Abstract
The current study tested the “critical window” hypothesis of menopause that postulates that the timing and duration of hormone treatment determine their potential outcomes. Our focus was genes in the rat hypothalamus involved in social and affiliative behaviors that change with aging and/or estradiol (E2): Avp, Avpr1a, Oxt, Oxtr, and Esr2 in the paraventricular nucleus (PVN) and supraoptic nucleus (SON). Rats were reproductively mature or aging adults, ovariectomized, given E2 or vehicle treatment of different durations, with or without a post-OVX delay. Our hypothesis was that age-related changes in gene expression are mitigated by E2 treatments. Contrary to this, PVN Oxtr increased with E2, and Avpr1a increased with age. In the SON, Avpr1a increased with age, Oxtr with age and timing, and Avp was by duration. Thus, chronological age and E2 have independent actions on gene expression, with the “critical window” hypothesis supported by the observed timing and duration effects.
Keywords: Vasopressin, Oxytocin, Menopause, Estradiol, Paraventricular nucleus, Supraoptic nucleus
1. Introduction
Menopause is the physiological cessation of ovarian function during which menstrual cyclicity ceases and concentrations of ovarian hormones such as estrogens and progesterone decline. Hormone replacement therapy (HRT) is currently the most commonly used therapy to treat menopausal symptoms. However, since the termination of the Women’s Health Initiative (WHI) study of conjugated equine estrogens and medroxyprogesterone acetate treatment, many women have been left asking questions about whether, when, and for how long to take hormone treatments for menopausal symptoms. The WHI initially reported that there was a small but significant increase in adverse incidents in women taking hormone therapy (Rossouw et al., 2002; Manson et al., 2013). Reanalysis suggested that there might be a critical window post-menopause during which hormone treatment is beneficial to neurobiological and other health-related endpoints in women (Klaiber et al., 2005; Bhupathiraju and Manson, 2014). Therefore, it is necessary to understand the risks and benefits of estrogen treatments in the context of timing and duration for women to be able to make informed decisions and to improve quality of life.
In addition to hot flashes, neurobehavioral changes such as anxiety and depression increase at menopause in humans, which can lead to social isolation and impact the quality of life (Uguz et al., 2011; Deeks and McCabe, 2004; Lanza di Scalea et al., 2012). Oxytocin (Oxt) and vasopressin (Avp) are modulators of these behaviors in mammalian species [de Kloet et al., 2005 (mice); Klenerova et al., 2009 (rat); Meyer-Lindenberg et al., 2011 (human); Lim and Young, 2006 (voles)]. Both of these neurohypophyseal peptides are synthesized in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) of the hypothalamus.
Vasopressin and oxytocin neurons are regulated by estrogens, predominantly through the estrogen receptor beta (ERβ) that is the dominant ER in these regions, as demonstrated in knockout mice (Winslow and Insel, 2004) and by gene and protein expression in rats (Hrabovszky et al, 1998). ERβ protein and mRNA is also colocalized with oxytocin- and to a lesser extent with vasopressin-expressing neurons of rats and mice (Hrabovszky et al., 1998; Alves et al., 1998; Patisaul et al., 2003). However, the effects of estradiol on the regulation of vasopressin and oxytocin are mixed, and most studies utilized short-term treatment regimes. Some studies showed up-regulation [Roy et al., 1999 (mRNA, monkey); Patisaul et al., 2003 (mRNA & protein, mouse)], others down-regulation [Shughrue et al., 2002 (protein, rat); Van Tol et al., 1988 (mRNA, rat); Nomura et al., 2002 (mRNA & protein, mouse)], and still others no effect [Peter et al., 1990 (mRNA, rat); Rhodes et al., 1981 (protein, rat); Akaishi & Sakuma, 1985 (protein, rat)] of estradiol treatment. This inconsistency in results was part of our motivation for conducting the work, and to hypothesize that the effects of estrogen would be influenced by different timings and durations of treatment.
Along with oxytocin and vasopressin, we also selected Oxtr and Avpr1a gene expression as endpoints because of their role in mediating effects of their respective nonapeptides on social behaviors such as anxiety and depression (Young, 1999; Bielsky et al., 2004; Sala et al., 2011). Previous studies using RT-PCR, in situ hybridization, immunohistochemistry and electrophysiology have shown that mRNA and protein of the vasopressin receptor 1a and the oxytocin receptor are expressed in the SON and PVN of rats (Hurbin et al., 1998, 2002; Gouzènes et al, 1999; Yoshimura et al., 1993). This study was designed to help fill the gap in research and to gain mechanistic insight into the molecular changes that occur during aging and in response to differential modes of treatment to assess the effects of timing and duration of E2. By studying outcomes of Avp, Avpr1a, Oxt, Oxtr and Esr2 in the PVN and SON of the hypothalamus, we can have a better understanding of the neural substrates involved in social behavior as a basis for future work testing the “critical window” hypothesis on the behaviors themselves.
2. Materials and Methods
2.1 Animals and Husbandry
All animal procedures were conducted in accordance with The Guide for the Care and Use of Experimental Animals following protocols approved by The University of Austin IACUC committee and NIH standards. Female Sprague Dawley rats (Harlan) were purchased as reproductively mature adults (MAT, ~3 months, sexually naïve) or aging adults (AG, ~11 months, retired breeders). These animals are the same as those used in a separate study (Yin et al., 2015) for analyses of different brain regions. Upon arrival, rats were pair housed on a 12-hour light, 12-hour dark cycle (lights on at 0700) and received water and food ad libitum. They were allowed to acclimate to the room for two weeks prior to surgery during which time estrous cyclicity was monitored daily by vaginal lavage of sterile saline. Only females with regular 4–5 day cycles were used. All rats received ovariectomy surgery under isofluorane inhalation anesthesia. A single injection of Rimadyl (5 mg/kg) was given at the start of surgery. Bilateral dorsolateral incisions were made through the skin, muscle, and peritoneum, and the ovaries were ligated and removed. Muscles were sutured and wound clips used to close the skin.
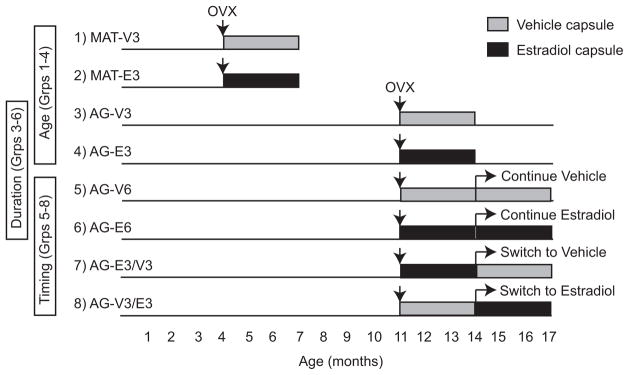
Animals were randomly assigned to one of eight treatment groups as illustrated in Figure 1 to test different timings and durations of hormone treatment based on those used in the WHI, in which the women experienced an average 12-year delay in treatment relative to the last menstrual period. We calculated based upon the rats’ life cycle compared to humans, that 3 months to an adult rat is equal to about 5 years in a woman (Sengupta, 2013; Quinn, 2005). Capsules containing either 100% cholesterol (Veh) or 5% 17β-estradiol/95% cholesterol (E2) were implanted subcutaneously between the shoulder blades at the time of surgery. Because the aim of this study was to address and reevaluate limitations of the WHI, we chose to use continuous exposure to E2 at a physiologically relevant dosage to mimic the type of treatment given in the Estrogen-alone WHI studies (The Women’s Health Initiative Study Group, 1998). This treatment regime is clinically relevant, as continuous E2 treatments are still in common use for subsets of postmenopausal women.
