Abstract
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer death in the United States. Cytotoxic therapies cause significant side effects for most patients and do not offer cure in many advanced cases of CRC. Immunotherapies are a promising new approach to harness the body’s own immune system and inflammatory response to attack and clear the cancer. Tryptophan metabolism along the kynurenine pathway is a particularly promising target for immunotherapy. Indoleamine 2,3 dioxygenase 1 (IDO1) is the most well studied of the enzymes that initiate this pathway and it is commonly overexpressed in CRC. Herein, we provide an in-depth review of how tryptophan metabolism and kynurenine pathway metabolites shape factors important to CRC pathogenesis including the host mucosal immune system, pivotal transcriptional pathways of neoplastic growth and luminal microbiota. This pathway’s role in other gastrointestinal malignancies such as gastric, pancreatic, esophageal and gastrointestinal stromal tumors (GIST) is also discussed. Finally, we highlight how currently available small molecule inhibitors and emerging methods for therapeutic targeting of IDO1 might be applied to colon, rectal and colitis associated cancer.
Keywords: IDO2, TDO, gastrointestinal disease, gut, inflammation, metabolome, metabolomics, Crohn’s disease, ulcerative colitis, biomarker, TLR, microbiome, colorectal
INTRODUCTION
Inflammation is a common feature of colorectal cancer (CRC).1 Despite immune cell infiltration, CRCs evade immune surveillance and resist immune mediated destruction. Metabolism of the essential amino acid tryptophan along the kynurenine pathway (KP) is one potential explanation for this phenomenon and is recognized as an important link between inflammation and neoplastic progression in many cancers.
Indoleamine 2,3 dioxygenase 1 (IDO1) is the most well studied of the enzymes that initiate tryptophan’s catabolism to kynurenine. High IDO1 expression is present in a subset of human CRC where it portends a worse prognosis.2, 3 IDO1 is also one of the most highly upregulated genes in human inflammatory bowel disease (IBD), a precancerous condition.4, 5 Local tryptophan depletion and the biologically active KP metabolites exert potent immunomodulatory effects to shape the tumor microenvironment and contribute to tumor immune escape.6 In CRC, IDO1 also directly supports tumor growth independent of effect on adaptive immunity.2 These features establish IDO1 and the KP as highly promising targets for immunotherapy of cancers including those of the gastrointestinal (GI) tract.
Herein, we provide an in-depth review of how tryptophan metabolism and KP metabolites shape factors important to CRC pathogenesis including the mucosal immune system, luminal microbiota and pivotal transcriptional pathways for neoplastic growth. More limited coverage is provided on how this pathway affects other GI malignancies. Finally, we highlight how currently available agents and emerging methods for therapeutic targeting of the IDO1-KP might be applied to CRC.
BACKGROUND ON COLON CANCER AND INFLAMMATION
CRC is the third most common cancer worldwide,7 and in the United States is the second leading cause of cancer related death (almost 50,000 per year).8 In most cases, the transition from normal colon epithelium to cancer is influenced by the acquisition of somatic mutations and environmental factors including diet and lifestyle.9, 10 The adenomatous polyposis coli (APC) gene is a key component to Wnt signaling and is mutated in most CRCs.10
Chronic inflammation is also a risk factor for CRC. Colitis-associated cancer (CAC) is a form of CRC that develops in patients with chronic inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis.5 In IBD, dysregulated activation of the gut mucosal immune system driven by genetic susceptibility loci and environmental factors leads to chronic inflammation.11 The risk of developing CAC correlates with the duration, extent and severity of IBD activity.5 While estimates vary, ~2% of individuals diagnosed with ulcerative colitis will develop CAC by 10 years after symptoms emerge, and 18% by 30 years, verses a 5.2% lifetime risk of developing CRC for the US population12, 13. Importantly, CAC often develops earlier in life and progresses more quickly than sporadic CRC, frequently affecting young persons in their prime productive years.
Several recent and excellent reviews highlight differences between CAC and sporadic CRC.5, 14, 15 Notably the molecular steps and sequence of acquired genetic mutations vary between the two. For example, the loss of APC gene function is considered a crucial early step in sporadic CRC, but occurs late in CAC tumors.10 In CAC, reactive oxygen species (ROS) and reactive nitrogen species (RNS) produced by both immune cells and the inflamed epithelium are a primary sources of DNA damage.9 Ultimately, mutations in the Wnt/APC and/or inflammatory signaling pathways such as PI3K/AKT support dysregulated β-catenin activity which in turn leads to the transcription of genes such as Cyclins, Axin2 and c-Myc that promote proliferation and tumor growth.16 While CAC is a model disease to examine links between chronic inflammation and neoplasia, inflammation and immune cell infiltration also comprise a component of all CRC.1 Once colon neoplasia begins to form, immune cells are invariably recruited to the tumor site. This immune cell infiltration can be somewhat paradoxical, as immune cells both contribute to tumorigenesis and participate in clearance of the tumor. Cytokines produced by the tumor-associated immune cells, including TNF-α, IFNγ, IL-1β and IL-6, induce ROS and RNS in the tumor which potentiate genetic damage and tumor progression.9 Conversely, the presence of natural killer and T lymphocytes in tumors correlates with better clinical outcome and longer survival in patients with CRC17–19. Despite the infiltration of natural killer and T cells, tumors are often able to evade cytolysis, indicating that mechanisms of immune suppression are present in the tumor milieu.20 These findings highlight as an important focus in CRC research to target interruption of this process, known as immunoediting, with immunotherapies.
CURRENT THERAPEUTICS ILLUSTRATE A LINK BETWEEN INFLAMMATION AND COLON CANCER
In setting the stage for a detailed examination of tryptophan metabolism’s role in inflammation and CRC, it is useful to have perspective on how current therapeutics fit into this paradigm. To date no immunotherapy is approved for CRC. However, observations from currently available therapeutics provide support for this concept and insight into the complex relationship between colon inflammation and cancer. Aspirin and NSAIDs, common anti-inflammatory drugs, reduce the risk of sporadic CRC in some individuals.21 Aminosalycilates (mesalamine), integral maintenance anti-inflammatories for IBD, decrease the risk of CAC.22 NF-κB modulation, may contribute to mesalamine’s chemo-preventative properties. TNFα inhibitors, another potent mainstay of IBD therapy, reduces tumorigenesis in experimental CAC models,23 but it is not yet clear if they do so in humans.
Stimulating the immune system is also recently recognized as an important additional function of some cytotoxic CRC therapies. Chemotherapy regimens for cancer proximal to the rectum are based on the pyrimidine analog 5-Fluorouracil (5-FU) and leucovorin. Radiation therapy is added for rectal cancers and works in synergy with 5-FU. 5-FU was recently demonstrated to also shape tumor immunity by reducing myeloid derived suppressor cells which are known to promote colon tumorigenesis.24–26 The recently reported “abscopal effect” of radiation therapy in melanoma suggests that locally applied radiation can enhance anti-tumor immunity against metastatic sites.27 These findings suggest that combining cytotoxic therapies with immunotherapy may be the key to unlocking the potential of both therapies. This concept is discussed again later in this review.
