Abstract
Objective
In the first days after cardiac arrest, accurate prognostication is challenging. Serum biomarkers are a potentially attractive adjunct for prognostication and risk stratification. Our primary objective in this exploratory study was to identify novel early serum biomarkers that predict survival after cardiac arrest earlier than currently possible.
Design
Prospective, observational study
Setting
A single academic medical center
Subjects
Adult subjects who sustained cardiac arrest with return of spontaneous circulation
Intervention
None
Measurements and Main Results
We obtained blood samples from each subject at enrollment, 6, 12, 24, 48 and 72 hours after return of spontaneous circulation. We measured serum levels of novel biomarkers including neutrophil gelatinase-associated lipocalin (NGAL), high-mobility group protein B1 (HMGB), intracellular cell adhesion molecule 1 (ICAM-1) and leptin, as well as previously characterized biomarkers including neuron specific enolase (NSE) and S100B protein. Our primary outcome of interest was survival to hospital discharge. We compared biomarker concentrations at each time point between survivors and non-survivors and used logistic regression to test the unadjusted associations of baseline clinical characteristics and enrollment biomarker levels with survival. Finally, we constructed a series of adjusted models to explore the independent association of each enrollment biomarker level with survival.
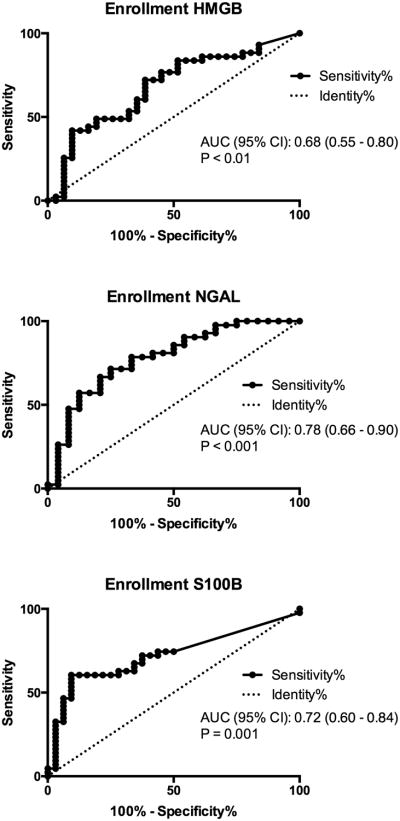
A total of 86 subjects were enrolled. Enrollment levels of HMGB, NGAL and S100B were higher in non-survivors than survivors. Enrollment leptin, NSE and ICAM-1 levels did not differ between non-survivors and survivors. The discriminatory power of enrollment NGAL level was greatest (c-statistic 0.78 (95%CI 0.66 – 0.90), and remained stable across all time points. In our adjusted models, enrollment NGAL level was independently associated with survival even after controlling for development of acute kidney injury and its addition to clinical models improved overall predictive accuracy.
Conclusion
Serum NGAL levels are strongly predictive of survival to hospital discharge after cardiac arrest.
MeSH Headings: Biomarker, cardiac arrest, outcome, acute kidney injury, prognosis
Introduction
Over 500,000 Americans suffer a cardiac arrest (CA) each year and more than 100,000 achieve return of spontaneous circulation (ROSC) and are cared for in the hospital (1, 2). Of these, 50-89% die in the hospital (3, 4). In the first 72 hours after ROSC, no known clinical finding or diagnostic test has adequate specificity for poor outcome or anticipated clinical course to inform clinical care or accurate neurological prognostication (5-7). Thus, it is difficult to prospectively identify and intervene upon high-risk patients prior to deterioration, and in most cases at least 3 days of critical care are necessary before accurate prognostication is possible, creating a burden to families and the healthcare system.
Serum biomarkers are a potentially attractive adjunct for prognostication and risk stratification. The best-described serum biomarkers after CA are neuron specific enolase (NSE) and S100B protein, which are released into the blood following anoxic brain injury and may reflect the severity of injury (8, 9). Unfortunately, NSE levels peak between 48-72 hours after ROSC, and so do not predict outcomes early after ROSC, and recent consensus guidelines have concluded that S100B lacks sufficient precision and discrimination to be clinically useful (8, 9).
Our primary objective in this exploratory study was to identify novel early serum biomarkers that predict survival after cardiac arrest earlier than currently possible. The post-arrest syndrome is characterized by multiple organ dysfunction secondary to global ischemia-reperfusion injury and a systemic inflammatory response (10-12). As such, rather than testing markers of brain injury as have been previously evaluated, we selected candidate biomarkers from molecular pathways related to systemic inflammation (T cell activation, leukocyte adhesion and activation, and toll-like receptor-mediated innate immunity) that might reflect the extent of total body injury.
