Abstract
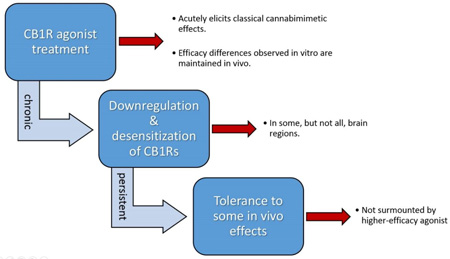
These studies probed the relationship between intrinsic efficacy and tolerance / cross-tolerance between Δ9-THC and synthetic cannabinoid drugs of abuse (SCBs) by examining in vivo effects and cellular changes concomitant with their repeated administration in mice. Dose-effect relationships for hypothermic effects were determined in order to confirm that SCBs JWH-018 and JWH-073 are higher efficacy agonists than Δ9-THC in mice. Separate groups of mice were treated with saline, sub-maximal hypothermic doses of JWH-018 or JWH-073 (3.0 mg/kg or 10.0 mg/kg, respectively) or a maximally hypothermic dose of 30.0 mg/kg Δ9-THC once per day for 5 consecutive days while core temperature and locomotor activity were monitored via biotelemetry. Repeated administration of all drugs resulted in tolerance to hypothermic effects, but not locomotor effects, and this tolerance was still evident 14 days after the last drug administration. Further studies treated mice with 30.0 mg/kg Δ9-THC once per day for 4 days, then tested with SCBs on day 5. Mice with a Δ9-THC history were cross-tolerant to both SCBs, and this cross-tolerance also persisted 14 days after testing. Select brain regions from chronically treated mice were examined for changes in CB1 receptor expression and function. Expression and function of hypothalamic CB1Rs were reduced in mice receiving chronic drugs, but cortical CB1R expression and function were not altered. Collectively, these data demonstrate that repeated Δ9-THC, JWH-018 and JWH-073 can induce long-lasting tolerance to some in vivo effects, which is likely mediated by region-specific downregulation and desensitization of CB1Rs.
Keywords: cannabinoid, tolerance, cross-tolerance, CB1R, downregulation, desensitization
Graphical abstract
1. Introduction
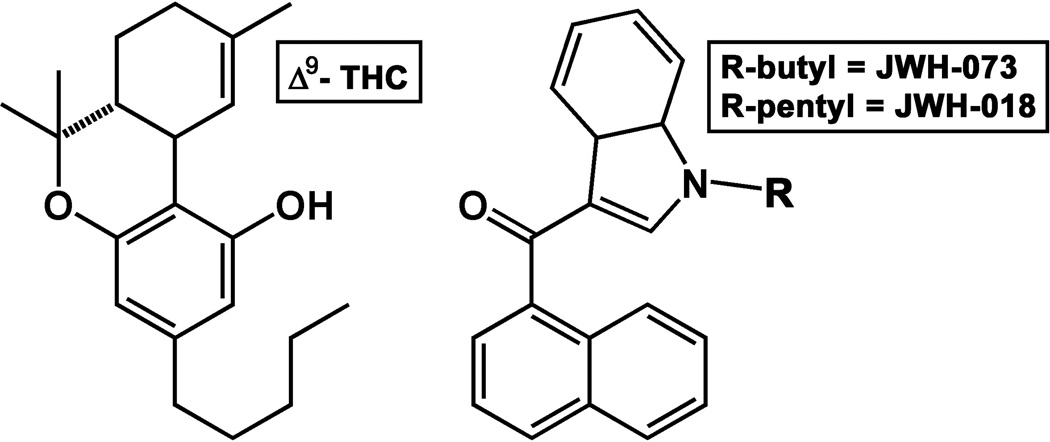
In recent years, high efficacy synthetic cannabinoids (SCBs) have proliferated as psychoactive constituents in commercial smoking preparations, typically advertised as “marijuana alternatives” or “herbal incense” and usually branded as K2 or Spice. Product surveillance and analytical testing have detected numerous formulations for these cannabinoids, including powders, capsules, liquids, and smoking mixtures (Verster, 2010; Seely et al., 2013). Among the first synthetic cannabinoids detected in these products were JWH-018 (1-pentyl-3-(1-naphthoyl)indole) and JWH-073 (1-butyl-3-(1-naphthoyl)indole), both of which bind with high affinity and act as full agonists at cannabinoid type 1 receptors (CB1Rs) (Huffman et al., 1994). Since the initial appearance of these products on the illicit market, product composition has rapidly changed in order to stay ahead of drug scheduling laws. Thus, in addition to a number of Huffman aminoalkylindoles (the “JWH” series), other synthetic cannabinoids including the Δ9-THC analogue HU-210 (1,1-dimethylheptyl-11-hydroxytetrahydrocannabinol), a number of cyclohexylphenols (including CP-47,497 and CP-55,940), and several other indole-derived cannabinoids (such as the fluorinated analogue of JWH-018, AM-2201) have also been detected (Seely et al., 2012). Despite the diversity of the psychoactive constituents of these products, a common feature has been their higher CB1R efficacy, as compared to the partial CB1R agonist Δ9-THC.
Repeated administration of cannabinoid agonists has been shown to result in tolerance to several central and peripheral effects in laboratory animals (Dewey, 1986; Abood and Martin, 1992; Maldonado and Rodriguez de Fonseca, 2002), and to cellular effects observed in vitro (reviewed by Pertwee, 1991). In human marijuana users, tolerance to numerous cannabinoid effects has also been reported following smoked (Jones et al., 1981; Hollister, 1986; Ramaekers et al., 2011) and oral (Benowitz and Jones, 1975; Hunt and Jones, 1980; Gorelick et al., 2013) administration of Δ9-THC. This raises the possibility that a history of Δ9-THC administration might also render individuals less sensitive to some effects of the higher efficacy SCBs through the phenomenon of cross-tolerance. However, much of what is known regarding tolerance to drug effects comes from the study of opioids, where intrinsic efficacy is a critical factor in both the development of tolerance and the degree of cross-tolerance observed across in vivo effects (Paronis and Holtzman, 1992; Walker and Young, 2001). In this regard, tolerance to specific opioid effects induced by repeated treatment with a high efficacy agonist elicit even greater cross-tolerance when low efficacy drugs are tested, while tolerance to specific drug effects produced by treatment with a low efficacy agonist can be at least partially surmounted by administration of a high efficacy compound. But whether this relationship between tolerance, cross-tolerance, and intrinsic efficacy extends to cannabinoids remains largely unknown.
In order to better understand the relationship between intrinsic efficacy and tolerance/cross-tolerance among the cannabinoids, the present studies utilized radiotelemetry to simultaneously monitor core temperature and locomotor activity in mice receiving daily treatments of Δ9-THC, JWH-018 or JWH-073 (Figure 1) to determine the development and expression of tolerance to these two classical effects of the well-characterized “cannabinoid tetrad” (Compton et al., 1992). In separate cross-tolerance experiments, the hypothermic and locomotor effects of JWH-018 and JWH-073 were assessed in mice with or without a prior history of Δ9-THC administration. Mechanisms for changes in drug effects following repeated administration were investigated using brain tissue harvested from drug-naïve or Δ9-THC-treated mice. Ex vivo assays of CB1R expression and function were performed in hypothalamus and cortex, as these brain regions at least partially mediate the thermoregulatory and locomotor effects, respectively, studied in mice. As reports of toxicity and mortality related to illicit use of synthetic cannabinoids accumulate (Auwarter et al., 2009; Zimmermann et al., 2009; Every-Palmer, 2010; Muller et al., 2010; Vardakou et al., 2010; Vearrier and Osterhoudt, 2010; Schneir et al., 2011; Gunderson et al., 2012; Seely et al., 2012; Nacca et al., 2013), it is critical to better understand the mechanisms of tolerance associated with repeated cannabinoid exposure. As with other classes of abused drugs, including benzodiazepines (Lalive et al., 2011), opioids (Morgan and Christie, 2011), and psychostimulants (Bradberry, 2002), tolerant users of synthetic cannabinoids may also attempt to overcome diminished drug effects by escalating dose, thereby dramatically increasing exposure and the concomitant risk of toxicity.
Fig. 1.
Structures of Δ9-THC (left) and the aminoalkylindole cannabinoids JWH-018 and JWH-073 (right).
