Abstract
Background
Early outcomes after HIV+ liver transplantation (LT) are encouraging, but data are lacking regarding long-term outcomes and comparisons with matched HIV− patients.
Methods
We examined outcomes among 180 HIV+ LT, and compared outcomes to matched HIV− counterfactuals (SRTR 2002–2011). Iterative expanding radius matching (1:10) on recipient age, race, BMI, hepatitis C infection (HCV), MELD score, and acute rejection; and donor age and race, CIT, and year of transplant. Patient survival and graft survival were estimated using Kaplan-Meier methodology and compared using log-rank and Cox proportional hazards. Subgroup analyses were performed by transplant era (early: 2002–2007 vs. modern: 2008–2011) and HCV-infection status.
Results
Compared to matched HIV− controls, HIV+ LT recipients had a 1.68-fold increased risk for death (aHR: 1.68, 95% CI: 1.28–2.20, p<0.001), and a 1.70-fold increased risk for graft loss (aHR: 1.70, 95% CI: 1.31–2.20, p<0.001). These differences persisted independent of HCV-infection status. However, in the modern transplant era risk for death [aHR: 1.11; 95% CI: 0.52–2.35, p=0.79] and graft loss [aHR: 0.89; 95% CI: 0.42–1.88, p=0.77] were similar between monoinfected and uninfected LT recipients. In contrast, independent of transplant era, coinfected LT recipients had increased risk for death [aHR: 2.24; 95% CI: 1.43–3.53, p < 0.001] and graft loss [aHR: 2.07, 95% CI: 1.33–3.22, p=0.001] compared to HCV+ alone LT recipients.
Conclusion
These results suggest that outcomes among monoinfected HIV+ LT recipients have improved over time. However, outcomes among HIV+ LT recipients coinfected with HCV remain concerning, and motivate future survival benefit studies.
INTRODUCTION
Improvements in the care of human immunodeficiency virus infected (HIV+) patients, including potent anti-retroviral therapy (ART), development of integrase inhibitors, and once a day ART regimen, have resulted in the decline of HIV-related deaths; as such, the life expectancy of HIV+ individuals has increased (1–3). Chronic diseases, such as end stage liver disease (ESLD), have now surpassed opportunistic infections as the leading causes of death among HIV+ individuals (4, 5). Liver transplantation is now offered as an acceptable treatment option for HIV+ ESLD patients, and is being offered outside of clinical trials. While this marks a new era in the care of the HIV+ ESLD patient, experience with HIV+ liver transplantation remains limited.
Recently, the largest prospective US cohort study reported outcomes among 89 HIV+ liver transplant recipients coinfected with hepatitis C virus (HCV+): three-year patient and graft survival were 60% and 53%, respectively, both significantly lower than survival rates among a cohort of unmatched HCV+ liver transplant recipients (6). Donor age over 50 years, low recipient body mass index (BMI), need for simultaneous kidney transplant, and the use of HCV+ donor livers were found to be predictors of worse outcomes among coinfected recipients. When these high risk factors were avoided, HIV+/HCV+ liver transplant recipients experienced similar outcomes to an unmatched cohort of elderly (over 65 years) HIV−/HCV− liver recipients. The median length of follow-up among study participants was 1.8 years (IQR: 0.7–3.4), but fewer than 25 patients had 3 years of follow-up. While these findings are encouraging, concerns remain about the high incidence of acute rejection and acceleration of cirrhosis after HIV+ liver transplantation, and the possible impact on long-term outcomes (6–9). Furthermore, both control cohorts (unmatched HCV+ recipients and unmatched elderly HIV−/HCV− recipients) failed to provide an appropriate counterfactual comparison.
To date no study has examined outcomes among HIV+ monoinfected recipients, quantified long-term outcomes in monoinfected or coinfected recipients, nor compared monoinfected or coinfected recipients to their appropriately matched HIV− counterparts. Furthermore, current practice is based upon results from a highly scrutinized clinical protocol used within the prospective NIH trial. The trial was limited to high volume centers, involved only 89 participants, and was limited to HIV+ recipients coinfected with HCV+, representing a highly selected subset of the more than 180 HIV+ liver transplants that have been performed in the US (6). Going beyond the confines of the NIH trial to study the entire US experience with HIV+ liver transplantation is necessary to properly assess the generalizability of findings and provides the sample size necessary for risk factor estimation, allowing for comparison to properly matched controls.
To better understand outcomes in this unique population, we conducted a retrospective cohort study to: 1) examine long-term patient survival (PS) and graft survival (GS) among the US HIV+ liver transplant population in its entirety, including both mono and coinfected HIV+ recipients, and 2) compare these outcomes to those of matched HIV− recipients.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), on all donors, waitlisted candidates, and transplant recipients in the US. The Health Services Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors. This study received approval from the University of Alabama at Birmingham Institutional Review Board.
Study Population
All adult first-time liver transplant patients with known HIV status who were transplanted between January 1, 2002 and December 31, 2011 were identified [HIV+: 180, HIV−: 34,020]. Patients were excluded from the adjusted multivariable and matched controlled analyses if they were missing information on cold ischemia time (CIT) (n=8), BMI (n=7), HCV status (n=10), or a combination of the three (n=3).