TRYPTOPHAN METABOLISM AND INFLAMMATION
Tryptophan metabolizing enzymes
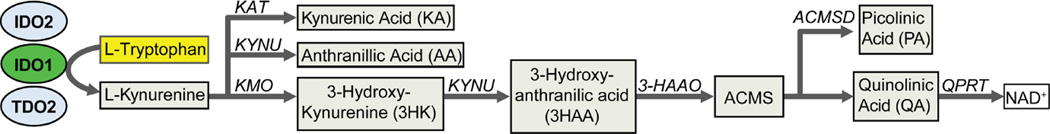
Tryptophan metabolism is one important mechanism exploited by cancers to evade immune surveillance.28–31 Tryptophan is the most essential amino acid and 95% of dietary tryptophan is metabolized along the kynurenine pathway (KP, Figure 1).32 Indoleamine 2,3 dioxygenase 1 (IDO1), the first and rate limiting step in this pathway, has important roles in limiting adaptive immune responses in a variety of both inflammatory and malignant diseases.
Figure 1. Tryptophan metabolism along the kynurenine pathway.
The principle enzymes (IDO1, IDO2 and TDO) are shown. The bioactive metabolites downstream of kynurenine are shown with the respectively required enzymes. KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase; KYNU, kynureninase; 3-HAAO, 3-hydroxyanthranillic acid oxygenase; ACMS, 2-Amino-3-carboxymuconate semialdehyde; ACMSD, Aminocarboxymuconate-semialdehyde decarboxylase; QPRT, quinolinic acid phosphoribosyl transferase. NAD+, nicotinamide adenine dinucleotide (oxidized form)
Two other enzymes, indoleamine 2, 3 dioxygenase 2 (IDO2) and tryptophan dioxygenase (TDO or TDO2) also metabolize tryptophan along the KP.33 IDO1 and IDO2 are similar in both structure, while TDO is structurally unique34. At baseline, IDO1 is widely expressed across most tissue and cells types, while tissue distribution is much more limited for IDO2 (epididymis and kidney) and TDO (Liver). However, all three have been shown to be expressed in a variety of cancers35–37. As IDO1 is the most well studied of these enzymes and is the principle enzyme expressed in the inflamed and malignant gut, it will be the primary focus of this review.
IDO1: the immune modulator
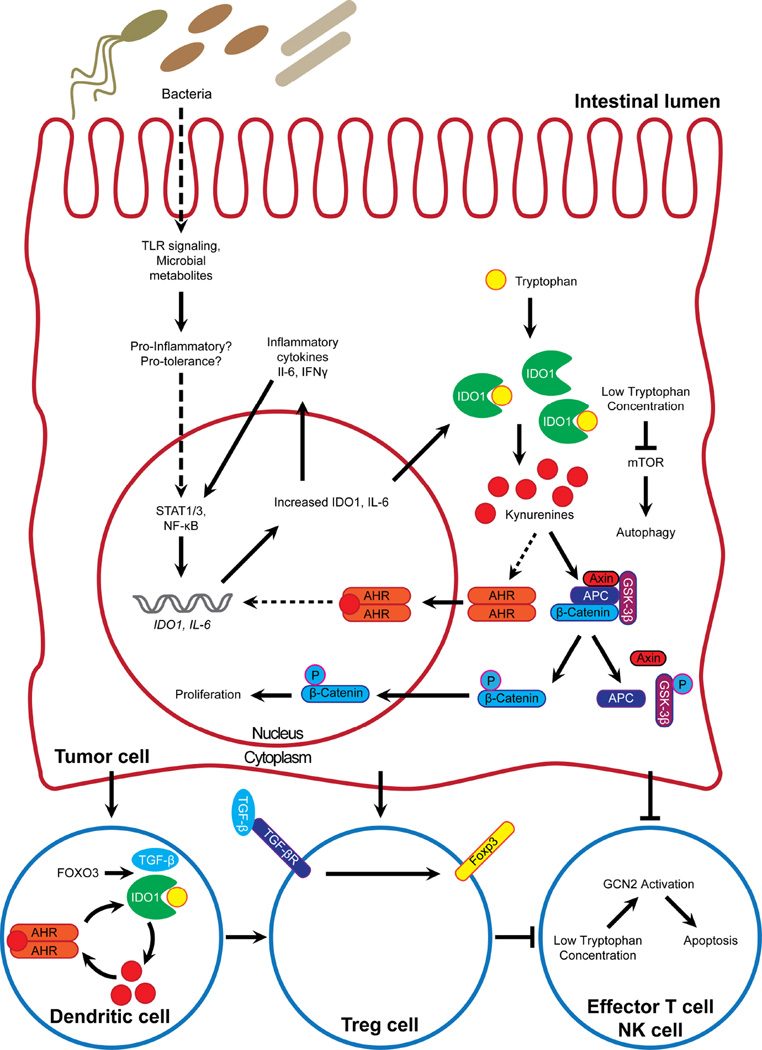
IDO1 as a mediator of immune tolerance was first described for its role at the maternal-fetal interface by Munn et al. in 1998.38, 39. This group described the now classical mechanism whereby IDO1 expression in professional antigen presenting cells (APCs, monocytes/macrophages, dendritic cells) reduces local tryptophan concentrations, which reduces T cell proliferation and thus inhibits T cell-mediated immune response (Figure 2). Mechanistic studies eventually determined that local tryptophan depletion is itself immunosuppressive, but that these effects are also mediated by several KP metabolites30, 40. Low levels of tryptophan in the local microenvironment activate stress-response pathways, including GCN2 kinase and mTOR41, 42. Some KP metabolites bind the aryl hydrocarbon receptor (AHR) to promote forkhead box (FOX) P3+ regulatory T cell (Tregs) differentiation43–46. The function of kynurenines as promoters of tolerance is a theme reflected in numerous immunological contexts, including protection of transplanted tissues and reduction in immune response to pathogen infection47–50.
Figure 2. Schematic of confirmed and postulated roles and interactions of IDO1 in colorectal cancer.
IDO1 modulates signaling pathways in both cell autonomous and non-autonomous fashion. Solid lines represent interactions demonstrated in colorectal cancer, while dotted lines represent interactions extrapolated from other cell or tissue contexts.
A non-enzymatic mechanism by which IDO1 functions as a tolerogenic signaling molecule in plasmacytoid DCs has also been described46, 51. This mechanism is mediated by phosphorylation of IDO1’s immunoreceptor tyrosine-based inhibitory motifs by Src family kinases and requires co-stimulation with TGF-β46, 51, 52. These signals in turn activate non-canonical NF-κB signaling to induce type I IFNs46, 52.
IDO1 in colitis
The colon is a site of high IDO1 expression even in the homeostatic state. In this basal state, IDO1 is predominately in myeloid derived cells of the lamina propria. IDO1 expression is strongly induced by inflammatory cytokines including IFNγ, TNFα and IL-1β and, as such, is thus one of the most upregulated genes in mouse models of colitis and human inflammatory bowel disease (IBD)4. In the inflamed colon, epithelial cells become a major IDO1 expressing cell type. Functionally relevant IDO1 gene polymorphisms correlate with a more severe disease phenotype in Crohn’s disease.53 Genetic or pharmacological inhibition of IDO1 worsens the severity of colitis in mouse models54, 55. Induction of IDO1 by a toll-like receptor (TLR) 9 agonist limits murine disease severity in both the T-cell driven 2,4,6-Trinitrobenzenesulfonic acid (TNBS) and epithelial cell disruption driven dextran sodium sulfate (DSS) models of colitis56. These studies indicate IDO1 expression acts as a natural brake in limiting colitis. However, mice with germline deletion of IDO1 do not demonstrate spontaneous colitis, suggested the existence of complementary tolerance promoting pathways exist.