2. Materials and Methods
2.1 Animals
Prior to surgery (see section 2.2), male NIH Swiss mice (Harlan Sprague Dawley Inc., Indianapolis, IN), weighing approximately 25–30 g, were housed 3 animals per Plexiglas cage (15.24 × 25.40 × 12.70 cm) in a temperature-controlled room at the University of Arkansas for Medical Sciences. Room conditions were maintained at an ambient temperature of 22 ± 2°C at 45–50% humidity. Lights were set to a 12-h light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet #5001, PMI Feeds, Inc., St. Louis, MO) and water ad libitum until immediately before testing. Animals were acclimated to the laboratory environment 2 days prior to experiments and were tested in groups of 5–6 mice per condition. All studies were carried out in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
2.2 Core Temperature and Locomotor Activity
Following appropriate anesthetization with inhaled isoflurane, the abdominal area of each mouse was shaved and sanitized with iodine swabs. A rostro-caudal cut approximately 1.5 cm in length was made with skin scissors, providing access to the intraperitoneal cavity. A cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter, Mini Mitter, Bend, OR, USA) was then inserted, and the incision was closed using absorbable 5–0 chromic gut suture material. At least 7 days were imposed between surgery and experimental observation of drug effects to allow incisions to heal and mice to recover to normal body weights. Following surgery, implanted mice were individually housed in Plexiglas mouse cages (15.24 × 25.40 × 12.70 cm) for the duration of all temperature and locomotor activity experiments. Implanted transmitters produced activity- and temperature-modulated signals that were transmitted to a receiver (model ER-4000 Receiver, Mini Mitter Co., Inc.) underneath each mouse cage. Receivers were housed in light- and sound-attenuating cubicles (Med Associates model ENV-022MD, St. Albans, VT) equipped with exhaust fans, which further masked ambient laboratory noise. Temperature and locomotor activity data were collected simultaneously at regular 5-min intervals and processed by the Vital View data acquisition system (Mini Mitter Co., Inc.).
For dose-effect determinations, mice were weighed, marked, and returned to their individual cages during which at least 1 hr of baseline data were collected. Cannabinoid doses were then calculated and drugs prepared for injection. Animals were subsequently removed from their cage and injected with saline, or various doses of Δ9-THC, JWH-018 or JWH-073. Mice were placed into a new cage with fresh bedding to stimulate exploratory behavior, providing an elevated activity baseline from which a drug-induced suppression of locomotor activity could be characterized, then returned to the telemetry chambers for approximately 24 hrs of data collection. Separate groups of mice (n=5) were tested at each dose condition for each compound.
To assess tolerance to hypothermic and locomotor effects, four groups of mice (n=5) were injected daily with either saline, 30 mg/kg Δ9-THC, 3 mg/kg JWH-018, or 10 mg/kg JWH-073 for 5 consecutive days, and data were collected continuously until 24 hrs after the last injection. After 5 days of data collection was completed, injections were suspended for 14 days, then mice were retested with the same dose of the same cannabinoid to assess the persistence of tolerance. For studies involving cross-tolerance among Δ9-THC and the SCBs, mice were injected daily with 30 mg/kg Δ9-THC for 4 consecutive days, then tested with either 3 mg/kg JWH-018 or 10 mg/kg JWH-073 on day 5, and again after 14 days of drug abstinence.
2.3 Membrane Preparation
Four groups of mice (n=6) were injected daily with drug vehicle (see section 2.6), 30 mg/kg Δ9-THC, 3 mg/kg JWH-018, or 10 mg/kg JWH-073 for 4 consecutive days. Twenty four hours after the last injection, mice were euthanized by cervical dislocation and decapitation. Whole brains were immediately removed and hypothalamus and cortex regions were hand dissected out on ice, then snap-frozen in liquid nitrogen and stored at −80°C. Thus, all in vitro studies were performed in brain homogenates at a drug state equivalent to that in mice used in tolerance studies just prior to the 5th consecutive cannabinoid injection (see section 2.2).
To prepare membrane homogenates, brains were thawed on ice, pooled and suspended in ice-cold homogenization buffer (50 mM HEPES pH 7.4, 3 mM MgCl2, and 1 mM EGTA) (Prather et al., 2000). Suspended brain regions were then subjected to 10 complete strokes employing a 40 mL Dounce glass homogenizer, and centrifuged at 40,000 × g for 10 min at 4°C. Supernatants were discarded and pellets were resuspended in ice cold homogenization buffer, homogenized and centrifuged similarly twice more. Following the final centrifugation step, pellets were resuspended in ice-cold 50 mM HEPES, pH 7.4, to a concentration of approximately 2 mg/mL and aliquoted for storage at −80°C. Protein concentration was determined using BCA™ Protein Assay (Thermo Scientific, Rockford, IL).
2.4 Competition Receptor Binding
Fifty µg of mouse hypothalamic or cortical membrane homogenates (containing a relatively pure source of CB1Rs) were incubated with 1.0 nM of the radiolabeled cannabinoid agonist [3H]CP-55,940 for 90 min at room temperature in an assay buffer containing 5 mM MgCl2, 50 mM Tris, 0.05% bovine serum albumin (BSA). Assays were performed in triplicate, in a final volume of 1 mL, as previously described (Shoemaker et al., 2005). Total binding was defined as the amount of radioactivity observed when 1.0 nM [3H]CP-55,940 was incubated in tissues from vehicle-treated mice. Nonspecific binding was defined as the amount of [3H]CP-55,940 binding remaining in the presence of 1 µM of the non-radioactive CB1/CB2R agonist WIN-55,212-2. Specific binding was calculated by subtracting non-specific from total binding. Reactions were terminated by rapid filtration through Whatman GF/B glass fiber filters, followed by five washes with an ice-cold buffer containing 50 mM Tris and 0.05% bovine serum albumin (BSA). Filters were punched out into 7 mL scintillation vials and immersed in 4 mL of ScintiVerse™ BD Cocktail scintillation fluid. After overnight extraction, bound radioactivity was determined by liquid scintillation spectrophotometry. Specific binding is expressed as a percentage of binding occurring in vehicle samples (e.g., binding in the absence of any competitor).
2.5 [35S]GTPγS Binding
[35S]GTPγS binding was performed as previously described (Liu and Prather, 2001), with minor modifications. JWH-018 (10 µM) was incubated with 25 µg of mouse hypothalamus or cortical membrane homogenates, 10 µM GDP, 0.1 nM [35S]GTPγS and assay buffer (20 mM HEPES, 10 mM MgCl2, 100 mM NaCl, 20 units/L adenosine deaminase, 0.05% BSA). Assays were performed in triplicate in a final volume of 1 mL for 30 min at 30°C. Total binding was defined as the amount of radioactivity observed when 0.1 nM [35S]GTPγS was incubated in membranes from vehicle-treated mice. Nonspecific binding was defined as the amount of [35S]GTPγS binding remaining in the presence of 10 µM of non-radioactive GTPγS. Specific binding was calculated by subtracting non-specific from total binding. Reactions were terminated by rapid filtration through Whatman GF/B glass fiber filters, followed by five washes with an ice-cold buffer containing 20 mM HEPES and 0.05% BSA. Filters were punched out into 7 mL scintillation vials and immersed in 4 mL of ScintiVerse™ BD Cocktail scintillation fluid. After overnight extraction, bound radioactivity was determined by liquid scintillation spectrophotometry. Specific binding is expressed as picomoles of [35S]GTPγS bound per mg of protein.
2.6 Drugs
All drugs used for in vitro assays were diluted to a stock concentration of 10−3 M with 100% ethanol and stored at −20°C. JWH-018 and JWH-073 were synthesized by Thomas Prisinzano, Ph.D. (University of Kansas, Lawrence, KS) and provided to the investigators free of charge. Δ9-THC was supplied by the National Institute on Drug Abuse (NIDA, Bethesda, MD). GTPγS and GDP used in the [35S]GTPγS assay were purchased from EMD Chemical (Gibbstown, NJ), and Sigma Aldrich (St. Louis, MO), respectively. Both chemicals were diluted to a stock concentration of 10−2 M with water and stored at −20°C. [3H]CP-55,940 (174.6 Ci/mmol) used for competition receptor binding was purchased from PerkinElmer (Waltham, MA) and [35S]GTPγS (1250 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). For in vivo studies, all drugs were dissolved to the appropriate concentrations in a ratio of 1:1:18 of absolute ethanol:emulphor:physiological saline vehicle and stored at 4°C until used. All drugs were administered intraperitoneally in mice.
2.7 Data Analysis
All data are presented as group means ± SEM. Points without error bars indicate that the variance is contained within the data point. For dose-effect determinations of effects on core body temperature, the lowest core temperature measured within 8 hours after injection was averaged across animals in a given dose condition, then compared by a Kruskal-Wallis one way analysis of variance (ANOVA) on ranks because data were not normally distributed. To determine significant differences in hypothermic responses elicited by 30 mg/kg Δ9-THC, 3.0 mg/kg JWH-018 or 10.0 mg/kg JWH-073 after saline or 30.0 mg/kg Δ9-THC pretreatment, an ANOVA was conducted, then all groups were compared to the saline + Δ9-THC using a Dunnett’s test. For single dose tolerance and cross tolerance studies, repeated measures one way ANOVAs were performed on the lowest temperatures achieved by each subject, as well as on the times at which core temperatures returned to pre-injection baseline (operationally defined as three consecutive readings ≥ 36°C.) Locomotor data were analyzed by summing all locomotor counts between 0 and 8 hrs after injection, calculating group means, then statistically comparing groups using one way repeated measures ANOVA. For consistency in statistical testing across in vivo studies, the Student-Newman-Keuls method was always used to test all pairwise comparisons since this test can be conducted after ANOVA or repeated measures ANOVA, and is applicable to normally- and abnormally-distributed data sets. All in vivo statistical calculations were performed using SigmaStat 3 (Systat Software, Inc., San Jose, CA), and significance was judged at the level of p < 0.05.
