Abstract
Stress responses entail neuroendocrine, autonomic, and behavioral changes to promote effective coping with real or perceived threats to one’s safety. While these responses are critical for the survival of the individual, adverse effects of repeated exposure to stress are widely known to have deleterious effects on health. Thus, a considerable effort in the search for treatments to stress-related CNS disorders necessitates unraveling the brain mechanisms responsible for adaptation under acute conditions and their perturbations following chronic stress exposure. This paper is based upon a symposium from the 2014 International Behavioral Neuroscience Meeting, summarizing some recent advances in understanding the effects of stress on adaptive and maladaptive responses subserved by limbic forebrain networks. An important theme highlighted in this review is that the same networks mediating neuroendocrine, autonomic, and behavioral processes during adaptive coping also comprise targets of the effects of repeated stress exposure in the development of maladaptive states. Where possible, reference is made to the similarity of neurobiological substrates and effects observed following repeated exposure to stress in laboratory animals and the clinical features of stress-related disorders in humans.
INTRODUCTION
One of the major limitations in stress research related to disease etiology is the difficulty in distinguishing between what are considered as adaptive versus maladaptive coping mechanisms. Characterization of each of these can be a matter of perspective and context (Herman, 2013). Stress responses promote survival by helping organisms meet the demands of a variety of acute challenges in the short-term (Huether, 1996; Levine and Ursin, 1991; McEwen, 2004; Ursin and Olff, 1993), yet they are also linked with impaired functioning and the development of pathology under repeated activation or extended conditions (Chrousos, 2000; Mann, 1999; McEwen, 2000; Pasternac and Talajic, 1991; Paykel, 1976; Shively et al., 2009; Vanitallie, 2002). One popular formulation of maladaptation is allostatic load, and refers to the cumulative effects or "costs" generated following repeated stress exposure on multiple physiologic systems (Brindley and Rolland, 1989; McEwen and Stellar, 1993). In a similar vein, stress responses can also be thought of as representative of the recruitment of a global response systems (i.e., neuroendocrine, autonomic, and behavioral) to any threat, real or perceived, that overwhelms selective homeostatic systems (Day, 2005). In this framework, the prolonged induction of stress responses in the face of repeated challenges are unable to prevent the adverse consequences associated with the breakdown of specific homeostatic systems. Over time, the cumulative effects of chronic stress are linked to a variety of adverse health consequences, such as hypertension, atherosclerosis, metabolic syndrome, diabetes, infertility, immunosuppression, osteoporosis, psychopathologies, and even neurodegenerative diseases.
The physiological response to stress entails activation of sympathoadrenal, (i.e., “fight-or-flight”) and hypothalamo-pituitary-adrenal (HPA) systems. Over the years, glucocorticoids (cortisol in primates, corticosterone in rodents), the end-products of HPA axis output, have taken center stage as primary mediators of stress-related disorders (Herman, 2013; McEwen, 2004). Under acute conditions, glucocorticoids redirect metabolism for increased energy demands, bias the response properties of other physiological systems, and promote cognitive adjustments associated with adaptive responses to environmentally salient experiences. Importantly, in the aftermath of a stressful experience, adrenocortical hormones help to restore HPA functioning to pre-stress levels via receptor mediated negative feedback. Nevertheless, heightened or prolonged glucocorticoid levels such as during chronic stress exposure are known to be predictive of, and contribute to, the development of various disease states (Aguilera, 1994; Post, 1992; Post and Weiss, 1998; Ursin, 2014).
Differential responsiveness of stress systems are also influenced by early-life experience, hereditary factors, and stress history, and have been shown to predict susceptibility to neuropsychiatric and somatic diseases in humans (Biondi and Picardi, 1999; Claessens et al., 2011; Dickerson and Kemeny, 2004; McEwen and Stellar, 1993; Reiss et al., 2013). Robust sex differences in the prevalence of stress-related psychiatric disorders highlight the role of gonadal steroids in differentially altering stress responses and susceptibility under repeated conditions (Kessler, 2003; Kessler et al., 1993; Kessler et al., 1995; Ter Horst et al., 2009; Weissman et al., 1993). Taken together, elucidating the neural mechanisms of stress responses, their alterations under chronic conditions, individual or population differences influencing these processes, and how these factors influence susceptibility for psychiatric illness, has been a dauntingly complex undertaking for stress neurobiologists. Nevertheless, significant progress has been made in laying the groundwork for understanding these issues, and this review will summarize the current state of knowledge and some recent developments from a basic research perspective.
STRESS AND LIMBIC NETWORK PLASTICITY
Stress triggers autonomic, endocrine, and behavioral responses regulated by multiple brain circuits and neurochemical systems (Cullinan et al., 1995; Dallman, 2000; Dayas et al., 2001; Herman and Cullinan, 1997; Herman et al., 2003; Joels et al., 2012; Li and Sawchenko, 1998; Radley and Sawchenko, 2011). Whereas, physiological stressors involve more targeted challenges that overwhelm selective homeostatic systems, such as hemorrhage, hypoxia, or immunogenic stimuli, emotional (a.k.a., psychogenic, psychological, processive) stressors require interpretation by exteroceptive sensory modalities and integration with distinct cognitive (comparison with past experience) and affective (positive or negative valence) information processing systems in the brain (Dayas et al., 2001; Herman and Cullinan, 1997; Sawchenko et al., 2000). Commonly employed animal models of emotional stress are restraint, footshock, noise, and social defeat. Whereas each class of stressor enlists brainstem and hypothalamic effectors for activation of the sympathoadrenal and HPA axis output, emotional stressors manifest widespread activation in the limbic forebrain, and correspond to a broad array of behavioral changes (e.g., vigilance, fear) that help to facilitate adaptive coping as required by the specific environmental demand (Campeau and Watson, 1997; Cullinan et al., 1995; Dayas et al., 2001; Li and Sawchenko, 1998). Emotional stressors are further distinguished by their capacity to induce long-term alterations in limbic forebrain networks that are associated with cognitive and affective symptoms in stress-related disorders.
The HPA axis is often regarded as the canonical stress response system (Munck et al., 1984; Selye, 1980). Early investigations into glucocorticoid functions arose from observations that their receptors are widely expressed and distributed in the entire body and brain, and are especially enriched in many of the limbic forebrain networks involved in the genesis of stress responses (Gerlach and McEwen, 1972; McEwen et al., 1986; McEwen et al., 1968; Sapolsky et al., 1983b). Evidence from animal studies and post mortem brains of patients that suffered from mood disorders show decreases in density and/or expression levels of corticosteroid receptors throughout the limbic forebrain (Alt et al., 2010; de Kloet, 1987; Furay et al., 2008; Klok et al., 2011; Matsubara et al., 2006; Patel et al., 2008; Perlman et al., 2004; Sapolsky et al., 1983a; Sapolsky et al., 1984b). This diminished sensitivity to glucocorticoids is likely to impair the capacity of these networks to optimally respond to subsequent challenges for promoting cognitive and affective adjustments for adaptation. Alterations in the forebrain distribution of this receptor population also disrupts negative feedback regulation of HPA axis (Dallman and Jones, 1973; de Kloet, 1987; Keller-Wood and Dallman, 1984; Sapolsky et al., 1984a), and underlies HPA axis abnormalities in major depression and post-traumatic stress disorder (PTSD; (de Kloet et al., 2005; Holsboer, 2000; Mason et al., 1986; Mathew et al., 2008; Pariante and Miller, 2001; Pariante et al., 1995; Strohle and Holsboer, 2003; Yehuda, 2002)). Below, we shall highlight the involvement of several key forebrain regions - amygdala, hippocampus, and prefrontal cortex that are implicated in the modulation of stress responses, with specific reference to how neuroplasticity in these networks may contribute to maladaptive changes.
Amygdala
The amygdaloid complex is implicated in a wide array of behavioral, autonomic, and neuroendocrine responses relevant to stress, such as aversive learning, associative fear conditioning, and anxiety (Allen and Allen, 1974a; Davis, 1992; LeDoux, 2000; Loewy, 1991; McGaugh, 2002; Ressler, 2010; Shekhar et al., 2003). Information flow through the amygdala is generally considered to begin by afferent input into the lateral amygdala, and through several intrinsic pathways in the basolateral complex (BLA) before relaying to central nucleus (CeA), which provides the principal source of extrinsic connections with behavioral, autonomic and endocrine effector systems (Pitkänen and Amaral, 1998; Pitkänen et al., 1997; Pitkanen et al., 1995). The amygdala exhibits the capacity to facilitate most of these functions under both acute and chronic stress conditions, and in some instances may even show augmentation or sensitization following repeated stress exposure. These experimental data are consistent with human imaging studies showing increased amygdaloid volume (Frodl et al., 2002; Lange and Irle, 2004; Lupien et al., 2011; Shin and Liberzon, 2010; Weber et al., 2013) and functional activity (Breiter et al., 1996; Drevets et al., 1992; Siegle et al., 2002; Thomas et al., 2001) in individuals suffering from depression or anxiety disorders, and suggest that its dysfunction or hyperactivity may be a key contributor in the pathogenesis of anxiety disorders, PTSD, and related symptomatologies in other types of mental illnesses.
