and post-dose 3 in the FHlow baselin group was statistically significant. **p=0.0065.
3.4. Serum anti-FHbp antibodies elicited by MenB-4C-vaccination enhance binding of FH to FHbp
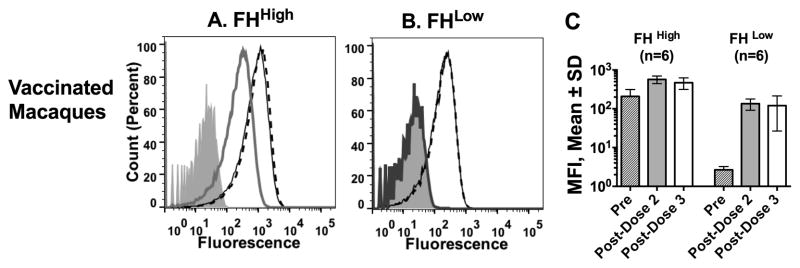
In human FH transgenic mice, binding of FH to the FHbp antigen redirected the anti-FHbp antibody repertoire to FHbp epitopes outside of the FH binding site, which did not inhibit binding of FH to FHbp [8, 9, 24]. For reasons that remain unknown, the anti-FHbp antibodies enhanced FH binding [8]. In contrast, in immunized wild-type mice whose mouse FH didn’t bind to the FHbp vaccine antigen, the serum anti-FHbp antibodies inhibited FH binding [8]. An unexpected result in our previous macaque study was that the post-dose 2 sera enhanced FH binding irrespective of baseline FHhigh or FHlow phenotypes. In the present study, we also observed enhanced FH binding by serum anti-FHbp antibodies after dose 3, which was similar in both groups of macaques, and similar to the respective FH enhancement by serum obtained after dose 2 (Figure 4).
Figure 4. Effect of post-immunization sera on binding of macaque FH to live bacteria.
All sera were tested at a dilution of 1:150 with serogroup B strain H44/76. A and B. Sera obtained before vaccination (gray line) and after dose 2 of vaccine (solid line) or dose 3 (dashed line). A. Representative macaque with FHhigh baseline phenotype B. Representative macaque with FHlow phenotype. By dose 2, the animal with the FHlow baseline phenotype has high FH binding, which did not increase further after dose 3. C. Mean median fluorescence intensities (MFIs) ± SD of 6 macaques with FHhigh and 6 macaques with FHlow to FHbp.
3.5. Alternative pathway amplification of anti-FHbp bactericidal activity despite enhanced FH binding
Because of enhanced FH binding with the post-immunization macaque sera, we expected the alternative complement pathway to be inhibited and for anti-FHbp bactericidal activity to depend largely on the classical complement pathway. To investigate this hypothesis, we determined the effect of blocking the alternative pathway with an anti-Bb mAb on anti-FHbp bactericidal activity. In previous experiments, we showed that this concentration (100 μg/ml) of the anti-Bb mAb completely inhibited C3b deposition on zymosan particles under conditions that blocked the classical and lectin pathways but were permissive for the alternative pathway [24]. In the present study, the addition of the anti-Bb mAb to post-dose 2 or post-dose 3 macaque sera markedly decreased anti-FHbp bactericidal activity (Figure 5). This result indicated that the alternative pathway remained active despite the bound FH. As expected, the anti-Bb mAb had no effect on bactericidal activity of a control mouse anti-PorA mAb or anticapsular mAb, which, because of the greater abundance of the respective target antigens, did not require alternative pathway amplification for classical pathway bacteriolysis [16].