Materials and Methods
Study Design
We performed a prospective, observational study enrolling consecutive subjects at a single academic medical center. The University of Pittsburgh Institutional Review Board approved all aspects of this study, and either the subject or a legally authorized proxy provided written informed consent prior to enrollment.
Patients and Setting
The UPMC Presbyterian Hospital is a 795-bed tertiary care referral center. Our Post-Cardiac Arrest Service (PCAS) provides standardized care to over 250 patients admitted after cardiac arrest annually. During the study period, this included routine use of therapeutic hypothermia for comatose patients, defined as not following commands within 6 hours of ROSC, regardless of initial arrest rhythm or arrest location (13). Our standard practice was to induce hypothermia as rapidly as possible with intravenous boluses of 4°C crystalloid, application of ice to the neck, axillae and groins, and surface or endovascular cooling systems, targeting a core temperature of 33°C. We maintained hypothermia for 24 hours, then rewarmed patients to normothermia over 16 hours at a rate of 0.25°C/hr to a goal temperature of 37°C which we maintained until 5 days after ROSC or awakening. We included subjects in the present study who suffered an in-hospital cardiac arrest (IHCA) or out-of-hospital cardiac arrest (OHCA) with return of spontaneous circulation (ROSC) and were admitted to the emergency department or intensive care unit between January 1, 2007 and December 31, 2009. We excluded subjects for age <18 years, presentation >6 hours after ROSC, pregnant, prisoner, withdrawal of life-sustaining therapy within 6 hours, and surgical or traumatic etiology of arrest. We defined “cardiac arrest” as a subject having received chest compressions or defibrillation from a health care provider, and considered arrests in the ED to be OHCA.
Measurements
We obtained blood samples from each subject at enrollment, 6, 12, 24, 48 and 72 hours after ROSC. We collected samples in 2.7mL plastic tubes with 3.2% buffered sodium citrate additive and 10mL plastic serum tubes without additives (Becton, Dickinson and Co, Franklin Lakes, NJ) that we rested for 20 minutes prior to centrifugation at 3000rpm for 10 minutes. We removed the supernatant and stored it in Eppendorf tubes at -80°C until analysis. We measured serum levels of novel biomarkers in the post-arrest population including neutrophil gelatinase-associated lipocalin (NGAL), high-mobility group protein B1 (HMGB), intracellular cell adhesion molecule 1 (ICAM-1) and leptin, as well as previously characterized biomarkers including neuron specific enolase (NSE) and S100B protein. We used Luminex immunofluorescence assays (Millipore, Billerica, MA) and analyzed samples according to manufacturer instructions (available at www.millipore.com).
We prospectively recorded baseline characteristics including subject age, sex, presenting arrest rhythm [categorized as shockable (ventricular tachycardia/fibrillation (VT/VF)], or non-shockable (pulseless electrical activity, asystole, or unknown), arrest location (IHCA or OHCA), Sequential Organ Failure Assessment (SOFA) score, use of therapeutic hypothermia, and Pittsburgh Cardiac Arrest Category (PCAC). The PCAC is a clinical prediction tool that stratifies CA survivors by their risk of subsequent death or neurological deterioration based on clinical characteristics during the first 6h after ROSC (14). This tool divides survivors of CA into four categories that are predictive of survival and functional outcome. Because it could potentially affect biomarker clearance, we recorded each subject's temperature hourly for the first 36 hours, and then at 48 and 72 hours. As a measure of ongoing cardiovascular dysfunction and the severity of the post-cardiac arrest inflammatory syndrome, we recorded cumulative vasopressor index (CVI) hourly for the first 24 hours. The CVI is a method of standardizing vasopressor dosing (15, 16).
We defined acute kidney injury (AKI) according to the AKI Network (AKIN) criteria(17) as a rise in SCr of ≥0.3mg/dL within 48 hours of ROSC or ≥1.5-fold from baseline, or the need for renal replacement therapy, and treated AKI as a dichotomous outcome. We recorded serum creatinine (SCr) level at presentation and the peak value within 72 hours (+/- 12 hours) of ROSC, as well as each subject's pre-arrest baseline SCr. When a pre-arrest baseline was not available within the prior 6 months, we treated the lowest SCr after arrest as that subject's baseline. SCr was checked at the discretion of the treating clinician, and measurements were performed by the hospital's clinical laboratory. For patients who were discharged from the hospital less than 5 days after ROSC, we assumed that renal function did not deteriorate after discharge and so if they had not developed AKI prior to discharge they were classified as having no AKI.