In human IBD, IDO1 activity tracks with disease activity and is expressed in both epithelial and myeloid derived cells.57 In IBD, chronic inflammation can cause DNA damage by oxidative and nitrosative stress, resulting in genetic and epigenetic alterations10, 58 placing these patients at a higher risk for developing CAC9, 10. As such, in IBD patients with chronic uncontrolled colitis, chronic IDO1 overexpression may take on a pathogenic function to promote CAC.
TRYPTOPHAN METABOLISM PROMOTES TUMOR IMMUNE ESCAPE
IDO1’s function in tempering host response to both allogenic and pathogenic antigens hastened the discovery of its role in tumoral escape from immune surveillance. The majority of tumors are infiltrated by immune cells, indicating tumor cells are able to illicit an immune response19. However, tumor cells are often able to evade cytolysis, suggesting mechanisms of immune suppression are present in the tumor milieu59. IDO1 expression has been described as one such mechanism in several cancers60–62. IDO1-expressing tumor-associated dendritic cells inhibit cytotoxic T lymphocyte proliferation and activity62, 63. In dendritic cells of tumor-draining lymph nodes, the transcription factor FOXO3 induces expression of both IDO1 and TGF-β, indicating a tolerance pathway parallel to normal immune function63. In ovarian cancer, tumor-associated macrophages were shown to produce CCL-22, which recruits Tregs to the tumor microenvironment64. Additionally, expression of cytotoxic T-lymphocyte associated protein 4 (CTLA-4) by Tregs has been shown to induce IDO1 expression in DCs, suggesting a potential immunosuppressive feedback loop in cancer-associated immune cells64, 65.
Inhibition of IDO1 increases anti-tumor immunity in a number of tumor models60, 61, 66, 67. Inhibition of IDO1 by 1-methyltryptophan (1mT) reverses T cell inhibition in dendritic cells derived from tumor-draining lymph nodes in a murine model of melanoma and breast cancer68. These effects have also been reported for TDO and for the IDO2 inhibitor or D-1mT (indoximod).6, 31
IDO1 expression in cancer is not limited to tumor-associated immune cells. A survey of both cancer cell lines and human cancer tissue sections revealed that the majority of carcinoma cells also express IDO166. Theate and colleagues recapitulated these findings by surveying a collection of normal and cancerous human tissues, although their findings illustrate that the cellular source of IDO1 in tumors can include cells from the stroma, endothelium, and the primary neoplastic epithelial cells69. IDO1 expression in these tumors are predicted to contribute to an immunosuppressive tumor microenvironment by tryptophan depletion60, 66, 69, 70.
Tryptophan metabolism in colon cancer
Colon cancers frequently exhibit IDO1 expression in primary tumor and infiltrating myeloid derived cells3, 66, 69. Several studies have shown reduced tryptophan levels and increased kynurenine pathway metabolites in colon cancer patients, indicating an increased IDO1 activity71–73. High IDO1 expression at the tumor invasion front is an independent adverse prognostic factor for overall survival and metachronous CRC metastases, and high density of IDO1 expressing cells in the tumor draining lymph nodes was associated with a reduced 5 year survival rates in colon cancer patients3, 74, 75.
IDO1 expression appears to be an important early event in the development of colon dysplasia and may be driven by the genetic changes associated with CRC. Rats exposed to the carcinogen azoxymethane (AOM) express IDO1 in early aberrant crypt foci (ACF).76 The development of these tumor precursor lesions was reduced with 1mT. KRAS and β-Catenin mutations are common in AOM-induced pre-neoplastic lesions in rats. Though it is not known if these mutations drive the expression of IDO177 in CRC, this has been in a model of KRAS driven lung carcinoma.78
Constitutive expression of IDO1 is also present in some CRC cell lines like HCT-116 and HT-29, but not in Caco-22, 74, 79. As this expression occurs in the absence of an inflammatory tumor milieu, it suggests IDO1 expression may be related to the genetic changes that drive CRC.80, 81 In support of this, our lab has found that IDO1 expression is higher in the colon epithelial cells from Apcmin/+ mice compared to WT controls (unpublished data). Studies performed on tumor tissues with genetic alterations in APC, KRAS or p53 alone could help identify the precise regulation of IDO1 by these genetic alterations82.
Other studies also identify links between cancer associated genes and IDO1 expression. In a mouse model of esophageal carcinoma, overexpression of the extracellular matrix protein periostin and a mutant p53 induced STAT1, which in turn induced IDO183. IDO1 is also a known genetic target of the transcriptional repressor BIN167. Only one published study has evaluated this relationship in CRC and did not identify a significant correlation.75 Understanding the mechanisms responsible for IDO1 expression in CRC should facilitate the identification of novel ways to target this pathway in cancer and thus deserves further investigation.
In CRC and CAC, inflammatory pathways likely also drive IDO1 expression. The IDO1 promoter region contains two interferon-sensitive response elements and two interferon-γ activated sites, and therefore is upregulated in response to a number of inflammatory stimuli84, 85. Litzenburger and colleagues recently identified an autocrine signaling loop whereby IDO1 sustains its own expression via AHR-mediated IL-6 expression and STAT3 activation85. Though not this study’s focus, this pathway is certainly relevant in CRC and especially so in CAC.
IDO1 expression directly promotes proliferation of colon cancer
We identified a key role for IDO1 in promoting tumorigenesis in the AOM/DSS mouse model of CAC.2 Germline IDO1−/− mice developed tumors that were smaller, had a lower tumor proliferation index and reduced nuclear β-catenin. These findings were recapitulated in mice treated with the IDO1 inhibitor 1mT. We went on to identify that this effect was independent of IDO1’s impact on adaptive immunity as the phenotype was also observed Rag1 null mice. Moreover, we found a specific role for epithelial IDO1 by demonstrating that gene silencing reduced proliferation in CRC cell lines which constitutively expressed IDO1. Proliferation could be normalized by addition of exogenous kynurenines, though the upstream signaling events were not yet identified. These findings together support a new paradigm for dual functions of IDO1 in colon neoplasia to both promote adaptive immune-tolerance and to directly promote tumor cell growth.
IDO1 functions at the host-microbe interface
Luminal microbiota can have a profound influence GI health including the development and progression of colitis and colon cancer.86, 87 Resident microbes interact with host cells directly by binding pattern recognition receptors (PRRs) and indirectly by secreted metabolites88, 89. An increasing body of evidence indicates IDO1 is an important player in host-microbe crosstalk, which may impact the pathophysiology of gastrointestinal cancer. One way IDO1 is involved in host-microbe interactions is at the level of PRRs. Expression of IDO1 is induced in response to activation of several PRRs, especially TLR4 and TLR956, 90. CpG oligonucleotides (CpG-ODNs), a Toll-like receptor (TLR) 9 agonist induces IDO1 throughout the GI tract and protect from murine colitis56. Additionally, activation of TLR4 by lipopolysaccharide is important in promoting a pro-tolerance environment dependent on IDO1 expression52, 91.