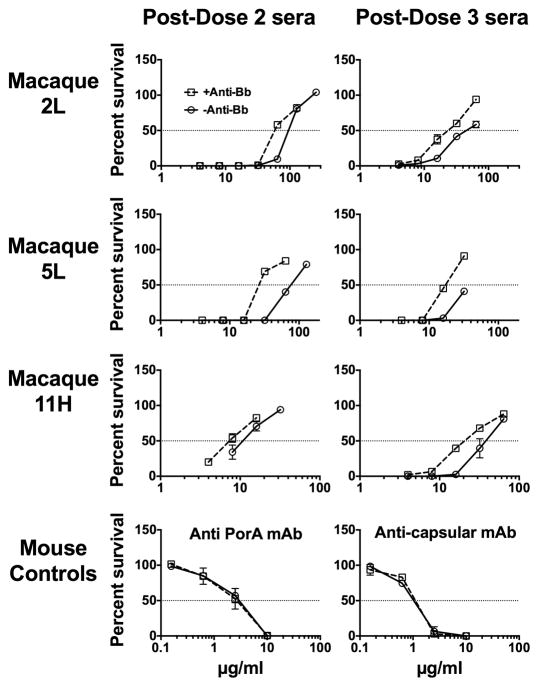
Figure 5. Effect of blocking the alternative complement pathway on serum bactericidal activity.
Open circles with solid lines, serum bactericidal activity without an anti-Bb mAb. Open squares with dashed line, serum bactericidal activity in the presence of an anti-Bb mAb, 100 μg/ml, which blocked the alternative complement pathway. Data are shown for three macaques, designated 2L, 5L and 11H. Error bars represent ranges from two independent experiments.
3.6. Transient serum IgG autoantibodies to FH
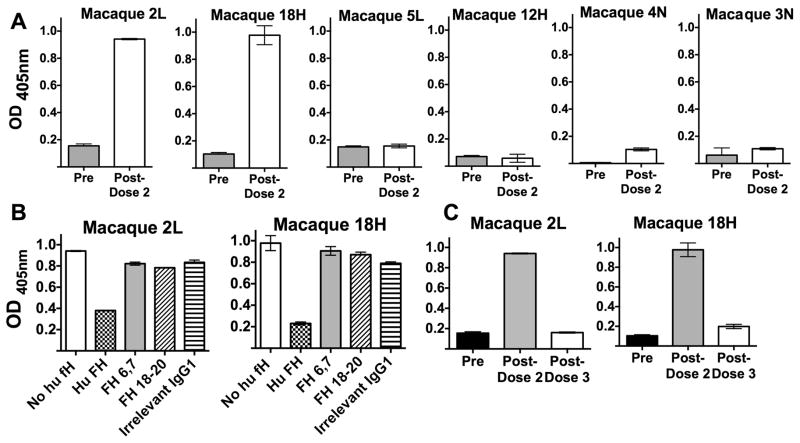
In a previous study, two of eleven human FH transgenic mice immunized with MenB-4C developed serum IgM auto-antibodies to human FH [8]. We therefore investigated FH autoantibody activity in serum samples from the immunized macaques. By ELISA, none of the 12 vaccinated animals had IgM anti-FH antibody (data not shown). However, two macaques (designated “2L” with a FHlow phenotype, and “18H” with a FHhigh phenotype) were positive for IgG anti-FH antibody in post-dose 2 immunization sera (Figure 6, Panel A). Binding was inhibited by the addition of purified human FH to the reaction mixture (Panel B). However, there was no inhibition of anti-FH binding by recombinant human FH domain fragments (6,7 or 18–20, each fused to a murine IgG1 Fc). None of the animals had anti-FH antibodies detected in sera obtained after the third MenB-4C dose (data shown for the two macaques positive for anti-FH antibody after dose 2; Panel C).
Figure 6. Serum IgG autoantibody to human FH.
Two of 12 vaccinated animals developed IgG anti-FH antibody after dose 2. A. Serum IgG anti-FH antibodies in the two positive vaccinated macaques (2L and 18H) and in four representative negative animals, two vaccinated macaques (5L and 12H) and two unvaccinated macaques (4N and 3N). All sera were tested at a dilution of 1:100. B. Effect of soluble inhibitors (50 μg/ml) on binding of serum anti-FH. Inhibitors tested were purified human FH, recombinant human FH fragments 6,7 or 18–20, each expressed with mouse Fc IgG1, and, as a negative control, an irrelevant control mouse IgG1. C. Serum IgG anti-FH antibodies in the two macaques positive after dose 2 had declined to baseline in serum obtained 4 weeks after dose 3.