Figure 1.
The eight treatment groups are shown that were used to test age, and duration and timing of E2 treatment. Reproductively mature adult (MAT) were 4 months old at the time of ovariectomy (OVX) surgery and aging rats (AG) were 11 months old at the time of OVX, and received either vehicle (cholesterol; VEH) or 17β-estradiol capsule implantation at the time of surgery. Both groups of MAT rats and two groups of AG rats received treatment for a duration of 3 months (Groups 1–4). Two groups of AG rats received treatment for duration of 6 months (Group 5 & 6). There were two AG “switch” groups that received either VEH or E2 initially at the time of OVX and were switched to the opposite treatment after 3 months, and continued on the new treatment for a 3-month duration (Group 7 & 8). This model enabled us to differentiate effects of age (Groups 1–4), duration (Groups 3–6) and timing (Groups 5–8).
2.2 Tissue processing
Rats were euthanized 3 or 6 months after OVX and hormone treatment, when mature adults (MAT) were ~7 months, and aging adults (AG) were ~14 or ~17 months of age. All animals were weighed and euthanized by rapid decapitation starting at 1330 hours with the last animal killed not later than 1600 hours; therefore they were all killed during the lights on period. Although there is a diurnal rhythm of vasopressin gene expression in the SCN, previous studies have shown that within in SON and PVN, the areas used in the present study, there is no diurnal change in vasopressin or oxytocin gene expression in rats (Uhl & Reppert, 1986, Burbach et al., 1988). In addition, when we used time of euthanasia as a covariate in statistical analyses, no time of day effect was found. Brains were removed and sectioned in 1-mm coronal sections using an ice-cold stainless steel brain matrix. Sections were placed into cryogenic storage vials that contained RNAlater (Life technologies, Grand Island, NY). Tubes were stored at 4°C overnight, then mounted onto chilled slides and placed in a −20°C freezer for storage. Using Palkovits punches and the Paxinos and Watson (2009) rat brain atlas (all coordinates are based on that atlas), bilateral micropunches were taken under a dissecting microscope. The PVN punch (1.22 mm diameter) began rostrally at ~Bregma = −0.84 mm, and extended caudally 1 mm. The SON punches (0.96 mm diameter) started rostrally at ~Bregma = −0.60 mm and extended caudally for 1 mm. The choice of sampling areas was based on previous studies showing large concentrations of magnocellular neurons containing both vasopressin and oxytocin protein in rats at this range of coordinates (Rhodes et al., 1981; Swanson & Sawchenko, 1983). The PVN has several subdivisions (Swanson & Sawchenko, 1983), and the location of our punches includes the part that lies ventromedial to the descending column of the fornix between Bregma = −1.44 to −1.56), and the lateral magnocellar subdivision (Krieg, 1932; Swanson & Sawchenko, 1983) from Bregma = −1.72 to −1.92. Punches for each region were placed in a frozen Eppendorf tube for storage at −80°C. At the time of decapitation trunk blood was collected and allowed to clot, serum was separated and centrifuged (2300 X g for 5 minutes) then stored in Eppendorf tubes at −80°C.
2.3 Real-time PCR assays
RNA was extracted from frozen PVN and SON punches using an Allprep RNeasy mini kit (Qiagen, Valencia, California), according to the manufacturer’s protocol. Although we started with n=10 rats per group, loss of some tissues during freezer storage caused some attrition (a subset of samples were frozen with too much liquid RNAlater that made it difficult to visualize brain landmarks; only samples were included with which we were confident in the landmarks), resulting in n=7 for most gene assays. After extraction the quality of RNA was checked on an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA) and all RIN values fell in the range of 8.6 to 9.9. The quantity of RNA was measured using a GloMax-Multi Detection System (Promega, Madison, WI), and ranged from 71 to 741 ng/ul in SON, and from 207 to 1550 ng/ul in the PVN. Using a high-capacity cDNA reverse transcription kit (Life Technologies, Grand Island, NY), mRNA (PVN 200 ng, SON 150 ng) was converted to single-stranded cDNA. Samples were stored at −20°C until use. Quantitative real-time PCR was used to analyze 5 genes in the PVN and 4 genes in the SON: Avp, Oxt, Avpr1a, Oxtr, Esr2 (FAM) in tandem with Gapdh (VIC). Esr2 could not be run in SON due to the smaller amounts of RNA extracted from this smaller dissection limiting us to 4 genes maximum for analysis. All samples were run in triplicates with probe and primer predesigned gene expression assays (Life Technologies, Grand Island, NY). Target genes were amplified using Taqman universal mastermix (Life Technologies, Grand Island, NY) and detected on a ViiA7 Real time PCR machine (Applied Biosystems, Life Technologies, Grand Island, NY) with the following run parameters: 0°C for 2 minutes, 95°C for 10 minutes, 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Relative expression was determined for each sample using the comparative cycle threshold method (Pfaffl, 2001; Schmittgen and Livak, 2008). All samples were normalized to the housekeeping gene Gapdh and then calibrated to the median δ-cycle threshold of the young 3-month vehicle treated group. Any of the triplicates with a value of 2 SD above the mean of an individual animal was removed. Samples that amplified at or above 35ct were excluded from analysis.
2.4 Gene expression Analysis
Expression of each gene was analyzed using SPSS (version 22). For age (groups 1–4, Figure 1), two-way ANOVA was used to analyze the effects of age (MAT vs. AG) and hormone (VEH vs. E2). For duration (groups 3–6, Figure 1), two-way ANOVA was used to analyze effects of treatment duration (3 mo vs. 6 mo) and hormone (VEH vs. E2). Interactions among variables were also analyzed. A one-way ANOVA with a Bonferroni post hoc was performed to examine the effect of timing (groups 5–8, Figure 1). For all of these analyses, alpha was set at 0.05 and significant main or interaction effects were followed by two-tailed independent sample t-tests. Those data that did not pass the assumptions of normality and/or variance were transformed using either a square root or log transformation. Avpr1a in the PVN and Oxtr in the SON data did not meet assumptions even after transformation; therefore a non-parametric analysis was performed followed up with Mann-Whitney t-test.
2.5 Hormone assays and correlations
Twelve serum hormones were measured, for which detailed methods and results were previously reported on these same rats in a separate study evaluating other endpoints (Yin et al., 2015). For the current study, values for serum estradiol (E2), progesterone (P4), corticosterone (Cort), brain-derived neurotrophic factor (BDNF) were used for correlations with each of the target genes using Pearson’s correlation on raw or square-root transformed data when a dataset did not meet assumptions of normality. For those data sets that did not meet the assumptions even after transformation, a Spearman’s nonparametric correlation was used.