However, the physiologic impact of IDO1 and TLR signaling is dependent on both cell type and disease context. For example, contrary to these findings, in a mouse model of sepsis, IDO1 activity exacerbates inflammation and disease activity by increasing the sensitivity of TLR492. This is important in considering the suggested role of TLR signaling in cancer. One study of colon polyps from 70 human patients revealed that, while TLR7 and TLR9 expression is higher in hyperplastic or adenomatous polyps, TLR9 expression in polyps from patients which formed CRC was decreased93. Treatment with CpG-ODNs has also been shown to enhance the efficacy of chemotherapeutic agents in several mouse tumor models90, 94, 95. Additionally, treatment with an IDO1 inhibitor augments the anti-tumor effects of the TLR7 agonist imiquimod in mice inoculated with colon carcinoma cells96. These findings suggest TLR signaling, and perhaps the associated IDO1 expression, has differential roles in normal and cancer tissues90, 97.
Secreted bioactive metabolites represent another way in which microbial populations interact with the host and has likely relevance to CRC88, 89, 98–100. Two classes of microbial metabolites with particular relevance to tryptophan metabolism and CRC are short chain fatty acids and indole compounds101. Short chain fatty acids such as acetic, propionic, and butyric acid play significant roles in intestinal homeostasis and resistance to tumorigenesis102–105. Butyrate-producing microbial populations are reduced in CRC-associated microbial dysbiosis106. While not yet evaluated in CRC, butyrate reduces IDO1 expression in some epithelial carcinomas which could contribute to its tumor suppressive properties102, 104. Indole compounds are generated from dietary tryptophan by commensal colonic microbiota. Several of these indole metabolites can in turn activate the aryl hydrocarbon receptor (AHR)89, 107, which has important roles in several cancers and is discussed in greater detail below. The emerging field of microbial metabolomics further highlights the importance of the gut microbiota in gastrointestinal cancer pathophysiology108. Figure 2 illustrates potential mechanisms by which IDO1 may be involved in host-microbial interactions.
COLON CANCER RELEVANT PATHWAYS THAT INTERSECT WITH IDO1
The aryl hydrocarbon receptor
First identified as a dioxin detoxifying enzyme, AHR is a cytoplasmic transcription factor which is activated by a variety of compounds109. A number of tryptophan metabolites produced by both host and microbes act as agonists for this receptor35, 36. Activation of AHR is an important mechanism by which IDO1 expression promotes immune tolerance43, 44. AHR effect on pathology appears to depends on the cancer type109. Constitutive AHR activation leads to the spontaneous gastric tumors110. In brain tumors, kynurenines derived from TDO2 activate AHR to promote clonogenic survival and malignant progression35, 111. A number of studies have shown that AHR activation leads to enhanced tumor cell proliferation109. However, the role of ligand-activated AHR in colon cancer pathogenesis is less clear.107, 112 One study suggested that AHR may actually be a tumor suppressor in CRC as its genetic ablation led to β-catenin accumulation and increased cecal tumor formation in both wildtype and ApcMin/+ mice107. Finally, it is intriguing to note that in breast and ovarian cancer AHR activation and IDO1 induction appear to be in a feedback loop with one another (Figure 2)43, 85. Further defining of the interactions between IDO1 and AHR in CRC is warranted113, 114.
Quinolinic acid dependent NAD+ production offers resistance to apoptosis
An important characteristic of cancer cells is their ability to counter cellular stress in the setting of hypoxia and high proliferative activity. Nicotinamide adenine dinucleotide (NAD+) plays a key role as both a substrate and an enzyme cofactor in maintaining cellular integrity under these conditions.115 As such, cancer cells rapidly consume NAD+ and require alternative synthesis pathways (other than via nicotinamide). The tryptophan-kynurenine pathway generates Quinolinic Acid (QA) is an alternative substrate for NAD+ synthesis in a step involving the enzyme quinolinate phosphoribosyltransferase (QPRT). QA, as a precursor to NAD+, has recently been identified as an important factor in conferring resistance of gliomas to oxidative stress.116 QPRT expression was induced in glioblastoma patients who underwent chemotherapy. High QPRT expression was associated with poor prognosis, indicating glioma cells resisted apoptosis to chemotherapy by inducing de novo NAD+ synthesis116. Colon carcinoma cells also express QPRT117. If colon carcinoma cells produce NAD+ from QA, antitumor efficacy of IDO1 inhibitors could potentially be enhanced by concurrent blockade of other enzymes involved in NAD+ synthesis such as nicotinamide phosphoribosyltransferase using the specific inhibitor FK866.
Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2)
iNOS and COX-2 are known mediators of colon carcinoma progression118–121. iNOS expression is reported in aberrant crypt foci (ACF) of both rats and humans121, 122. In humans, enhanced iNOS staining was found in ACF transitioning from hyperplasia to dysplasia78. Several studies have shown that iNOS can up-regulate COX-2 expression123–127 and COX-2 has been shown to upregulate IDO1 expression in some cases128–130. Clarifying the relationship between the complementary pathways may lead to the identification of novel and synergistic was to target CRC.
OTHER GASTROINTESTINAL CANCERS
Tryptophan metabolism along the kynurenine pathway is relevant in other gastrointestinal tumors, though the data is less well developed. We will briefly discuss this pathway in gastric, pancreatic, esophageal and stromal tumors. As each of these tumors is typically diagnosed at a late stage and has a poor prognosis, it is exciting to think that inhibitors of this pathway may have a role in improving therapeutic outcomes.
Gastric carcinoma
IDO1 is overexpressed in up to 90% of human gastric carcinomas66, 131. Zhang et al reported the expression of IDO1 correlated significantly with tumor invasion depth, lymph node positivity and reduced tumor infiltration with CD4+ and CD8+ T cells132. Other human specimen and cell culture studies indicate that gastric tumor cells may promote immune escape via IDO1 activity133, 134. One of these studies intriguingly tied expression of FoxP3 in gastric carcinoma cells to IDO1 expression.133
Pancreatic carcinoma
Enhanced expression of IDO1 is reported in pancreatic carcinoma in humans66. Overexpression of IDO1 in metastatic pancreatic ductal adenocarcinoma (PDA) was shown to be associated with increased T cell numbers135. This study also reported that IDO1 expression was present in both primary and metastatic tumors in all the patients with lymph node metastasis, and suggested that PDA use IDO1 mediated immune escape mechanisms to survive in the lymph node. The same group later published another study showing that IDO2 was also expressed in PDA and should be considered as a target for inhibition along with IDO1136. IDO1 inhibitor INCB023483 was shown to inhibit the growth of murine PAN02 pancreatic cell-derived tumors in WT and IDO1−/− mice, highlighting that tumor-derived IDO1 contributes to pancreatic tumor growth and immune evasion.137
Esophageal carcinoma
Enhanced expression of IDO1 is observed in esophageal carcinoma and its expression correlates with poor survival138, 139. IDO1 expression correlated with a lower number of tumor infiltrating lymphocytes139. In a mouse model of p53 mutant esophageal cancer, the expression of IDO1 was shown to be associated with expression of periostin, an extracellular matrix protein involved in esophageal carcinoma metastasis. In this model, periostin induced expression of IDO1 via STAT1 activation, indicating an intersection between inflammatory signaling pathways and malignancy in esophageal carcinoma83.