Under acute conditions, stress-induced HPA activation is generally facilitated or attenuated by CeA stimulation or lesions, respectively (Allen and Allen, 1974b; Beaulieu et al., 1986; Carter et al., 2004; Prewitt and Herman, 1994, 1997; Van de Kar et al., 1991; Prewitt and Herman, 1994; cf. Prewitt et al., 1997; Carter et al., 2004). The circuitry accounting for how stress-stimulatory influences from the amygdala reach HPA-effector neurons in the paraventricular hypothalamic nucleus (PVH) remains to be tested, it likely involves disynaptic relays via components of the bed nuclei of the stria terminalis (Sun et al., 1991; Tsubouchi et al., 2007). Amygdala activation following acute stress exposure is also critically important for memory consolidation (Roozendaal and McGaugh, 1997; Wolff et al., 2014), and may be enhanced by a variety of neurochemicals, most notably corticosteroids and brainstem aminergic inputs into BLA (McGaugh, 2002; Roozendaal et al., 2009; Roozendaal and McGaugh, 2011). These effects are produced via widespread activation of the limbic forebrain brought about by the extensive and divergent projection systems emanating from BLA, although the specific neural circuits likely depend on the particular type of learning or memory systems undergoing modulation (McGaugh, 2006).
Evidence from in vitro and in vivo physiological studies suggests that amygdala responses following acute stressors are also excitatory, although some studies suggest inhibitory effects (Buffalari and Grace, 2007; Chen and Sara, 2007; Correll et al., 2005; Garcia et al., 1998; Kavushansky and Richter-Levin, 2006; Kavushansky et al., 2006; Pelletier et al., 2005; Shors, 1999; Vouimba et al., 2006; Vouimba et al., 2004). Direct manipulations of corticosteroid or noradrenergic receptors within BLA mimics some of the excitatory effects observed in acute stress studies, via the induction of high-voltage activated calcium currents (Karst et al., 2002; Liebmann et al., 2008), reducing GABAA receptor mediated currents (Duvarci and Pare, 2007) and increasing the amplitude and frequency of AMPA receptor-mediated responses (Karst et al., 2010; Liebmann et al., 2009). Nevertheless, contrasting patterns of amygdala activation is likely accounted for by differences in the recording methodologies and the fact that there is a substantial degree of intrinsic processing within this region. For instance, the application of optogenetic techniques to this problem have generally verified that activation of BLA promotes anxiety-like responses in rodents, whereas stimulation of BLA-projecting inputs into CeA were observed to reduce these behaviors (Tye et al., 2011). Moreover, both BLA and CeA contain heterogeneous intrinsic subpopulations of interneurons that differentially modulate adaptive behavioral responses (Ciocchi et al., 2010; Haubensak et al., 2010; Wolff et al., 2014). Collectively, these studies highlight the fact that amygdala activation of autonomic, endocrine, and behavioral outputs are mediated via intrinsic disinhibitory mechanisms within BLA and CeA.
The amygdala plays a similar role in modulating these same response systems following repeated stress exposure, albeit typically to a greater degree than under acute conditions. Many of these sensitizing actions can be understood in terms of the apparent anabolic effects produced by repeated stress exposure on amygdala plasticity and activity. Interestingly, these anabolic-like effects of amygdala functioning have yet to be reconciled with the fact that the increased metabolic demands associated with prolonged glucocorticoid exposure are mostly catabolic in nature. Increases in intrinsic BLA neural electrotonic properties (larger membrane resistance and time constants) and excitability have been observed in vivo following repeated restraint stress (Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012). Enhanced excitatory synaptic drive into BLA principal neurons has also been reported after repeated restraint (Padival et al., 2013), which is simultaneously associated with reductions in tonic GABAergic control (Liu et al., 2014; Suvrathan et al., 2014) and increased expression and activation of NMDA receptors (Mozhui et al., 2010; Suvrathan et al., 2014). Indeed, whereas amygdala excitability under acute conditions involves a larger AMPA receptor-mediated component (Karst et al., 2010; Liebmann et al., 2009), sustained activity following chronic stress appears more closely associated with NMDA receptor functioning (Mozhui et al., 2010; Suvrathan et al., 2014).
Studies examining the effects of chronic stress on structural plasticity in the amygdala have identified substantial increases in excitatory synaptic input into BLA principal neurons. Repeated restraint stress in rats increased both dendritic branching and spine density in BLA pyramidal-like neurons (Mitra et al., 2005; Vyas et al., 2002). This neuronal hypertrophy was observed to correspond specifically with a repeated stress regimen resulting in elevated plasma levels of glucocorticoids (Vyas et al., 2002), and subsequent studies have shown that adrenal steroid administration also increases dendritic spine density in BLA neurons (Mitra and Sapolsky, 2008). In fact, increases in spine density in amygdala neurons, whether induced by stress or glucocorticoids as in the aforementioned studies, or as a preexisting genetic condition (Adamec et al., 2012), are each linked with increased anxiety-like behaviors. In addition to glucocorticoids, tissue plasminogen activator enzyme, corticotropin-releasing hormone (CRH) and brain derived neurotrophic factor have also been implicated in stress-related structural plasticity in the amygdala, and recent evidence suggests that epigenetic mechanisms may mediated their induction (for reviews, see (Bennett and Lagopoulos, 2014; Gray et al., 2013; Maddox et al., 2013; McEwen et al., 2012). Differential susceptibility of mice to display anxiety-like behaviors has also been linked to epigenetic marks on the CRHR1 receptor gene promoter region (Sotnikov et al., 2014).
Collectively, the evidence suggest that durable increases in amygdala plasticity may be initiated by, and maintained long after the abatement of stress exposure. These studies help to envisage how structural and functional alterations in the amygdala could play an important role in the sequelae of events initiated by stress, that, while adaptive for survival (i.e., heightened vigilance and arousal, enhancement of aversive memories to harmful stimuli and contexts), may also lead to anxiety and states of hypervigilance that are maladaptive.
Hippocampal Formation
Over the past several decades, the hippocampus has been the quintessential limbic region implicated in stress regulation. Attention was first directed to this, when autoradiographic evidence revealed prominent steroid binding in hippocampal cell layers, relative to other limbic regions (McEwen, 1994; McEwen et al., 1969). Since then, numerous studies have shown that the hippocampal formation has the capacity to inhibit the HPA axis under both basal and stress conditions, is a prominent site for glucocorticoid receptor-mediated feedback, and is essential for restoring glucocorticoids to baseline levels following the cessation of stress ((Herman et al., 1992; Herman, 1992; Herman et al., 1995b; Herman et al., 1989; Jacobson and Sapolsky, 1991; Sapolsky et al., 1984a) cf.(Tuvnes et al., 2003)). The circuitry accounting for how hippocampal influences are regulated over HPA effectors in PVH has been anatomically characterized (Cullinan et al., 1993), and functionally, is relayed in part via extrinsic projections from the ventral subiculum that project to GABAergic neurons in the anterior division of the bed nucleus of the stria terminalis, and in turn, to PVH (Radley and Sawchenko, 2011)(see Box 1).
Both acute and repeated stress are associated with the disruption of hippocampal memory functions in many species (Conrad, 2008; Conrad et al., 1996; Kim and Diamond, 2002; Luine et al., 1994; McLaughlin et al., 2007; Sousa et al., 2000; Venero et al., 2002), and can be recapitulated by increasing glucocorticoid levels (Bodnoff et al., 1995; Dachir et al., 1993; Luine et al., 1993; McLay et al., 1998). By contrast, there is also evidence that hippocampal-dependent functions may be promoted by acute stress exposure (McGaugh, 2006; Shors and Servatius, 1997; Shors et al., 1992), although these examples tend to involve emotionally salient forms of learning that have a strong amygdala component associated with them. Rapid effects of glucocorticoid on physiological plasticity are relayed via non-genomic steroid receptors, that are probably localized on or near post-synaptic membranes in hippocampal neurons (Joels et al., 2012; Johnson et al., 2005; Karst et al., 2005). Although the precise mechanisms have yet to be clarified (for review, see (Groeneweg et al., 2011)), glucocorticoids may rapidly alter neuronal excitability to enhance responsiveness to contextually relevant information. During chronic stress, long-term increases in glucocorticoids impair both structural and functional plasticity in the hippocampus (Conrad, 2008; Herman et al., 1995a; McEwen, 2001, 2004; McLaughlin et al., 2009; Mizoguchi et al., 2003; Roozendaal and McGaugh, 2011; Sapolsky et al., 1985; Vaher et al., 1994; Vyas et al., 2002; Young et al., 1990). These structural alterations are most prominently featured by dendritic retraction and spine loss in CA3, and to a lesser extent, CA1 pyramidal neurons (Magariños et al., 1997; Sandi et al., 2003; Sapolsky et al., 1985; Sousa et al., 2000; Stewart et al., 2005; Vyas et al., 2002; Watanabe et al., 1992). These findings parallel human neuroimaging studies reporting reduced hippocampal volumes in individuals suffering from stress-related disorders (Bremner, 1999, 2002; Lindauer et al., 2006; Lupien et al., 2007; Sheline et al., 2003; Sheline et al., 1996; cf. Fink, 2011).
One unique feature of the hippocampal formation is its ability to undergo neuronal replacement throughout the lifespan of rodents and primates (Eriksson et al., 1998; Gould et al., 1999a; Kaplan and Bell, 1983). Neurogenesis is reliably decreased by exposure to glucocorticoids following a variety of repeated stress paradigms (Gould et al., 1992; Gould et al., 1997; Mirescu et al., 2004; Pham et al., 2003; Tanapat et al., 2001). Newly-generated granule neurons are known to migrate into the granule cell layer, extend axons to their appropriate targets in CA3 and become functionally integrated into the hippocampal network. Evidence supports a role for the survival of these newly-generated neurons in certain forms of hippocampal-dependent learning (Cameron et al., 1993; Gould et al., 1999b; Hastings and Gould, 1999; Markakis and Gage, 1999; Shors et al., 2001; Shors et al., 2002; van Praag et al., 2002), and HPA restraining influences during chronic stress exposure (Snyder et al., 2011).