Outcome Ascertainment
The primary outcome measures were GS and PS. GS was defined as the time from transplantation to graft loss or death; PS was defined as the time from transplantation to death. Death indicators were supplemented by linkage to the Social Security Death Master File. Both GS and PS were censored for administrative end of study.
Statistical Analyses
Exploratory Data Analysis
Donor and recipient variables were analyzed using t tests or Wilcoxon rank-sum tests (based on distribution) and categorical variables were examined using chi-square or Fisher’s exact tests of independence (based on sample size).
Survival Analyses
GS and PS were estimated among HIV+ recipients and compared between HIV+ recipients and HIV− recipients from the general population using Kaplan-Meier methods, log rank tests, and Cox proportional hazards models. Risk factors for graft loss and death within the HIV+ cohort were identified using univariate Cox proportional hazards with statistical significance set at 0.1. GS and PS were also estimated among the entire cohort of HIV+ recipients matched to appropriate HIV− controls (1:10) using expanded radius matching. HIV+ and HIV− liver transplant recipients were matched on recipient age, race, BMI, HCV infection, MELD score, and acute rejection; and donor age and race, CIT, and year of transplant. Subgroup analyses by transplant era (early: 2002–2007 vs. modern: 2008–2011) and HCV-infection status were also performed.
Sensitivity Analyses
The same covariates used to create the matched cohort were used to build the full multivariate models. Results from these models confirmed inferences reported from the matched (1:10) analyses. Matched control analyses must balance introduction of bias with reduction in variability (i.e. with increasing numbers of controls per patient, more bias is potentially introduced, yet variability is theoretically reduced). Given this, the analyses were performed among 4 distinct matched cohorts (1:1; 1:3; 1:5; and 1:10); inferences did not change. For the purposes of simplicity, results comparing outcomes among HIV+ and HIV− liver transplant recipients are from the 1:10 matched cohort.
RESULTS
Study Population
Between 2002–2011, there were 180 first-time liver transplants performed among HIV+ adult recipients [median follow-up (f/u): 3.1 years, IQR 1.6–6.0] and 34,020 performed among HIV− recipients [median f/u: 4.5 years, IQR 2.5–6.9]. Since 2002, there has been a 4-fold increase in the number of transplant performed in HIV+ recipients (Figure 1). Compared to HIV− recipients, HIV+ recipients were younger (≥ 50 years old: 55% vs. 72.7%, p < 0.001), more often male (77.8% vs. 67.5%, p=0.003), and more likely to be African American (25% vs. 9.4%, p < 0.001). Additionally, they were more often underweight (4.1% vs. 1.9%, p < 0.001), and more likely to be infected with HCV (64.9% vs. 45.8%, p < 0.001) and hepatitis B virus (HBV) (64.1% vs 45.8%, p < 0.001) (Table 1).
Figure 1.
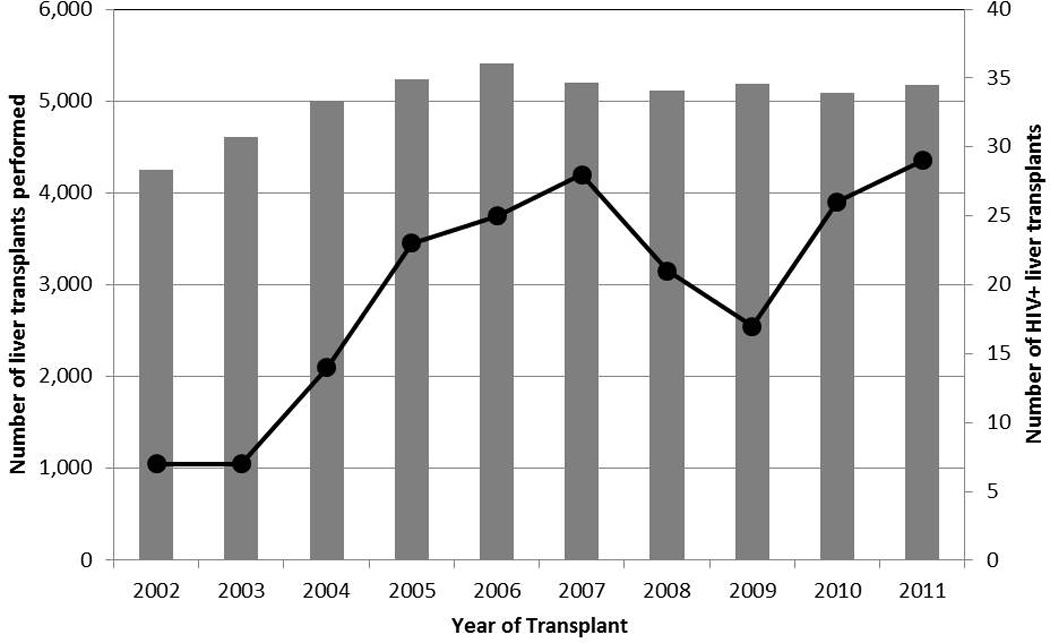
The number of liver transplants performed among the general population and the number of liver transplants performed among HIV+ patients between 2002–2011.
Table 1.
