Abstract
Many medications induce diarrhea as a side effect, which can be a major obstacle to therapeutic efficacy and also a life-threatening condition. Secretory diarrhea can be caused by excessive fluid secretion in the intestine under pathological conditions. The cAMP/cGMP-regulated cystic fibrosis transmembrane conductance regulator (CFTR) is the primary chloride channel at the apical membrane of intestinal epithelial cells and plays a major role in intestinal fluid secretion and homeostasis. CFTR forms macromolecular complexes at discreet microdomains at the plasma membrane, and its chloride channel function is regulated spatiotemporally through protein-protein interactions and cAMP/cGMP-mediated signaling. Drugs that perturb CFTR-containing macromolecular complexes in the intestinal epithelium and upregulate intracellular cAMP and/or cGMP levels can hyperactivate the CFTR channel, causing excessive fluid secretion and secretory diarrhea. Inhibition of CFTR chloride-channel activity may represent a novel approach to the management of drug-induced secretory diarrhea.
Keywords: Drug-induced diarrhea, CFTR chloride channel, intestinal fluid secretion, CFTR inhibitor, CFTR-containing macromolecular complex
Graphical Abstract
1. Introduction
Diarrhea is a common side effect for many medications and accounts for approximately 7% of all drug-induced adverse effects, with over 700 drugs indicated to cause diarrhea [1-3]. Drugs including laxatives, antacids and heartburn medications, antibiotics, chemotherapy medication, anti-inflammatories as well as many supplements frequently cause diarrhea [4-6]. Drug-induced diarrhea can be acute or chronic, the severity of which is dictated by drug dosage and duration and frequency of administration [7]. In addition to causing dehydration, electrolyte imbalance, renal insufficiency, and immune dysfunction, drug-induced diarrhea decreases the efficiency of therapeutic interventions. Currently, standard approaches to mitigate a diarrheal side effect include dose reductions, treatment delays, discontinuation of therapy, and rehydration [8]. These approaches may temporarily relieve the diarrhea; however, they do not resolve the ‘root cause’ nor benefit the efficiency of ongoing therapeutic interventions.
Drugs can induce different types of diarrheas, including osmotic diarrhea, secretory diarrhea, inflammatory diarrhea, exudative diarrhea, fatty diarrhea, and motility diarrhea [1, 9]. For this review, we focus on the current understanding of drug-induced secretory diarrhea, particularly the molecular mechanisms underlying secretory diarrhea pathogenesis and the role of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel in this process. Identification of a treatable target will facilitate the development of therapies that not only mitigate drug-induced diarrheal side effects, but increase the efficiency of the drug being used.
2. CFTR chloride channel and its role in intestinal fluid secretion and homeostasis
2.1 The domain structure of CFTR and channel regulation
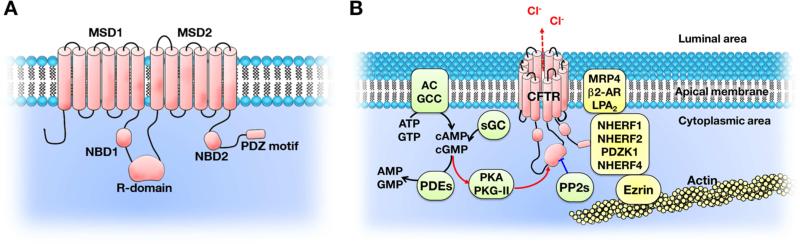
CFTR is a member of the ATP-binding cassette (ABC) transporter superfamily and has two repeated membrane-spanning domains (MSD). Each of these domains contains six helices and is associated with a cytoplasmic nucleotide-binding domain (NBD) that can bind and hydrolyze ATP. Two halves of CFTR are linked by a cytoplasmic regulatory domain (R-domain) that has several consensus phosphorylation sites. N-linked glycosylation sites are located on the extracellular loop between the 7th and 8th transmembrane helices [10]. CFTR has been shown to form macromolecular complexes with a wide variety of binding partners, particularly through the C-terminal PDZ-binding motif [11]. The formation of these macromolecular complexes enables the spatial-temporal regulation of CFTR channel function (Fig 1. A) [12].
Figure 1. A. The domain structure of cystic fibrosis transmembrane conductance regulator (CFTR).
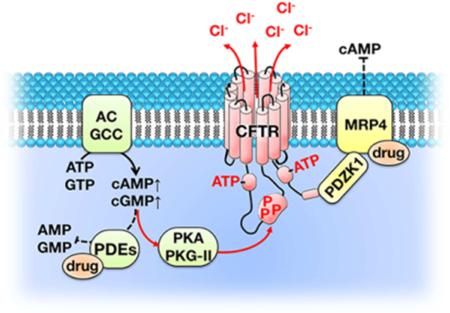
The CFTR chloride channel is activated by phosphorylation of the R domain and ATP binding to, and hydrolysis by, the two NBD. NBD, nucleotide binding domain; R-domain, regulatory domain; MSD, membrane-spanning domain; PDZ, PSD-95, Dlg, and ZO-1. B. CFTR forms macromolecular complexes at discrete domains underneath the apical plasma membrane. CFTR channel activity is regulated by dynamic protein-protein interactions and cAMP- and cGMP-mediated signaling. AC, adenylyl cyclase; GCC, guanylyl cyclase C; sGC, soluble guanylyl cyclase; PKG-II, protein kinase G-II; PDE, phosphodiesterase; PP2, protein phosphatase 2; MRP4, multidrug resistance protein 4; β2-AR, β2-adrenergic receptor; LPA2, lysophosphatidic acid receptor 2; NHERF1, Na+/H+ exchanger regulatory factor 1; PDZK1, PDZ domain-containing protein in kidney 1. The two red arrows to and from PKA PKG-II show activation of PKA/PKG-II and CFTR. The blue line indicates dephosphorylation of the R domain by PP2.