3. Results
3.1 Gene expression
3.1.1 Paraventricular nucleus
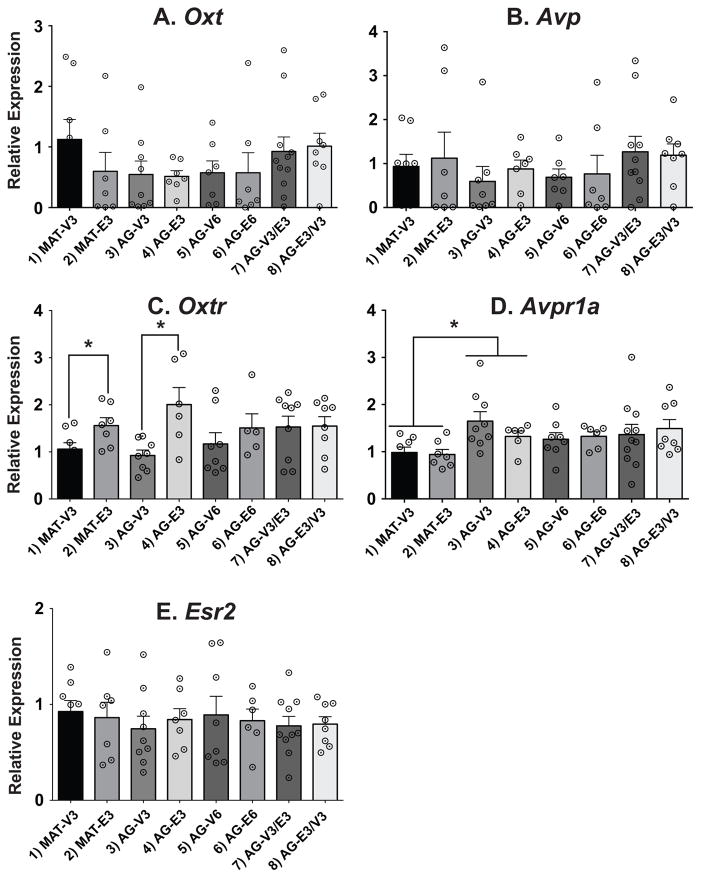
Two genes, Oxtr and Avpr1a, were significantly affected only in comparisons of the 3-month treatment groups 1–4 (Figure 2). In these animals, there was a main effect of treatment on Oxtr expression. Expression was increased by estradiol compared to vehicle in both the 3-month MAT and AG groups, an effect that was not seen in any of the 6-month duration groups (Figure 2C; p < 0.05). For Avpr1a, there was a significant main effect of age. In the comparison of the 3-month post-OVX groups, the AG animals had significantly higher gene expression than the corresponding MAT rats, but no hormone effects (Figure 2D; p < 0.05). There were no effects of age, timing, or duration on Oxt, Avp, and Esr2 expression in the PVN (Figure 2A, B, E).
Figure 2.
Relative gene expression data are shown in the PVN. Note that the scale of the y-axis varies depending on the gene. Comparisons of age were made in groups 1–4, duration of hormone in groups 3–6, and timing of hormone in groups 5–8. Oxt, Avp and Esr2 were unaffected by treatment, age, duration, or timing. For Oxtr there were significant hormone effects in the 3-mo treated groups, with expression higher in the E2 groups. For Avpr1a, there was a significant age effect in those same groups, with levels increasing from the mature to the aging animals. White circles with black center dots indicate individual rat data, and data shown are mean ± SEM. *, p < 0.05.
3.1.2 Supraoptic Nucleus
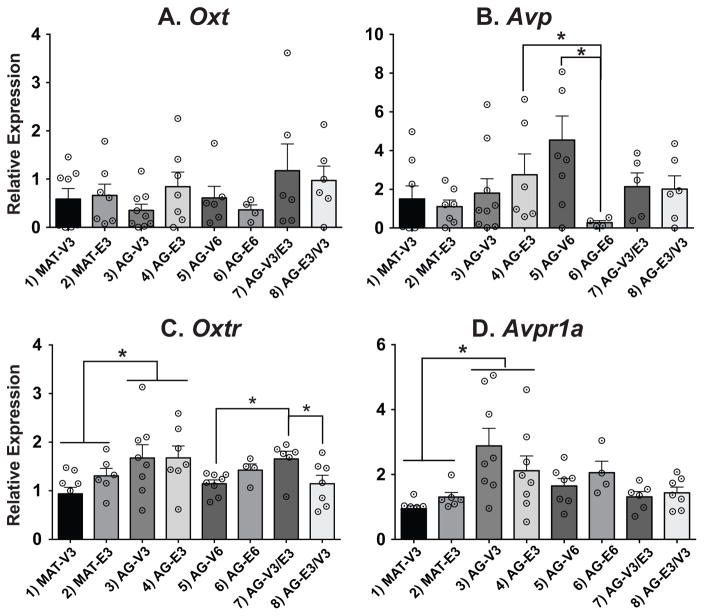
Three of the four genes measured, Avp, Oxtr, and Avpr1a, were significantly affected, each with a unique expression pattern (Figure 3). For Avp, two-way ANOVA of Groups 3–6 found no main effects of duration or treatment; however there was a significant interaction (p < 0.05). Specifically, Avp was significantly lower in the AG-E6 than the AG-E3 and AG-V6 (Figure 3B; p < 0.05). For Oxtr, a main effect of age was found in the 3-month treatment groups (1–4), with levels higher in the AG than the MAT rats (Figure 3C; p < 0.05). In addition, a timing effect was found for groups 5–8, with Oxtr higher in the AG-V3/E3 rats than the AG-V6 or AG-E3/V3 rats. Avpr1a expression also had a main effect of age in groups 1–4, with levels higher in the AG than the MAT rats (Figure 3D; p<0.05). Oxt gene expression was unaffected (Figure 3A).
Figure 3.
Relative gene expression data are shown in the SON. Note that the scale of the y-axis varies depending on the gene. Comparisons of age were made in groups 1–4, duration of hormone in groups 3–6, and timing of hormone in groups 5–8. Oxt was unaffected by treatment, age, duration, or timing. For Avp the E6 group had significantly lower expression than the V6 and E3 group. Oxtr had a significant age effect and a significant timing effect; the V3/E3 group had higher expression than the E3/V3 and E6 groups. For Avpr1a, there was also a significant age effect in the 3-month treatment groups. White circles with black center dots indicate individual rat data, and data shown are mean ± SEM. *, p < 0.05.
3.2 Correlation results
Correlation analysis was used as a hypothesis-generating method to identify relationships among genes, and between genes and hormones, as well as a way of validating the PCR results.