Gastrointestinal stromal tumors (GIST)
GIST account for less than 1% of all GI cancers but are the most common non-epithelial GI tumor. Imatinib is a drug which targets mutated KIT oncoproteins and produces clinical response in 80% of patients. In a mouse model of spontaneous GIST, activation of CD8+ T cells was found to be key to the antitumor effects of Imatinib and that this effect involved IDO1140. Moreover, concomitant CTLA-4 blockade of T cell co-stimulation further enhanced Imatinib efficacy suggesting a role for dual immunotherapies as a strategy for GIST.
THERAPEUTIC TARGETING OF TRYPTOPHAN METABOLISM PATHWAYS FOR COLORECTAL CANCER
IDO1 is a well-established target for drug discovery in cancer immunotherapy. Outstanding recent reviews have detailed IDO1 inhibitors and other inhibitors of tryptophan metabolism.31 Here we will briefly summarize the topic with a focus on application to CRC. We also highlight that IDO1 inhibitors’ role in clinical oncology will most likely be as a combined, rather than monotherapy.
Pharmacologic Inhibitors
The most commonly used inhibitor of IDO1 in preclinical studies is the methylated tryptophan molecule termed 1-methyl Tryptophan (1mT). Studies have used the L and D enantiomers alone or in combination. While the L-enantiomer is more specific for blocking IDO1 activity, its practical implementation for human clinical applications it limited by low potency, poor solubility and likely off target effects.4, 31 The D-enantiomer of 1mT is being evaluated in clinical trials (indoximod, NLG8189). IDO2 appears to be its main target as an enzymatic inhibitor.79, 141 However, IDO2 has also been demonstrated to impact IDO1 function and T-cell suppression.142 NewLink Genetics also has an IDO1 inhibitor, NLG919, which increased the efficacy of Indoximod against a preclinical model of melanoma.
A novel hydroxyamidine small molecule (INCB24360) was recently described as a potent specific inhibitor of IDO1. It does not affect IDO2, TDO or tryptophan transporter THP-1 and has a more favorable pharmacokinetic profile than 1mT with better oral bioavailability.137, 143 INCYTE, the maker of this compound, sponsored a Phase I clinical trial with INCB024360 in 52 patients with advanced solid tumors which had failed prior therapies.143, 144 29 (55.8%) of enrolled patients had colon cancer. This compound demonstrated safety and a maximum tolerable dose was not established. However, at doses ≥300 mg BID produced biochemical efficacy by lowering the serum Kyn/Trp ratio, a marker of IDO1 activity.143 No patients in this study demonstrated complete or partial response, but nearly 30% did demonstrate stable disease for eight weeks. Based on these results INCB24360 is currently being evaluated as a combination with Ipilimumab for melanoma in a phase Ib/2 study and advanced ovarian cancer as a monotherapy. This agent is not currently being targeted for CAC or CRC, but these results would suggest that it will likely need to be combined with another therapeutic.
TDO expression is another way by which some tumors pathogenically exploit tryptophan metabolism.36 The TDO pathway is important because IDO1 inhibitors typically do not target its activity and it is possible that IDO1 inhibition could enhance TDO expression or function. Some studies suggest that TDO is also expressed in a subset of CRC,35 a finding corroborated by our query of TCGA datasets145, 146 (Table 1). However, even in CRC, the expression of TDO appears to be ~100 fold less than in the normal liver.36 TDO specific inhibitors are described in preclinical models,36 but have not yet moved clinical trials. Hepatotoxicity is a potential concern given that TDO is constitutively expressed in the liver where it plays a role in maintaining systemic tryptophan levels. Specific gene silencing of both IDO1 and TDO in specific cell types may be one way to get around this problem.
Table 1. Correlation of IDO1 and related gene expression in colon cancer.
Gene expression profiling of colorectal adenocarcinoma samples relative to IDO1 expression. Gene expression data from colon and rectal adenocarcinomas (cbioportal) were analyzed using Graphpad Prism 5 to identify correlations in gene expression patterns relative to IDO1. Data generated by the TCGA Research Network: http://cancergenome.nih.gov/. TCGA provisional Colorectal Adenocarcinoma Dataset, Illumina HiSeq V2 RNA expression data. N=365. Two-tailed P values are reported for Pearson’s correlation coefficient (r).
| GENE | PEARSON (r) | CORRELATION WITH IDO1 | P VALUE |
|---|---|---|---|
| IDO2 | 0.265 | Positive | <0.0001 |
| TDO2 | −0.013 | Negative | 0.8115 |
| IL10 | 0.171 | Positive | 0.001 |
| STAT1 | 0.621 | Positive | <0.0001 |
| TGFB1 | 0.248 | Positive | <0.0001 |
| AHR | 0.139 | Positive | 0.0079 |
Recently, investigators probed CRC tissue micro-arrays using a new and proprietary L-kynurenine specific antibody.147 Positivity was noted in the neoplastic epithelium of 20% of 69 CRC samples and was not detected in normal colon epithelial cells. Positivity of IDO1 and kynurenine staining overlapped in only 42% of cases. Immunostaining for IDO2 and TDO were not reported, though it is inferred that these may be the source of kynurenine production. Bacterial produced kynurenine may be another consideration. There are inherent limitations to immunohistochemistry based scoring and selection bias confounds interpretation of tissue micro-arrays. However, if confirmed, these findings suggest that IDO1 inhibition alone may not be sufficient to counter the pathogenic functions of the kynurenine pathway in CRC. It is conceptually intriguing that antibodies against kynurenine could fill that gap, but physiologic proof of efficacy is not yet available.
Gene silencing of IDO1
Genetic manipulation is another promising option for targeting tryptophan metabolism in cancer. Using RNA interference, Zheng and colleagues illustrated that silencing IDO1 reduced melanoma tumor growth both in vitro and in vivo.148 This same group subsequently demonstrated that silencing IDO1 in dendritic cells which have been loaded with murine breast cancer antigens significantly enhanced the efficacy of this DC-based vaccine approach.149 An exciting feature of this approach is that it can be targeted to specific cell types and can inhibit not only IDO1, but other tryptophan metabolizing enzymes concurrently. Furthermore, unlike pharmacologic inhibitors, gene silencing also eliminates any non-enzymatic tolerance promoting capabilities of the IDO1 protein.46 The startup company formed based on these discoveries was recently bought by a large pharmaceutical company.
IDO inhibitors as Combinatorial Therapy
How IDO1 inhibitors will most effectively be employed in the clinical setting is an active area of investigation. It is likely that these agents will be most effective when used in combination with other immune checkpoint inhibitors or with traditional cytotoxic therapies. In most preclinical models, just as with the Phase I clinical trials, IDO1 inhibition alone slowed neoplastic growth but did not eliminate or prevent the tumors. This slowing of tumor growth was found in a preclinical model of a CRC using a syngeneic tumor implant and IDO1 inhibition with INCB024360.137
There are currently no clinical trials specifically targeting IDO1 in combination with other cytotoxic or immunotherapies for CRC. However, in breast cancer cell lines IDO1 gene silencing enhanced the effect of radiation therapy, suggesting this may be an effective option.150 Additionally, INCB24360 is being evaluated in combination with programmed cell death (PD-1) inhibitors in ongoing Phase I/II clinical trials for other solid tumors. One of these two trials does include metastatic CRC patients who have progression on or intolerance of approved standard therapies. The recent report identifying that an anti-PD1 inhibitor had some effect in subgroups of sporadic CRC suggests promise for this approach151.