A number of parallels have been identified between results obtained from animal studies of repeated stress and observations made in clinically depressed, PTSD and Cushing's syndrome patients (Conrad, 2008). A large proportion of these patients show hippocampal volume reductions, hippocampal-based cognitive impairments and abnormal glucocorticoid secretory properties (Bremner, 2002; Dalgleish et al., 2007; Gurvits et al., 1996; Karl et al., 2006; Rigucci et al., 2010; Sheline et al., 2003; Sheline et al., 1996; Starkman et al., 1992; Starkman et al., 2001). Impaired glucocorticoid receptor-mediated feedback has been widely documented in individuals suffering from major depressive illness (Carroll et al., 1976; Holsboer, 1983; Young et al., 2004). Some evidence supports the idea that normalization of circulating glucocorticoid levels leads to an amelioration of hippocampal volumetric cognitive impairments (Bourdeau et al., 2005; Starkman et al., 1999; Starkman et al., 2003; Starkman et al., 1986). However, many inconsistencies linger in the human literature with respect to the association of stress, hippocampal volume and cognitive functions (Fink, 2011; Lindauer et al., 2006; Pederson et al., 2004; Stein et al., 1999). Difficulties with generalizing laboratory animal studies to clinical populations derive from determining the direction of causality between glucocorticoid secretion during stress exposure and adverse effects of these stress hormones that alter hippocampal plasticity. Another difficulty entails the fact that individual differences in hippocampal structure may predict vulnerability to stress and related disorders (Conrad, 2008; McLaughlin et al., 2009).
Prefrontal cortex
The prefrontal cortical regions are implicated in a wide array of complex functions that distinguish human cognitive processes from other species, including cognitive control, behavioral flexibility, emotional regulation and working memory. The prefrontal cortex (PFC) is anatomically positioned to modulate both amygdala and hippocampal activity, providing a means for top-down regulation of limbic information processing. Moreover, the medial prefrontal cortex (mPFC) exhibits the capacity to modulate endocrine and physiologic adaptive functions during acute challenges. Given these diverse cognitive and homeostatic functions regulated by PFC, it is not surprising that frontal cortical regions have commanded increased interest in recent years for their roles in the maladaptive effects of stress and in stress-related psychopathologies (for reviews, see (Arnsten, 2011; Bremner, 2002; Drevets et al., 1997; Liberzon and Sripada, 2008; Price and Drevets, 2012; Rigucci et al., 2010; Sheline, 2003). An important feature of PFC is the capacity to provide top-down control over homeostatic-like responses during stress exposure, yet it is also a target of the adverse effects of repeated stress. Although it is difficult to pinpoint exactly how this process is initiated, the available evidence suggests a feed-forward cycle whereby repeated stress impairs PFC regulated functions, thereby leading to the further endangerment of cognitive and homeostatic functions subserved by PFC.
Evidence gathered in humans suggests that long-term stress exposure induces deficits in decision-making processes and may lead to reductions in prefrontal volume (Soares et al., 2012). Mild acute stressors have even been found to reliably disrupt working memory functions (Arnsten et al., 2012; Arnsten and Goldman-Rakic, 1998). Similar to the hippocampal formation, morphometric analyses of prefrontal regions in depressed and PTSD patients show volume reductions in anterior cingulate, orbital, medial and ventrolateral subregions of the prefrontal cortex (Li et al., 2010; Salvadore et al., 2011; Weber et al., 2013; Yamasue et al., 2003). Post mortem histological analyses of brains from depressed individuals generally corroborate volumetric data in observing reductions in neuron and glial cell numbers in the same cortical subfields (Cotter et al., 2002; Cotter et al., 2001; Rajkowska et al., 1999; Stockmeier and Rajkowska, 2004). Abnormal prefrontal activity, often in the form of hypoactivity, and diminished executive functioning, are common features of mood and anxiety disorders (Austin et al., 2001; Davidson et al., 2002; Fossati et al., 2002; Matsuo et al., 2003; Merriam et al., 1999; Phan et al., 2006; Rogers et al., 2004). These observations, coupled with an increasing body of evidence from animal studies, implicate the prefrontal cortex as a set of brain structures that are exquisitely sensitive to the effects of environmental stress, such that the modulatory functions on adaptive responses under acute conditions are intricately intertwined with chronic effects that feedback and alter the response properties of these cortical networks.
Acute stress has been shown to induce a relatively consistent diminution in prefrontal processing and related cognitive functions (Arnsten et al., 2012; Arnsten and Goldman-Rakic, 1998). One underlying idea is that the complexity and time demands required for prefrontal cognitive processing exceed the necessity for more immediate responses required for adaptive responding. Experimental results from animal studies of repeated or chronic challenges highlight the same general theme that stress interferes with cognitive flexibility (Birrell and Brown, 2000; Bondi et al., 2010; Bondi et al., 2008; Kim and Ragozzino, 2005; Liston et al., 2006; McAlonan and Brown, 2003; Schoenbaum et al., 2002). Working memory is also sensitive to the effects of stress. Macaque monkeys subjected to acute noise stress demonstrated impairments in a spatial working memory task, whereas several studies have shown that rats demonstrate a similar type of cognitive impairment following exposure to stressful stimuli (Anderson et al., 2014; Arnsten and Goldman-Rakic, 1998; Hains et al., 2009).
A variety of neurotransmitters and signaling pathways have been shown to underlie prefrontal cognitive alterations during stress, and several examples will be highlighted here. Catecholamine influences in PFC have been demonstrated to play an important role during stress (for review, see Arnsten, 1997). In a series of studies, Arnsten and colleagues have shown that inputs into PFC (dorsolateral area 46 in primates; PL in rodents) activate a cellular cascade in principal neurons, inducing cyclic AMP and increases in protein kinase C signaling, ultimately resulting in dampened neuronal firing patterns and impaired behavioral output (Arnsten, 2009; Arnsten et al., 2012; Hains et al., 2009; Ramos and Arnsten, 2007). Whereas excessive increases in noradrenergic signaling may take the PFC “off-line” to allow for more reflexive responses (Arnsten and Goldman-Rakic, 1998; Radley et al., 2008b), under normal conditions elevations in noradrenergic signaling in PFC promotes cognitive flexibility (Aston-Jones et al., 1999, 2000; Cole and Robbins, 1992; Devauges and Sara, 1990; Page and Lucki, 2002; Ramos and Arnsten, 2007), and may even enhance attentionrelated task processing during periods when heightened vigilance is required (Aston-Jones et al., 1999, 2000). During repeated stress, the relationship between noradrenergic signaling and PFC function appears to be more straightforward, and contributes to the development of prefrontal cognitive deficits (Bondi et al., 2010; Bondi et al., 2008; Jett and Morilak, 2013). Elevated glucocorticoids have also been documented to adversely impact prefrontal-related cognitive functions in a variety of developmental contexts (Gourley et al., 2009; Anderson et al., 2014), and increases in these hormones are likely to play an important role in prefrontal dysfunction following chronic stress exposure (Cerqueira et al., 2007; Dias-Ferreira et al., 2009; Liston et al., 2006). Recent evidence suggests that endocannabinoids may play a countervailing role in limiting glucocorticoid-mediated impairment of prefrontal function (Hill and McEwen, 2010; McLaughlin et al., 2014). However, under repeated stress, decrements in endocannabinoid signaling are also implicated in the exacerbation of glucocorticoid effects.
Structural plasticity in PFC is a widely documented phenotype resulting from chronic stress exposure, and provides additional insight into the possible mechanisms accounting for the impairment of prefrontal-related cognitive functions. Morphometric studies in laboratory animals have generally shown regressive structural alterations in pyramidal neurons in multiple PFC subregions in rodents (anterior cingulate, prelimbic, and infralimbic cortices), such as reduced apical dendritic length and branching, and decreases in spine density in the apical dendritic tree (Cook and Wellman, 2004; Liston et al., 2006; Liu and Aghajanian, 2008; Martin and Wellman, 2011; Radley et al., 2005; Radley et al., 2006b; Radley et al., 2008a; Radley et al., 2004; Shansky and Lipps, 2013). Glucocorticoids and their agonists are capable of recapitulating the effects of repeated stress on mPFC structure (Cerqueira et al., 2007; Cook and Wellman, 2004; Wellman, 2001), and interfering with GR receptors may block these structural alterations (Liu and Aghajanian, 2008). Regressive structural plasticity in PL following repeated stress or glucocorticoid exposure has also been correlated with the impairment of prefrontal functioning, and some of these effects may be reversed by protein kinase C inhibition (Anderson et al., 2014; Cerqueira et al., 2005; Dias-Ferreira et al., 2009; Hains et al., 2009; Liston et al., 2006). With regard to regressive synaptic effects, a subclass of dendritic spines denoted as “thin” (i.e., long neck, small head diameter) have been shown to be particularly vulnerable to the effects of repeated stress, glucocorticoids (Anderson et al., 2014; Bloss et al., 2013; Liston et al., 2013; Liston and Gan, 2011; Radley et al., 2008a), and aging (Anderson et al., 2014; Bloss et al., 2011; Dumitriu et al., 2010). Recent evidence suggests that thin spines play a critical role in maintaining optimal prefrontal network function and working memory (for review, see (Arnsten et al., 2010)). Despite the adverse effects of chronic stress on structural synaptic function in PFC, these alterations have been suggested to provide an adaptive mechanism to restrain adverse glutamatergic influences that would otherwise result in neurotoxicity (Bruno et al., 1993; Holmes and Wellman, 2009; Radley et al., 2008a). If true, then this line of reasoning provides an alternative view that helps to explain the constellation of maladaptive effects of stress on prefrontal functioning; however, more direct evidence for this view has not yet been forthcoming.