Patient Characteristics by HIV Status
| HIV+ | HIV− | ||
|---|---|---|---|
| (n=180) | (n=34,020) | P | |
| Follow-up time in years, median (IQR) | 3.1 (1.6–6.0) | 4.5 (2.5–6.9) | < 0.001 |
| Recipient Characteristics | % | % | |
| Age ≥ 50 years | 55.0 | 72.7 | < 0.001 |
| Male Gender | 77.8 | 67.5 | 0.003 |
| Race | |||
| African American | 25.0 | 9.4 | < 0.001 |
| Caucasian | 60.6 | 72.1 | |
| Other | 14.4 | 18.5 | |
| BMI (kg/m2) | |||
| < 18.5 | 4.1 | 1.9 | < 0.001 |
| 18.5–24.9 | 44.4 | 27.7 | |
| 25–29.9 | 33.9 | 35.8 | |
| ≥ 30 | 17.5 | 34.6 | |
| Cause of liver failure | |||
| Acute hepatic necrosis | 9.4 | 5.4 | 0.02 |
| Cirrhosis | 72.8 | 71.6 | |
| Metabolic disease | 0.6 | 2.2 | |
| Primary liver malignancy | 11.7 | 10.5 | |
| Primary sclerosing cholangitis | 0.6 | 4.4 | |
| Other | 5.0 | 6.0 | |
| Pre-transplant GFR | |||
| ≥ 90 | 32.8 | 28.8 | 0.75 |
| 60–89 | 29.4 | 31.0 | |
| 30–59 | 26.7 | 26.7 | |
| 15–29 | 7.8 | 9.8 | |
| < 15 | 3.3 | 3.7 | |
| MELD score, median (IQR) | 19 (12.5–29) | 19 (13–27) | 0.7 |
| HCV Infection | 64.9 | 45.8 | < 0.001 |
| HBV Infection | 64.1 | 22.7 | < 0.001 |
| Acute rejection within 1 year | 16.1 | 12.1 | 0.09 |
| Donor characteristics | |||
| Age ≥ 50 years | 34.4 | 36.7 | 0.53 |
| Race | |||
| African American | 21.7 | 17.2 | 0.12 |
| Caucasian | 60.0 | 67.2 | |
| Other | 18.3 | 15.7 | |
| CIT ≥ 6 hours | 64.1 | 63.3 | 0.82 |
Long-term Patient Survival (PS)
Among all HIV+ recipients, 5-year PS was 55.8% and 10-year PS was 41.0% (Table 2). PS was not statistically different among monoinfected (HIV+/HCV−) compared to coinfected (HIV+/HCV+) recipients at 5 years (64.8% (HIV+/HCV−) vs. 51.8% (HIV+/HCV+), p=0.15) or 10 years (43.9% (HIV+/HCV−) vs. 44.1% (HIV+/HCV+), p=0.2) post-transplant. Among HIV+ recipients, the risk of death was greater in the presence of a pre-transplant glomerular filtration rate less than 30cc/min (HR: 2.44, 95%CI: 1.12–5.31, p=0.03) (Table 3).
Table 2.
Survival Rates by HIV and HCV Status
| Patient Survival | Graft Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | 10-year | 1-year | 3-year | 5-year | 10-year | |
| HIV+ | 77.2% | 62.2% | 55.8% | 41.0% | 73.3% | 58.2% | 50.7% | 35.4% |
| HIV− general | 88.2%a | 79.6%a | 73.4%a | 60.9%a | 85.4%a | 76.5%a | 70.1%a | 57.8%a |
| HIV− matched | 87.7%b | 78.8%a | 72.1%a | 57.1%a | 84.9%b | 75.3%a | 68.4%a | 53.7%a |
| HIV+/HCV− | 81.4% | 71.9% | 64.8% | 43.9% | 76.3% | 66.6% | 59.3% | 35.1% |
| HIV−/HCV− | 88.8% | 82.6%b | 77.3%b | 65.3%b | 85.9%b | 79.3%b | 73.8%b | 62.2%b |
| HIV+/HCV+ | 77.1% | 58.5% | 51.8% | 44.1% | 73.4% | 54.8% | 46.4% | 41.1% |
| HIV−/HCV+ | 87.5%a | 76.3%a | 69.1%a | 55.9%a | 84.9%a | 73.4%a | 66.0%a | 53.0%a |
| HIV+/HCV− | 81.4% | 71.9% | 64.8% | 43.9% | 76.3% | 66.6% | 59.3% | 35.1% |
| HIV+/HCV+ | 77.1% | 58.5% | 51.8% | 44.1% | 73.4% | 54.8% | 46.4% | 41.1% |
significant at an alpha level of 0.001;
significant at an alpha level of 0.05
Table 3.