We recorded each subject's outcomes including, survival to hospital discharge, neurological function at hospital discharge measured using the Pittsburgh Cerebral Performance Category (CPC), development of AKI and intensive care unit (ICU) length of stay. Finally, for non-survivors, we recorded each subject's mode of death, which we categorized as withdrawal of life-sustaining therapy based on anticipated neurological prognosis; brain death; withdrawal of life-sustaining therapy based on prior advanced directives or a surrogate's representation of the subject's wishes; or multiple system organ failure, hemodynamic instability, or re-arrest without successful resuscitation.
Statistical methods
Serum biomarker levels were not normally distributed and no transformation consistently minimized skewness. Therefore, we used nonparametric tests for all analyses. We summarized baseline clinical characteristics and report medians with interquartile ranges. Our primary outcome of interest was survival to hospital discharge. To determine whether each biomarker profile differed between those who survived to discharge and those who did not, we compared the level between survivors and non-survivors using Wilcoxon rank-sum tests at each time point, and report medians, interquartile ranges, and P values for these comparisons. Since the accuracy of early predictions of outcome is of greatest clinical interest, the remainder of our analyses focused on enrollment biomarker levels. First, we used unadjusted logistic regression to test the association between each baseline clinical characteristics and biomarker level with survival. We quantified the discriminatory power of each biomarker that was associated with survival at enrollment by generating receiver operating characteristic curves (ROC) and report area under the curve (AUC) with associated 95% confidence intervals (CI) and P values. Next, we sequentially explored the independent association of each enrollment biomarker with after survival adjusting for baseline clinical characteristics associated with survival at a level of P < 0.1. We excluded SOFA score and therapeutic hypothermia from these models, as they are collinear with PCAC.
Based on these models, enrollment NGAL level demonstrated the most favorable performance characteristics, so we used three additional approaches to further explore its utility. Since biomarker levels could be influenced by the presence of AKI, (18, 19) we forced “development of AKI within 72 hours” as a binary predictor into the adjusted model constructed above. Next, since a combination of multiple biomarkers and clinical characteristics might perform better than a model including only a single biomarker, we used forward selection to determine the most predictive combination of baseline clinical characteristics and biomarkers. We selected a probability of entry and removal of 0.1 and 0.2, respectively, and included all baseline clinical characteristics and enrollment biomarker levels as potential covariates. We evaluated the predictive performance of each of these models by computing c-statistics. Finally, we assessed the contribution of enrollment NGAL to each model by comparing c-statistics and integrated discrimination improvement (IDI) for each adjusted model with and without NGAL. We performed all analyses using Stata Version 13.1 (StataCorp, College Station, TX).
Results
Baseline characteristics and outcomes
A total of 86 subjects were enrolled. Median age was 56 (IQR 45-70) years, and 58% were male (Table 1). Most patients (69%) were enrolled after an OHCA, and multiple organ system dysfunction was common at presentation (median SOFA score 5 (IQR 7-10)). AKI was present at presentation in 53 (62%) subjects, and a majority of subjects met criteria for AKI within the first 5 hospital days (Table 2). Overall, 69 (80%) of subjects survived at least 72 hours after ROSC, 39 (45%) survived to hospital discharge and 7 (18%) of survivors were discharged with a CPC of 1-2.
Table 1. Baseline clinical characteristics after cardiac arrest in the biomarker cohort.
| Characteristic | Survivors (n = 39) | Non-survivors(n = 47) | Overall cohort (n = 86) |
|---|---|---|---|
| Age, years | 56 [43 – 56] | 56 [45 – 56] | 56 [45 – 70] |
| Male sex | 21 (54) | 29 (62) | 50 (58) |
| Presenting rhythm | |||
| VT/VF | 24 (62) | 15 (32) | 39 (45) |
| PEA | 8 (21) | 12 (26) | 20 (23) |
| Asystole | 4 (10) | 12 (26) | 16 (19) |
| Unknown | 3 (8) | 8 (17) | 11 (13) |
| Out-of-hospital arrest | 28 (72) | 31 (66) | 59 (69) |
| Pittsburgh Cardiac Arrest Category | |||
| I | 9 (23) | 0 (0) | 9 (10) |
| II | 18 (46) | 12 (26) | 30 (35) |
| III | 7 (18) | 8 (17) | 15 (17) |
| IV | 5 (13) | 27 (57) | 32 (27) |
| Baseline SOFA score | 6 [4 – 6] | 9 [7 – 9] | 7 [5 – 10] |
| Central nervous system | 3 [2 – 3] | 4 [4 – 4] | 4 [3 – 4] |
| Respiratory | 2 [1 – 2] | 3 [2 – 3] | 3 [1 – 3] |
| Cardiovascular | 0 [0 – 0] | 0.5 [0 – 0.5] | 0 [0 – 1] |
| Liver | 0 [0 – 0] | 1 [0 – 1] | 0 [0 – 0] |
| Renal | 0 [0 – 0] | 0 [0 – 0] | 0 [0 – 2] |
| Coagulation | 0 [0 – 0] | 0 [0 – 0] | 0 [0 – 0] |
| Cumulative vasopressor index | 0 [0 – 4] | 2 [0 – 38] | 0 [0 – 30] |
| Baseline SCr, mg/dL | 1 [0.9 – 1] | 1.4 [0.8 – 1.4] | 0.7 [0.6 – 1.0] |
| Therapeutic hypothermia | 26 (67) | 45 (98) | 71 (84) |
Data are presented as median [interquartile range] or raw number with corresponding percentages.