Statistical analyses for ex vivo experiments were performed using GraphPad Prism version 4.0b (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± S.E.M. A one-way ANOVA, followed by Tukey’s Multiple Comparison post-hoc Test, was used to determine statistical significance (P < 0.05) between three or more groups.
3. Results
3.1 Dose-effect determinations of hypothermic effects: apparent in vivo efficacy differences between Δ9-THC, JWH-018 and JWH-073
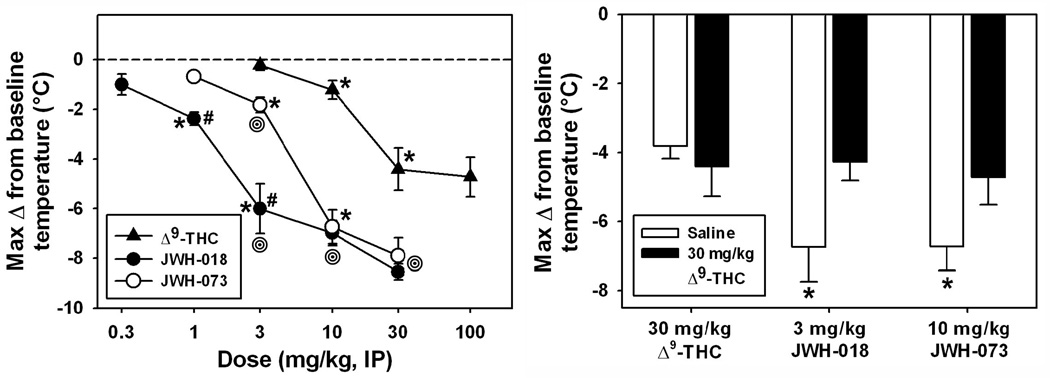
All three cannabinoids decreased core temperature in a dose-dependent manner, with a relative order of potency of JWH-018 > JWH-073 > Δ9-THC (Figure 2, left). For all compounds, peak hypothermic effects typically occurred within 60–75 minutes after injection. The overall ANOVA indicated a significant difference between median values (H=55.608, df=12, p<0.05), and significant pairwise comparisons were found within drug (between doses, see asterisks in Figure 2, left panel). Importantly, hypothermic effects of 30.0 and 100.0 mg/kg Δ9-THC induced similar hypothermic effects (p>0.05), indicating a maximal effect had been reached, so no further doses were tested.
Fig. 2.
Left panel: Maximal hypothermic effects following Δ9-THC, JWH-018 or JWH-073 in mice (n=5 per drug, per dose). Abscissa: dose of drug, in mg/kg, expressed on a log scale. Ordinate: lowest core temperature achieved, in °C, as measured via radiotelemetry. Absence of error bars indicates an instance where the variability is contained within the point. Asterisks indicate significant differences from lower doses, within drug. Hash marks indicate significant differences from JWH-073, within dose. Bullseyes indicate significant differences from Δ9-THC, within dose. Right panel: Maximal hypothermic effects of 30 mg/kg Δ9-THC, 3 mg/kg JWH-018 or 10 mg/kg JWH-073 in mice (n=5 per group) pretreated with saline (white) or 30 mg/kg Δ9-THC (black) 60 min prior. Asterisks indicate significantly different values from the saline+Δ9-THC group.
Significant differences were also detected in pairwise comparisons between drugs (within dose, see bullseyes in Figure 2, left panel). At 3.0 mg/kg, hypothermia elicited by JWH-018 was significantly different from that induced by JWH-073 (q=6.563) and by Δ9-THC (q=6.793, p<0.05 for both comparisons), while hypothermic effects of JWH-073 were different from those elicited by Δ9-THC (q=5.560, p<0.05). At 10.0 mg/kg, there was no significant difference between hypothermic effects of JWH-018 and JWH-073 (p>0.05), but both JWH-018 (q=6.255) and JWH-073 (q=6.961) induced hypothermic effects which differed from those of Δ9-THC (p<0.05 for both comparisons.) At 30.0 mg/kg, again there was no significant difference between hypothermic effects of JWH-018 and JWH-073 (p>0.05), but both JWH-018 (q=5.499) and JWH-073 (q=4.851) again induced hypothermic effects which differed from those of Δ9-THC (p<0.05 for both comparisons.) Importantly, the maximal hypothermic effects of both SCBs (observed at 3.0 and 10.0 mg/kg) were significantly different from the maximal hypothermic effects of Δ9-THC (observed at 30.0 and 100.0 mg/kg). Therefore, maximally-effective hypothermic doses of 30.0 mg/kg Δ9-THC, 3.0 mg/kg JWH-018 and 10.0 mg/kg JWH-073 were used for all subsequent studies on tolerance, cross-tolerance and receptor expression and function were conducted with these doses.
In blockade studies, mice pretreated with saline (Figure 2, right, open bars) all exhibited hypothermic effects when challenged 60 min later with either 30.0 mg/kg Δ9-THC (a maximally-effective dose), 3.0 mg/kg JWH-018 or 10.0 mg/kg JWH-073. Importantly, pretreatment with 30.0 mg/kg Δ9-THC (Figure 2, right, filled bars) significantly attenuated hypothermic effects of JWH-018 and JWH-073, but not those of Δ9-THC. After saline pretreatment, 3.0 JWH-073 (q’=3.286) and 10.0 JWH-073 (q’=3.268, p<0.05 for both comparisons) induced significantly different hypothermic effects than 30.0 mg/kg Δ9-THC. However, after pretreatment with Δ9-THC, neither 3.0 mg/kg JWH-018 nor 10.0 mg/kg JWH-073 induced hypothermic effects which were different from those of 30.0 mg/kg Δ9-THC.
3.2 Rapid and persistent tolerance to the hypothermic effects of Δ9-THC, JWH-018 and JWH-073
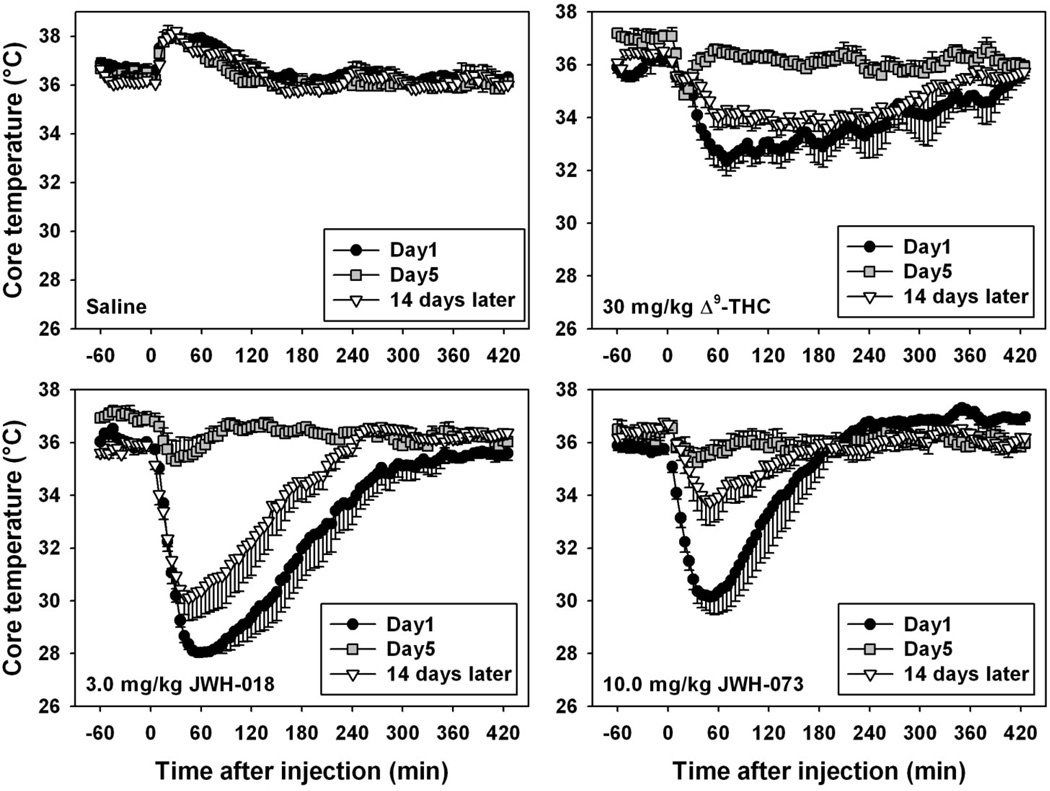
Handling, injection of saline, and placement into a new cage with fresh bedding elicited mild and relatively brief hyperthermic effects, which were consistently expressed throughout the treatment period (Figure 3, upper left). In contrast, the initial administration of all three cannabinoids elicited marked hypothermic effects (filled circles in Figure 3, upper right and both bottom panels), lasting between 3 and 8 hours, depending on the drug. Importantly, with daily administration, a progressive tolerance developed to hypothermic effects of all three cannabinoids. For repeated 30.0 mg/kg Δ9-THC administration (Figure 3, upper right), significant differences were found in the lowest temperature reached and the time required to return to pre-injection baseline temperature (χ2=28.095 and 21.714, respectively, df=5 and p<0.05 for both tests.) The lowest temperature reached on day 5 was significantly different from that observed on day 1 (q=6.110), as was the time required for temperatures to return to pre-injection baseline (q=5.237, p<0.05 for both comparisons). Two weeks after the fifth Δ9-THC administration, the lowest