3.2.1 Gene correlations within the PVN and SON
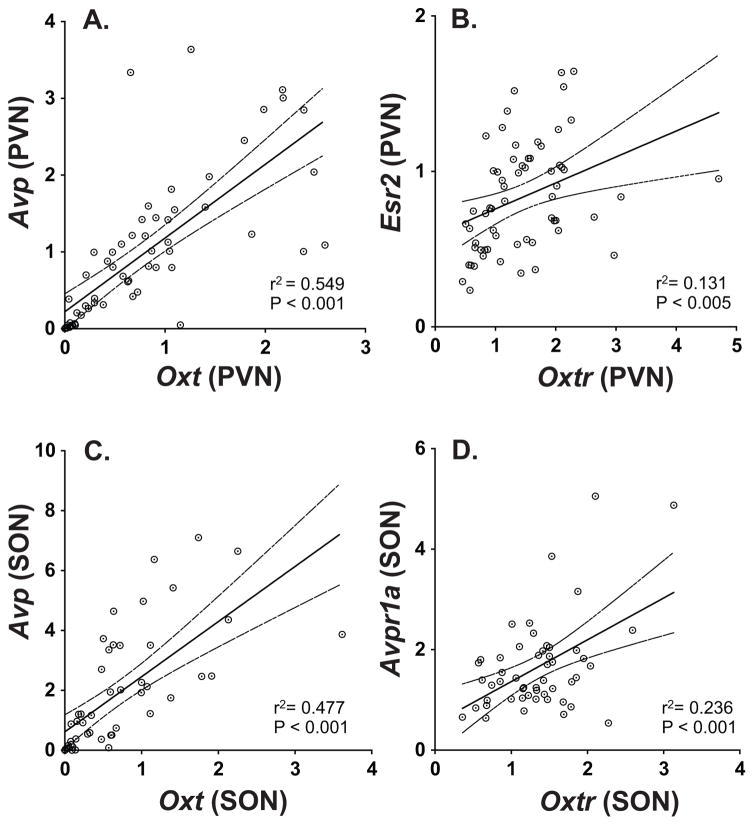
No genes in the PVN correlated significantly with any of the genes in the SON, but within each region there were positive correlations. Within the PVN, Oxt was significantly correlated with Avp (Figure 4A), and Oxtr was significantly correlated with Esr2 (Figure 4B). Within the SON, Oxt was significantly correlated with Avp (Figure 4C). In addition, Oxtr was significantly correlated with Avpr1a (Figure 4D). Detailed statistics for all of the gene correlations are provided in Supplemental Table S1.
Figure 4.
Correlations are shown between expression of genes. In the PVN, significant positive correlations were found for (A) Oxt and Avp, and for (B) Oxtr and Esr2. In the SON, positive correlations were found for (C) Oxt and Avp, and for (D) Oxtr and Avpr1a.
3.2.2 Hormone and gene expression correlations
Serum hormone concentrations and assay characteristics were reported in a companion paper (Yin et al., 2015) and we refer readers to that study for graphic presentation. As published, concentrations (means ± SEM) used for the correlations were as follows for groups 1–8. Estradiol (pg/ml): 1) 24 ± 3; 2) 105 ± 11; 3) 20 ± 1; 4) 63 ± 7; 5) 17 ± 2; 6) 63 ± 5; 7) 64 ± 6; 8) 20 ± 2. The estradiol treated groups all had significantly higher E2 concentrations than their matched vehicle groups. BDNF (ng/ml): 2) 4.8 ± 0.4; 2) 3.2 ± 0.3; 3) 3.4 ± 0.3; 4) 3.2 ± 0.4; 5) 4.4 ± 0.7; 6) 2.7 ± 0.3; 7) 2.6 ± 0.3; 8) 4.0 ± 0.6. The estradiol groups had lower BDNF concentrations than their matched vehicle groups, and there was a trend for an age-related decrease in BDNF. Corticosterone (ng/ml): 1) 36 ± 8; 2) 105 ± 21; 3) 37 ± 9; 4) 47 ± 11; 5) 53 ± 9; 6) 65 ± 8; 7) 59 ± 7; 8) 39 ± 8. Reproductively mature rats had significantly higher corticosterone concentrations in the E2 group compared to vehicle, but there were no such effects in the aging rats. Progesterone (ng/ml): 1) 0.7 ± 0.1; 2) 3.8 ± 1.2; 3) 1.1 ± 0.2; 4) 1.5 ± 0.2; 5) 1.4 ± 0.2; 6) 3.5 ± 0.5; 7) 3.3 ± 0.7; 8) 1.4 ± 0.4. The estradiol-treated groups had higher P4 than their matched vehicle groups.
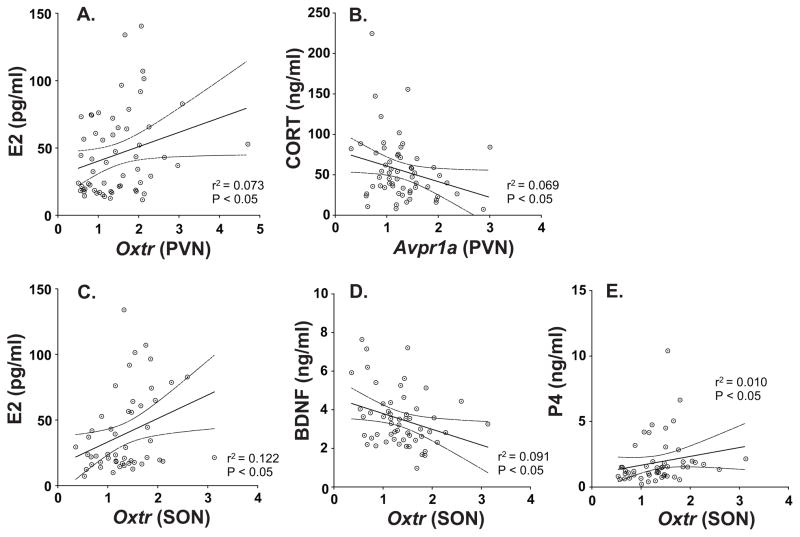
Two genes in the PVN were significantly correlated with serum hormones. Oxtr was positively correlated with serum estradiol concentrations (Figure 5A), and Avpr1a was negatively correlated with corticosterone (Figure 5B). In the SON, Oxtr was positively correlated with estradiol and progesterone, and negatively correlated with brain-derived neurotrophic factor (Figure 5C, D, E). Detailed statistics for these correlations can be found in Supplemental Table S2.
Figure 5.
Correlations are shown for genes and hormones. In the PVN a significant positive correlation was found for Oxtr and serum E2 (A), and a negative correlation for Avpr1a and corticosterone (B). In the SON, Oxtr positively correlated with serum E2 (C) and P4 (E), and negatively correlated with BDNF (D). CORT = corticosterone; P4 = progesterone.
4. Discussion
Since the publication of the results from the WHI, which suggested an increase in adverse health outcomes in women taking hormone therapy, the question of whether there is a critical window post-menopause during which the body and brain may respond positively to hormone replacement has become paramount (Rossouw et al., 2002; Anderson et al., 2004). This study utilized a pre-clinical rat model to determine how chronological age, and timing and duration of estrogen therapy, affected genes in the PVN and SON that, to our knowledge, have not systematically been studied in this context. Oxytocin and vasopressin are regulators of social behavior in a variety of animal species and humans [reviewed in (Young and Flanagan-Cato, 2012; Winslow et al., 1993; Caldwell al., 2008; Ebstein et al., 2012)] so understanding how they change, and estrogen regulation, are translationally relevant.
Our major finding was that it was the receptors for oxytocin and vasopressin, rather than the nonapeptides themselves, which were most sensitive to hormones and age. The presence and functional importance of these receptors in PVN and SON is far less well-studied than that of the peptides themselves, but the literature in general suggests that the proteins for these receptors are indeed expressed in PVN and SON of rats (Bealer et al., 2006; Ophir et al., 2013), and that there is co-expression of the vasopressin receptor 1a protein in vasopressinergic neurons in both of these regions (Hurbin et al., 2002). Of the nonapeptides oxytocin and vasopressin, the only effect we found was an interaction between treatment and duration for Avp in the SON. In rats oxytocin and vasopressin affect sexual (Arletti and Bertolini, 1985; Pedersen and Boccia, 2006), aggressive (Bosch and Neumann, 2012; Elkabir et al., 1990) and affiliative (Bosch and Neumann, 2012) behaviors, and also influence social interaction and memory (Dantzer et al., 1987; Lukas et al., 2011). Therefore, changes in expression of their receptors with age and estradiol treatment have potential functional implications and may relate to affective and social changes that can occur in some perimenopausal women (Schmidt and Rubinow, 2009).