Univariate Hazard Ratios (HR) of Mortality and Graft Loss
| Among HIV+ Recipients | |||||
|---|---|---|---|---|---|
| Risk of Mortality | Risk of Graft Loss | ||||
| Recipient Characteristics | HR (95% CI) | p-value | Recipient Characteristics | HR (95% CI) | p-value |
| Age ≥ 50 years | 1.41 (0.90–2.20) | 0.13 | Age ≥ 50 years | 1.27 (0.83–1.93) | 0.28 |
| Male Gender | 1.32 (0.76–2.28) | 0.32 | Male Gender | 1.57 (0.91–2.60) | 0.10 |
| Race | Race | ||||
| Caucasian | Ref | Caucasian | Ref | ||
| African American | 0.90 (0.53–1.53) | 0.70 | African American | 0.96 (0.59–1.59) | 0.90 |
| Other | 1.10 (0.60–2.03) | 0.76 | Other | 1.01 (0.55–1.85) | 0.98 |
| BMI (kg/m2) | BMI (kg/m2) | ||||
| Normal weight | Ref | Normal weight | Ref | ||
| Underweight | 0.54 (0.13–2.23) | 0.39 | Underweight | 0.42 (0.10–1.74) | 0.23 |
| Overweight | 0.90 (0.54–1.51) | 0.70 | Overweight | 0.74 (0.45–1.20) | 0.22 |
| Obese | 0.93 (0.50–1.76) | 0.83 | Obese | 0.80 (0.44–1.47) | 0.47 |
| Pre-transplant GFR | Pre-transplant GFR | ||||
| ≥ 90 | Ref | ≥ 90 | Ref | ||
| 60–89 | 1.45 (0.82–2.55) | 0.20 | 60–89 | 1.34 (0.78–2.31) | 0.30 |
| 30–59 | 1.35 (0.75–2.43) | 0.31 | 30–59 | 1.34 (0.78–2.33) | 0.30 |
| 15–29 | 2.44 (1.12–5.31) | 0.03 | 15–29 | 2.00 (0.93–4.31) | 0.07 |
| < 15 | 1.12 (0.26–4.75) | 0.88 | < 15 | 0.91 (0.22–3.84) | 0.90 |
| Last MELD > 25 | 0.82 (0.501.33) | 0.42 | Last MELD > 25 | 0.87 (0.54–1.38) | 0.55 |
| HCV Infection | 1.39 (0.84–2.28) | 0.20 | HCV Infection | 1.29 (0.81–2.07) | 0.28 |
| HBV Infection | 1.42 (0.87–2.32) | 0.16 | HBV Infection | 1.45 (0.90–2.31) | 0.12 |
| Acute rejection w/i 1 year | 0.82 (0.45–1.52) | 0.54 | Acute rejection w/i 1 year | 0.69 (0.38–1.27) | 0.24 |
| Donor characteristics | Donor characteristics | ||||
| Age ≥ 50 years | 1.43 (0.92–2.23) | 0.11 | Age ≥ 50 years | 1.70 (1.11–2.60) | 0.01 |
| Race | Race | ||||
| Caucasian | Ref | Caucasian | Ref | ||
| African American | 1.20 (0.70–2.07) | 0.51 | African American | 1.23 (0.72–2.09) | 0.45 |
| Other | 1.16 (0.66–2.04) | 0.61 | Other | 1.27 (0.74–2.17) | 0.38 |
| CIT ≥ 6 hours | 0.96 (0.61–1.54) | 0.88 | CIT ≥ 6 hours | 1.00 (0.64–1.56) | 0.99 |
PS Compared to HIV− Population
Compared to the general HIV− population, 5 and 10-year survival were significantly worse among HIV+ recipients [5-yr: 55.8% vs. 73.4%, p < 0.001; 10-yr: 41.0% vs. 60.9%, p < 0.001]. While PS was higher among monoinfected recipients (5yr: 64.8%; 10yr: 43.9%), it remained statistically lower than the uninfected (HIV−/HCV−) general transplant population (p=0.05). Moreover, coinfected patients (HIV+/HCV+) had significantly lower PS (5yr: 51.8%; 10yr: 44.1%) compared to HCV+ recipients from the general transplant population (5yr: 69.1%; 10yr: 55.9%, p<0.001) (Table 2). These differences persisted even when compared to appropriately matched HIV− controls (5yr: 55.8% vs. 72.1%, p<0.001; 10yr: 39.3% vs. 57.1%, p<0.001; adjusted hazard ratio (aHR): 1.68, 95% CI: 1.28–2.20, p<0.001) (Figure 2), and across transplant era (early era, aHR: 1.66, 95%CI: 1.11–2.50, p<0.001; modern era, aHR: 1.88, 95%CI: 1.26–2.82, p<0.001) (Table 4).
Figure 2.