Abbreviations: VT/VF – Ventricular tachycardia/fibrillation; PEA – Pulseless electrical activity; SOFA – Sequential Organ Failure Assessment; SCr – Serum creatinine.
Table 2. In-hospital outcomes after cardiac arrest within the overall cohort.
| Outcome | Overall cohort (n = 86) |
|---|---|
| Survival to hospital discharge | 39 (45) |
| Discharge CPC (% of survivors) | |
| 1-2 | 7 (18) |
| 3-4 | 32 (37) |
| ICU length of stay, days | 5 [3 – 11] |
| Non-survivors | 4 [3 – 9] |
| Survivors | 7 [4 – 12] |
|
| |
| Hospital length of stay, days | 9 [4 – 14] |
|
| |
| Non-survivors | 4 [3 – 9] |
|
| |
| Survivors | 12 [9 – 20] |
Data are presented as median [interquartile range] or raw number with corresponding percentages.
Abbreviations: CPC – Cerebral Performance Category; ICU – Intensive Care Unit
Unadjusted analysis
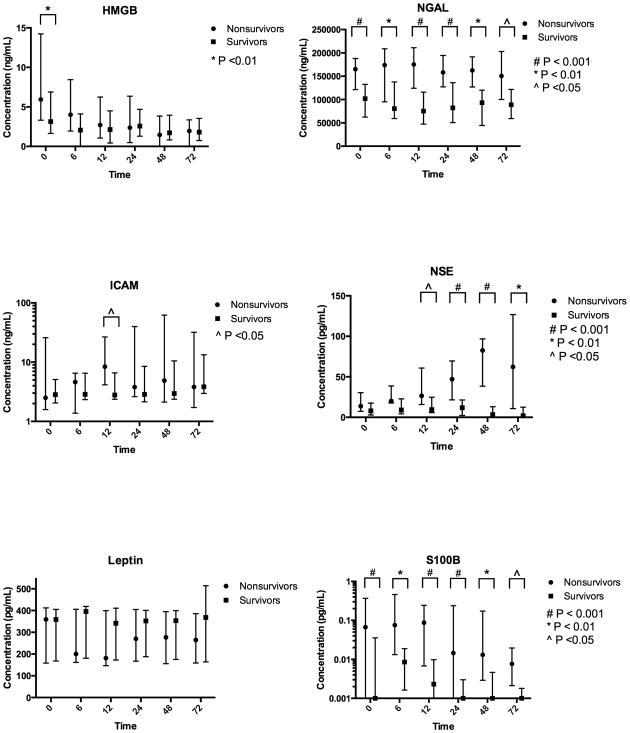
In unadjusted analysis, an initial shockable rhythm was associated with improved survival (OR 3.41 (95%CI 1.40-8.31)) and higher baseline SOFA score (OR 0.76 (95%CI 0.62 – 0.93) per unit increase) and PCAC (OR 0.30 (95% CI 0.18 – 0.51)) were associated with reduced survival (Table 3). Presence of AKI at baseline, within 72 hours and within 5 days of ROSC was not associated with outcome. Enrollment levels of HMGB, NGAL and S100B were higher in non-survivors than survivors (Figure 1). The AUC of the ROC curve for enrollment NGAL level was 0.78 (95%CI 0.66 – 0.90, and remained similar at all time points (Figure 2, Table 5). Enrollment NGAL below 58,000 ng/mL was perfectly specific for survival to hospital discharge (Figure 2). Conversely, enrollment NGAL above 188,000 ng/mL was 96% specific for non-survival. Consistent with previous work, (9) NSE levels in non-survivors began to rise after 12-24 hours and were maximally separated at 48 hours, while S100b levels began to fall after 12 hours.