4.1 Gene expression in the PVN – effects of estradiol and age
The current findings show age and hormone effects on gene expression in a region-specific manner. In the PVN, of the five genes measured, only the two-peptide receptors, Oxtr and Avpr1a, differed across the groups, each with a unique expression pattern. We were surprised not to see effects on of estradiol on Oxt, Avp and Esr2 based on the literature on such effects on mRNA and protein in rats (Patisaul et al., 1999; Suzuki and Handa, 2004; Akaishi and Sakuma, 1985; Patchev et al., 1995), and age-related effects of E2 on vasopressin protein in the human PVN (Ishunina and Swaab, 1999; Fliers et al., 1985). Our lack of age-related changes in Oxt or Esr2 is in line with previous research showing no E2 effect on mRNA levels in rats (Wilson et al., 2002; Yamaguchi-Shima and Yuri, 2007) or protein levels in humans (Ishunina and Swaab, 1999; Wierda et al., 1991). Differences are probably attributable to species as well as experimental design details, especially the relatively long-term E2 treatments and the specific ages of our animals in the current study.
4.1.1 Oxytocin receptor in PVN is upregulated by estradiol in 3-month treated MAT and AG rats
For Oxtr, mature and aging OVX rats given estradiol for 3 months had higher expression than their vehicle treated counterparts, when euthanized at ~7 or ~14 months, respectively. No differences were found in any of the 6-month treated groups, whether given constant hormone or vehicle, or switched after 3 months. There are several interpretations of these results, including the fact that the chronological age differences between the aging rats at 14 (groups 3 and 4) and 17 months (groups 5–8) may reflect a functional transition when Oxtr neurons no longer respond to estradiol. Alternatively or in addition, the longer hormone treatment paradigm in the 6-month treatment group (E6), the removal of estradiol at the 3-month mark (E3/V3 group), or the delay of estradiol treatment for 3 months (V3/E3 group) may represent some adaptation to the longer treatment period post-ovariectomy.
The interactions of estradiol and aging on Oxtr in the PVN have not been widely studied, and there are limited studies looking at these factors independently. Young and colleagues (1997) reported that short-term (4 day) E2 treatment after given one week after ovariectomy did not have a significant effect on Oxtr expression in the PVN. Another study found similar results in protein expression of oxytocin receptor in OVX female rats given E2 or E2+P starting at the time of OVX and continuing for a 16-day duration (Bealer et al., 2006). It is important to note that our treatment duration (3 or 6 months) is considerably longer than that of these published studies. Also, the current study demonstrated that estradiol treatment was only effective in increasing Oxtr when animals were earlier in the aging process (~14 mo) compared to older (~17 mo) rats. However, studies have found that estradiol increases oxytocin receptor protein expression in other areas of the hypothalamus such as the supraoptic nucleus, ventromedial nucleus and medial preoptic area of rats (Bealer et al., 2006; Tribollet et al., 1990; Patchev et al., 1993) as well as the medial amygdala and bed nucleus of the stria terminalis (Patchev et al., 1993; de Kloet et al., 1986). In a study previously published study utilizing the same rats as the current study we found the Oxtr is upregulated by E2 in the medial preoptic area (mPOA) (Yin et al., 2015).
The literature examining Oxtr in the context of aging is even more limited. There are no studies, to our knowledge, which have looked at the effects of aging on Oxtr in the PVN. However, there is one study that reported age-related increase in Oxtr mRNA in in the POA of aging female rats (Mobbs, 1994).
4.1.2 Vasopressin receptor in PVN is upregulated by age in 3-month treated MAT and AG rats
For Avpr1a expression in the PVN, we found an increase with age in the 3-month treated groups, but no further differences in any of the 6-month aging rats, suggesting that a plateau may be reached at 14 months of age. One interpretation of this is that the amount of Avpr1a is increasing as the animal ages, much as the AVP gene does in the human PVN (Ishunina and Swaab, 1999; Fliers et al., 1985; Swaab et al., 1985). The fact that we do not see a response to E2 in both MAT and AG rats could be due to this elevated baseline and not necessarily an impaired response to estrogen.
To our knowledge this is the first study to look at the effects of estradiol and aging on Avpr1a in the PVN, although it was studied during pregnancy in prairie voles (Ophir et al., 2013). In other areas of the hypothalamus Avpr1a is influenced by both age and/or E2 treatment. In a previous study published by our lab using the same animals as the current study we found that E2 down-regulated Avpr1a expression in all groups despite age, timing and duration (Yin et al., 2015). This same study also found an interaction between age and E2 treatment in the mPOA of MAT and AG 3-month groups (Yin et al., 2015). In addition, Kalamatianos and colleagues (2004) found that 4 days of estradiol treatment led to an increase in Avpr1a expression in the anteroventral periventricular nucleus of female rats. There is one study that has looked at the interaction of estradiol and aging in the preoptic area of the hypothalamus. Their results revealed that 4 days of E2 injections in young (2 months) and middle-aged (8 months) rats led to young estrogen-treated OVX rats having significantly higher Avpr1a mRNA than their untreated counterparts, but estrogen treatment did not increase expression in the middle-aged rats (Funabashi et al., 2000). However, they did find that the middle-aged OVX animals had significantly higher Avpr1a expression than young OVX rats (Funabashi et al., 2000).
4.2 Gene expression in the SON – effects of estradiol and age
In the SON, three out of the four genes measured, Avp, Oxtr and Avpr1a, but not Oxt, differed across the groups. The literature on whether or not estradiol has an effect on Oxt in the SON is mixed, with some finding an increase or decrease, as reported for mRNA levels in rats (Peter et al., 1990; Van Tol et al., 1988), and others finding no effects on protein levels in rats (Shughrue et al., 2002; Rhodes et al., 1981) and mice (Alves et al., 1998). One possible explanation for why we see changes in Avp but not in Oxt with E2 treatment is that co-expression of ERβ protein and mRNA is higher in vasopressin than oxytocin neurons of rats (Shughrue et al., 2002; Isgor et al., 2003) and mice (Alves et al., 1998). Double-labeling studies would be needed to test this hypothesis in our model.
In addition, Oxt expression was not affected by age, which in part is supported by past studies. In post-menopausal women oxytocin neuronal volume and cell numbers are not altered by age (Wierda et al., 1991; Ishunina and Swaab, 1999; Hoogendijk et al., 1985), which is in line with the current study, and suggesting that there is not higher activity of oxytocin neurons when steroid hormones are lost or replaced. However, we were surprised that age did not affect Avp, due to the findings in humans of an increased somatic size of vasopressin neurons, as well as increased numbers of these neurons in the SON, although these studies did not taken into account hormone replacement therapy (Ishunina and Swaab, 1999; Fliers et al., 1985; Hoogendijk et al., 1985). Yet, we did see age-related changes in both Oxtr and Avpr1a expression. It is possible that this is due to species differences, with age playing a larger role in regulating the receptors in rodents and the genes themselves in humans.