temperature reached was significantly different from that observed on both day 1 (q=4.041) and day 5 (q=5.422, p<0.05 for both comparisons), as was the time required for temperatures to return to pre-injection baseline (q=2.887 for the comparison with day 1, q=4.906 for the comparison with day 5, p<0.05 for both comparisons). For repeated administration of 3.0 mg/kg JWH-018 (Figure 3, bottom left), significant differences were found in the lowest temperature reached and the time required to return to pre-injection baseline temperature (F=22.361 and χ2=18.529, df=5 and p<0.05 for both tests.) The lowest temperature reached on day 5 was significantly different from that observed on day 1 (q=12.256), as was the time required for temperatures to return to pre-injection baseline (q=4.781, p<0.05 for both comparisons). Two weeks after the fifth JWH-018 administration, the lowest temperature reached was significantly different from that observed on day 5 (q=8.887, p<0.05) but not from that observed on day 1 (p>0.05), although the time required for temperatures to return to pre-injection baseline was significantly different from that observed on both day 1 (q=3.795) and day 5 (q=3.960, p<0.05 for both comparisons). For repeated administration of 10.0 mg/kg JWH-073 (Figure 3, bottom right), significant differences were found in the lowest temperature reached and the time required to return to pre-injection baseline temperature (F=9.810 and 13.588, df=5 and p<0.05 for both tests.) The lowest temperature reached on day 5 was significantly different from that observed on day 1 (q=6.428), as was the time required for temperatures to return to pre-injection baseline (q=5.376, p<0.05 for both comparisons). Two weeks after the fifth JWH-073 administration, the lowest temperature reached was significantly different from that observed on day 5 (q=5.421, p<0.05) but not from that observed on day 1 (p>0.05), although the time required for temperatures to return to pre-injection baseline was significantly different from that observed on both day 1 (q=2.176) and day 5 (q=7.552, p<0.05 for both comparisons).
Fig. 3.
Effects of daily administration of saline (upper left), 30 mg/kg Δ9-THC (upper right), 3 mg/kg JWH-018 (bottom left) or 10 mg/kg JWH-073 (bottom right) on core temperature in mice (n=5 per group). Only data from days 1 (black circles) and 5 (grey squares), and after 14 days of cannabinoid abstinence (inverted triangles) are shown. Abscissa: time after drug injection, in minutes. Ordinate: core temperature, in °C, as measured via radiotelemetry. Absence of error bars indicates an instance where the variability is contained within the point. For statistical comparisons, please see section 3.2.
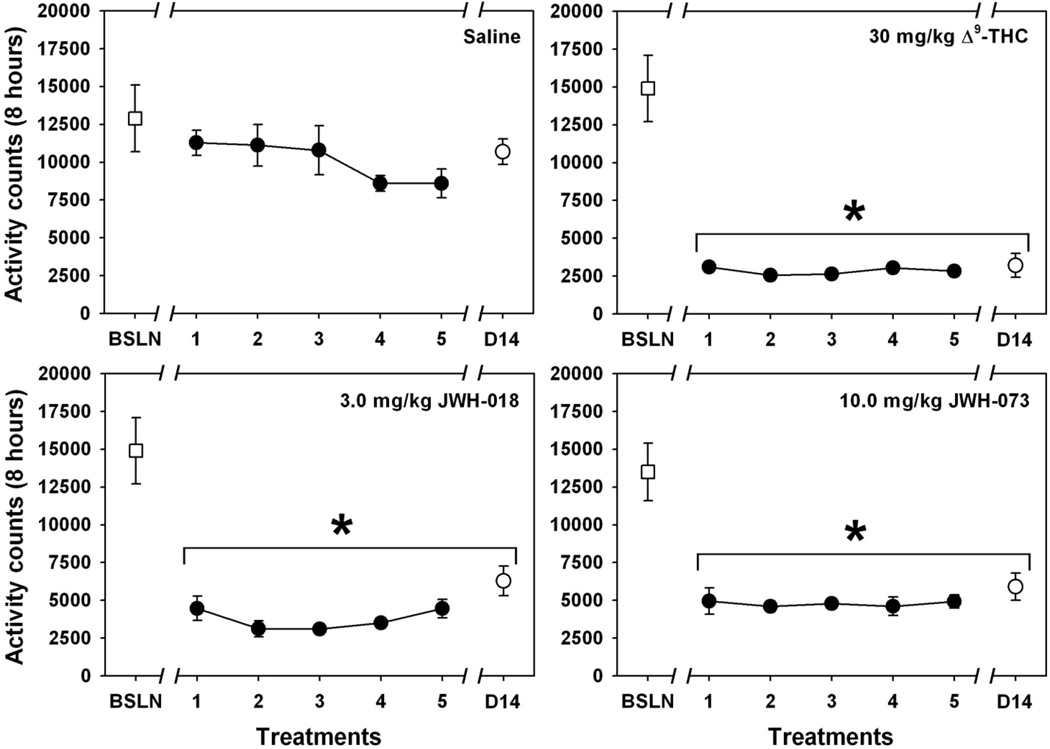
3.3 Lack of tolerance to hypolocomotion induced by Δ9-THC, JWH-018 and JWH-073
Handling, injection of saline, and placement into a new cage with fresh bedding elicited relatively high levels of motor activity and exploratory behavior during the first 8 hours of the baseline observation (“BSLN” point in all panels of Figure 4), one day prior to initiation of daily cannabinoid (or saline control) injections. For mice treated daily with saline across the treatment period, (Figure 4, upper left) no significant differences from the baseline level of motor activity were detected across the treatment period (χ2= 11.929, df=6, p>0.05). In contrast, the initial administration of all three cannabinoids elicited marked suppression of motor activity (Figure 4, upper right and both bottom panels), and no apparent tolerance to this effect developed across the chronic treatment period for any drug. For repeated 30.0 mg/kg Δ9-THC administration (Figure 4, upper right), the overall ANOVA was significant (χ2=25.429, df=5, p<0.05) because every treatment day was significantly different from baseline trial (q=5.674, 5.669, 6.957, 8.165 and 5.680 for days 1–5, respectively, and q=3.464 for the trial after 2 week abstinence; p<0.05 for all comparisons.) For repeated 3.0 mg/kg JWH-018 administration (Figure 4, bottom left), the overall ANOVA was significant (χ2=21.929, df=5, p<0.05) because every treatment day was significantly different from baseline trial (q=5.376, 8.083, 6.047, 6.532 and 5.422 for days 1–5, respectively, and q=5.674 for the trial after 2 week abstinence; p<0.05 for all comparisons.) For repeated 10.0 mg/kg JWH-073 administration (Figure 4, bottom right), the overall ANOVA was significant (χ2=18.214, df=5, p<0.05) because every treatment day was significantly different from baseline trial (q=6.325, 5.422, 7.348, 5.237 and 8.083 for days 1–5, respectively, and q=5.480 for the trial after 2 week abstinence; p<0.05 for all comparisons.)
Fig. 4.
Effects of daily administration of saline (upper left), 30.0 mg/kg Δ9-THC (upper right), 3.0 mg/kg JWH-018 (bottom left) or 10.0 mg/kg JWH-073 (bottom right) on locomotor activity in mice (n = 6 per group). Data from all 5 days of injection are presented (black circles), along with a vehicle injection control point (open square) and a point obtained after 14 days of cannabinoid abstinence (open circle.) Abscissa: injection condition. Ordinate: mean total activity counts for 8 hours after injection, as measured via radiotelemetry. Absence of error bars indicates an instance where the variability is contained within the point. Asterisks indicate significant differences from baseline.