PFC is also implicated in the regulation of HPA axis and autonomic functions (Crane et al., 2003; Diorio et al., 1993; Neafsey, 1990; Radley et al., 2006a; Radley et al., 2009; Radley and Sawchenko, 2011; Sullivan and Gratton, 1999; Van Eden and Buijs, 2000). Whereas mPFC effects on these more homeostatic-like responses are generally inhibitory, evidence suggests some differentiation by cortical subfield that also accounts for excitatory modulation of these responses (Radley, 2012; Radley et al., 2006a; Radley et al., 2009). Several recent studies have shown that HPA-inhibitory influences from PFC are mediated by PL, and reach neuroendocrine effector cells in the hypothalamus via a disynaptic relay in aBST ((Radley et al., 2009; Radley and Sawchenko, 2011); see Box 1). Furthermore, pyramidal neurons in PL giving rise to aBST outputs show diminished structural and functional plasticity following chronic stress exposure (Radley et al., 2013). Taken together, these findings underscore that adaptive responses are highly integrative, involving a continuum of multiple cortical projections, in addition to hippocampus-bearing relays to the neuroendocrine hypothalamus. Thus, compromises along any part of this continuum could effectively contribute to maladaptive alterations in HPA axis regulation.
Box 1. Circuit organization providing for limbic forebrain control of the HPA axis
Over the years, attempts to understand top-down inhibitory modulation of stress-induced HPA activation from the limbic forebrain has been complicated by the fact that these influences are likely to be achieved via indirect, or even multisynaptic relays to HPA effector cell groups in PVH. Extrinsic projections from mPFC and hippocampal formation are largely excitatory glutamatergic in nature, implicating a disynaptic GABAergic relay interceding between upstream excitatory forebrain regions and downstream HPA effector cell groups in PVH. Combined pathway tracing and immediate-early gene mapping studies have helped to identify a number of candidate cell groups that could serve as inhibitory relays interfacing between forebrain regulators and PVH (Cullinan et al., 1993; Roland and Sawchenko, 1993; van de Kar and Blair, 1999; Herman et al., 2003), nevertheless, the picture that has emerged is one involving a complex network of higher-order structures interconnected in parallel with PVH.
Work from our laboratory has identified a discrete cluster of GABAergic neurons interposed throughout anterior aspects of the bed nuclei of the stria terminalis (aBST; i.e., within subcommissural, dorsomedial, and fusiform subdivisions of Dong et al., 2001) that forms the missing link in this circuit model (Radley et al., 2009; Radley and Sawchenko, 2011). Our data suggest that stress-inhibitory influences of the mPFC and hippocampal formation are exerted principally via convergence onto the same population of GABAergic neurons, and that these cell groups have the capacity to integrate stress-inhibitory signals from the forebrain (Radley and Sawchenko 2011; Figure 1). This conclusion is based on three lines of evidence: (1) anatomical tracing experiments indicate that extrinsic projections from the hippocampal formation and the mPFC converge onto stress-sensitive, PVH-projecting neurons in the aBST; (2) GABAergic PVH-projecting cell groups in the aBST show diminished functional activation following acute stress in animals bearing excitotoxin lesions of either the hippocampal formation or mPFC; (3) the aBST plays a more prominent inhibitory role than the hippocampal formation over stress-induced increases in plasma corticosterone levels.
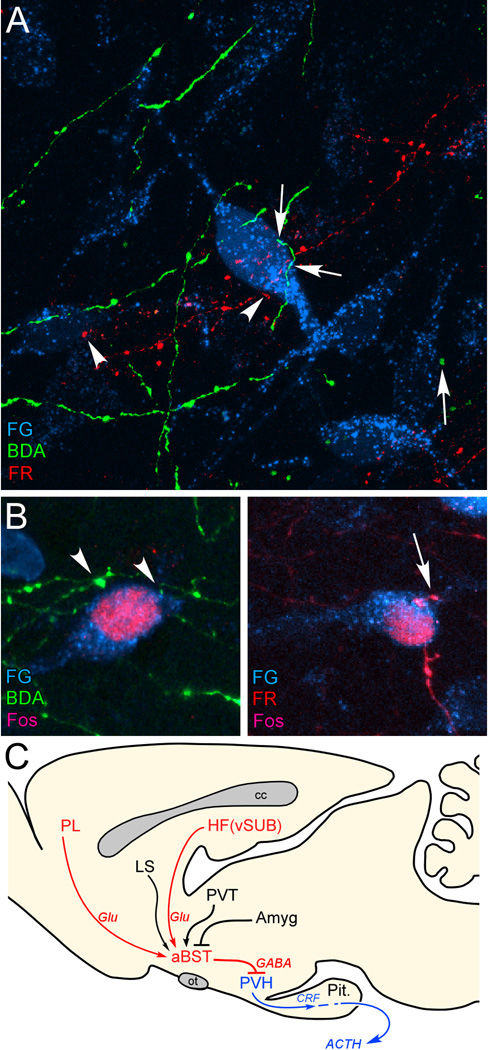
Figure 1.
A: Confluence of labeling in aBST following retrograde tracer injections in PVH (Fluoro-Gold, FG; cyan), and anterograde tracers in PL (biotinylated dextran amine, BDA; green) and vSUB (FluoroRuby, FR; red). Instances of BDA- (arrows) and FR-labeled (arrowhead) terminals make appositions onto single PVH-projecting neurons in aBST, by laser-scanning confocal microscopic analysis. B: After a single stress exposure, numerous instances of Fos-labeled nuclei are evident in PVH-projecting neurons containing appositions from BDA- (left) and FR-labeled (right) terminals. C: Previous work of ours supports the pathways highlighted in red, with aBST providing an important source of GABAergic innervation of PVH, and relaying limbic cortical influences. Other forebrain cell groups known to influence HPA output (highlighted in black) also project to aBST, whose integrated output targets PVH directly. Like vSUB and PL, these regions do not provide any appreciable innervation of PVH, but do issue projections to the aBST. ACTH, adrenocorticotropic hormone; Amyg, amygdala; cc, corpus callosum; CRF, corticotropin-releasing factor; Glu, glutamate; HF, hippocampal formation; LS, lateral septum; ot, optic tract; Pit., pituitary gland; PL, prelimbic cortex; PVH, paraventricular nucleus of the hypothalamus; PVT, paraventricular thalamic nucleus; vSUB, ventral subiculum. Data are based upon Radley and Sawchenko (2011).
There are a number of questions that derive from this work. Is this subpopulation of aBST GABAergic neurons the principal clearinghouse for prefrontal and hippocampal influences? If not, to what extent or under which contexts, are other relays involved? Do other limbic forebrain regions, such as the amygdala, also relay HPA-modulatory influences through these same cell groups? Can neuroplasticity in these pathways help to understand the mechanisms of chronic stress-induced perturbations in endocrine systems? Future studies will help to better understand how stress leads to altered behavioral, physiological and endocrine responses, and how perturbations in key neural pathways leads to maladaptive states.
CAVEATS AND FUTURE CONSIDERATIONS
The preceding implicates several candidate mediators and neural circuits capable of responding in a context- or situation-specific manner to meet the demands of stress. While modifications in these limbic forebrain regions are widely implicated in maladaptive effects following chronic stress, less well understood is the extent to which recruitment patterns within individual network elements are actually required for our survival. Perhaps some level of protection may be afforded by such redundancy, if at all true. However, this might also explain why lesions (i.e. experimental, disease, ageing) of any one limbic structure (or principal afferents) are known to produce widespread and overlapping effects, including those related to mood, metabolic and cardiovascular disorders. Thus, in order to understand or dissociate adaptive from maladaptive responses, incorporating acutely exposed animals in studies of chronic stress is crucial, but all too often remains a neglected comparator.
Large variations in individual cortisol release patterns feature prominently in repeated challenge experiments in humans (Kirschbaum et al., 1995; Kudielka and Wust, 2010). As discussed elsewhere, the biological determinants for individual variations are poorly understood, and extensive phenotyping remains essential (Kudielka et al., 2009). Evidence drawn from animal models of chronic stress, nevertheless, continues to shape our understanding of just how disruptive homeostatic threats may be, as well as for identifying key contributions of different neural substrates. Similar to humans, and depending on the characteristics of stimuli employed in animal models, stressors do not invariably lead to pathology or maladaptive responses (Anisman and Matheson, 2005). Thus, individual differences in resilience, defined as an individual's ability for properly adapting to stress, form an important basis for this variability. Studies exploring this possibility, including models of stressor controllability and repeated homotypic stress (Frank et al., 2013; Grissom and Bhatnagar, 2009; Jaferi and Bhatnagar, 2006; Maier et al., 2006; Maier and Watkins, 2010), for example, show that the reactivity of the HPA axis can readily decline during successive stimulus exposures. This response is thought to be adaptive, insofar as it would limit exposure to circulating glucocorticoids. Moreover, this response does not reflect an exhaustion of the biosynthetic and/or secretory capacity of the HPA axis, nor is it passive. Decrements in neuroendocrine responses are met by global decreases in stress-induced activation of the PVH and its extended circuitries, and various ablation methods (pharmacological, physical, genetic) can reverse the expression of stress HPA axis habituation (Day et al., 2009; Herman, 2013; Masini et al., 2012; Masini et al., 2008; Weinberg et al., 2010)(Box 2). What has emerged from these studies is that similar to chronic stress, the process of stress habituation also requires a sizeable amount of neural substrate. On this point, and if one takes notice, brain regions and/ or neural mechanisms thought to represent antecedents for pathology (in the face of chronic stress) may also be the same as those enlisted during stress HPA axis habituation. Despite the differential effects of uncontrollable and controllable stressors on a variety of experimental endpoints, it is conceivable that these too may share overlapping neurobiological substrates. As discussed elsewhere (De Boer and Koolhaas, 2003; Koolhaas et al., 2010), careful appraisal or analysis of this possibility is requisite for developing better models of individual differences in stress reactivity.