4.0 Discussion
FHbp is relatively sparsely exposed [25, 26], and there may be insufficient bound anti-FHbp antibody on the meningococcal surface to activate bacteriolysis via the classical complement pathway without alternative pathway amplification [1, 16, 25]. In FHbp-immunized wild-type mice, serum anti-FHbp antibodies inhibited binding of FH to FHbp [8]. With less bound FH, there was less down-regulation of the alternative pathway, and greater anti-FHbp bactericidal activity than by anti-FHbp antibodies that did not inhibit FH binding [8, 16, 19].
At the time vaccine trials with the new serogroup B vaccines were started in humans, the function of FHbp in binding FH [1], and the specificity for binding only to human and some non-human primate FH [4–6], were not known. These findings raised questions about the possible effects of binding of a host protein to a vaccine antigen on immunogenicity and safety. To investigate these questions, we performed immunogenicity studies in a human FH transgenic mouse model. Our most important findings were that human FH lowered serum anti-FHbp bactericidal responses of transgenic mice compared to wild-type mice whose mouse FH did not bind to FHbp [7, 8]. Further, in human FH transgenic mice, mutant FHbp antigens with decreased FH binding elicited higher serum bactericidal antibody responses than FHbp vaccines that bound human FH [7, 27–29]. Finally, two human FH transgenic mice immunized with MenB-4C developed serum IgM autoantibodies to human FH [8], which raised safety concerns. These findings led us to the current investigations of the effect of FH binding on FHbp immunogenicity in infant macaques [9].
Our initial macaque study investigated the responses of infant macaques to two doses of MenB-4C. Macaques with the FHlow phenotype had higher serum anti-FHbp bactericidal responses than macaques with the FHhigh phenotype [9]. These results were consistent with earlier results in MenB-4C-immunized wild-type and human FH transgenic mice [8]. However, the anti-FHbp antibody responses of the wildtype mice, but not the transgenic mice, inhibited FH binding to FHbp, while the anti-FHbp antibodies in both groups of macaques did not inhibit FH binding. Although it is possible that the lack of FH inhibition by the anti-FHbp antibodies of the macaques with the low baseline FH binding phenotype reflected intrinsic differences between mouse and macaque immunoglobulin gene diversity, we think another explanation is more likely. In particular, mouse FH does not bind FHbp whereas even in the macaques with the baseline low FH binding phenotype there was some FH binding to the FHbp vaccine (albeit of lower affinity than human FH, or macaque FH from animals with the high FH binding phenotype) [27]. Our hypothesis is that in FHbp-immunized wild-type mice, because the FHbp antigen does not bind to mouse FH, the anti-FHbp antibodies are directed at FHbp epitopes in the FH binding site, which inhibit binding of FH to FHbp [7, 8]. In contrast, even in the macaques with low-FH binding baseline phenotype, there was sufficient low affinity FH binding to the vaccine to skew the anti-FHbp antibody repertoire to FHbp epitopes outside of the FH binding site, which did not inhibit binding of FH to FHbp.
In the present study, the macaques with the FH high binding baseline phenotype were analogous to human infants whose FH binds strongly to FHbp. In Europe, MenB-4C is licensed for three doses in infants immunized beginning at ages 2 to 5 months. However, in a study of infants given three doses at 2, 4 and 6 months of age, the post-dose 3 anti-FHbp serum bactericidal titers were similar to those after dose 2 (i.e., no clear booster responses) [30]. Thus, the lack of booster responses to dose 3 in the macaques with the FH high-binding baseline phenotype were similar to those of vaccinated human infants.