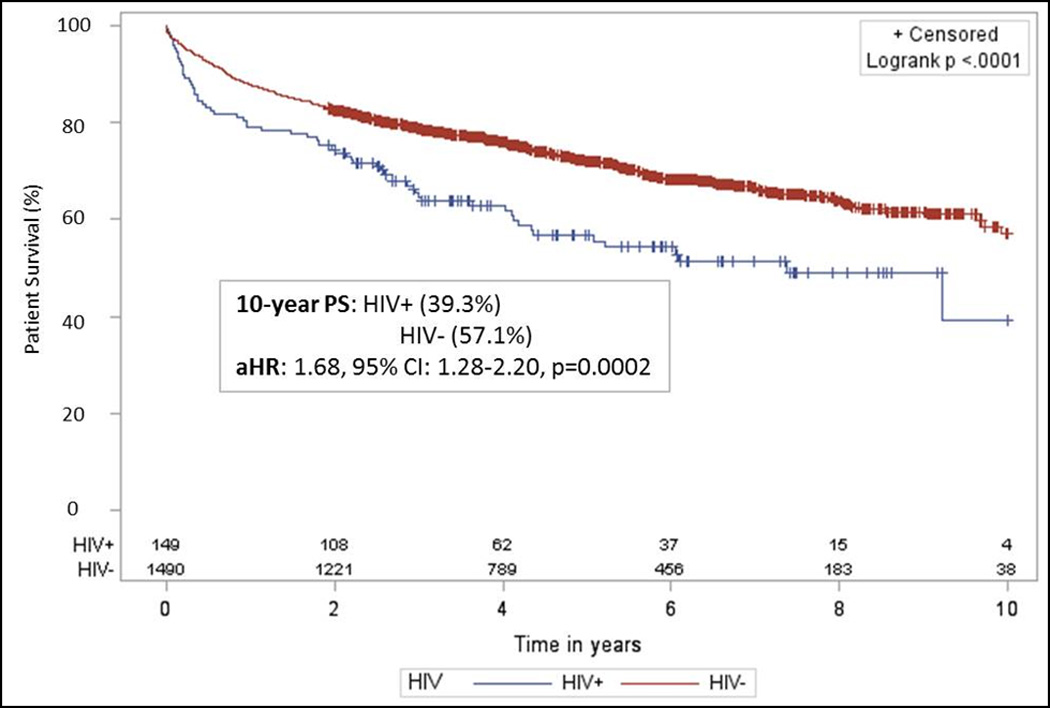
Patient survival among a matched case controlled cohort of HIV+ and HIV− liver transplant recipients. HIV+ recipients were matched 1:10 with HIV− recipients on recipient age, race, BMI, MELD, HCV status, acute rejection; donor age and race, cold ischemia time, and year of transplant. Compared to appropriate matched HIV− controls, patient survival was statistically lower at 5 years [55.8% vs. 72.1%, p < 0.001] and 10 years [39.3% vs. 57.1%, p < 0.001] post–transplant [aHR=1.68; 95% CI=1.28–2.20, p = 0.0002].
Table 4.
Adjusted Hazard Ratios (aHR) by Transplant Era and HCV Status. Risk for graft loss and patient death for monoinfected patients have improved over time. However, outcomes among coinfected patients remain poor even in the modern transplant era.
| Early (2002–2007) | Modern (2008–2011) | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Graft loss | ||
| All HIV+ | 1.86 (1.23–2.70) | 1.58 (1.06–2.34) |
| All HIV− | ref | ref |
| Mono-infected (HIV+/HCV−) | 3.26 (1.61–6.67) | 0.89 (0.42–1.88) |
| No infection (HIV−/HCV−) | ref | ref |
| Co-infected (HIV+/HCV+) | 1.56 (1.02–2.39) | 2.07 (1.33–3.22) |
| HCV alone (HIV−/HCV+) | Ref | ref |
| Death | ||
| All HIV+ | 1.66 (1.11–2.50) | 1.88 (1.26–2.82) |
| All HIV− | ref | ref |
| Mono-infected (HIV+/HCV−) | 3.58 (1.62–7.91) | 1.11 (0.52–2.35) |
| No infected (HIV−/HCV−) | ref | Ref |
| Co-infected (HIV+/HCV+) | 1.37 (0.86–2.19) | 2.24 (1.43–3.53) |
| HCV alone (HIV−/HCV+) | ref | ref |
Stratification by HCV-status within each era demonstrated improved outcomes over time among monoinfected patients, such that in the modern transplant era there was no longer a difference in risk for death between monoinfected and uninfected recipients (aHR: 1.11; 95% CI: 0.52–2.35, p=0.79). In contrast, survival did not improve over time among coinfected patients, such that, compared to HCV+ alone recipients, increased risk for death persisted over time (aHR: 2.24; 95% CI: 1.43–3.53, p < 0.001) (Table 4).
Long-term Graft Survival (GS)
Among all HIV+ recipients, 5-year GS was 50.7%, and 10-year GS was 35.4% (Table 2). There was no statistically significant difference in GS among monoinfected (HIV+/HCV−) compared to coinfected (HIV+/HCV+) recipients at 5 years (59.3% (HIV+/HCV−) vs. 46.4% (HIV+/HCV+), p=0.19) and 10 years (35.1% (HIV+/HCV−) vs. 41.1% (HIV+/HCV+), p=0.28) post-transplant. Among HIV+ recipients, the risk for graft loss was greater with the use of liver grafts from donors over the age of 50 years (HR=1.70, 95% CI=1.11–2.60, p=0.01) (Table 3).