Box 2. Neural mechanisms of stress habituation
An important, and often overlooked aspect of many animal models of repeated stress is the fact that the activity of several systems, including neuroendocrine, behavioral and autonomic responses, tend to decline upon repeated stimulus exposures, a process known as habituation (Armario et al., 1984; Campeau et al., 2002; De Boer et al., 1988; Masini et al., 2008; van Raaij et al., 1997). Work in our laboratory has helped to advance understanding of the neural pathways involved in this process, highlighting the auditory thalamus as an apex responsible for the activation of a several subcortical pathways subserving adaptive responses to repeated audiogenic stress (Campeau et al., 2002). Loud noises are usually associated with danger signals in many species and readily induce HPA, autonomic, and behavioral responses (Bao et al., 1999; Borrell et al., 1980; Campeau and Watson, 1997; De Boer et al., 1989; Gamallo et al., 1992; Henkin and Knigge, 1963; Masini et al., 2008; Overton et al., 1991; Saha et al., 1996; Segal et al., 1989). Although a significant research effort has been devoted to understanding limbic forebrain plasticity following repeated stress exposure, there is a dearth of information available regarding its role in stress habituation. Audiogenic stress affords a significant advantage over other commonly employed paradigms (e.g., social defeat, restraint, footshock) that are multisensory and multimodal, since it provides an opportunity for clarifying how a single sensory modality may come to enlist brain regions encoding stress habituation. In this design, our combined connectivity and functional studies highlight the posterior hypothalamus as a candidate nodal point for distributing audiogenic information to downstream effectors of HPA, autonomic and behavioral responses (Bailey and Dimicco, 2001; Nyhuis et al., 2010, 2011; Nyhuis et al., 2012) (Figure 2). As the limbic forebrain can gain access to several aspects of this circuitry (and vice versa), this audiogenic model also provides an important entry point for critically examining and affixing changes in PFC and amygdaloid plasticity to adaptive and maladaptive outcomes in neuroendocrine, autonomic and behavioral responses to stress.
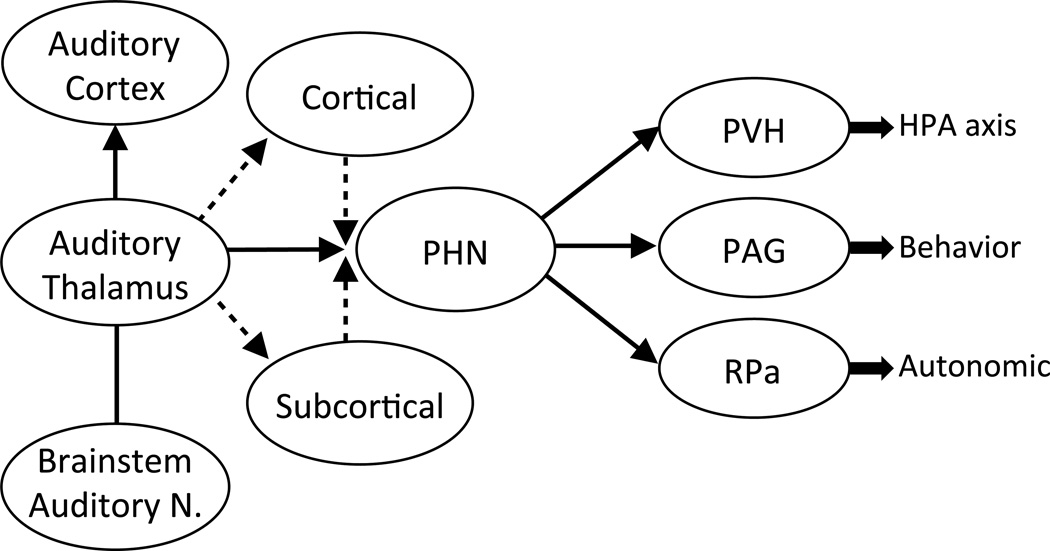
Figure 2.
Working model of neuroendocrine and autonomic responses following audiogenic stress. In response to repeated loud noise exposure, animals reliably show reduced corticosterone and autonomic responses. Based on previous functional-lesion, connectivity and immediate early gene experiments, audiogenic stress is capable of eliciting neuroendocrine, autonomic and behavioral responses to and through various forebrain, hindbrain and midline thalamic and hypothalamic candidate nuclei. The posterior hypothalamus (PHN) stands out in this regard, based on our current understanding of its functional and anatomical connectivity to HPA effector neurons in the paraventricular hypothalamic nucleus (PVN), as well as the periaqueductal gray (PAG) and the Raphe Pallidus (RPa), important mediators of flight or flight responses and emotional arousal, respectively. Several cortical and subcortical regions known to send reciprocal connections with sensory, motor and limbic-related cortices, have yet to be fully elucidated (dashed lines) in the context of repeat audiogenic stimuli and stress habituation.
Finally, but by no means an end to a large list of candidate factors (Anisman and Matheson, 2005; Anisman and Zacharko, 1990)(Table 1), consideration of sex differences and sex steroid hormone influences is of paramount importance. Males and females would seem to require or develop different neuroendocrine and behavioral coping strategies in response to stress (Babb et al., 2013a, b, 2014; Carvalho-Netto et al., 2011; Mashoodh et al., 2008; McEwen and Milner, 2007; Solomon et al., 2012; ter Horst et al., 2012), which could form a basis for the gender bias in the onset, type and relative risk of different types of disease (Kokras and Dalla, 2014; McCarthy et al., 2012). Manipulating gonadal status in humans and rodents makes clear that androgens and estrogens can potently inhibit and stimulate, respectively, neuroendocrine stress responses, operating on both feed-forward and glucocorticoid-mediated negative feedback regulation of the HPA axis (Goel et al., 2014; Young, 1995). Females express marked variations in stress neuroendocrine and behavioral reactivity in response to cyclic changes in ovarian status, and it is perhaps this variability that deters investigators from including females in their studies. This notion is slightly misdirected, however, as males show circadian variations in testosterone secretion that are as dynamic as the hormonal changes in females (Viau, 2002). Moreover, male rodents show marked changes in testosterone synthesis and release under acute or chronic stress conditions, whereas estrous cyclicity in females remains relatively intact.
Table 1.
Factors influencing the stress response
| Stressor type |
| Processive (neurogenic or psychogenic) |
| Systemic (immune insults) |
| Stressor characteristics |
| Controllability |
| Predictability |
| Ambiguity/uncertainty |
| Chronicity |
| Intermittence |
| Organismic variables |
| Species |
| Strain |
| Age |
| Sex |
| Experiential variables |
| Previous stressor experiences (sensitization) |
| Early life events (maternal factors, trauma) |
| Resource characteristics |
| Personal characteristics |
| Coping skills |
| Self-esteem |
| Self-efficacy |
| Personality (hardiness, optimism, neuroticism) |
| Social characteristics |
| Social support (perceptions) |
| Attachment (bonding) |
Reprinted from (Anisman and Matheson, 2005) with permission.
Sex steroid hormone receptors are not only distributed within brain regions regulating the gonadal axis, but also within those involving the HPA axis (Bingham et al., 2006; Handa et al., 1994; Handa and Weiser, 2014; Williamson et al., 2005; Williamson and Viau, 2007). These would include the PVH and its extended circuitries, several of the same forebrain regions discussed above, in addition to ascending hindbrain modulators of emotional and somatosensory processing. The onset of depression and anxiety is frequently associated with major disruptions in reproductive endocrine function in females, and in some cases with hypogonadism in males. As previously argued (Rubinow and Schmidt, 2002), changes in gonadal status may not be the root cause of disease onset, but just like glucocorticoid hormones (Bourke et al., 2012; Oitzl et al., 2010; Quinn et al., 2014), may provide a context for understanding how the brain responds to stress. Based on their propensities for directing the HPA axis across all stages of the life span (Gobinath et al., 2014; McCarthy and Arnold, 2011; McCormick et al., 1998; McCormick and Mathews, 2007; Toufexis et al., 2014), as well as other stress response systems, where and how the sex and adrenal steroid hormones intersect in the brain to alter the development of stress habituation remains worthy of pursuit (Bangasser and Valentino, 2012; Goel and Bale, 2009), and promises to explain the bases for both individual and gender based differences in stress-related pathology (Figure 3).
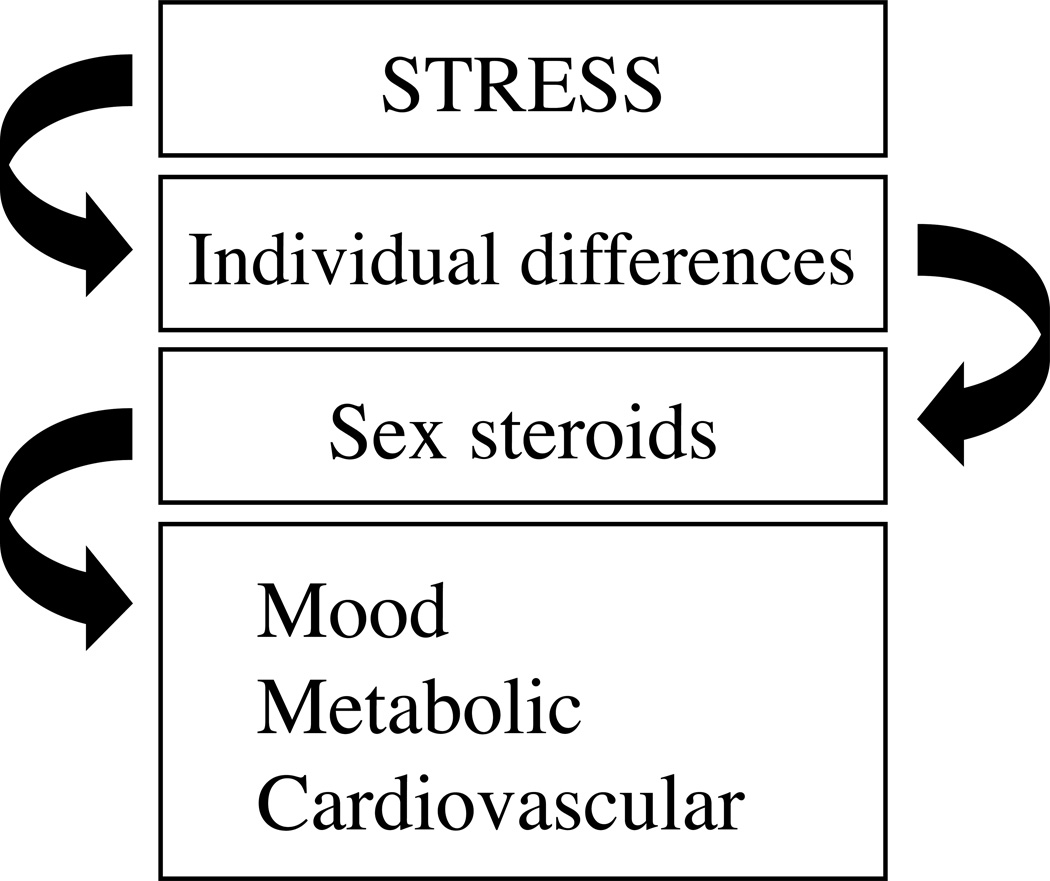
Figure 3.