4.2.1 Oxytocin receptor in SON is regulated by age in 3-month treated MAT and AG rats and by timing of estradiol treatment in AG rats
Oxtr expression in the SON was affected by age, with mature animals having significantly less than their aged counterparts. In addition, the aged animals that had delayed estradiol treatment had significantly more Oxtr than the aged animals with 6 months of vehicle treatment and those who were deprived of treatment for 3 months prior to euthanasia. This demonstrates that even after being deprived of steroid hormones for 3 months, estradiol replacement is still effective in increasing Oxtr. The delayed treatment brought the aged animals back to the level similar to those who were euthanized at ~14 months. This is an indication that there is not a critical window for the effectiveness of estradiol on regulation of this gene in the SON.
The few studies published on the effects of estradiol on oxytocin receptor in the SON have reported mixed results. Young and colleagues (1997) found that there were no changes of Oxtr in the SON of rats during the different stages of the estrous cycle or during pregnancy. Yet, a study looking at the effects of E2 and E2+P treatment after OVX in female rats found that both treatments increased protein expression of oxytocin receptor in the SON compared to the control group (Bealer et al., 2006). The same study also showed that levels of the oxytocin receptor are increased during late gestation compared to early gestation and OVX control animals (Bealer et al., 2006). Similar results have been seen in pregnant prairie voles, with Oxtr being unregulated during late pregnancy, a time when steroid hormones such as estradiol are high (Ophir et al., 2013). Our results support the latter findings that estradiol does indeed play a role in the regulation of Oxtr in the SON. In regard to aging, Oxtr in the SON has not been studied. However, findings from other nuclei do indicate that aging does play a role in regulating oxytocin receptor mRNA and protein expression in the hypothalamus of the rat (Mobbs, 1994; Arsenijevic et al., 1995).
4.2.2 Vasopressin receptor in SON is regulated by age in 3-month treated MAT and AG rats
In addition to seeing age effects of Avpr1a in the PVN we also saw age-related changes in the SON. We found an increase with age in the 3-month treated groups, but no differences in any of the 6-month aging rats. As previously mentioned, this could be due to species difference, with age affecting Avpr1a expression in rats and Avp in humans. To our knowledge, this is the first study to examine the effects of age and estradiol on Avpr1a expression in the SON. Previous studies indicate both age and estradiol treatment affects other parts of the hypothalamus (Kalamatianos et al., 2004; Funabashi et al., 2000).
4.2.3 Vasopressin in SON is down regulated by estradiol in 6-month treated AG rats
The SON was the only region in which Avp changed, decreased by E2 in the 6-month treatment group. As the SON ages from 14 to 17 months the responsiveness to E2 treatment appears to change. Literature on the effects of estradiol on Avp is mixed, with some showing no effects and other showing increases. E2 did not alter vasopressin protein or gene expression in the SON of mice (Nomura et al., 2002); nor did it have an effect on mRNA in the monkey (Roy et al., 1999). However, a study looking at the effects of E2 post OVX in female mice found that 3 days of consecutive E2 treatment led to an increase in vasopressin protein in the SON (Grassi et al., 2010). E2 has also been shown to increase expression of Avp in the bed nucleus of the stria terminalis of rats (Han & De Vries, 2003), and decrease mRNA and protein in the medial amygdala and lateral septum of mice (Nomura et al., 2002).
Again, we were surprised to not see any age effects in Avp expression. As stated previously this may be due to species differences, because previous changes in expression have been found in humans but not rodents. In addition, the findings in humans of an increased somatic size of vasopressin neurons, as well as increased numbers of these neurons did not take into account hormone replacement therapy (Ishunina and Swaab, 1999; Fliers et al., 1985). The decrease in Avp in our aged rats but not our mature rats could be an indication that long-term estradiol treatment reverses the protective effects that accompany normal aging.
4.2.4 Correlation results and new hypotheses
We conducted the gene and hormone correlations as a way to generate future hypothesis based on the relationships among genes, and between genes and hormones. The Avp and Oxt genes were positively correlated with each other in both brain regions. More surprising was the significant positive correlation between Avpr1a and Oxtr in the SON; while relatively weak, it poses the possibility that these receptors are co-regulated by age and hormones. In fact, both Avpr1a and Oxtr were upregulated by age in the SON, consistent with this hypothesis. Correlations conducted between genes and hormones also identified positive and negative relationships. For example, there was a significant negative correlation between corticosterone and Avpr1a. There are studies showing that stress can increase corticosterone levels and Avp expression in the parvocellular neurons in the PVN (Pinnock & Herbert, 2001; Ma et al., 1997; Makino et al., 1995), but relationships between corticosterone and Avpr1a are not as well known and merit future research. Serum E2 correlated positively with Oxtr in both brain regions and suggests that the oxytocin receptor is an important target for menopause research.
4.3 Conclusions, Clinical Implications, and Limitations
The current study is the first to look at the affects of age, and timing and duration of estradiol on social behavior related genes in the PVN and SON of female rats. Results on Avpr1a and Oxtr show that most differences occurred in animals with shorter treatment durations and of younger chronological ages. There were exceptions though, as Avp and Oxtr in the SON showed some unexpected duration (Avp) and timing (Oxtr) effects. Estradiol’s ability to increase Oxtr even after the animal has been deprived of steroid hormones for 3-months and that timing did not affect any other genes is an indication that the “critical window” hypothesis is supported for specific genes (Avpr1a and Oxtr) but not others.
These findings could also have clinical implications for women taking hormone replacement therapy. Both Oxt and Avp have been implicated in regulating a variety of behaviors such as anxiety and depression, in mice (de Kloet et al., 2005), rats (Klenerova et al., 2009), humans (Meyer-Lindenberg et al., 2011), and voles (Lim and Young, 2006). Oxtr and Avpr1a knockout mice show aberrant social and emotional behaviors, including increasesd aggression, stress and deficits in social memory (Bielsky et al., 2004; Sala et al., 2011; Takayanagi et al., 2005). The decrease of Oxtr that is experienced when estradiol levels drop may contribute to these behavioral changes. However, whereas shorter-term treatment with E2 causes an increase in expression of some genes, long-term treatment did not have any effects on any of the genes except Avp, which was down-regulated. According to the “critical window” hypothesis there is a point in which hormone replacement therapy because more detrimental than it is helpful. This decrease in expression of Avp may be indicative of such a negative effects of long-term treatment.
There were several limitations to the current study. We were not able to include a delayed treatment group in our MAT animals due to the fact that they would have been entering reproductive senescence, which would have introduced a confound to our study. There were also technical pitfalls: we were only able to obtain enough RNA to assay 5 genes in PVN and 4 in SON. In addition, while the study was adequately powered to detect significant group differences, sample sizes for some of the genes were small. It is also notable that there is quite a bit of individual variability of gene expression within groups. This is not surprising considering the complex experimental model and the inevitable differences between outbred rats of strains such as Sprague-Dawley. This may be an advantage though from a clinical perspective, as humans are not inbred and symptoms and other characteristics of menopause vary enormously from woman to woman.
There are several areas that merit future investigation to enhance the clinical relevance of the work. Here, we only tested continuous E2 and we did not replace progesterone, though women with a uterus may use cyclic regimes of hormones that include P4. Including P4 treatments in our study would have doubled the number of animals needed, thus we decided to focus on addressing and reevaluating the limitations of the WHI estrogen-alone trials. Currently, we are determining direct relationships between the timing and duration of E2 treatment on neurobehavioral outcomes of animals that are behaviorally characterized. This will give us a better understanding of links between gene regulation and behavior, to inform treatment options for menopausal women.