Table 3. Unadjusted predictors of survival to hospital discharge after cardiac arrest.
| Characteristic | Odds ratio (95% CI) | P value |
|---|---|---|
| Baseline clinical characteristic | ||
| Age | 0.99 (0.97 – 1.02) | 0.54 |
| Male sex | 0.72 (0.31 – 1.71) | 0.46 |
| Shockable rhythm | 3.41 (1.40 – 8.31) | 0.007 |
| Out-of-hospital arrest | 1.31 (0.53 – 3.30) | 0.56 |
| Pittsburgh Cardiac Arrest Category | 0.30 (0.18 – 0.51) | <0.001 |
| Baseline SOFA score | 0.76 (0.62 – 0.93) | 0.008 |
| Therapeutic hypothermia | 0.04 (0.01 – 0.34) | 0.004 |
| Acute kidney injury | ||
| Immediate post-arrest | 1.21 (0.50 – 2.91) | 0.67 |
| Within 72 hours | 0.51 (0.17– 1.49) | 0.22 |
| Within 5 days | 0.58 (0.20 – 1.74) | 0.34 |
| Biomarkers at enrollment | ||
| NGAL, per 25,000 ng/mL | 0.58 (0.42 – 0.79) | <0.001 |
| ICAM-1, per 10 ng/mL | 0.73 (0.52 – 1.02) | 0.07 |
| Leptin, per 100 pg/mL | 1.10 (0.81 – 1.50) | 0.53 |
| HMGB, per 10 ng/mL | 0.95 (0.77 – 1.17) | 0.61 |
| NSE, per 10 pg/mL | 0.85 (0.67 – 1.07) | 0.17 |
| S100B, per 0.1 pg/mL | 0.65 (0.45 – 0.92) | 0.01 |
Abbreviations: NGAL - Neutrophil gelatinase-associated lipocalin; ICAM-1 – Intracellular cell adhesion molecule 1; HMGB – High-mobility group protein B1; NSE – Neuron specific enolase.
Figure 1.
Biomarker profiles after cardiac arrest versus time versus time from return of spontaneous circulation, stratified by survival to hospital discharge.
Figure 2.
Receiver operating characteristic curves for enrollment biomarker levels associated with survival to hospital discharge after cardiac arrest.
Table 5. Receiver operating curve characteristics for the association of serum NGAL level with survival to hospital discharge.
| Time point | ROC AUC (95%CI) | P value |
|---|---|---|
| Enrollment | 0.78 (0.66 – 0.90) | 0.0002 |
| 6 hours | 0.80 (0.66 – 0.95) | 0.003 |
| 12 hours | 0.82 (0.68 – 0.96) | 0.0008 |
| 24 hours | 0.79 (0.67 – 0.90) | <0.0001 |
| 48 hours | 0.79 (0.62 – 0.95) | 0.005 |
| 72 hours | 0.79 (0.61 – 0.97) | 0.02 |
Abbreviation: ROC AUC – Receiver operating curve area under the curve.
Adjusted analysis
In our adjusted models, enrollment NGAL level was independently associated with survival, regardless of whether we forced development of AKI into the model (Table 4). When we removed enrollment NGAL from each of these models, the overall model fit and discriminatory power declined (Table 4). Addition of enrollment NGAL to each clinical model resulted in a significant increase in IDI and predictive accuracy (Table 4). No other enrollment biomarker was independently associated with outcome after controlling for PCAC and shockable rhythm (all P >0.05, data not shown). Using forward selection, enrollment NGAL and PCAC remained significant and had stable point estimates and confidence intervals when compared with the other methods of model building. Consistent with previous reports, NGAL levels rose early in subjects who went on to develop clinically apparent AKI within 72 hours of ROSC, but was not elevated in those who already exhibited AKI at presentation (data not shown).
Table 4.
Enrollment NGAL level improves the discriminatory value of adjusted models predicting survival after cardiac arrest, regardless of the methods used to build the model or adjustment for acute kidney injury.