Schematic to illustrate that the sex steroid hormones (e.g. androgens and estrogens) have an important basis for individual differences in stress reactivity under normal conditions, and under those that produce pathological changes in mood, metabolic and cardiovascular function, for examples. This schematic underscores that the sex steroid hormones can redirect the strength of influence of stress on the body. Note that sex steroid hormone secretion and signaling (as with other factors listed in Table 1) are also themselves subject to homeostatic threat, and in this design can be placed either above or below “individual differences”; implying that relationships between gonadal status and stress can change in a situation- and context-dependent manner.
Highlights.
Adaptive and maladaptive processes associated with repeated stress are reviewed
Amygdala, hippocampus and prefrontal cortex are differentially modulated by stress Adaptive processes associated with habituation to stress are discussed
Gonadal hormone variations mediate individual differences to stress
Importance of individual context in characterizing adaptive vs. maladaptive outcome
Acknowledgments
Support for some of the work presented in this manuscript came from National Institute of Health grants MH053851, MH072672 (DM), MH095972 (JR) and MH077152 (SC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adamec R, Hebert M, Blundell J, Mervis RF. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav. Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front. Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Allen JP, Allen CF. Role of the amygdaloid complexes in the stress-induced release of ACTH in the rat. Neuroendocrinology. 1974a;15:220–230. doi: 10.1159/000122310. [DOI] [PubMed] [Google Scholar]
- Allen JP, Allen CF. Role of the amygdaloid complexes in the stress-induced release of ACTH in the rat. Neuroendocrinology. 1974b;15:220–230. doi: 10.1159/000122310. [DOI] [PubMed] [Google Scholar]
- Alt SR, Turner JD, Klok MD, Meijer OC, Lakke EA, Derijk RH, Muller CP. Differential expression of glucocorticoid receptor transcripts in major depressive disorder is not epigenetically programmed. Psychoneuroendocrinology. 2010;35:544–556. doi: 10.1016/j.psyneuen.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, Radley JJ. Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. J. Neurosci. 2014;34:8387–8397. doi: 10.1523/JNEUROSCI.1385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Multiple neurochemical and behavioral consequences of stressors: implications for depression. Pharmacol. Ther. 1990;46:119–136. doi: 10.1016/0163-7258(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behav. Neural Biol. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn. Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys - Evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br. J. Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013a;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Stressor-specific effects of sex on HPA axis hormones and activation of stress-related neurocircuitry. Stress. 2013b;16:664–677. doi: 10.3109/10253890.2013.840282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, Campeau S. Habituation of hypothalamic-pituitary-adrenocortical axis hormones to repeated homotypic stress and subsequent heterotypic stressor exposure in male and female rats. Stress. 2014;17:224–234. doi: 10.3109/10253890.2014.905534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R8–R15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell. Mol. Neurobiol. 2012;32:709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao G, Metreveli N, Fletcher EC. Acute and chronic blood pressure response to recurrent acoustic arousal in rats. Am. J. Hypertens. 1999;12:504–510. doi: 10.1016/s0895-7061(99)00032-1. [DOI] [PubMed] [Google Scholar]
- Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–254. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog. Neurobiol. 2014;112:80–99. doi: 10.1016/j.pneurobio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-beta distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J. Comp. Neurol. 2006;499:911–923. doi: 10.1002/cne.21151. [DOI] [PubMed] [Google Scholar]
- Biondi M, Picardi A. Psychological stress and neuroendocrine function in humans: the last two decades of research. Psychother. Psychosom. 1999;68:114–150. doi: 10.1159/000012323. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J. Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Puri R, Yuk F, Punsoni M, Hara Y, Janssen WG, McEwen BS, Morrison JH. Morphological and molecular changes in aging rat prelimbic prefrontal cortical synapses. Neurobiol. Aging. 2013;34:200–210. doi: 10.1016/j.neurobiolaging.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff S, Humphreys A, Lehman J, Diamond D, Rose G, Meaney M. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Borrell J, Torrellas A, Guaza C, Borrell S. Sound stimulation and its effects on the pituitary-adrenocortical function and brain catecholamines in rats. Neuroendocrinology. 1980;31:53–59. doi: 10.1159/000123050. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Bard C, Forget H, Boulanger Y, Cohen H, Lacroix A. Cognitive function and cerebral assessment in patients who have Cushing's syndrome. Endocrinol. Metab. Clin. North Am. 2005;34:357–369. ix. doi: 10.1016/j.ecl.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Harrell CS, Neigh GN. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr. Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin. Sci (Lond) 1989;77:453–461. doi: 10.1042/cs0770453. [DOI] [PubMed] [Google Scholar]
- Bruno V, Scapagnini U, Canonico PL. Excitatory amino acids and neurotoxicity. Funct. Neurol. 1993;8:279–292. [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J. Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Campeau S, Dolan D, Akil H, Watson SJ., Jr c-fos mRNA induction in acute and chronic audiogenic stress: Possible role of the orbitofrontal cortex in habituation. Stress. 2002;5:121–130. doi: 10.1080/10253890290027895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Carroll, Curtis GC, Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch Gen Psychiatry. 1976;33:1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- Carter RN, Pinnock SB, Herbert J. Does the amygdala modulate adaptation to repeated stress? Neuroscience. 2004;126:9–19. doi: 10.1016/j.neuroscience.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Myers B, Jones K, Solomon MB, Herman JP. Sex differences in synaptic plasticity in stress-responsive brain regions following chronic variable stress. Physiol. Behav. 2011;104:242–247. doi: 10.1016/j.physbeh.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J. Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17:1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int. J. Obes. Relat. Metab. Disord. 2000;24:S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl) 2011;214:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Forebrain norepinephrine: role in controlled information processing in the rat. Neuropsychopharmacology. 1992;7:129–142. [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Crane JW, Ebner K, Day TA. Medial prefrontal cortex suppression of the hypothalamicpituitaryadrenal axis response to a physical stressor, systemic delivery of interleukin-1. Eur. J. Neurosci. 2003;17:1473–1481. doi: 10.1046/j.1460-9568.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dachir S, Kadar T, Robinzon B, Levy A. Cognitive deficits induced in young rats by long-term corticosterone administration. Behav Neural Biol. 1993;60:103–109. doi: 10.1016/0163-1047(93)90173-f. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Williams JM, Golden AM, Perkins N, Barrett LF, Barnard PJ, Yeung CA, Murphy V, Elward R, Tchanturia K, Watkins E. Reduced specificity of autobiographical memory and depression: the role of executive control. J. Exp. Psychol. Gen. 2007;136:23–42. doi: 10.1037/0096-3445.136.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF. Glucocorticoid negative feedback, Encyclopedia of Stress. Academic Press; 2000. pp. 224–229. [Google Scholar]
- Dallman MF, Jones MT. Corticosteroid feedback control of ACTH secretion: Effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology. 1973;92:1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. Reversible inactivation of the auditory thalamus disrupts HPA axis habituation to repeated loud noise stress exposures. Brain Res. 2009;1276:123–130. doi: 10.1016/j.brainres.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1195–1200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Medullary neurones regulate hypothalamic corticotropin-releasing factor cell responses to an emotional stressor. Neuroscience. 2001;105:707–719. doi: 10.1016/s0306-4522(01)00213-5. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur. J. Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Slangen JL, van der Gugten J. Adaptation of plasma catecholamine and corticosterone responses to short-term repeated noise stress in rats. Physiol. Behav. 1988;44:273–280. doi: 10.1016/0031-9384(88)90149-7. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol. Behav. 1989;45:789–795. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Reul JMHM. Feedback action and tonic influence of glucocorticoids on brain function: a concept arising from heterogeneity of brain receptor systems. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res. 1990;39:19–28. doi: 10.1016/0166-4328(90)90118-x. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J. Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids Enhance the Excitability of Principal Basolateral Amygdala Neurons. J. Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fink G. Stress controversies: post-traumatic stress disorder, hippocampal volume, gastroduodenal ulceration*. J. Neuroendocrinol. 2011;23:107–117. doi: 10.1111/j.1365-2826.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- Fossati P, Ergis AM, Allilaire JF. [Executive functioning in unipolar depression: a review] Encephale. 2002;28:97–107. [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain. Behav. Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallo A, Alario P, Gonzalez-Abad MJ, Villanua MA. Acute noise stress, ACTH administration, and blood pressure alteration. Physiol Behav. 1992;51:1201–1205. doi: 10.1016/0031-9384(92)90309-p. [DOI] [PubMed] [Google Scholar]
- Garcia R, Paquereau J, Vouimba RM, Jaffard R. Footshock stress but not contextual fear conditioning induces long-term enhancement of auditory-evoked potentials in the basolateral amygdala of the freely behaving rat. Eur. J. Neurosci. 1998;10:457–463. doi: 10.1046/j.1460-9568.1998.00027.x. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS. Rat brain binds adrenal steroid hormone: Radioautography of hippocampus with corticosterone. Science. 1972;175:1133–1136. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Gobinath AR, Mahmoud R, Galea LA. Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Front. Neurosci. 2014;8:420. doi: 10.3389/fnins.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J. Neuroendocrinol. 2009;21:415–420. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Innala L, Viau V. Sex differences in serotonin (5-HT) 1A receptor regulation of HPA axis and dorsal raphe responses to acute restraint. Psychoneuroendocrinology. 2014;40:232–241. doi: 10.1016/j.psyneuen.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: Get used to it. Neurobiol. Learn. Mem. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J. Comp. Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin RI, Knigge KM. Effect of sound on the hypothalamic-pituitary-adrenal axis. Am. J. Physiol. 1963;204:910–914. doi: 10.1152/ajplegacy.1963.204.4.710. [DOI] [PubMed] [Google Scholar]