Alternatives Approaches to Targeting IDO1
Beyond direct inhibition of IDO1, Platten and colleagues recently proposed five conceptually distinct hubs that may serve as potential therapeutic targets for interfering with tryptophan catabolism in the context of cancer.31 These include: 1) TDO and IDO2 inhibitors which may have enhanced function when IDO1 is inhibited. 2) Upstream regulators of IDO1 such as KIT, STAT3 and BIN1 3) Downstream targets affected by IDO1 mediated tryptophan depletion including mTOR and GCN2. 4) Kyurenine pathway receptors such as AHR and GPR35. 5) Cell membrane tryptophan transporters which could modify intra-and extracellular levels of tryptophan.
CONCLUSIONS AND FUTURE DIRECTIONS
Activated tryptophan metabolism is a common feature in colorectal cancer and preclinical data indicates that this pathway promotes neoplastic disease progression. Orally administered small molecule inhibitors of IDO1, the most prevalent enzyme in this pathway, have demonstrated safety and tolerability in Phase I human trials. These agents appeared to slow, but not halt progression of solid tumors including colorectal cancer. Thus, key questions remain as to how these agents might be used in CRC. Should patients be selectively chosen based on demonstrated IDO1 overexpression? Could quantitative grading of IDO1 expression in tumor biopsy specimens be used to identify the subset of patients in which inhibitors might be most effective? What is the clinical prevalence and distribution of high IDO1 expression among CRC subtypes? Could IDO1 inhibitors have enhanced efficacy by combining them with currently available cytotoxic therapies or other immune checkpoint inhibitors in development. Do alternative tryptophan metabolizing enzymes including IDO2 or TDO also have important functional roles in CRC? Clarifying the answers to these questions should guide the application of a novel and low toxicity therapeutic option for patients with advanced colon cancer.
ACKNOWLEDGEMENTS
Support for our research as described herein comes from W. M. Keck Fellowship (DMA) and grants DK100737, AI095776, DK089016 and from P30 DK052574 to the Washington University Digestive Diseases Research Cores Center. Additional support comes from the Givin’ It All For Guts Foundation (www.givinitallforguts.weebly.com). Colorectal adenocarcinoma data from cBioportal.org was generated by the TCGA Research Network: http://cancergenome.nih.gov/.
ABBREVIATIONS
- CRC
colorectal cancer
- CAC
colitis-associated cancer
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- DNA
deoxyribonucleic acid
- APC
adenomous polyposis coli
- KRAS
Kirsten rat sarcoma
- GSK-3β
glycogen synthase kinase 3 beta
- TNF
tumor necrosis factor
- IFN
interferon
- IL
interleukin
- DC
dendritic cell
- KP
kynurenine pathway
- IDO1
indoleamine 2,3 dioxygenase 1
- IDO2
indoleamine 2,3 dioxygenase 2
- TDO2
tryptophan dioxygenase
- APCs
antigen presenting cells
- GCN2
general control nonderepressible 2
- mTOR
mammalian target of rapamycin
- AHR
aryl hydrocarbon receptor
- TGF-β
transforming growth factor β
- Tregs
regulatory T cells
- NF-κB
nuclear factor kappa B
- TLR
toll-like receptor
- TNBS
2,4,6-Trinitrobenzenesulfonic acid
- DSS
dextran sodium sulfate
- AOM
azoxymethane
- 1mT
1-methyltryptophan
- CCL
CC chemokine ligand
- CD
cluster of differentiation
- CTLA-4
cytotoxic T-lymphocyte associated protein 4
- STAT
signal transducers and activators of transcription
- QPRT
quinolinate phosphoribosyltransferase
- MSCs
Mesenchymal stem cells
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- COX-2
cyclooxygenase 2
- ACF
aberrant crypt foci
- PDA
pancreatic ductal adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors declare that no conflicts of interest to disclose and all authors have read the journal's policy on disclosure of potential conflicts of interest.
REFERENCES
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaker AI, Rao MS, Bishnupuri KS, et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology. 2013;145:416–425. e1–e4. doi: 10.1053/j.gastro.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferdinande L, Decaestecker C, Verset L, et al. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141–147. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–152. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–1452. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 6.Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014 doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 8.Xiang B, Snook AE, Magee MS, et al. Colorectal cancer immunotherapy. Discov Med. 2013;15:301–308. [PMC free article] [PubMed] [Google Scholar]
- 9.Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horner MJRL, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK. SEER Cancer Statistics Review. Vol. 2009. Bethesda, MD: National Cancer Institute; 1975–2006. SEER Stat Fact Sheets - Cancer of the Colon and Rectum. 2009:based on November 2008 SEER data submission. [Google Scholar]
- 14.Low D, Mino-Kenudson M, Mizoguchi E. Recent advancement in understanding colitis-associated tumorigenesis. Inflamm Bowel Dis. 2014;20:2115–2123. doi: 10.1097/MIB.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldner MJ, Neurath MF. Mechanisms of Immune Signaling in Colitis-Associated Cancer. Cellular and Molecular Gastroenterology and Hepatology. 1:6–16. doi: 10.1016/j.jcmgh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 17.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 21.Nan H, Hutter CM, Lin Y, et al. Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313:1133–1142. doi: 10.1001/jama.2015.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velayos FS, Loftus EV, Jr, Jess T, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941–1949. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, DuBois RN. Myeloid-derived suppressor cells link inflammation to cancer. Oncoimmunology. 2014;3:e28581. doi: 10.4161/onci.28581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katoh H, Wang D, Daikoku T, et al. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanterman J, Sade-Feldman M, Biton M, et al. Adverse immunoregulatory effects of 5FU and CPT11 chemotherapy on myeloid-derived suppressor cells and colorectal cancer outcomes. Cancer Res. 2014;74:6022–6035. doi: 10.1158/0008-5472.CAN-14-0657. [DOI] [PubMed] [Google Scholar]
- 27.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast GC. Cancer: Why tumours eat tryptophan. Nature. 2011;478:192–194. doi: 10.1038/478192a. [DOI] [PubMed] [Google Scholar]
- 30.Lob S, Konigsrainer A, Rammensee HG, et al. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 31.Platten M, von Knebel Doeberitz N, Oezen I, et al. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. 1991;294:345–358. doi: 10.1007/978-1-4684-5952-4_32. [DOI] [PubMed] [Google Scholar]
- 33.Austin CJ, Rendina LM. Targeting key dioxygenases in tryptophan-kynurenine metabolism for immunomodulation and cancer chemotherapy. Drug Discov Today. 2014 doi: 10.1016/j.drudis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Ball HJ, Jusof FF, Bakmiwewa SM, et al. Tryptophan-catabolizing enzymes - party of three. Front Immunol. 2014;5:485. doi: 10.3389/fimmu.2014.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 36.Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109:2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platten M, Wick W, Van den Eynde BJ. Tryptophan Catabolism in Cancer: Beyond IDO and Tryptophan Depletion. Cancer Res. 2012;72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 38.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Shafizadeh E, Attwood JT, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fougeray S, Mami I, Bertho G, et al. Tryptophan depletion and the kinase GCN2 mediate IFN-gamma-induced autophagy. J Immunol. 2012;189:2954–2964. doi: 10.4049/jimmunol.1201214. [DOI] [PubMed] [Google Scholar]