| Model | Covariate | Adjusted OR (95% CI) | P value | Model performance | Full model | Reduced model (NGAL removed) | Integrated discrimination improvement with NGAL | |
|---|---|---|---|---|---|---|---|---|
| Estimate | P value | |||||||
| 1 | Enrollment NGAL | 0.65 (0.44 – 0.96) | 0.03 | C statistic | 0.90 | 0.79 | 0.13 | <0.001 |
| Shockable rhythm | 1.28 (0.28 – 5.89) | 0.76 | Efron's R2 | 0.49 | 0.30 | |||
| PCAC | 0.20 (0.08 – 0.48) | <0.001 | BIC | -210 | -280 | |||
|
| ||||||||
| 2 | Enrollment NGAL | 0.64 (0.43 – 0.95) | 0.03 | C statistic | 0.90 | 0.83 | 0.12 | 0.002 |
| AKI within 72 hours | 1.76 (0.41 – 7.55) | 0.45 | Efron's R2 | 0.50 | 0.33 | |||
| Shockable rhythm | 1.36 (0.29 – 6.45) | 0.70 | BIC | -206 | -279 | |||
| PCAC | 0.20 (0.09 – 0.49) | <0.001 | ||||||
|
| ||||||||
| 3 | Enrollment NGAL | 0.52 (0.30 – 0.89) | 0.02 | C statistic | 0.94 | 0.81 | 0.18 | <0.001 |
| Enrollment HMGB | 1.37 (1.02 – 1.86) | 0.04 | Efron's R2 | 0.59 | 0.35 | |||
| Enrollment ICAM-1 | 0.53 (0.28 – 1.02) | 0.06 | BIC | -214 | -230 | |||
| PCAC | 0.12 (0.04 – 0.38) | <0.001 | ||||||
Abbreviations: NGAL – Neutrophil gelatinase-associated lipocalin; PCAC – Pittsburgh Cardiac Arrest Category; AKI – Acute kidney injury; HMBG – High-mobility group protein B1; ICAM-1 – intracellular cell adhesion molecule 1; BIC – Bayesian information criterion.
Adjusted Model 1: Built including significant clinical predictors with unadjusted P <0.1; Adjusted Model 2: Development of AKI is forced into Model 1; Adjusted Model 3: Built with forward selection. All models are presented with (full) and without (reduced) inclusion of NGAL. Odds ratios for NGAL are expressed per 25,000ng/mL change; odds ratios for HMGB and ICAM-1 are expressed per 10ng/mL change.
Discussion
The main finding of our study is that serum NGAL level is strongly predictive of outcome after cardiac arrest. At enrollment, NGAL performed better than the other candidate biomarkers, including NSE and S100b, which have been better investigated after cardiac arrest. Moreover, addition of enrollment NGAL to clinical models resulted in a significant increase in the discriminatory power of these models. Notably, the association between NGAL and outcome remained stable at all time points up to 72 hours after ROSC. Thus, even in the case of inter-facility transfer, where time from arrest to presentation at the final treating hospital may be considerably delayed, the prognostic value of NGAL is preserved.
NGAL is expressed in a variety of tissues including kidney, liver and lung (20). Plasma levels may be elevated in a variety of pathological inflammatory states including heart failure and malignancy (20). In AKI, NGAL is secreted both by neutrophils that contribute to tubular injury and directly by tubular epithelial cells themselves (21). Compared to serum creatinine, NGAL rises earlier and may be a more sensitive marker of AKI; moreover, in risk-adjusted analyses, development of AKI independently predicts mortality (18, 19). Thus, it is possible that the association between NGAL and outcomes in this cohort is related to underlying, subclinical AKI. Overt AKI detected by change in serum creatinine was not associated with mortality after cardiac arrest in past studies (22), nor did we find an association between AKI and outcome in this cohort. However, serum creatinine is relatively insensitive for AKI and it is possible that the NGAL is a more sensitive marker for subclinical but important AKI. Alternatively, as a nonspecific inflammatory marker (20), NGAL may also reflect the overall severity of systemic inflammation in the post-cardiac arrest syndrome (10-12). In this regard, NGAL may be an easily measurable surrogate measure of covariates such as duration of pulselessness, quality of CPR and total burden of anoxic and ischemia-reperfusion injury, metrics which are at present are unreliably available or difficult to quantify for clinical or research purposes (23-25).
Both NSE and S100B have been proposed to be specific markers of brain injury and evaluated in the CA population. NSE is an intracellular enzyme involved in glucose metabolism found in neurons and neuroendocrine cells, but also platelets and erythrocytes (26). To our knowledge, the largest study NSE as a prognostic biomarker after CA was the PROPAC study, which was conducted before the widespread implementation of targeted temperature management (27). This study suggested that an NSE value above 33mcg/L uniformly predicted poor outcome. However, hypothermia attenuates the rise in NSE level, which limits the applicability of the PROPAC results to current clinical care. Furthermore, as re-demonstrated by our results in the present study, NSE rises slowly after CA and is not predictive of outcome in the first days after ROSC, limiting its utility during the first 48-72 hours after ROSC when accurate prognostication is most challenging (28). S100B is an intracellular calcium binding protein found in glial cells, and may be more specific to brain injury that NSE (26). Our results are consistent with previous studies that have shown that S100B rises early in non-survivors, although the variability of levels among survivors and non-survivors is large. (27, 29). Previous studies have suggested that S100B may not have sufficient discriminatory power to be clinically useful (27, 29).