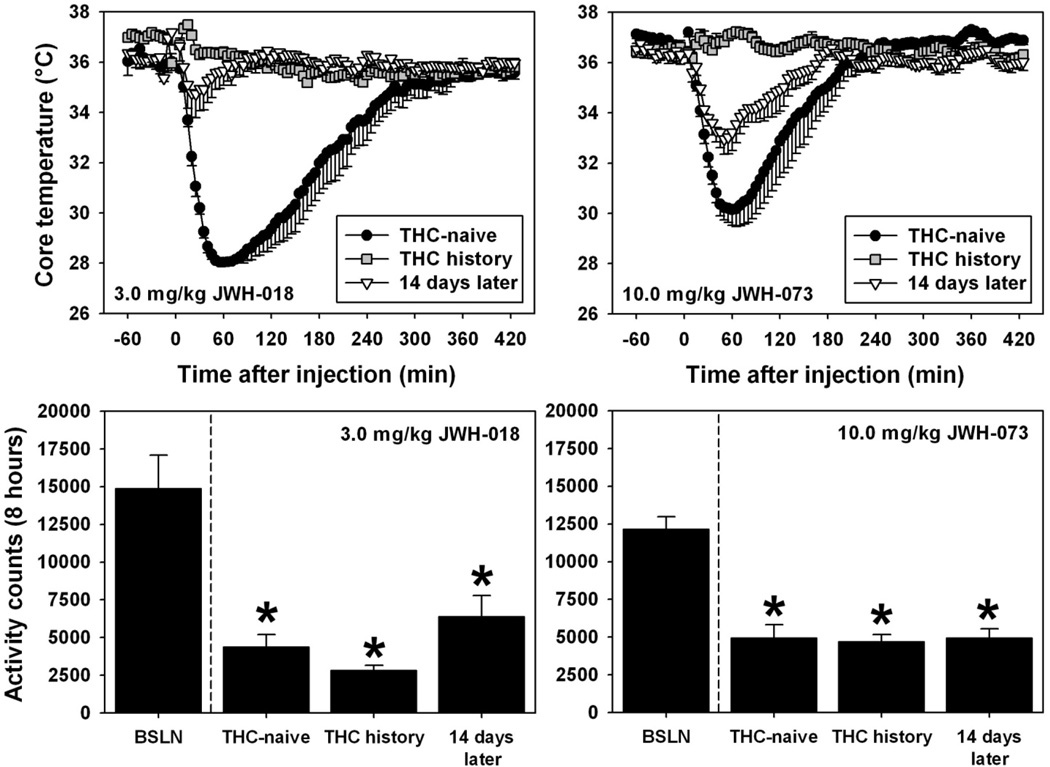
3.4 Cross-tolerance to hypothermic effects of SCBs in mice previously treated with Δ9-THC
As previously demonstrated (data replotted from Figure 3), Δ9-THC-naïve mice acutely administered 3.0 mg/kg JWH-018 or 10.0 mg/kg JWH-073 exhibited dramatic hypothermic effects which resolved within 3 to 5 hours (Figure 5, filled circles in top panels). In contrast, a profound attenuation of these hypothermic effects was observed in mice previously treated with 30.0 mg/kg Δ9-THC once per day for 4 consecutive days (Figure 5, grey squares in top panels) then tested with 3.0 mg/kg JWH-018 (F=87.905, df=2, p<0.05) or 10.0 mg/kg JWH-073 (F=41.345, df=2, p<0.05) on day 5. For Δ9-THC-treated mice tested with 3.0 mg/kg JWH-018 (Figure 5, top left), the lowest temperature recorded was never below the species-typical range, and was significantly different from that observed in Δ9-THC-naïve mice (q=17.191, p<0.05). When these same mice were re-tested with 3.0 mg/kg JWH-018 after a 14-day drug abstinence period, the lowest temperature recorded remained significantly different from that observed in Δ9-THC-naïve mice (q=15.081, p<0.05), but was not different from that observed during the initial cross-tolerance test (p>0.05). Similarly, for Δ9-THC-treated mice tested with 10.0 mg/kg JWH-073 (Figure 5, top right), the lowest temperature recorded was never below the species-typical range, and was significantly different from that observed in Δ9-THC-naïve mice (q=12.801, p<0.05). When these same mice were re-tested with 10.0 mg/kg JWH-073 after a 14-day drug abstinence period, the lowest temperature recorded remained significantly different from that observed in Δ9-THC-naïve mice (q=5.332, p<0.05), and was also different from that observed during the initial cross-tolerance test (q= 7.469, p<0.05).
Fig. 5.
Top - Effects of acute administration of 3.0 mg/kg JWH-018 (left) or 10.0 mg/kg JWH-073 (right) on core temperature in mice (n = 5 or 6), as a function of Δ9-THC history. Black circles represent hypothermia elicited by the SCBs in previously drug-naïve mice, while grey squares depict the analogous data obtained from mice previously treated with Δ9-THC (see section 2.2) Inverted triangles illustrate the thermoregulatory effects of a second injection of 3.0 mg/kg JWH-018 (left) or 10.0 mg/kg JWH-073 (right) in mice previously treated with Δ9-THC after 14 days of cannabinoid abstinence. All other graph properties as described in Figure 3. Please see section 3.4 and 3.5 for statistical comparisons. Bottom - Effects of acute administration of 3.0 mg/kg JWH-018 (left) or 10.0 mg/kg JWH-073 (right) on locomotor activity in mice (n = 6), as a function of Δ9-THC history. Bars represent mean total activity quantified for 8 hours after injection. Asterisks indicate significant differences from baseline observations.
3.5 Lack of cross-tolerance to hypolocomotor effects of SCBs in mice previously treated with Δ9-THC
As previously demonstrated, Δ9-THC-naïve mice acutely administered 3.0 mg/kg JWH-018 or 10.0 mg/kg JWH-073 exhibited profound suppression of locomotor activity during the 8 hour post-injection period (Figure 5, bottom panels). Consistent with the previous findings of no tolerance to hypolocomotor effects, mice tested with either of the SCBs exhibited suppressed locomotor behavior relative to the baseline observation, regardless of Δ9-THC treatment history. For mice tested with 3.0 mg/kg JWH-018 (Figure 5, bottom left), motor activity was significantly different from that observed under the baseline condition in Δ9-THC-naïve animals (q=7.682), in animals with a Δ9-THC history (q=8.824), and in these same animals when re-tested with 3.0 mg/kg JWH-018 after a 14-day drug abstinence period (q=6.215, p<0.05 for all comparisons.) No other between-group comparisons were statistically-significant (p>0.05 for all comparisons). Similarly, for mice tested with 10.0 mg/kg JWH-073 (Figure 5, bottom right), motor activity was significantly different from that observed under the baseline condition in Δ9-THC-naïve animals (q=10.175), in animals with a Δ9-THC history (q=10.513), and in these same animals when re-tested with 10.0 mg/kg JWH-073 after a 14-day drug abstinence period (q=10.187, p<0.05 for all comparisons.) No other between-group comparisons were statistically-significant (p>0.05 for all comparisons).
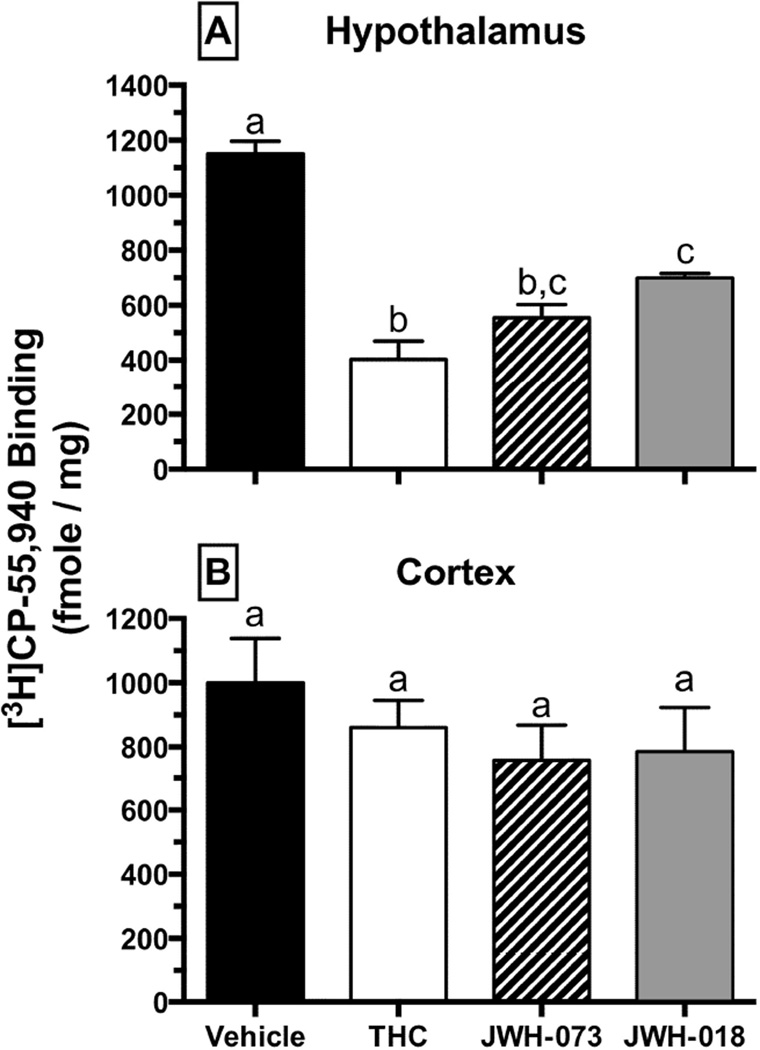
3.6 Downregulation of CB1Rs in the hypothalamus but not the cortex after repeated CB treatment
To provide a mechanistic framework for behavioral findings, complementary ex vivo studies evaluating changes in CB1R expression (Figure 6) and function (Figure 7) in select brain regions were performed. Mice were treated for 4 days with either vehicle, Δ9-THC (30 mg/kg), JWH-073 (10 mg/kg) or JWH-018 (3 mg/kg). One day after the last dose, brains were harvested, dissected into discrete regions, and membrane homogenates prepared to examine CB1R receptor binding (Figure 6). To estimate CB1R density, specific binding of the CB1R radioligand [3H]CP-55,940 (1nM) was determined in hypothalamic (Figure 6A) and cortical (Figure 6B) membranes. CB1Rs in hypothalami from untreated mice bound 1151 ± 45 fmole/mg of [3H]CP-55,940. Consistent with CB1R downregulation, CB1Rs in hypothalami obtained from mice treated for 4 days with either Δ9-THC, JWH-073 or JWH-018 bound significantly less [3H]CP-55,940 (Figure 6A; p>0.05). In contrast, CB1R binding levels in mouse cortex homogenates did not change significantly from vehicle regardless of cannabinoid pretreatment (Figure 6B). These results indicate that CB1R downregulation does occur and it transpires in a region-specific manner.