- 42.Metz R, Rust S, Duhadaway JB, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mezrich JD, Fechner JH, Zhang X, et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 47.Zaher SS, Germain C, Fu H, et al. 3-hydroxykynurenine suppresses CD4+ T-cell proliferation, induces T-regulatory-cell development, and prolongs corneal allograft survival. Invest Ophthalmol Vis Sci. 2011;52:2640–2648. doi: 10.1167/iovs.10-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romani L, Puccetti P. Controlling pathogenic inflammation to fungi. Expert Rev Anti Infect Ther. 2007;5:1007–1017. doi: 10.1586/14787210.5.6.1007. [DOI] [PubMed] [Google Scholar]
- 49.Cobbold SP, Adams E, Farquhar CA, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howie D, Waldmann H, Cobbold S. Nutrient Sensing via mTOR in T Cells Maintains a Tolerogenic Microenvironment. Front Immunol. 2014;5:409. doi: 10.3389/fimmu.2014.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pallotta MT, Fallarino F, Matino D, et al. AhR-Mediated, Non-Genomic Modulation of IDO1 Function. Front Immunol. 2014;5:497. doi: 10.3389/fimmu.2014.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee A, Kanuri N, Zhang Y, et al. IDO1 and IDO2 Non-Synonymous Gene Variants: Correlation with Crohn's Disease Risk and Clinical Phenotype. PLoS One. 2014;9:e115848. doi: 10.1371/journal.pone.0115848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gurtner GJ, Newberry RD, Schloemann SR, et al. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 55.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 56.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn's disease activity. Inflamm Bowel Dis. 2012;18:1214–1220. doi: 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 60.Friberg M, Jennings R, Alsarraj M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 61.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 62.von Bergwelt-Baildon MS, Popov A, Saric T, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 63.Watkins SK, Zhu Z, Riboldi E, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 65.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 66.Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 67.Muller AJ, DuHadaway JB, Donover PS, et al. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 68.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 69.Theate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 70.Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889–3900. doi: 10.1038/onc.2008.35. [DOI] [PubMed] [Google Scholar]
- 71.Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 72.Walczak K, Dabrowski W, Langner E, et al. Kynurenic acid synthesis and kynurenine aminotransferases expression in colon derived normal and cancer cells. Scand J Gastroenterol. 2011;46:903–912. doi: 10.3109/00365521.2011.579159. [DOI] [PubMed] [Google Scholar]
- 73.Engin AB, Karahalil B, Karakaya AE, et al. Helicobacter pylori and serum kynurenine-tryptophan ratio in patients with colorectal cancer. World J Gastroenterol. 2015;21:3636–3643. doi: 10.3748/wjg.v21.i12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 75.Gao YF, Peng RQ, Li J, et al. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. doi: 10.1186/1479-5876-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ogawa K, Hara T, Shimizu M, et al. Suppression of azoxymethane-induced colonic preneoplastic lesions in rats by 1-methyltryptophan, an inhibitor of indoleamine 2,3-dioxygenase. Cancer Sci. 2012;103:951–958. doi: 10.1111/j.1349-7006.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith C, Chang MY, Parker KH, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012;2:722–735. doi: 10.1158/2159-8290.CD-12-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo-but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flatmark K, Maelandsmo GM, Martinsen M, et al. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–1598. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed D, Eide PW, Eilertsen IA, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith G, Carey FA, Beattie J, et al. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong GS, Lee JS, Park YY, et al. Periostin cooperates with mutant p53 to mediate invasion through the induction of STAT1 signaling in the esophageal tumor microenvironment. Oncogenesis. 2013;2:e59. doi: 10.1038/oncsis.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon-gamma-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–17252. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- 85.Litzenburger UM, Opitz CA, Sahm F, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ciorba MA. A gastroenterologist's guide to probiotics. Clin Gastroenterol Hepatol. 2012;10:960–968. doi: 10.1016/j.cgh.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arthur JC, Gharaibeh RZ, Muhlbauer M, et al. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat Commun. 2014;5:4724. doi: 10.1038/ncomms5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wells JM, Rossi O, Meijerink M, et al. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Sommariva M, De Cecco L, De Cesare M, et al. TLR9 agonists oppositely modulate DNA repair genes in tumor versus immune cells and enhance chemotherapy effects. Cancer Res. 2011;71:6382–6390. doi: 10.1158/0008-5472.CAN-11-1285. [DOI] [PubMed] [Google Scholar]
- 91.Fallarino F, Pallotta MT, Matino D, et al. LPS-conditioned dendritic cells confer endotoxin tolerance contingent on tryptophan catabolism. Immunobiology. 2015;220:315–321. doi: 10.1016/j.imbio.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 92.Hoshi M, Osawa Y, Ito H, et al. Blockade of indoleamine 2,3-dioxygenase reduces mortality from peritonitis and sepsis in mice by regulating functions of CD11b+ peritoneal cells. Infect Immun. 2014;82:4487–4495. doi: 10.1128/IAI.02113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eiro N, Gonzalez L, Gonzalez LO, et al. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol. 2012;32:848–854. doi: 10.1007/s10875-012-9666-3. [DOI] [PubMed] [Google Scholar]
- 94.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Furi I, Sipos F, Germann TM, et al. Epithelial toll-like receptor 9 signaling in colorectal inflammation and cancer: clinico-pathogenic aspects. World J Gastroenterol. 2013;19:4119–4126. doi: 10.3748/wjg.v19.i26.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ito H, Ando T, Arioka Y, et al. Inhibition of indoleamine 2,3-dioxygenase activity enhances the anti-tumor effects of a TLR7 agonist in an established cancer model. Immunology. 2014 doi: 10.1111/imm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sommariva M, De Cecco L, Tagliabue E, et al. Modulation of DNA repair genes induced by TLR9 agonists: A strategy to eliminate "altered" cells? Oncoimmunology. 2012;1:258–259. doi: 10.4161/onci.1.2.18343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nugent JL, McCoy AN, Addamo CJ, et al. Altered tissue metabolites correlate with microbial dysbiosis in colorectal adenomas. J Proteome Res. 2014;13:1921–1929. doi: 10.1021/pr4009783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen W, Liu F, Ling Z, et al. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weir TL, Manter DK, Sheflin AM, et al. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8:e70803. doi: 10.1371/journal.pone.0070803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raman M, Ambalam P, Kondepudi KK, et al. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. 2013;4:181–192. doi: 10.4161/gmic.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He YW, Wang HS, Zeng J, et al. Sodium butyrate inhibits interferon-gamma induced indoleamine 2,3-dioxygenase expression via STAT1 in nasopharyngeal carcinoma cells. Life Sci. 2013;93:509–515. doi: 10.1016/j.lfs.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 103.Wong JM, de Souza R, Kendall CW, et al. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 104.Jiang GM, He YW, Fang R, et al. Sodium butyrate down-regulation of indoleamine 2, 3-dioxygenase at the transcriptional and post-transcriptional levels. Int J Biochem Cell Biol. 2010;42:1840–1846. doi: 10.1016/j.biocel.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 105.Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu N, Yang X, Zhang R, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66:462–470. doi: 10.1007/s00248-013-0245-9. [DOI] [PubMed] [Google Scholar]