With the exception of NGAL, the remaining novel biomarkers we investigated in the present study were inconsistently or never associated with clinical outcome. HMGB is a ubiquitous nuclear protein involved in chromatin structure, and also found in cytosol, where it is involved in sterile inflammation and autophagy (30). This offers some face validity as a marker of the severity of the post-arrest syndrome. Indeed, HMGB concentration was significantly higher in non-survivors than survivors at enrollment. However, the discriminatory power of enrollment HMGB level was lower than that of enrollment NGAL, and in contrast to NGAL, subsequent HMGB levels did not predict outcome. Leptin is a peptide expressed in neuronal tissue and extracranial adipose, and has been evaluated as a prognostic marker after traumatic brain injury (31), but did not correlate with outcome in this cohort. ICAM-1 is a marker of endothelial dysfunction and pathological central nervous system inflammatory states where it facilitates leukocyte adhesion and recruitment (32). It also did not correlate with outcome in this cohort.
Our study has several important limitations. As a tertiary care referral center, many of our patients are received in transfer from other facilities. As such, “enrollment” biomarker levels were not obtained immediately after cardiac arrest. However, all “enrollment” biomarker levels were obtained within 6 hours of ROSC. For all subsequent time points, samples were obtained at fixed intervals from return of spontaneous circulation, but there was some variability in timing of the initial sample that reflects real-world clinical practice. In the case of NGAL, this variability does not alter our conclusions since the relationship with outcome was stable over time. Also, although we used several methods of model building to generate multivariable models, we could not adjust for several potentially important covariates such as time intervals from collapse-to-CPR and CPR-to-ROSC, which we have previously shown are unreliably documented in our care system (23). It is possible that unmeasured confounders biased our findings, a limitation inherent to all observational studies.
Like many biomarker studies, we followed subjects' clinical outcomes to hospital discharge. While this is an important marker of overall recovery, in patients with acquired brain injury, survival to hospital discharge is not necessarily a patient-centered outcome as compared to a favorable neurological outcome. Future investigations may focus on subjects' neurological recovery and long-term outcomes. As a single center study, it is possible that some of our the correlations we observed were influenced by our local standard of care. For example, the rise in many biomarkers after cardiac arrest is influenced by therapeutic hypothermia. While we systematically treated all comatose subjects in this study with therapeutic hypothermia, other local care practices may represent unmeasured confounders. Since comatose patients in this cohort were treated with hypothermia and also had a greater severity of illness, we are not able to differentiate the effects of hypothermia on biomarker levels from the effect of illness severity. The results of the recent Target Temperature Management trial, which support hypothermia to a goal of either 33 or 36°C, offer a future opportunity to disentangle this confounding in these most severely injured patients. Finally, while our local standard of care has not changed significantly since the recruitment period for the present study, it is possible that recent changes in resuscitation and general critical care management and guidelines limit the generalizability of our results to other centers' current clinical practice. Contemporary, external validation of our findings by other groups is a necessary next step in exploring the utility of NGAL as a prognostic marker in this population.
Conclusion
In this exploratory study, it appears that serum NGAL levels are strongly predictive of survival to hospital discharge after cardiac arrest. This relationship is stable over time, and addition of enrollment NGAL to clinical models improve overall model fit and discriminatory power. We believe NGAL is an appealing candidate that merits further investigation in this patient population.
Acknowledgments
Disclosures and COI: Dr. Elmer's research effort is supported by the National Institutes of Health through grant number 5K12HL109068. Dr. Rittenberger's research effort is supported by the National Institutes of Health through grant number 1KL2RR024154.