Fig. 6.
Region-specific downregulation of CB1Rs in mouse hypothalamus (top), but not cortex (bottom), following administration of vehicle (black), 30 mg/kg Δ9-THC (white), 10 mg/kg JWH-073 (striped) or 3 mg/kg JWH-018 (grey) in mice (n = 4 per group), once per day for 4 days. Values designated with different letters above the error bars are significantly different (P<0.05, one-way ANOVA followed by a Tukey’s post-hoc test, mean ± SEM).
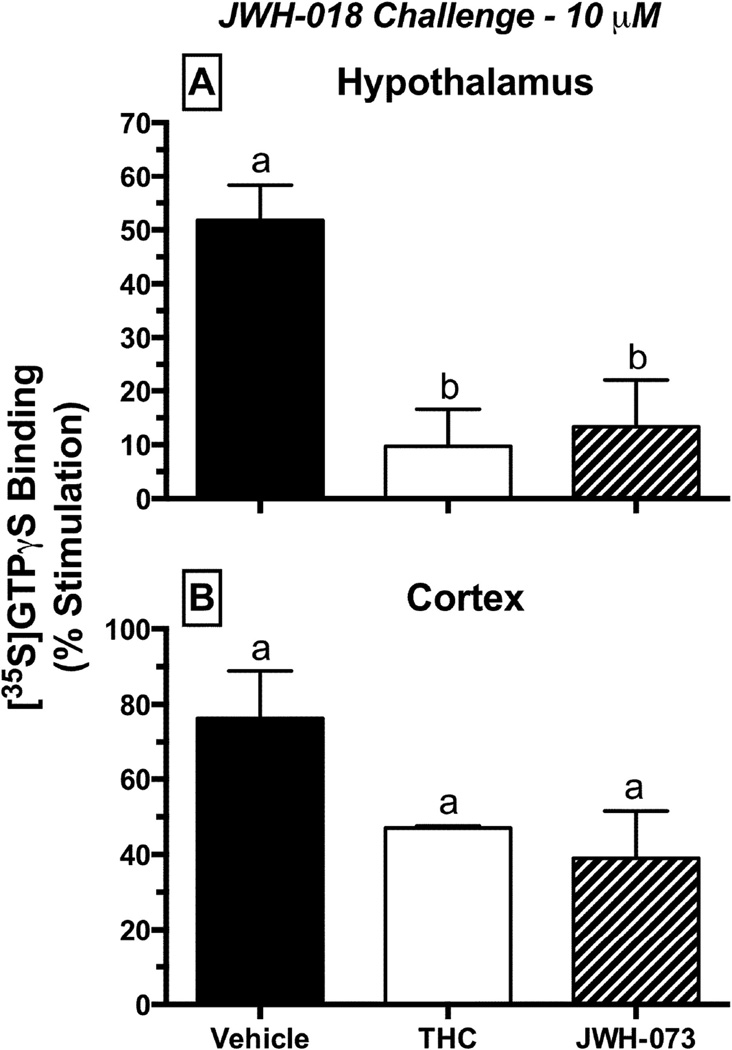
Fig. 7.
Desensitization of CB1Rs in mouse hypothalamus (top), but not cortex (bottom), when stimulated with a receptor-saturating concentration (10 µM) of JWH-018. Prior to tissue harvesting, mice (n = 4 per group) were treated with vehicle (black), 30 mg/kg Δ9-THC (white) or 10 mg/kg JWH-073 (striped), once per day for 4 days. Values designated with different letters above the error bars are significantly different (P<0.05, one-way ANOVA followed by a Tukey’s post-hoc test, mean ± SEM).
3.7 Desensitization of CB1Rs in the hypothamlamus but not the cortex after repeated CB treatment
To determine the effects of repeated cannabinoid exposure on G-protein activation by CB1Rs, the [35S]GTPγS binding assay was employed (Figure 7). The capacity of a receptor saturating concentration of the full CB1R agonist JWH-018 (10 µM) to activate G-proteins was evaluated in hypothalamic (Figure 7A) and cortical (Figure 7B) membranes of chronically treated mice. Indicative of G-protein activation, JWH-018 produced a 51.8 ± 6.5% increase in binding of [35S]GTPγS to G-proteins in hypothalami from drug naïve mice. In contrast, JWH-018 produced less G-protein activation in hypothalami isolated from mice chronically treated with Δ9-THC or JWH-073, consistent with receptor desensitization (Figure 7A). Importantly, treatment of mice with Δ9-THC or JWH-073 did not desensitize CB1Rs in striatal membranes (Figure 7B), indicating that the adaptive changes produced by chronic treatment with phytocannabinoids or synthetic cannabinoids occur in a brain region specific manner. G-protein activation produced by JWH-018 (100 nM) in both brain regions was CB1R-dependent because it was completely blocked by co-incubation with the neutral CB1R antagonist O-2050 (1 µM, data not shown). Collectively, ex vivo receptor binding and functional data suggest that chronic treatment of mice with cannabinoids produces brain region-specific CB1R downregulation and desensitization.
4. Discussion
In these studies, complementary in vivo and ex vivo assays were used to compare the effects of repeated exposure to the low efficacy phytocannabinoid Δ9-THC and two high efficacy SCB drugs of abuse, JWH-018 and JWH-073. Initially, dose effect curves for hypothermic effects were generated after acute exposure to various doses of each drug in mice. Not surprisingly, all three cannabinoids produced dose-dependent hypothermic responses, similar to those previously reported for various CB1R agonists (e.g., CP-55,940 and WIN 55,212-2) (Pertwee et al., 1993). Consistent with partial CB1 agonist effects, Δ9-THC-induced hypothermia plateaued at 30 mg/kg, and 100 mg/kg Δ9-THC did not produce a greater response. In contrast, doses of JWH-018 and JWH-073 induced more extreme hypothermic effects than were ever observed with Δ9-THC, which is consistent with their relatively higher CB1 efficacy, as has been previously demonstrated ex vivo (Brents et al., 2011; Brents et al., 2012). In addition, pretreatment with the low efficacy agonist Δ9-THC attenuated the hypothermic effects induced by subsequent administration of either higher efficacy SCB. Thus, congruent with previous work in other mouse strains (McMahon and Koek, 2007; Paronis et al., 2012), Δ9-THC exhibited characteristics of a partial agonist when administered alone, and when co-administered with a full agonist. Our present in vivo findings therefore replicate similar studies from other laboratories, and recapitulate the ex vivo profiles of Δ9-THC and the SCBs from our own previous experiments (Brents et al., 2011; Brents et al., 2012).