GS Compared to HIV− Population
Compared to the general HIV− population, 5 and 10-year survival were significantly worse among HIV+ recipients [5-yr: 50.7% vs. 70.1%, p < 0.001; 10-yr: 35.4% vs. 57.8%, p < 0.001]. Moreover, monoinfected recipients had significantly lower GS (5yr: 59.3%; 10yr: 35.1%) compared to the uninfected transplant population (5yr: 73.8%; 10yr: 62.2%, p=0.05). Further, compared to HCV+ recipients (5yr: 66.0%; 10yr: 53.0%), coinfected patients (HIV+/HCV+) had significantly lower GS (5yr: 46.4%; 10yr: 41.1%, p<0.001) (Table 2). These differences in GS remained even when comparing HIV+ recipients with appropriately matched HIV− controls (5yr: 51.3% vs. 68.4%, p<0.001; 10 yr: 34.2% vs. 53.7%, p<0.001; aHR: 1.70, 95% CI: 1.31–2.20, p<0.001) (Figure 3), and across transplant era (early era aHR: 1.86, 95% CI: 1.23–2.70, p=0.001; modern era aHR: 1.58, 95% CI: 1.06–2.34, p=0.02) (Table 4).
Figure 3.
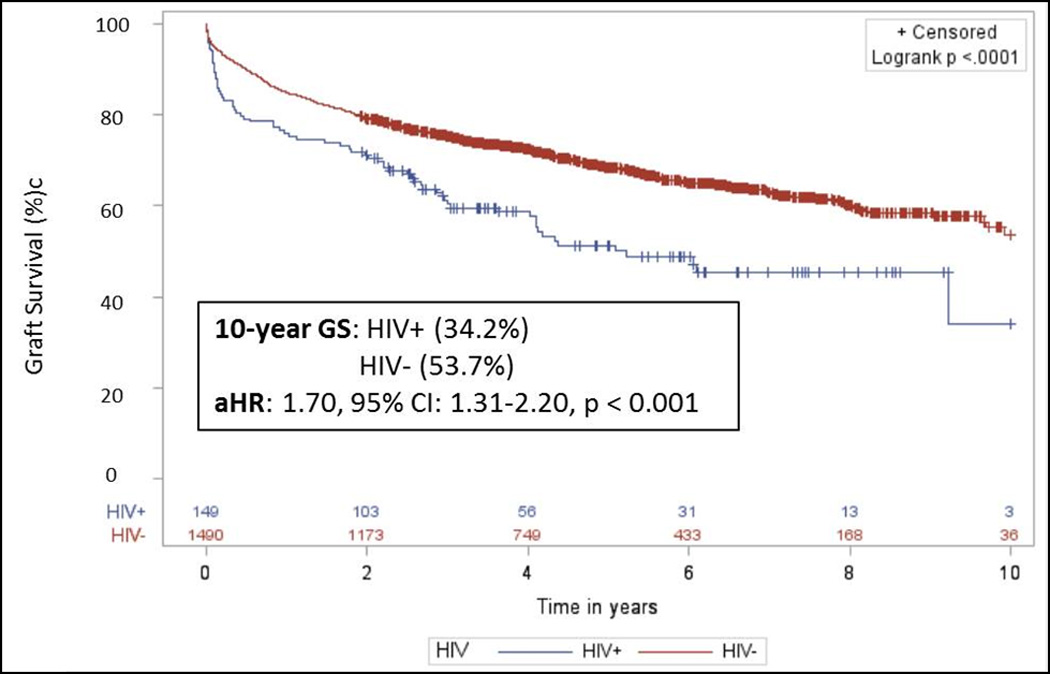
Graft survival among a matched case controlled cohort of HIV+ and HIV− liver transplant recipients. HIV+ recipients were matched 1:10 with HIV− recipients on recipient age, race, BMI, MELD, HCV status, acute rejection; donor age and race, cold ischemia time, and year of transplant. Compared to appropriate matched HIV− controls, graft survival was statistically lower at 5 years [51.3% vs. 68.4%, p < 0.001] and 10 years [34.2% vs. 53.7%, p < 0.001] post–transplant [aHR=1.70; 95% CI=1.31–2.20, p < 0.001].
Stratification by HCV-status within each era demonstrated improved outcomes over time among monoinfected patients, such that in the modern transplant era there was no longer a difference in risk for graft loss between monoinfected and uninfected recipients (aHR: 0.89; 95% CI: 0.42–1.88, p=0.77). Differences in risk for graft loss persisted in the modern era among coinfected patients when compared to recipients with HCV alone (aHR: 2.07, 95% CI: 1.33–3.22, p=0.001) (Table 4).
DISCUSSION
In this national study of long-term outcomes among HIV+ liver transplant recipients, we found that, compared to the general HIV− liver recipient population, long-term GS and PS were significantly lower among HIV+ recipients, particularly among those recipients coinfected with HCV. In fact, at 10 years post-transplant, only 35.4% of HIV+ recipients had functioning grafts and 41.0% were still living compared to 57.8% and 60.9%, respectively, among the general population of HIV− recipients. Significant differences in baseline characteristics of HIV+ recipients compared to the general population of HIV− recipients were identified, including factors known to be associated with increased risk of graft loss and death, such as co-infection with HCV and hepatitis B and African American race. Interestingly, however, even after adjusting for these potential confounders, HIV+ liver recipients continued to have lower GS and PS at 5 and 10 years post-transplant compared to their appropriately matched HIV− counterparts.