An important question in the present study is why the macaques with the baseline FHlow phenotype showed lower serum anti-FHbp bactericidal titers after dose 3 than after dose 2 (Figure 2). As noted above, after dose 2, serum anti-FHbp antibodies in the animals with the baseline FHlow phenotype enhanced binding of FH to FHbp [9], which functionally converted them to a FHhigh phenotype. Thus, at the time of dose 3 the FHbp vaccine antigen could form a complex with FH, mask important FHbp epitopes, and skew the serum anti-FHbp antibody repertoire to epitopes giving less protective activity than after dose 2. Thus, the importance of the lower anti-FHbp bactericidal antibody responses after dose 3 than after dose 2 in the FHlow baseline group is that they underscore the effect of binding of FH to the vaccine antigen on anti-FHbp repertoire and functional antibody activity.
Note also that the enhanced binding of FH to the bacterial surface by the serum anti-FHbp antibodies in the macaques would be expected to inhibit the alternative complement pathway. However, despite bound FH, the macaque serum anti-FHbp antibodies utilized the alternative complement pathway for bacteriolysis as shown by decreased bactericidal activity in the presence of an anti-Bb mAb that functionally blocked the alternative complement pathway (Figure 5) [16]. In a recent study, we observed similar results with affinity-purified human anti-FHbp antibodies; despite enhancing binding of FH to the bacteria, anti-FHbp bactericidal activity was decreased by an anti-Bb mAb [24]. Collectively, these results indicate that macaque or human anti-FHbp antibody enhancement of FH binding to the bacteria is not fully functional in down-regulating the alternative pathway. Conceivably, the anti-FHbp antibodies interfere with FH coFactor and/or decay acceleration activities. Further studies are needed to understand the basis for enhanced FH binding by anti-FHbp antibodies and the reason why the bound FH is not more effective in down-regulating the alternative pathway.
A theoretical risk of immunizing with a vaccine antigen that binds to a host antigen is developing autoantibodies to the host antigen. In the present study, two MenB-4C-vaccinated macaques developed serum IgG autoantibodies to FH, which was consistent with previous data in human FH transgenic mice [8]. In both studies the anti-FH reactivity was specific in that the majority of the activity was inhibited by the addition of full-length human FH. The clinical importance of the serum autoantibodies to FH is unknown. In the mice, the isotype was limited to IgM, and in the macaques the IgG serum anti-FH antibodies were present only after dose 2 and not after dose 3. An important limitation of the present study is that we lacked serum samples from the macaques taken immediately before dose 3. Therefore we do not know whether the serum anti-FH antibodies present one month after dose 2 were still present two months later at the time of dose 3 and declined after vaccination, or had declined to undetectable levels before dose 3.
Serum IgG anti-FH autoantibodies are found in patients with two rare diseases, atypical hemolytic uremic syndrome [31–34] and C3 glomerulopathies [35]. In patients with anti-FH autoantibodies, these diseases may not manifest clinically for many years. Therefore, the occurrence of these diseases in relation to FHbp vaccination may be difficult to ascertain. To date, we are not aware of studies of development of serum anti-FH autoantibodies in humans immunized with MenB-4C. The experimental “signals” of autoantibody activity to FH in two animal models of FHbp immunization suggest the potential for FHbp vaccines to elicit anti-FH autoantibodies in humans, and underscore the need to investigate the presence of these antibodies in sera from immunized humans.
Finally, a promising strategy to decrease the potential for FHbp vaccines to elicit anti-FH autoantibodies and to increase vaccine immunogenicity is to develop mutant FHbp vaccines with decreased FH binding. While we have investigated such mutant FHbp antigens in human FH transgenic mice [7, 27–29, 36], future studies will be needed to establish the safety and immunogenicity of these antigens in non-human primates and humans.
Highlights.
Infant rhesus macaques were boosted with a third dose of a licensed meningococcal serogroup B vaccine that contained FHbp
After dose 2, anti-FHbp antibodies in macaques that at baseline had FH with low binding to FHbp enhanced binding of FH to FHbp
The anti-FHbp antibody repertoire to dose 3 in this group was skewed to less protective epitopes than after dose 2.