Supplementary Material
Table S1: P-values are shown for the gene correlations, all of which were positive. In the PVN, significant correlations were found for Oxt and Avp, and for Oxtr and Esr2. In the SON, correlations were found for Oxt and Avp, and for Oxtr and Avpr1a. All significant p values are indicated with an asterisk.
Table S2: P-values for correlations between the hormone assays and the target genes in both the PVN and SON. In the PVN, a significant negative correlation was found for Avpr1a and corticosterone, and a positive correlation for Oxtr and serum E2. In the SON, Oxtr correlated positively with serum E2 and P4, and negatively with BDNF. All significant p values are indicated with an asterisk.
Vasopressin receptor gene expression increased with age in PVN and SON.
Oxytocin receptor gene expression increased with shorter-term E2 in the PVN.
In SON, oxytocin receptor gene had complex age and hormone regulation.
The oxytocin gene was unaffected by age or estradiol in PVN and SON.
The vasopressin gene had complex timing/duration effects of E2 in the SON.
Acknowledgments
We thank Kelsey Bezner and Mercedes Munselle for excellent technical assistance. Work was supported by funding from the NIH AG16765 (ACG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akaishi T, Sakuma Y. Estrogen excited oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Research. 1985;335(2):302–305. doi: 10.1016/0006-8993(85)90481-0. [DOI] [PubMed] [Google Scholar]
- 2.Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Waddertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. The Journal of the American Medical Association. 2004;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 4.Arletti R, Bertolini A. Oxytocin stimulated lordosis behavior in female rats. Neuropeptides. 1985;6(3):247–253. doi: 10.1016/0143-4179(85)90095-2. [DOI] [PubMed] [Google Scholar]
- 5.Arsenijevic Y, Dreifuss JJ, Vallet P, Marguerat A, Tribollet E. Reduced binding of oxytocin in the rat brain during aging. Brain Research. 1995;698(1–2):275–279. doi: 10.1016/0006-8993(95)01020-v. [DOI] [PubMed] [Google Scholar]
- 6.Bealer SL, Lipschitz DL, Ramoz G, Crowley WR. Oxytocin receptor binding in the hypothalamus during gestation in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291(1):R53–R58. doi: 10.1152/ajpregu.00766.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 8.Bhupathiraju SN, Manson JE. Menopausal hormone therapy and chronic disease risk in the Women’s Health Initiative: Is timing everything? Endocrine Practice. 2014;20(11):1201–1213. doi: 10.4158/EP14205.RA. [DOI] [PubMed] [Google Scholar]
- 9.Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior. 2012;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Burbach JPH, Liu B, Voorhuis TAM, Huber HM, Tol V. Diurnal variation in vasopressin and oxytocin messenger RNAs in hypothalamic nuclei of the rat. Molecular Brain Research. 1988;464(2):157–160. doi: 10.1016/0169-328X(88)90007-1. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell HK, Lee HJ, Macbeth AH, Young WS. Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91(3):363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- 13.Deeks AA, McCabe MP. Well-being and menopause: An investigation of purpose in life, self-acceptance and social role in premenopausal, perimenopausal, and postmenopausal women. Quality of Life Research. 2004;13(2):389–398. doi: 10.1023/B:QURE.0000018506.33706.05. [DOI] [PubMed] [Google Scholar]
- 14.De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 15.De Kloet ER, Voorhuis DA, Boschma Y, Elands J. Estradiol modulates density of putative “oxytocin receptors” in discrete rat brain regions. Neuroendocrinology. 1986;44(4):415–421. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 16.Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior. 2012;61(3):359–379. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Elkabir DR, Wyatt ME, Vellucci SV, Herbert J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behavior in the rat. Regulatory Peptides. 1990;28(2):199–214. doi: 10.1016/0167-0115(90)90018-R. [DOI] [PubMed] [Google Scholar]
- 18.Fliers E, Swaab DF, Pool CW, Verwer RW. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Research. 1985;342(1):45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- 19.Funabashi T, Shinohara K, Mitsushima D, Kimura F. Estrogen increases arginine-vasopressin V1a receptor mRNA in the preoptic area of young but not of middle-aged female rats. Neuroscience Letters. 2000;285(3):205–208. doi: 10.1016/S0304-3940(00)01069-7. [DOI] [PubMed] [Google Scholar]
- 20.Gouzènes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V(1a)- and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. Journal of Physiology. 1999;517(3):771–779. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi D, Amorim AM, Garcia-Segura LM, Panzica G. Estrogen receptor alpha is involved in the estrogenic regulation of arginine vasopressin immunoreactivity in the supraoptic and paraventricular nuclei of ovariectomized rats. Neuroscience Letters. 2010;474(3):135–139. doi: 10.1016/j.neulet.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. Journal of Neurobiology. 2003;54(3):502–510. doi: 10.1002/neu.10157. [DOI] [PubMed] [Google Scholar]
- 23.Hoogendijk JE, Fliers E, Swaab DF, Verwer RWH. Activation of vasopressin neurons in the human supraoptic and paraventricular nucleus in senescence and senile dementia. Journal of the Neurological Sciences. 1985;69(3):291–299. doi: 10.1016/0022-510X(85)90141-8. [DOI] [PubMed] [Google Scholar]
- 24.Hrabovszky E, Kalló I, Hajszán T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139(5):2600–2604. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 25.Hurbin A, Boissin-Agasse L, Orcel H, Rabié A, Joux N, Desarménien MG, Richard P, Moos FC. The V1a and V1b, but not V2, vasopressin receptor genes are expressed in the supraoptic nucleus of the rat hypothalamus, and the transcripts are essentially colocalized in the vasopressinergic magnocellular neurons. Endocrinology. 1998;139(11):4701–4707. doi: 10.1210/endo.139.11.6320. [DOI] [PubMed] [Google Scholar]
- 26.Hurbin A, Orcel H, Alonso G, Moos F, Rabíe A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143(2):456–466. doi: 10.1210/en.143.2.456. [DOI] [PubMed] [Google Scholar]
- 27.Isgor C, Shieh KR, Akil H, Watson SJ. Colocalization of estrogen beta-receptor messenger RNA with orphanin FQ, vasopressin and oxytocin in the rat hypothalamic paraventricular and supraoptic nuclei. Anatomy and Embryology. 2003;206(6):461–469. doi: 10.1007/s00429-003-0314-9. [DOI] [PubMed] [Google Scholar]
- 28.Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab. 1999;84(12):4637–4644. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- 29.Kalamatianos T, Kalló I, Goubillon ML, Coen CW. Cellular expression of V1a vasopressin receptor mRNA in the female rat preoptic area: Effects of oestrogen. Journal of Neuroendocrinology. 2004;16(6):525–533. doi: 10.1111/j.1365-2826.2004.01199.x. [DOI] [PubMed] [Google Scholar]
- 30.Klaiber EL, Vogel W, Rako S. A critique of the Women’s Health Initiative hormone therapy study. Fertility and Sterility. 2005;84(6):1589–1601. doi: 10.1016/j.fertnstert.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulatory effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. Journal of Physiology and Pharmacology. 2009;60(2):57–62. [PubMed] [Google Scholar]
- 32.Krieg WJS. The hypothalamus of the albino rat. Journal of Comparative Neurology. 1932;55(1):19–89. doi: 10.1002/cne.900550104. [DOI] [Google Scholar]
- 33.Lanza di Scalea T, Matthews KA, Avis NE, Thurston RC, Brown C, Harlow S, Bromberger JT. Role stress, role reward and mental health in a multiethnic sample of midlife women: Results from the study of Woman’s Health Across the Nation (SWAN) Journal of Women’s Health. 2012;21(5):481–489. doi: 10.1089/jwh.2011.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and Behavior. 2006;50(4):506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36(11):2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma XM, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: A study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138(10):4351–4357. doi: 10.1210/en.138.10.4351. [DOI] [PubMed] [Google Scholar]