While these findings likely reflect the natural history of HIV, it is also possible that residual confounding exists. In particular, cumulative viremia among HIV+ individuals has been identified as an important predictor of progression to liver fibrosis. A recent cohort study of 288 HIV/HCV coinfected participants demonstrated a 1.2-fold increased risk of fibrosis progression for each additional 1 log10 copies/ml HIV RNA cumulative exposure (10). Although an undetectable plasma HIV viral load is generally required for transplant candidacy, SRTR data lack granularity with regard to viral load at the time of transplant, time of suppressed viral load, and HIV suppression post-transplant. Moreover, similar effects of cumulative viremia have been observed among HCV+ individuals. While most HCV+ patients are viremic at the time of transplant, SRTR data do not quantify HCV viral load or document changes over time (11). Not surprisingly, multiple studies have demonstrated accelerated fibrosis pre- and post-transplant in the setting of HIV/HCV coinfection, as the individual viral effects appear to be additive in the setting of coinfection (7, 8).
Since their introduction in 2008, integrase-strand transfer inhibitors (INSTIs) have become the recommended ART regimen in the transplant setting, as they do not interact with standard maintenance immunosuppressants, such as calcineurin inhibitors (12–14). We further explored the matched cohort by transplant era (pre- and post-introduction of INSTIs) and observed that among monoinfected patients there was no longer a significant difference in GS or PS in the modern era, suggesting that outcomes among monoinfected patients have improved over time. In contrast, independent of transplant era, HIV+ recipients coinfected with HCV continued to have worse outcomes when compared to HIV−/HCV+ recipients, suggesting that HCV coinfection was an effect modifier for outcomes among HIV+ recipients.
While our findings among coinfected patients are worrisome, data suggest that HIV+ recipients do derive a significant survival benefit from transplant, as accelerated cirrhosis and high mortality rates among HIV+ ESLD patients have been well documented (15–17). A study of HIV+ liver transplant wait list candidates demonstrated that each unit increase in MELD score was associated with a 20% increase in the risk of pre-transplantation death (17). Moreover, the study found that detectable HIV RNA at baseline was associated with a 3.2-fold increased risk for death and a 2.79-fold increased risk for liver disease progression as measured by MELD. It is important to note that in the current ART era, liver disease is now the most frequent cause of death among HIV+ individuals, likely related to complications of HCV coinfection as well as hepatotoxicity secondary to ART. As such, the need for liver transplantation among this unique population is on the rise (18, 19). Therefore, the ability to assess the potential survival benefit of liver transplantation among HIV+ ESLD patients is of paramount importance. Given the small number of HIV+ liver transplants performed to date, pooled national data are needed to properly address this concern. Currently, HIV serostatus of wait list registrants/candidates is not reported to UNOS. This has hampered efforts to study survival benefit among this unique population on a national scale.
Although our study did not identify potentially modifiable recipient factors, we did find a significant increase in risk for graft loss among HIV+ recipients of liver grafts from older donors (≥50 years). Our findings parallel results from studies within the general HCV+/HIV− transplant population that have demonstrated an association between older donor age and increased risk of graft loss and patient death (20, 21). Results from our study and others suggest that the effect of older donor age on outcomes after liver transplantation is more pronounced among HIV+ recipients compared to their HIV− counterparts (6, 15). These findings motivate a focus in clinical practice to avoid grafts from donors over the age of 50 years among HIV+ recipients, which may serve to improve outcomes among this vulnerable population.
Inferences based on our findings must take into account a number of important limitations. SRTR data lack granularity with regard to CD4 count, viral loads, infections, and malignancies – factors that are known to influence long-term outcomes among HIV+ patients. However, the NIH protocol used relatively restricted criteria for HIV+ ESLD patients to be considered candidates for transplantation, requiring CD4 counts of >100. It is unlikely that there would be major deviations from this protocol in the national data. Moreover, immunosuppressant levels and antiviral regimen are not captured by SRTR data, and as such, it is impossible to determine whether drug interactions between HAART therapy and immunosuppressants led to subtherapeutic drug levels and higher rates of acute rejection and graft loss. While understanding these interactions would help inform the mechanism of our long-term findings, the absence of these data does not bias our findings. Finally, the sample size available for our subgroup analyses were relatively small, and may impact the accuracy of our estimates. However, these data represent the HIV+ liver transplant population in the United States in its totality, and therefore, contribute new and important information about long-term outcomes after liver transplantation in this unique population.
This is the first national study to examine long-term outcomes among the entire US cohort of HIV+ liver transplant recipients and to compare these outcomes to appropriately matched HIV− counterfactuals. We found long-term GS and PS to have improved among monoinfected patients in the modern transplant era, and were similar to matched HIV−/HCV− controls. In contrast, independent of transplant era, outcomes among HIV+ patients coinfected with HCV continue to be worrisome. These results motivate future studies of survival benefit in this vulnerable population.
ACKNOWELEDGMENTS
The research was presented in preliminary forms as abstracts at the 2014 American Society of Transplant Surgeons State of the Art Winter Symposium and the 2014 World Transplant Congress. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or US government. These analyses were performed by the UAB Comprehensive Transplant Institute Outcomes Research Center analytic team. This research was supported in part by NIH K24DK101828 (PI: Segev).