Two macaques developed transient serum IgG anti-FH autoantibodies
Mutant FHbp vaccines that eliminate FH binding may confer greater protection with less risk of inducing anti-FH autoantibodies than FHbp vaccines that bind FH.
Acknowledgments
This work was supported by grants R01 AI046464 (D.M.G.), R01 AI099125 (P.T.B.) and R01 AI114701 (D.M.G. and P.T.B) from the National Institute of Allergy and Infectious Diseases, NIH and by a Pilot Program grant (to D.M.G. and P.T.B.) from grant P51 OD011107 from the Office of Research Infrastructure Programs to the California National Primate Research Center. The work was performed in a facility funded by the Research Facilities Improvement Program grant C06 RR016226 from the National Center for Research Resources, NIH. Dan M. Granoff, Serena Giuntini and Peter T. Beernink declare no conflicts of interest. We are grateful to Dr. Sanjay Ram, University of Massachusetts School of Medicine, Worcester MA for critical review of the data, and to Koen Van Rompay and Paul-Michael Sosa, California National Primate Research Center, for supervising immunization of the macaques and blood collection, and to Patricia Zuno-Mitchell for performing the serum bactericidal assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence. 2014;5:98–126. doi: 10.4161/viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–75. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 4.Beernink PT, Shaughnessy J, Stefek H, Ram S, Granoff DM. Heterogeneity in rhesus macaque complement Factor H binding to meningococcal Factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines. Clin Vaccine Immunol. 2014;21:1505–11. doi: 10.1128/CVI.00517-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–9. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konar M, Beernink PT, Granoff DM. A Newly-Identified polymorphism in rhesus macaque complement Factor H modulates binding affinity for meningococcal FHbp. PLoS One. 2015;10:e0135996. doi: 10.1371/journal.pone.0135996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, et al. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol. 2011;186:3606–14. doi: 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa I, Pajon R, Granoff DM. Human Factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enchance FH binding. mBio. 2014;5 doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. Binding of complement Factor H (FH) decreases protective anti-FH binding protein antibody responses of infant rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis. 2015;212:784–92. doi: 10.1093/infdis/jiv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–9. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR Centers for Disease C. Use of Serogroup B Meningococcal Vaccines in Persons Aged >/=10 Years at Increased Risk for Serogroup B Meningococcal Disease: Recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608–12. [PMC free article] [PubMed] [Google Scholar]
- 12.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of labortory animals. 8. Washington DC: 2011. [Google Scholar]
- 13.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: Immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis. mBio. 2013;4:e00339–13. doi: 10.1128/mBio.00339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hourcade DE, Wagner LM, Oglesby TJ. Analysis of the short consensus repeats of human complement factor B by site-directed mutagenesis. J Biol Chem. 1995;270:19716–22. doi: 10.1074/jbc.270.34.19716. [DOI] [PubMed] [Google Scholar]
- 16.Giuntini S, Reason DC, Granoff DM. Combined roles of human IgG subclass, alternative complement pathway activation, and epitope density in the bactericidal activity of antibodies to meningococcal factor H binding protein. Infect Immun. 2012;80:187–94. doi: 10.1128/IAI.05956-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Jarvis GA, Achtman M, Rosenqvist E, Michaelsen TE, Aase A, et al. Functional activities and immunoglobulin variable regions of human and murine monoclonal antibodies specific for the P1.7 PorA protein loop of Neisseria meningitidis. Infect Immun. 2000;68:1871–8. doi: 10.1128/iai.68.4.1871-1878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, et al. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J Immunol. 1998;160:5028–36. [PubMed] [Google Scholar]