- 37.Makino S, Smith M, Gold P. Corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: Association. Endocrinology. 1995;136(8):3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended potstopping phase of the Women’s Health Initiative randomized trials. The Journal of the American Medical Association. 2013;310(13):1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 40.Mobbs CV. Molecular hysteresis: Residual effects of hormones and glucose on genes during aging. Neurobiology of Aging. 1994;15:523–534. doi: 10.1016/0197-4580(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 41.Nomura M, McKenna E, Korach KS, Pfaff DW, Ogawa S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Research Molecular Brain Research. 2002;109(1–2):84–94. doi: 10.1016/S0169-328X(02)00525-9. [DOI] [PubMed] [Google Scholar]
- 42.Ophir AG, Sorochman G, Evans BL, Prounis GS. Stability and dynamics of forebrain vasopressin receptor and oxytocin receptor during pregnancy in prairie voles. Journal of Neuroendocrinology. 2013;25(8):719–728. doi: 10.1111/jne.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. The Journal of the Federation of American Societies for Experimental Biology. 1995;9(5):419–423. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- 44.Patchev VK, Schlosser SF, Hassan AH, Almeida OF. Oxytocin binding sites in rat limbic and hypothalamic structures: Site-specific modulation by adrenal and gonadal steroids. Neuroscience. 1993;57(3):537–543. doi: 10.1016/0306-4522(93)90003-x. [DOI] [PubMed] [Google Scholar]
- 45.Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. Journal of Neuroendocrinology. 2003;15(16):787–793. doi: 10.1046/j.1365-2826.2003.01061. [DOI] [PubMed] [Google Scholar]
- 46.Patisaul HB, Whitten PL, Young LJ. Regulation of estrogen receptor beta mRNA in the brain: Opposite effects of 17β-estradiol and the phytoestrogen, coumestrol. Molecular Brain Research. 1999;67(1):165–171. doi: 10.1016/S0169-328X(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates: Compact sixth edition. San Diego, CA: Elsevier Inc; 2009. [Google Scholar]
- 48.Pedersen CA, Boccia ML. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience. 2006;139(3):843–851. doi: 10.1016/j.neuroscience.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Peter J, Burbach H, Adan RA, Tol HH, Verbeeck MA, Axelson JF, Leeuwen FW, Beekman JM, Ab G. Regulation of the rat oxytocin gene by estradiol. Journal of Neuroendocrinology. 1990;2(5):633–639. doi: 10.1111/j.1365-2826.1990.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 50.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinnock SB, Herbert J. Corticosterone, differentially modulated expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. The European Journal of Neuroscience. 2001;13(3):576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinn R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition. 2005;21(6):775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Rhodes CH, Morrell JI, Pfaff DW. Changes in oxytocin content in the magnocellular neurons of the rat hypothalamus following water deprivation or estrogen treatment. Quantitative immunohistological studies. Cell and Tissue Research. 1981;216(1):47–55. doi: 10.1007/BF00234544. [DOI] [PubMed] [Google Scholar]
- 54.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risk and benefits of estrogen plus progestin in healthy postmenopausal women: Principle results form the Women’s Health Initiative randomized controlled trial. The Journal of the American Medical Association. 2002;288(3):321–333. doi: 10.1001/jama.288.3.366. [DOI] [PubMed] [Google Scholar]
- 55.Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140(5):2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- 56.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biological Psychiatry. 2011;69(9):875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Annals of the New York Academy of Sciences. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 59.Sengupta P. The laboratory rat: Relating its age with human’s. International Journal of Preventive Medicine. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 60.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-β. Progress in Brain Research. 2002;139:15–29. doi: 10.1016/S0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: Differential effects of dexamethasone and estradiol. Endocrinology. 2004;145(8):3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 62.Swaab DF, Fliers E, Fisser B. The vasopressin containing neurons in the human brain; changes during ageing and senile dementia. British Journal of Clinical Practice. 1985;39:7–10. [PubMed] [Google Scholar]
- 63.Swanson LW, Sawchenko PE. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annual Review of Neuroscience. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 64.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials. 1998;19(1):61–109. doi: 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 66.Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Research. 1990;511(1):129–140. doi: 10.1016/0006-8993(90)90232-Z. [DOI] [PubMed] [Google Scholar]
- 67.Uguz F, Sahingoz M, Gezhinc K, Ayhan MG. Quality of life in postmenopausal women: The impact of depressive and anxiety disorders. The International Journal of Psychiatry in Medicine. 2011;41(3):281–292. doi: 10.2190/PM.41.3.g. [DOI] [PubMed] [Google Scholar]
- 68.Uhl GR, Reppert SM. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986;232(4748):390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- 69.Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypopshyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122(3):945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- 70.Wierda M, Goudsmit E, Van Der Woude PF, Purba JS, Hofman MA, Bogte H, Swaab DF. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiology of Aging. 1991;12(5):511–516. doi: 10.1016/0197-4580(91)90081-T. [DOI] [PubMed] [Google Scholar]
- 71.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mechanisms of Ageing and Development. 2002;123(6):593–601. doi: 10.1016/S0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 72.Winslow JT, Hastings N, Cater CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 73.Winslow JT, Insel TR. Neuroendocrine basis of social recognition. Current Opinion in Neurobiology. 2004;14(2):248–53. doi: 10.1016/j.conb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Research. 2007;1155(1):34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 75.Yin W, Maguire SM, Pham B, Garcia AN, Dang N, Liang J, Wolfe A, Hofmann HA, Gore AC. Testing the critical window hypothesis of timing and duration of estradiol treatment on hypothalamic gene networks in reproductively mature and aging female rats. Endocrinology. 2015;156(8):2918–2933. doi: 10.1210/en.2015-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshimura R, Kiyama H, Kimura T, Araki T, Maeno H, Tanizawa O, Tohyama M. Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology. 1993;133(3):1239–1246. doi: 10.1210/endo.133.3.8396014. [DOI] [PubMed] [Google Scholar]
- 77.Young LJ. Oxytocin and vasopressin receptors and species-typical social behaviors. Hormones and Behavior. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- 78.Young LJ, Flanagan-Cato LM. Editorial comment: oxytocin, vasopressin and social behavior. Hormones and Behavior. 2012;61(3):227–229. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. Journal of Neuroendocrinology. 1997;9(11):859–865. doi: 10.1046/j.1365-2826.1997.00654.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: P-values are shown for the gene correlations, all of which were positive. In the PVN, significant correlations were found for Oxt and Avp, and for Oxtr and Esr2. In the SON, correlations were found for Oxt and Avp, and for Oxtr and Avpr1a. All significant p values are indicated with an asterisk.
Table S2: P-values for correlations between the hormone assays and the target genes in both the PVN and SON. In the PVN, a significant negative correlation was found for Avpr1a and corticosterone, and a positive correlation for Oxtr and serum E2. In the SON, Oxtr correlated positively with serum E2 and P4, and negatively with BDNF. All significant p values are indicated with an asterisk.