Abbreviations
- HIV+0
human immunodeficiency virus
- ART
antiretroviral therapy
- ESLD
end stage liver disease
- HCV
hepatitis C virus
- BMI
body mass index
- PS
patient survival
- GS
graft survival
- SRTR
Scientific Registry of Transplant Recipients
- OPTN
Organ Procurement and Transplantation Network
- CIT
cold ischemia time
- MELD
model for end stage liver disease
- f/u
follow-up
- IQR
interquartile range
- aHR
adjusted hazard ratio
Footnotes
Disclosure: The authors declare no conflicts of interest
Authorship: Locke, Durand, and Segev: participated in research design, writing of the manuscript, and performance of the research; Reed and MacLennan: participated in data analysis; Massie: contributed new analytic tool; Mehta, Nellore and DuBay: participated in writing of the manuscript.
Contributor Information
Jayme E. Locke, Email: jlocke@uabmc.edu.
Christine Durand, Email: ChristineDurand@jhmi.edu.
Rhiannon D. Reed, Email: rdeierhoi@uabmc.edu.
Paul MacLennan, Email: pmaclennan@uabmc.edu.
Shikha Mehta, Email: smehta@uabmc.edu.
Allan Massie, Email: amassie1@jhmi.edu.
Anoma Nellore, Email: anellore@uabmc.edu.
Derek DuBay, Email: ddubay@uabmc.edu.
Dorry L. Segev, Email: dorry@jhmi.edu.
REFERENCES
- 1. [June 9, 2014];HIV in the United States: A Glance. 2013 Available from: http://www.cdc.gov/hiv/statistics/basics/ataglance.html.
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Rao TK. Human immunodeficiency virus infection in end-stage renal disease patients. Semin Dial. 2003;16(3):233–244. doi: 10.1046/j.1525-139x.2003.16047.x. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg EA, Stock P Practice ASTIDCo. Solid organ transplantation in the HIV-infected patient. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(Suppl 4):S131–S135. doi: 10.1111/j.1600-6143.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- 5.Stock PG, Roland ME. Evolving clinical strategies for transplantation in the HIV-positive recipient. Transplantation. 2007;84(5):563–571. doi: 10.1097/01.tp.0000279190.96029.77. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Roland ME, Schiano T, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(6):716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vera ME, Dvorchik I, Tom K, et al. Survival of liver transplant patients coinfected with HIV and HCV is adversely impacted by recurrent hepatitis C. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(12):2983–2993. doi: 10.1111/j.1600-6143.2006.01546.x. [DOI] [PubMed] [Google Scholar]
- 8.Duclos-Vallee JC, Feray C, Sebagh M, et al. Survival and recurrence of hepatitis C after liver transplantation in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2008;47(2):407–417. doi: 10.1002/hep.21990. [DOI] [PubMed] [Google Scholar]
- 9.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1- and 3-year outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(2):355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Rollet-Kurhajec K, Young J, et al. HIV virological rebounds but not blips predict liver fibrosis progression in antiretroviral-treated HIV/hepatitis C virus-coinfected patients. HIV medicine. 2014 doi: 10.1111/hiv.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA : the journal of the American Medical Association. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 12.Scholten EM, Cremers SC, Schoemaker RC, et al. AUC-guided dosing of tacrolimus prevents progressive systemic overexposure in renal transplant recipients. Kidney international. 2005;67(6):2440–2447. doi: 10.1111/j.1523-1755.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 13.Swinnen LJ. Outcomes of kidney transplantation in HIV-infected recipients. The New England journal of medicine. 2011;364(7):683. doi: 10.1056/NEJMc1014114. author reply 4. [DOI] [PubMed] [Google Scholar]
- 14.Tricot L, Teicher E, Peytavin G, et al. Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(8):1946–1952. doi: 10.1111/j.1600-6143.2009.02684.x. [DOI] [PubMed] [Google Scholar]
- 15.Mindikoglu AL, Regev A, Magder LS. Impact of human immunodeficiency virus on survival after liver transplantation: analysis of United Network for Organ Sharing database. Transplantation. 2008;85(3):359–368. doi: 10.1097/TP.0b013e3181605fda. [DOI] [PubMed] [Google Scholar]
- 16.Ragni MV, Eghtesad B, Schlesinger KW, Dvorchik I, Fung JJ. Pretransplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(11):1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian A, Sulkowski M, Barin B, et al. MELD score is an important predictor of pretransplantation mortality in HIV-infected liver transplant candidates. Gastroenterology. 2010;138(1):159–164. doi: 10.1053/j.gastro.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 19.Sulkowski MS. Viral hepatitis and HIV coinfection. Journal of hepatology. 2008;48(2):353–367. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Berenguer M, Prieto M, San Juan F, et al. Contribution of donor age to the recent decrease in patient survival among HCV-infected liver transplant recipients. Hepatology. 2002;36(1):202–210. doi: 10.1053/jhep.2002.33993. [DOI] [PubMed] [Google Scholar]
- 21.Grat M, Kornasiewicz O, Lewandowski Z, et al. Post-transplant outcomes of patients with and without hepatitis C virus infection according to donor age and gender matching. Annals of transplantation : quarterly of the Polish Transplantation Society. 2013;18:705–7415. doi: 10.12659/AOT.889537. [DOI] [PubMed] [Google Scholar]