Next, we sought to extend these in vivo profiles by assessing the development of tolerance and cross-tolerance to hypothermic and locomotor suppressant effects, two endpoints of the classical cannabinoid tetrad (i.e., Compton et al., 1992). Use of radiotelemetry probes allowed the simultaneous recording of core body temperature and locomotor activity in all subjects in order to assess tolerance to these two drug effects following repeated administration of low efficacy Δ9-THC or high efficacy SCBs, but the role of intrinsic efficacy in tolerance and cross-tolerance among the cannabinoids is underdeveloped and the data in this domain are often contradictory. For example, an early study demonstrated that mice repeatedly treated with Δ9-THC (20 mg/kg, once a day for 2 days) showed a 6-fold tolerance to the hypothermic effects of subsequent Δ9-THC, and a similar degree of tolerance (approximately 5-fold) was observed for the hypothermic effects of high efficacy cannabinoids CP-55,940 and WIN 55,212-2 (Pertwee et al., 1993). However, a different regimen of Δ9-THC (10 mg/kg, twice per day for 6 days) resulted in approximately 10-fold shifts in subsequent Δ9-THC dose effect curves for motor activity, hypothermia and antinociception in mice, but only moderate tolerance was apparent when high efficacy cannabinoids CP-55,940 or WIN 55,212-2 were tested, and only to some of these effects (Fan et al., 1994). Finally, and most relevant to the present study, Δ9-THC treatment (1 mg/kg, every day for 3 days) decreased sensitivity to the discriminative stimulus effects of Δ9-THC 3-fold, but did not alter the Δ9-THC-like interoceptive effects of high efficacy cannabinoids CP-55,940, JWH-073, or JWH-018 in rhesus monkeys, although prolonged Δ9-THC treatment reduced sensitivity to the Δ9-THC-like discriminative stimulus effects of all cannabinoids (Hruba et al., 2012). Importantly, the tolerance observed to the effects of high efficacy compounds was approximately 50% less than that observed for Δ9-THC itself (Hruba et al., 2012).
In the present study, cannabinoid efficacy did not appear to dramatically affect the development of tolerance to hypothermic effects. Whether mice were chronically treated with low efficacy Δ9-THC or with high efficacy JWH-018 or JWH-073, tolerance developed within 5 days of regular treatment and was still evident 2 weeks after the last cannabinoid injection. Previous reports show tolerance to Δ9-THC-induced hypothermia develops rapidly, and is often observed as early as the second treatment (Pertwee et al., 1993). In addition, McMahon and colleagues reported that tolerance to cannabinoid-induced hypothermia was still present 9 days after last treatment (Singh et al., 2011). Interestingly, tolerance does not develop to all cannabinoid effects. Here we demonstrated dramatic tolerance to hypothermic effects in the very same animals in which locomotor activity remained profoundly suppressed throughout the study. This finding is not necessarily consistent with previous studies, in which tolerance to locomotor suppressant effects is often observed. For instance, 14 days of continuous exposure to the synthetic Δ9-THC analogue HU-210 (3-(1,1’-dimethylheptyl)-6aR,7,10, 10aR-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol) resulted in clear tolerance to hypomotility in the rat (Caberlotto et al., 2004). It may be the case that tolerance to hypomotility develops at a slower rate than tolerance to hypothermia, in which case a longer treatment period than the one presently employed may be required to observe this effect. Another possibility is that our method of measuring activity using radiotelemetry probes in a home cage setting may be less sensitive than other devices that measure motor activity. Using our system, baseline activity levels are extremely low, and in order to observe dose-related suppression of motor activity we must place our subjects in new cages with fresh bedding immediately after injection. This stimulates exploratory behavior in control subjects, which is reliably suppressed by cannabinoid administration. The phenomenon of locomotor sensitization is often conceptualized as “reverse tolerance”, and the arousal state of an animal is an important determinant of locomotor sensitization (Martin-Iverson et al., 1988). Similarly, environmental novelty can also dramatically modulate locomotor sensitization, minimizing its expression in environments similar to the home cage, and maximizing its expression in environments distinct from the home cage (Badiani et al., 1995). We are not aware of studies directly testing whether these same relationships also apply to tolerance to locomotor effects, but assessment of acute and chronic drug effects on locomotor activity within a home cage environment is certainly likely to differ from similar experiments using environmentally distinct and novel activity chambers.
Much of our understanding of tolerance and cross-tolerance has been achieved by investigating these effects among opioids, where it has been determined that there is an inverse relationship between intrinsic efficacy and tolerance / cross-tolerance. For example, using an assay of morphine discrimination in rats, Young and colleagues (1991) found that a regimen of twice-daily treatment with morphine increased the ED50 for high efficacy opioids etorphine, methadone and buprenorphine by only 2- to 4-fold, while inducing an apparently insurmountable tolerance to the morphine-like interoceptive effects of the low efficacy opioid nalbuphine. In squirrel monkeys, daily morphine treatment shifted the dose effect curve for the rate-decreasing effects of morphine, but produced significantly smaller shifts in the dose effect curves for higher efficacy opioids such as etorphine, l-methadone, and sufentanil (Hughes et al., 1995). Similarly, daily administration of morphine in an assay of schedule-controlled responding in rats shifted the morphine dose-effect curve to the right, but produced a 3-fold larger shift in the dose-effect curve for the low efficacy opioid butorphanol (Hughes et al., 1995). Subsequent work (Smith and Picker, 1998) demonstrated that tolerance to rate-decreasing effects induced by repeated butorphanol treatments were surmounted by administration of high efficacy opioids fentanyl and sufentanil in the rat. The role of intrinsic efficacy in tolerance to the analgesic effects of opioids has also been investigated, and rats made tolerant to analgesic effects of morphine or fentanyl (as evidenced by shifts in their respective dose effect functions) were completely insensitive to analgesic effects of the low efficacy opioids buprenorphine, levorphanol and meperidine (Paronis and Holtzman, 1992). This same pattern of effects has also been observed with regard to respiratory depressant effects of opioids in mice, where tolerance to respiratory depression induced by implantation of subcutaneous morphine pellets was overcome by subsequent intracerebroventricular administration of high efficacy opioids etorphine and heroin (Roerig et al., 1987).
However, among the cannabinoids used in the present study, high efficacy agonists JWH-018 and JWH-073 were unable to induce hypothermia in mice previously made tolerant to hypothermic effects of low efficacy Δ9-THC, suggesting that cross-tolerance developed to the hypothermic effects of the high efficacy SCBs, despite the relatively large disparity in intrinsic activity. In other words, unlike what is typically observed with opioids, tolerance to an effect induced by low efficacy Δ9-THC was not surmounted by administration of either high efficacy SCB. Furthermore, cross-tolerance was still present 14 days after Δ9-THC cessation, suggesting that this cross-tolerance may be as persistent as the tolerance induced by repeated administration of the high efficacy SCBs themselves. In contrast, since tolerance to cannabinoid-induced hypomotility did not occur with repeated administration of any of the three drugs utilized in these experiments, it was not surprising that there was no cross-tolerance to hypomotility when JWH-018 and JWH-073 were administered after repeated Δ9-THC. Thus, our present work suggests that cross-tolerance does occur among cannabinoid agonists of differing efficacy; however, previous studies have demonstrated that the degree of cross-tolerance observed is often variable across different endpoints. In non-human primates, chronic Δ9-THC (1 mg/kg, every 12 hours) elicited marked tolerance (23- and 160-fold) to the effects of subsequent Δ9-THC on schedule-controlled responding, but induced lesser cross-tolerance (approximately 10-fold) to the effects of the full CB1 agonists CP-55,940 and WIN 55,212-2 on these same endpoints; however, while this regimen of Δ9-THC treatment reduced sensitivity to the discriminative stimulus effects of subsequent Δ9-THC, no cross-tolerance to these effects was observed when CP-55,940 and WIN 55,212-2 were tested (McMahon, 2011). In subsequent experiments in the mouse, chronic Δ9-THC treatment (32 mg/kg/day) resulted in tolerance to rate-decreasing and hypothermic effects of subsequent Δ9-THC, but cross-tolerance to CP-55,940 was only observed for hypothermic effects (Singh et al., 2011). McMahon (2011) has proposed that cannabinoid agonist efficacy has an inverse relationship with tolerance and cross-tolerance, similar to what is observed in the opioid system, although our present results fail to support that notion. Further investigation will be required to determine whether these disparate results are due to differences in dosing regimens, species-specific factors, pharmacokinetic variables, or the drugs themselves and how they may differ in their interactions with cannabinoid and non-cannabinoid binding sites.