- Herman J, Cullinan W, Young E, Akil H, Watson S. Selective forebrain fibertract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus CRH and AVP mRNA expression. Brain Res. 1992;592:228–238. doi: 10.1016/0006-8993(92)91680-d. [DOI] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front. Behav. Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995a;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995b;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Watson SJ. Involvement of the ventral hippocampus in regulation of the hypothalamo-pituitary- adrenocortical axis. Soc. Neurosci. Abstr. 1992;22:1541. [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK-H, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The dexamethasone suppression test in depressed patients: clinical and biochemical aspects. J. Steroid Biochem. 1983;19:251–257. [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Huether G. The central adaptation syndrome: Psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog. Neurobiol. 1996;48:569–612. doi: 10.1016/0301-0082(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamo-pituitary-adrenocortical axis. Endocrine Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone Can Act at the Posterior Paraventricular Thalamus to Inhibit Hypothalamic-Pituitary-Adrenal Activity in Animals that Habituate to Repeated Stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jett JD, Morilak DA. Too much of a good thing: blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology. 2013;38:585–595. doi: 10.1038/npp.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol. Rev. 2012;64:901–938. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, Ledoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Neuronal proliferation in the 9-month-old rodent-radioautographic study of granule cells in the hippocampus. Exp. Brain Res. 1983;52:1–5. doi: 10.1007/BF00237141. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. SciU.SA. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, Joèls M. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur. J. Neurosci. 2002;16(6):1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J. Neurosci. Res. 2006;84:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Vouimba RM, Cohen H, Richter-Levin G. Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus. 2006;16:35–42. doi: 10.1002/hipo.20130. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom. Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Klok MD, Alt SR, Irurzun Lafitte AJ, Turner JD, Lakke EA, Huitinga I, Muller CP, Zitman FG, de Kloet ER, Derijk RH. Decreased expression of mineralocorticoid receptor mRNA and its splice variants in postmortem brain regions of patients with major depressive disorder. J. Psychiatr. Res. 2011;45:871–878. doi: 10.1016/j.jpsychires.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Wust S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol. Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levine S, Ursin H. What is stress? In: Brown MR, Koob GF, Rivier C, editors. Stress: Neurobiology and Neuroendocrinology. New York: Marcel Dekker, Inc; 1991. pp. 3–21. [Google Scholar]
- Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, Lin WC, Su TP. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. 2010;50:347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Li HY, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in"neurogenic" stress: A comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J. Comp. Neurol. 1998;393:244–266. [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Liebmann L, Karst H, Joëls M. Effects of corticosterone and the beta-agonist isoproterenol on glutamate receptor-mediated synaptic currents in the rat basolateral amygdala. Eur. J. Neurosci. 2009;30:800–807. doi: 10.1111/j.1460-9568.2009.06882.x. [DOI] [PubMed] [Google Scholar]
- Liebmann L, Karst H, Sidiropoulou K, van Gemert N, Meijer OC, Poirazi P, Joels M. Differential effects of corticosterone on the slow after hyperpolarization in the basolateral amygdala and CA1 region: possible role of calcium channel subunits. J. Neurophysiol. 2008;99:958–968. doi: 10.1152/jn.01137.2007. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, van Meijel EP, Carlier IV, Gersons BP. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:171–177. doi: 10.1016/j.biopsych.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat. Neurosci. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZP, Song C, Wang M, He Y, Xu XB, Pan HQ, Chen WB, Peng WJ, Pan BX. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol. Brain. 2014;7:32. doi: 10.1186/1756-6606-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD. Forebrain nuclei involved in autonomic control. Prog. Brain Res. 1991;87:253–268. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen B. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Spencer RL, McEwen BS. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Res. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain and Cognition. 2007;65:209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, Pruessner JC, Seguin JR. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc. Natl Acad. Sci. U. S. A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE, Ressler KJ. Exploring epigenetic regulation of fear memory and biomarkers associated with post-traumatic stress disorder. Front. Psychiatry. 2013;4:62. doi: 10.3389/fpsyt.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Verdugo JMG, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann SJ. Severe paroxysmal hypertension (pseudopheochromocytoma): understanding the cause and treatment. Arch. Intern. Med. 1999;159:670–674. doi: 10.1001/archinte.159.7.670. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb. Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashoodh R, Wright LD, Hebert K, Perrot-Sinal TS. Investigation of sex differences in behavioural, endocrine, and neural measures following repeated psychological stressor exposure. Behav. Brain Res. 2008;188:368–379. doi: 10.1016/j.bbr.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Masini CV, Babb JA, Nyhuis TJ, Day HEW, Campeau S. Auditory cortex lesions do not disrupt habituation of HPA axis responses to repeated noise stress. Brain Res. 2012;1443:18–26. doi: 10.1016/j.brainres.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Day HE, Campeau S. Long-term habituation to repeated loud noise is impaired by relatively short interstressor intervals in rats. Behav. Neurosci. 2008;122:210–223. doi: 10.1037/0735-7044.122.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J. Nerv. Ment. Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am. J. Med. Genet. C Semin. Med. Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Funato H, Kobayashi A, Nobumoto M, Watanabe Y. Reduced Glucocorticoid Receptor alpha Expression in Mood Disorder Patients and First-Degree Relatives. Biol. Psychiatry. 2006;59:689–695. doi: 10.1016/j.biopsych.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Taneichi K, Matsumoto A, Ohtani T, Yamasue H, Sakano Y, Sasaki T, Sadamatsu M, Kasai K, Iwanami A, Asukai N, Kato N, Kato T. Hypoactivation of the prefrontal cortex during verbal fluency test in PTSD: a near-infrared spectroscopy study. Psychiatry Res. 2003;124:1–10. doi: 10.1016/s0925-4927(03)00093-3. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex Differences in the Brain: The Not So Inconvenient Truth. J. Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have 'organizational' effects on the hypothalamic-pituitary-adrenal axis of male rats. Dev. Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McEwen B. Corticosteroids and hippocampal plasticity. Ann NY Acad Sci. 1994;746:134–142. doi: 10.1111/j.1749-6632.1994.tb39223.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol. Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N.Y. Acad. Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene WH. Adrenal steroid receptors and actions in the nervous system. Physiol. Rev. 1986;66:1121–1150. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62:3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 1969;16:227–241. doi: 10.1016/0006-8993(69)90096-1. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol. Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 2014;42C:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol. Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, Sweeney JA. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. Am J Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–897. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J. Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrin. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog. Brain Res. 1990;85:147–166. doi: 10.1016/s0079-6123(08)62679-5. [DOI] [PubMed] [Google Scholar]
- Nyhuis TJ, Day HEW, Campeau S. Society for Neuroscience. San Diego, CA: 2010. Audiogenic stress activation of regions projecting to both the raphe pallidus and paraventricular hypothalamic nucleus. [Google Scholar]
- Nyhuis TJ, Day HEW, Campeau S. Society for Neuroscience. Washington, DC: 2011. Neurons in the posterior hypothalamus express Fos in response to audiogenic stress and project to both the raphe pallidus and paraventricular hypothalamic nucleus. [Google Scholar]
- Nyhuis TJ, Masini CV, Day HEW, Campeau S. Society for Neuroscience. New Orleans, LA: 2012. Reversible inactivation of a subregion of the posterior hypothalamus disrupts HPA axis habituation to repeated audiogenic stress exposures in rats. [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Overton JM, Kregel KC, Davis-Gorman G, Seals DR, Tipton CM, Fisher LA. Effects of exercise training on responses to central injection of CRF and noise stress. Physiol. Behav. 1991;49:93–98. doi: 10.1016/0031-9384(91)90237-i. [DOI] [PubMed] [Google Scholar]
- Padival M, Quinette D, Rosenkranz JA. Effects of repeated stress on excitatory drive of basal amygdala neurons in vivo. Neuropsychopharmacology. 2013;38:1748–1762. doi: 10.1038/npp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Lucki I. Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology. 2002;27:237–247. doi: 10.1016/S0893-133X(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Nemeroff CB, Miller AH. Glucocorticoid receptors in depression. Isr. J. Med. Sci. 1995;31:705–712. [PubMed] [Google Scholar]
- Pasternac A, Talajic M. The effects of stress, emotion, and behavior on the heart. Methods Achiev Exp Pathol. 1991;15:47–57. [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paykel ES. Life stress, depression and attempted suicide. J. Human Stress. 1976;2:3–12. doi: 10.1080/0097840X.1976.9936065. [DOI] [PubMed] [Google Scholar]