Copyright form disclosures: Dr. Elmer received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NHLBI, Zoll Foundation, and Laerdal Foundation. Dr. Abebe's institution received grant support from KL2 Multdisciplinary Clinical Research Scholars Award. Dr. Murugan received support for article research from the NIH. His institution received grant support from Bard Inc. (Received grant on intensity of monitoring in AKI and outcomes), CTSI (NIH grant on modeling renal recovery from sepsis induced AKI), Gambro Inc. (Timing of renal replacement therapy and outcomes), and HRSA (Monitoring Organ Donors to Improve Transplantation results). Dr. Callaway received support for article research from the NIH. His institution received grant support from the NHLBI K12 HL109068 and NHLBI U01 HL077871. Dr. Rittenberger received support for article research from the NIH (1KL2RR024154), is employed by the University of Pittsburgh/UPMC, and received support for article research from the NIH.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally B, Robb R, Mehta M, et al. Out-of-hospital cardiac arrest surveillance --- Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005--December 31, 2010. Morbidity and mortality weekly report Surveillance summaries. 2011;60(8):1–19. [PubMed] [Google Scholar]
- 3.Peberdy MA, Kaye W, Ornato JP, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 4.Stiell IG, Wells GA, Field B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. The New England journal of medicine. 2004;351(7):647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 5.Kamps MJ, Horn J, Oddo M, et al. Prognostication of neurologic outcome in cardiac arrest patients after mild therapeutic hypothermia: a meta-analysis of the current literature. Intensive care medicine. 2013;39(10):1671–1682. doi: 10.1007/s00134-013-3004-y. [DOI] [PubMed] [Google Scholar]
- 6.Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Annals of neurology. 2012;71(2):206–212. doi: 10.1002/ana.22632. [DOI] [PubMed] [Google Scholar]
- 7.Rossetti AO, Koenig MA. Prognostication after cardiac arrest: a tale of timing, confounders, and self-fulfillment. Neurology. 2011;77(14):1324–1325. doi: 10.1212/WNL.0b013e318231533b. [DOI] [PubMed] [Google Scholar]
- 8.Sandroni C, Cariou A, Cavallaro F, et al. Prognostication in comatose survivors of cardiac arrest: An advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive care medicine. 2014;40(12):1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderon LM, Guyette FX, Doshi AA, et al. Combining NSE and S100B with clinical examination findings to predict survival after resuscitation from cardiac arrest. Resuscitation. 2014;85(8):1025–1029. doi: 10.1016/j.resuscitation.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negovsky VA. The second step in resuscitation--the treatment of the post-resuscitation disease. Resuscitation. 1972;1(1):1–7. doi: 10.1016/0300-9572(72)90058-5. [DOI] [PubMed] [Google Scholar]
- 11.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 12.Negovsky VA. Postresuscitation disease. Critical care medicine. 1988;16(10):942–946. doi: 10.1097/00003246-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Rittenberger JC, Guyette FX, Tisherman SA, et al. Outcomes of a hospital-wide plan to improve care of comatose survivors of cardiac arrest. Resuscitation. 2008;79(2):198–204. doi: 10.1016/j.resuscitation.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittenberger JC, Tisherman SA, Holm MB, et al. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation. 2011;82(11):1399–1404. doi: 10.1016/j.resuscitation.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive care medicine. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Trzeciak S, McCoy JV, Phillip Dellinger R, et al. Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive care medicine. 2008;34(12):2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccinni P, Cruz DN, Gramaticopolo S, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva anestesiologica. 2011;77(11):1072–1083. [PubMed] [Google Scholar]
- 19.Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29(7):1301–1311. doi: 10.1093/ndt/gft510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Annals of clinical biochemistry. 2014;51(Pt 3):335–351. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martensson J, Xu S, Bell M, et al. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clinica chimica acta; international journal of clinical chemistry. 2012;413(19-20):1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Yanta J, Guyette FX, Doshi AA, et al. Renal dysfunction is common following resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2013;84(10):1371–1374. doi: 10.1016/j.resuscitation.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 23.Frisch A, Reynolds JC, Condle J, et al. Documentation discrepancies of time-dependent critical events in out of hospital cardiac arrest. Resuscitation. 2014;85(8):1111–1114. doi: 10.1016/j.resuscitation.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Rittenberger JC, Martin JR, Kelly LJ, et al. Inter-rater reliability for witnessed collapse and presence of bystander CPR. Resuscitation. 2006;70(3):410–415. doi: 10.1016/j.resuscitation.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Sasson C, Haukoos J. Learning to fly: lessons for the resuscitation community from the aviation industry. Circulation Cardiovascular quality and outcomes. 2013;6(2):135–136. doi: 10.1161/CIRCOUTCOMES.113.000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S-100, IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007;75(2):219–228. doi: 10.1016/j.resuscitation.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Zandbergen EG, Hijdra A, Koelman JH, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66(1):62–68. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 28.Tiainen M, Roine RO, Pettila V, et al. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke; a journal of cerebral circulation. 2003;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki K, Oda S, Sadahiro T, et al. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation. 2009;80(8):870–875. doi: 10.1016/j.resuscitation.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Tsung A, Tohme S, Billiar TR. High-mobility group box-1 in sterile inflammation. Journal of internal medicine. 2014;276(5):425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Huang SJ, Wang N, et al. Relationship between plasma leptin levels and clinical outcomes of pediatric traumatic brain injury. Peptides. 2012;35(2):166–171. doi: 10.1016/j.peptides.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Turowski P, Adamson P, Greenwood J. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation. Cellular and molecular neurobiology. 2005;25(1):153–170. doi: 10.1007/s10571-004-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]