- 107.Kawajiri K, Kobayashi Y, Ohtake F, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009;106:13481–13486. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Biedermann L, Rogler G. The intestinal microbiota: its role in health and disease. Eur J Pediatr. 2015;174:151–167. doi: 10.1007/s00431-014-2476-2. [DOI] [PubMed] [Google Scholar]
- 109.Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andersson P, McGuire J, Rubio C, et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci U S A. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gramatzki D, Pantazis G, Schittenhelm J, et al. Aryl hydrocarbon receptor inhibition downregulates the TGF-beta/Smad pathway in human glioblastoma cells. Oncogene. 2009;28:2593–2605. doi: 10.1038/onc.2009.104. [DOI] [PubMed] [Google Scholar]
- 112.Bertazzi PA, Consonni D, Bachetti S, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- 113.Xie G, Peng Z, Raufman JP. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1006–G1015. doi: 10.1152/ajpgi.00427.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Safe S, Lee SO, Jin UH. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci. 2013;135:1–16. doi: 10.1093/toxsci/kft128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garten A, Petzold S, Korner A, et al. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sahm F, Oezen I, Opitz CA, et al. The endogenous tryptophan metabolite and NAD+ precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res. 2013;73:3225–3234. doi: 10.1158/0008-5472.CAN-12-3831. [DOI] [PubMed] [Google Scholar]
- 117.Xiao X, Wang L, Wei P, et al. Role of MUC20 overexpression as a predictor of recurrence and poor outcome in colorectal cancer. J Transl Med. 2013;11:151. doi: 10.1186/1479-5876-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawamori T, Uchiya N, Sugimura T, et al. Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis. 2003;24:985–990. doi: 10.1093/carcin/bgg033. [DOI] [PubMed] [Google Scholar]
- 119.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cianchi F, Cortesini C, Fantappie O, et al. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol. 2003;162:793–801. doi: 10.1016/S0002-9440(10)63876-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takahashi M, Mutoh M, Kawamori T, et al. Altered expression of beta-catenin, inducible nitric oxide synthase and cyclooxygenase-2 in azoxymethane-induced rat colon carcinogenesis. Carcinogenesis. 2000;21:1319–1327. [PubMed] [Google Scholar]
- 122.Hao XP, Pretlow TG, Rao JS, et al. Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001;61:419–422. [PubMed] [Google Scholar]
- 123.Mei JM, Hord NG, Winterstein DF, et al. Expression of prostaglandin endoperoxide H synthase-2 induced by nitric oxide in conditionally immortalized murine colonic epithelial cells. FASEB J. 2000;14:1188–1201. doi: 10.1096/fasebj.14.9.1188. [DOI] [PubMed] [Google Scholar]
- 124.Zhu Y, Zhu M, Lance P. iNOS signaling interacts with COX-2 pathway in colonic fibroblasts. Exp Cell Res. 2012;318:2116–2127. doi: 10.1016/j.yexcr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 125.Marnett LJ, Wright TL, Crews BC, et al. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–13430. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 126.Salvemini D, Misko TP, Masferrer JL, et al. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salvemini D, Riley DP, Lennon PJ, et al. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br J Pharmacol. 1999;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gobel C, Breitenbuecher F, Kalkavan H, et al. Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis. 2014;5:e1568. doi: 10.1038/cddis.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee SY, Choi HK, Lee KJ, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32:22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 130.Iachininoto MG, Nuzzolo ER, Bonanno G, et al. Cyclooxygenase-2 (COX-2) inhibition constrains indoleamine 2,3-dioxygenase 1 (IDO1) activity in acute myeloid leukaemia cells. Molecules. 2013;18:10132–10145. doi: 10.3390/molecules180910132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Theate I, van Baren N, Pilotte L, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res. 2015;3:161–172. doi: 10.1158/2326-6066.CIR-14-0137. [DOI] [PubMed] [Google Scholar]
- 132.Zhang R, Liu H, Li F, et al. The correlation between the subsets of tumor infiltrating memory T cells and the expression of indoleamine 2,3-dioxygenase in gastric cancer. Dig Dis Sci. 2013;58:3494–3502. doi: 10.1007/s10620-013-2837-0. [DOI] [PubMed] [Google Scholar]
- 133.Yoshii M, Tanaka H, Ohira M, et al. Expression of Forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer. 2012;106:1668–1674. doi: 10.1038/bjc.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang R, Li H, Yu J, et al. Immunoactivative role of indoleamine 2,3dioxygenase in gastric cancer cells in vitro. Mol Med Rep. 2011;4:169–173. doi: 10.3892/mmr.2010.398. [DOI] [PubMed] [Google Scholar]
- 135.Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg. 2008;206:849–854. doi: 10.1016/j.jamcollsurg.2007.12.014. discussion 854-6. [DOI] [PubMed] [Google Scholar]
- 136.Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208:781–787. doi: 10.1016/j.jamcollsurg.2008.12.018. discussion 787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Lu G, Tang F, et al. Localization of indoleamine 2,3-dioxygenase in human esophageal squamous cell carcinomas. Virchows Arch. 2009;455:441–448. doi: 10.1007/s00428-009-0846-3. [DOI] [PubMed] [Google Scholar]
- 139.Zhang G, Liu WL, Zhang L, et al. Involvement of indoleamine 2,3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinoma. Clin Dev Immunol. 2011;2011:384–726. doi: 10.1155/2011/384726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 142.Metz R, Smith C, DuHadaway JB, et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–367. doi: 10.1093/intimm/dxt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Newton RC, Scherle PA, Bowman K, et al. Pharmacodynamic assessment of INCB024360, an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), in advanced cancer patients. J Clin Oncol. 2012;30 (Abstract 2500). [Google Scholar]
- 144.Beatty GL, O'Dwyer PJ, Clark J, et al. Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the oral inhibitor of indoleamine 2,3-dioxygenase (IDO1) INCB024360 in patients (pts) with advanced malignancies. J Clin Oncol. 2013;31(suppl) abstr 3025. [Google Scholar]
- 145.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Puccetti P, Fallarino F, Italiano A, et al. Accumulation of an Endogenous Tryptophan-Derived Metabolite in Colorectal and Breast Cancers. PLoS One. 2015;10:e0122046. doi: 10.1371/journal.pone.0122046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zheng X, Koropatnick J, Li M, et al. Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. J Immunol. 2006;177:5639–5646. doi: 10.4049/jimmunol.177.8.5639. [DOI] [PubMed] [Google Scholar]
- 149.Zheng X, Koropatnick J, Chen D, et al. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer. 2013;132:967–977. doi: 10.1002/ijc.27710. [DOI] [PubMed] [Google Scholar]
- 150.Maleki Vareki S, Rytelewski M, Figueredo R, et al. Indoleamine 2,3-dioxygenase mediates immune-independent human tumor cell resistance to olaparib, gamma radiation, and cisplatin. Oncotarget. 2014;5:2778–2791. doi: 10.18632/oncotarget.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]