- Pederson CL, Maurer SH, Kaminski PL, Zander KA, Peters CM, Stokes-Crowe LA, Osborn RE. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J. Trauma Stress. 2004;17:37–40. doi: 10.1023/B:JOTS.0000014674.84517.46. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Likhtik E, Filali M, Pare D. Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn. Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol. Psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Amaral DG. Organization of the intrinsic connections of the monkey amygdaloid complex: Projections originating in the lateral nucleus. J. Comp. Neurol. 1998;398:431–458. doi: 10.1002/(sici)1096-9861(19980831)398:3<431::aid-cne9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am. J. Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR. Sensitization and kindling phenomena in mood, anxiety, and obsessive-compulsive disorders: the role of serotonergic mechanisms in illness progression. Biol. Psychiatry. 1998;44:193–206. doi: 10.1016/s0006-3223(98)00144-9. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Lesion of the central nucleus of the amygdala decreases basal CRH mRNA expression and stress-induced ACTH release. Annals of the New York Academy of Sciences. 1994;746:438–440. doi: 10.1111/j.1749-6632.1994.tb39279.x. [DOI] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Hypothalamo-Pituitary-Adrenocortical Regulation Following Lesions of the Central Nucleus of the Amygdala. Stress. 1997;1:263–280. doi: 10.3109/10253899709013746. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Quinn M, Ramamoorthy S, Cidlowski JA. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci. 2014;1317:1–6. doi: 10.1111/nyas.12425. [DOI] [PubMed] [Google Scholar]
- Radley JJ. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front. Behav. Neurosci. 2012;6:7. doi: 10.3389/fnbeh.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J. Neurosci. 2013;33:14379–14391. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional Differentiation of the Medial Prefrontal Cortex in Regulating Adaptive Responses to Acute Emotional Stress. J. Neurosci. 2006a;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A Discrete GABAergic Relay Mediates Medial Prefrontal Cortical Inhibition of the Neuroendocrine Stress Response. J. Neurosci. 2009;29:7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex. 2006b;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008a;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A Common Substrate for Prefrontal and Hippocampal Inhibition of the Neuroendocrine Stress Response. J. Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic Innervation of the Dorsal Medial Prefrontal Cortex Modulates Hypothalamo-Pituitary-Adrenal Responses to Acute Emotional Stress. J. Neurosci. 2008b;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Leve LD, Neiderhiser JM. How genes and the social environment moderate each other. Am. J. Public Health. 2013;103(Suppl 1):S111–S121. doi: 10.2105/AJPH.2013.301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol. Psychiatry. 2010;67:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World. J. Biol. Psychiatry. 2010;11:165–180. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K, Fukuda M, Kato N. Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci. Res. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not the central amygdala modulates memory storage. Neurobiol. Learn. Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Memory Modulation. Behav. Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Gonadal steroids, brain, and behavior: role of context. Dialogues Clin Neurosci. 2002;4:123–137. doi: 10.31887/DCNS.2002.4.2/drubinow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Gandhi A, Das S, Kaur P, Singh SH. Effect of noise stress on some cardiovascular parameters and audiovisual reaction time. Indian. J. Physiol. Pharmacol. 1996;40:35–40. [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA, Jr, Drevets WC. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011;54:2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 1983a;289:235–240. doi: 10.1016/0006-8993(83)90024-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. Glucocorticoid sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl ACad Sci USA. 1984a;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. Prolonged exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984b;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS, Rainbow TC. Quantitative autoradiography of 3H- corticosterone receptors in rat brain. Brain res. 1983b;271:331–334. doi: 10.1016/0006-8993(83)90295-0. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, Swick D. Audiogenic stress response: behavioral characteristics and underlying monoamine mechanisms. J. Neural Transm. 1989;75:31–50. doi: 10.1007/BF01250642. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and holistic medicine. Fam. Community Health. 1980;3:85–88. doi: 10.1097/00003727-198008000-00009. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front. Hum. Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann. N. Y. Acad. Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. 2009;71:742–751. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport. 1999;10:2811–2815. doi: 10.1097/00001756-199909090-00021. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 1997;67:92–96. doi: 10.1006/nlme.1997.3763. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol. Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JM, Sampaio A, Ferreira LM, Santos NC, Marques F, Palha JA, Cerqueira JJ, Sousa N. Stress-induced changes in human decision-making are reversible. Transl. Psychiatry. 2012;2:e131. doi: 10.1038/tp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–143. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotnikov SV, Markt PO, Malik V, Chekmareva NY, Naik RR, Sah A, Singewald N, Holsboer F, Czibere L, Landgraf R. Bidirectional rescue of extreme genetic predispositions to anxiety: impact of CRH receptor 1 as epigenetic plasticity gene in the amygdala. Transl. Psychiatry. 2014;4:e359. doi: 10.1038/tp.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarski SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol. Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing's disease are associated with cognitive decrements. Psychosom. Med. 2001;63:985–993. doi: 10.1097/00006842-200111000-00018. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol. Psychiatry. 1999;46:1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Schteingart DE. Improvement in learning associated with increase in hippocampal formation volume. Biol. Psychiatry. 2003;53:233–238. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Schteingart DE, Schork MA. Cushing's syndrome after treatment: changes in cortisol and ACTH levels, and amelioration of the depressive syndrome. Psychiatry Res. 1986;19:177–188. doi: 10.1016/0165-1781(86)90096-x. [DOI] [PubMed] [Google Scholar]
- Stein MB, Hanna C, Vaerum V, Koverola C. Memory functioning in adult women traumatized by childhood sexual abuse. J. Trauma Stress. 1999;12:527–534. doi: 10.1023/A:1024775222098. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Rajkowska G. Cellular abnormalities in depression: evidence from postmortem brain tissue. Dialogues Clin. Neurosci. 2004;6:185–197. doi: 10.31887/DCNS.2004.6.2/cstockmeier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–S214. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain research bulletin. 1991;27:651–662. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130151. doi: 10.1098/rstb.2013.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J. Comp. Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: Focus on ovarian hormones. Physiol. Behav. 2009;97:239–249. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell. Mol. Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Rivarola MA, Lara H, Viau V. Stress and the reproductive axis. J. Neuroendocrinol. 2014;26:573–586. doi: 10.1111/jne.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi K, Tsumori T, Yokota S, Okunishi H, Yasui Y. A disynaptic pathway from the central amygdaloid nucleus to the paraventricular hypothalamic nucleus via the parastrial nucleus in the rat. Neuroscience research. 2007;59:390–398. doi: 10.1016/j.neures.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI. Selective hippocampal lesions do not increase adrenocortical activity. J. Neurosci. 2003;23:4345–4354. doi: 10.1523/JNEUROSCI.23-10-04345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin H. Brain sensitization to external and internal stimuli. Psychoneuroendocrinology. 2014;42:134–145. doi: 10.1016/j.psyneuen.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Ursin H, Olff M. The stress response. In: Stanford SC, Salmon P, editors. Stress: From Synapse to Syndrome. San Diego: Academic Press Ltd; 1993. pp. 3–22. [Google Scholar]
- Vaher P, Luine V, Gould E, McEwen B. Effects of adrenalectomy on spatial memory performance and dentate gyrus morphology. Brain Res. 1994;656:71–78. doi: 10.1016/0006-8993(94)91367-6. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog. Brain Res. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raaij MTM, Dobbe CJG, Elvers B, Timmerman A, Schenk E, Oortgiesen M, Wiegant VM. Hormonal status and the neuroendocrine response to a novel heterotypic stressor involving subchronic noise exposure. Neuroendocrinology. 1997;65:200–209. doi: 10.1159/000127273. [DOI] [PubMed] [Google Scholar]
- Vanitallie TB. Stress: a risk factor for serious illness. Metabolism. 2002;51:40–45. doi: 10.1053/meta.2002.33191. [DOI] [PubMed] [Google Scholar]
- Venero C, Tilling T, Hermans-Borgmeyer I, Schmidt R, Schachner M, Sandi C. Chronic stress induces opposite changes in the mRNA expression of the cell adhesion molecules NCAM and L1. Neuroscience. 2002;115:1211–1219. doi: 10.1016/s0306-4522(02)00543-2. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and - adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9:29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur. J. Neurosci. 2004;19:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen B. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weber M, Killgore WD, Rosso IM, Britton JC, Schwab ZJ, Weiner MR, Simon NM, Pollack MH, Rauch SL. Voxel-based morphometric gray matter correlates of posttraumatic stress disorder. J. Anxiety Disord. 2013;27:413–419. doi: 10.1016/j.janxdis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Johnson DC, Bhatt AP, Spencer RL. Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience. 2010;168:744–756. doi: 10.1016/j.neuroscience.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J. Affect. Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1239–1248. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J. Comp. Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- Wolff SB, Grundemann J, Tovote P, Krabbe S, Jacobson GA, Muller C, Herry C, Ehrlich I, Friedrich RW, Letzkus JJ, Luthi A. Amygdala interneuron subtypes control fear learning through disinhibition. Nature. 2014;509:453–458. doi: 10.1038/nature13258. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. N. Engl. J. Med. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Young EA. The role of gonadal steroids in hypothalamic-pituitary-adrenal axis regulation. Crit. Rev. Neurobiol. 1995;9:371–381. [PubMed] [Google Scholar]
- Young EA, Akana S, Dallman MF. Decreased sensitivity to glucocorticoid fast feedback in chronically stressed rats. Neuroendocrinology. 1990;51:536–542. doi: 10.1159/000125388. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Lopez JF, Kocsis JH, Schatzberg AF, DeBattista C, Zubieta JK. HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinology. 2004;29:1198–1204. doi: 10.1016/j.psyneuen.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]