- 19.Giuntini S, Reason DC, Granoff DM. Complement-mediated bactericidal activity of anti-factor H binding protein monoclonal antibodies against the meningococcus relies upon blocking factor H binding. Infect Immun. 2011;79:3751–9. doi: 10.1128/IAI.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Cohn A, Comanducci M, Andrew L, Zhao X, MacNeil JR, et al. Prevalence and genetic diversity of candidate vaccine antigens among invasive Neisseria meningitidis isolates in the United States. Vaccine. 2011;29:4739–44. doi: 10.1016/j.vaccine.2011.04.092. [DOI] [PubMed] [Google Scholar]
- 21.Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:379–89. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]
- 22.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuntini S, Pajon R, Ram S, Granoff DM. Binding of complement Factor H to PorB3 and NspA enhances resistance of Neisseria meningitidis to anti-FHbp bactericidal activity. Infect Immun. 2015 doi: 10.1128/IAI.02984-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beernink PT, Giuntini S, Costa I, Lucas AH, Granoff DM. Functional analysis of the human antibody response to meningococcal Factor H binding protein. mBio. 2015;6:e00842–15. doi: 10.1128/mBio.00842-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008;197:1053–61. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 26.Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine. 2010;28:6086–93. doi: 10.1016/j.vaccine.2010.06.083. [DOI] [PubMed] [Google Scholar]
- 27.Konar M, Rossi R, Walter H, Pajon R, Beernink PT. A mutant library approach to identify improved meningococcal Factor H binding protein vaccine antigens. PLoS One. 2015 doi: 10.1371/journal.pone.0128185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi R, Granoff DM, Beernink PT. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine. 2013;31:5451–7. doi: 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beernink PT, Shaughnessy J, Pajon R, Braga EM, Ram S, Granoff DM. The effect of human factor H on immunogenicity of meningococcal native outer membrane vesicle vaccines with over-expressed factor H binding protein. PLoS Pathog. 2012;8:e1002688. doi: 10.1371/journal.ppat.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127–37. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 31.Kim JJ, McCulloch M, Marks SD, Waters A, Noone D. The clinical spectrum of hemolytic uremic syndrome secondary to complement factor H autoantibodies. Clin Nephrol. 2015;83:49–56. doi: 10.5414/CN107777. [DOI] [PubMed] [Google Scholar]
- 32.Kopp A, Strobel S, Tortajada A, Rodriguez de Cordoba S, Sanchez-Corral P, Prohaszka Z, et al. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor H and factor H-related protein 1 to pentraxin 3. J Immunol. 2012;189:1858–67. doi: 10.4049/jimmunol.1200357. [DOI] [PubMed] [Google Scholar]
- 33.Strobel S, Hoyer PF, Mache CJ, Sulyok E, Liu WS, Richter H, et al. Functional analyses indicate a pathogenic role of factor H autoantibodies in atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2010;25:136–44. doi: 10.1093/ndt/gfp388. [DOI] [PubMed] [Google Scholar]
- 34.Blanc C, Roumenina LT, Ashraf Y, Hyvarinen S, Sethi SK, Ranchin B, et al. Overall neutralization of complement factor H by autoantibodies in the acute phase of the autoimmune form of atypical hemolytic uremic syndrome. J Immunol. 2012;189:3528–37. doi: 10.4049/jimmunol.1200679. [DOI] [PubMed] [Google Scholar]
- 35.Blanc C, Togarsimalemath SK, Chauvet S, Le Quintrec M, Moulin B, Buchler M, et al. Anti-factor H autoantibodies in C3 glomerulopathies and in atypical hemolytic uremic syndrome: one target, two diseases. J Immunol. 2015;194:5129–38. doi: 10.4049/jimmunol.1402770. [DOI] [PubMed] [Google Scholar]
- 36.van der Veen S, Johnson S, Jongerius I, Malik T, Genovese A, Santini L, et al. Nonfunctional variant 3 factor H binding proteins as meningococcal vaccine candidates. Infect Immun. 2014;82:1157–63. doi: 10.1128/IAI.01183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]