In this regard, to begin to understand the mechanisms behind our present findings, ex vivo CB1R binding and functional studies were also carried out in homogenates from discrete brain regions of mice chronically treated with Δ9-THC or the high efficacy SCBs. It has been previously demonstrated that adaptive cellular events including downregulation and desensitization of CB1Rs mediate tolerance to cannabinoid effects (Sim-Selley, 2003); therefore, our studies incorporated both competition receptor binding and [35S]GTPγS binding experiments to measure CB1R density and function, respectively. Repeated cannabinoid exposure induced a dramatic downregulation of CB1R expression in the hypothalamus, which is a critical brain region for thermoregulation. This reduction in CB1R availability in the hypothalamus may explain the profound tolerance presently observed to hypothermic effects of repeated cannabinoid administration. In contrast, CB1R expression in the cortex was not altered by repeated cannabinoid exposure, which may partially explain the lack of tolerance to hypomotility observed in mice. These observations are consistent with previous studies in humans demonstrating that prolonged marijuana exposure results in region-specific downregulation of central CB1Rs (Hirvonen et al., 2011). Since CB1R expression was profoundly reduced in the hypothalamus but not in the cortex, the function of remaining CB1Rs in these brain regions was further examined by agonist activation of G-proteins. As predicted by previous studies (Rinaldi-Carmona et al., 1998; Sim-Selley, 2003), CB1R-mediated G-protein activation was greatly desensitized by repeated cannabinoid exposure. Interestingly, the extent of CB1R downregulation and desensitization observed in the hypothalamus were not related to the efficacy of the cannabinoid used to induce these effects. Indeed, the effects of repeated Δ9-THC on CB1R expression and function were at least as pronounced as those of repeated exposure to the two high efficacy SCBs. Further mechanistic studies will be required to more fully understand the biochemical basis of CB1R downregulation and desensitization, but recent evidence suggests an important role for p-arrestin2 (Imperatore et al., 2015)
5. Conclusion
Finally, although the role of tolerance in drug abuse is incompletely understood, it is supposed that tolerant users may escalate drug dose to overcome reduced effectiveness of the drug of abuse. In the case of SCBs, use of these compounds is associated with significant toxicity (e.g., Fantegrossi et al., 2014), although the mechanisms for these adverse effects are still unclear. Nevertheless, regardless of mechanism, tolerance to drug effects might be expected to lead to increased drug exposure as users escalate their doses, thus also increasing their risk for serious adverse effects. Given that most college students who abuse SCBs also report use of marijuana (Hu et al., 2011; Vandrey et al, 2012), cross tolerance of the sort described in these studies may also be a critical factor in mediating dose selection of these emerging drugs of abuse. As abuse of these high efficacy SCBs continues, a thorough understanding of the acute and chronic effects of these substances is critical in order to inform both drug policy and clinical management of users who present with symptoms of SCB-induced toxicity.
Acknowledgements
We thank the UAMS Division of Laboratory Animal Medicine for expert husbandry services. The views expressed herein are those of the authors and do not necessarily represent the views of the University of Arkansas for Medical Sciences.
This research was supported, in part, by a NIGMS IDeA Program award [GM110702], by the UAMS Translational Research Institute [RR029884], by a Summer Undergraduate Research Fellowship from the American Society of Pharmacology and Experimental Therapeutics, and by a NIDA T32 [DA022981].
Abbreviations
- AM-2201
[1-(5-fluoropentyl)-1H-indol-3-yl]-1-naphthalenyl-methanone
- CB1Rs
Cannabinoid type 1 receptors
- CP-47,497
rel-2-[(1S,3R)-3-hydroxycyclohexyl]-5-(2-methylnonan-2-yl)phenol
- CP-55,940
5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol
- [35S]GTP8S
guanosine-5’-O-(3-[35S]thio)triphosphate
- HU-210
(1,1-dimethylheptyl-11-hydroxytetrahydrocannabinol)
- JWH-018
(1-pentyl-3-(1-naphthoyl)indole)
- JWH-073
(1-butyl-3-(1-naphthoyl)indole)
- Δ9-THC
Δ9-tetrahydrocannabinol
- WIN-55,212-2
[(3R)-2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflicts of interest involving this work.
Authorship Contributions
Participated in research design: Fantegrossi, Prather
Conducted experiments: Hyatt, Gu, Franks, Brents, Vasiljevik, Tai, Fantegrossi
Contributed new reagents or analytic tools: Vasiljevik
Performed data analysis: Tai, Fantegrossi, Prather
Wrote or contributed to the writing of the manuscript: Tai, Fantegrossi, Prather
References
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? Journal of mass spectrometry : JMS. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopha rmacology. 1995;117:443–52. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther. 1975;18:287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Dynamics of extracellular dopamine in the acute and chronic actions of cocaine. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2002;8:315–322. doi: 10.1177/107385840200800407. [DOI] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6:21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L, Rimondini R, Hansson A, Eriksson S, Heilig M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopha rmacology. 2004;29:15–22. doi: 10.1038/sj.npp.1300296. [DOI] [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacological reviews. 1986;38:151–178. [PubMed] [Google Scholar]
- Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105:1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta-9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Goodwin RS, Schwilke E, Schwope DM, Darwin WD, Kelly DL, McMahon RP, Liu F, Ortemann-Renon C, Bonnet D, Huestis MA. Tolerance to effects of high-dose oral delt?9-tetrahydrocannabinol and plasma cannabinoid concentrations in male daily cannabis smokers. J Anal Toxicol. 2013;37:11–16. doi: 10.1093/jat/bks081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL. “Spice” and “K2” herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2012;21:320–326. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol. Psychiatry. 2011;17:642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38:1–20. [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Primack BA, Barnett TE, Cook RL. College students and use of K2: an emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. Design, Synthesis and Pharmacology of Cannabimimetic Indoles. Bioorganic & Medicinal Chemistry Letters. 1994;4:556–566. [Google Scholar]
- Hughes CE, Picker MJ, Dykstra LA. Tolerance and cross-tolerance to the response rate-decreasing effects of micro opioids in morphine-maintained squirrel monkeys. Behav Pharmacol. 1995;6:776–784. [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Imperatore R, Morello G, Luongo L, Taschler U, Romano R, De Gregorio D, Belardo C, Maione S, Di Marzo V, Cristino L. Genetic deletion of monoacylglycerol lipase (MAGL) leads to impaired cannabinoid receptor CB1 R signaling and anxiety-like behavior. J Neurochem. 2015 doi: 10.1111/jnc.13267. in press. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Lalive AL, Rudolph U, Luscher C, Tan KR. Is there a way to curb benzodiazepine addiction? Sw iss medical weekly. 2011;141:13277. doi: 10.4414/smw.2011.13277. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol. 2001;60:53–62. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22:3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Iverson MT, Stahl SM, Iversen SD. Chronic administration of a selective dopamine D-2 agonist: factors determining behavioral tolerance and sensitization. Psychopharmacology. 1988;95:534–9. doi: 10.1007/BF00172969. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Chronic Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br J Pharmacol. 2011;162:1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007;569:70–76. doi: 10.1016/j.ejphar.2007.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. 2011;164:1322–1334. doi: 10.1111/j.1476-5381.2011.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophrenia research. 2010;118:309–310. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Nacca N, Vatti D, Sullivan R, Sud P, Su M, Marraffa J. The synthetic cannabinoid withdrawal syndrome. Journal of addiction medic ine. 2013;7:296–298. doi: 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- Paronis CA, Nikas SP, Shukla VG, Makriyannis A. Delta(9)-Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behav Pharmacol. 2012;23:802–805. doi: 10.1097/FBP.0b013e32835a7c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. In: Tolerance to and dependence on psychotropic cannabinoids, in The Biological Bases of Drug Tolerance and Dependence. Pratt J, editor. Academic Press; 1991. pp. 231–263. [Google Scholar]
- Pertwee RG, Stevenson LA, Griffin G. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol. 1993;110:1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Theunissen EL, de Brouwer M, Toennes SW, Moeller MR, Kauert G. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214:391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Le Duigou A, Oustric D, Barth F, Bouaboula M, Carayon P, Casellas P, Le Fur G. Modulation of CB1 cannabinoid receptor functions after a long-term exposure to agonist or inverse agonist in the Chinese hamster ovary cell expression system. J Pharmacol Exp Ther. 1998;287:1038–1047. [PubMed] [Google Scholar]
- Roerig SC, Fujimoto JM, Lange DG. Development of tolerance to respiratory depression in morphine- and etorphine-pellet-implanted mice. Brain Res. 1987;400:278–284. doi: 10.1016/0006-8993(87)90627-5. [DOI] [PubMed] [Google Scholar]
- Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. The Journal of emergency medicine. 2011;40:296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: A three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sc i Int. 2013;233:416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314:868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharm acology (Berl) 2011;215:665–675. doi: 10.1007/s00213-010-2162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Picker MJ. Tolerance and cross-tolerance to the rate-suppressing effects of opioids in butorphanol-treated rats: influence of maintenance dose and relative efficacy at the mu receptor. Psychopharmacology (Berl) 1998;140:57–68. doi: 10.1007/s002130050739. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Dunn KE, Fry JA, Girling ER. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2012;120:238–241. doi: 10.1016/j.drugalcdep.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatric emergency care. 2010;26:462–465. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- Verster JC. The popularity of “legal highs”. Curr Drug Abuse Rev. 2010;3:196. doi: 10.2174/1874473711003040196. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- Young AM, Kapitsopoulos G, Makhay MM. Tolerance to morphine-like stimulus effects of mu opioid agonists. J Pharmacol Exp Ther. 1991;257:795–805. [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Deutsches Arzteblatt international. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]