The CFTR chloride channel can be activated by two key processes: 1) Phosphorylation of the R-domain by various protein kinases. 2) Binding and hydrolysis of ATP at the two NBDs. There are multiple signaling-network proteins involved in phosphorylation of CFTR. Adenylyl cyclases and guanylyl cyclases catalyze the formation of cAMP (from ATP) and cGMP (from GTP), respectively. cAMP and cGMP activate cAMP-dependent protein kinase A (PKA), cAMP-dependent protein kinase C (PKC), and Type II cGMP-dependent protein kinase (PKG-II), which subsequently phosphorylate the R-domain of CFTR [13]. The CFTR chloride channel is deactivated by dephosphorylation of the R domain through protein phosphatase 2 (PP2) C and PP2 A [14]. Inhibition of cyclic nucleotide phosphodiesterases (PDE) also elicits high levels of cAMP and cGMP and activates the CFTR channel (Fig 1. B) [15].
2.2 CFTR-containing macromolecular complexes
A wide variety of proteins have been discovered to interact with CFTR, including transporters, ion channels, receptors, kinases, phosphatases, and cytoskeletal elements [16]. The PDZ-binding motif at the C-terminal tail of CFTR enables its binding to PDZ-domain-containing proteins, including Na+/H+ exchanger regulatory factors 1 and 2 (NHERF1 and NHERF2), PDZ-domain-containing protein in kidney 1 (PDZK1), NHERF4, SH3, ankyrin repeats containing protein 2 (SHANK2), and CFTR-associated ligand (CAL). Because these PDZ-domain-containing proteins typically have multiple PDZ domains and can dimerize, they also bind to other proteins that have PDZ-binding motifs and can bridge them to CFTR to form macromolecular complexes [17]. Examples of known CFTR-containing macromolecular complexes include the CFTR, NHERF1, and β2-adrenergic receptor (β2-AR) complex, the CFTR, NHERF2, lysophosphatidic acid receptor 2 (LPA2) complex, and the CFTR, PDZK1, multidrug resistance protein 4 (MRP4) complex [18-20]. The dynamic protein-protein interactions within these macromolecular complexes play important roles in regulating CFTR channel function and potentially could be targeted for treating diseases associated with altered CFTR channel function.
2.3 A role for CFTR in intestinal fluid secretion
CFTR is a chloride-ion channel protein at the apical membrane of epithelial cells lining several organs, including the intestine. CFTR has a critical role in transepithelial chloride transport and intestinal fluid secretion and homeostasis [21]. Chloride ions enter intestinal epithelial cells via the Na+-K+-2Cl− co-transporter at the basolateral side and exit at the apical side, primarily through CFTR, into the lumen [22]. Concurrently, water moves osmotically into the lumen through the paracellular transport mechanism, leading to fluid secretion. Hyperactivation of the CFTR channel causes excessive fluid secretion into the intestinal lumen, which could overwhelm the fluid reabsorption capacity of the colon and cause secretory diarrhea. Since CFTR is a major chloride channel at the apical side of intestinal epithelial cells, inhibition of CFTR channel function represents a promising approach to mitigating the drug-induced secretory diarrhea side effect.
2.4 A new model to study secretory diarrhea: intestinal enteroids
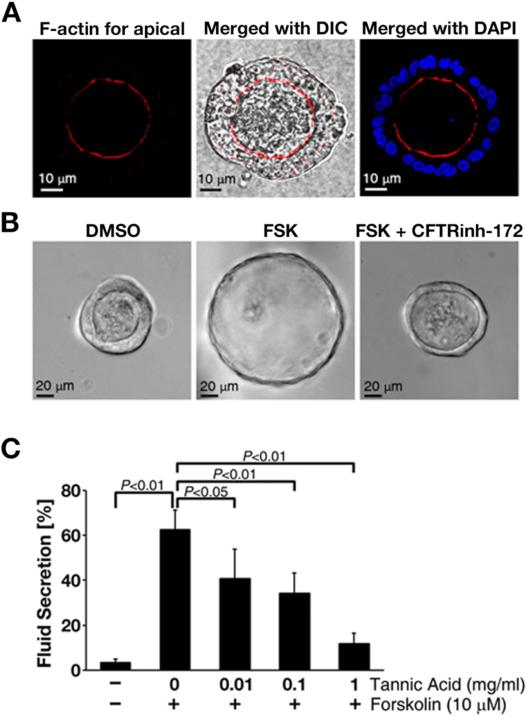
Until recently, limited models were available to study CFTR-mediated intestinal fluid secretion. Generally, the traditional two-dimensional epithelial cultures are not physiologically relevant to the structure and function of the intestine. Animal models are physiologically relevant, but are not suitable for certain applications such as early-stage drug screening. A new model system, three-dimensional cultures of primary intestinal enteroids, has been developed to recapitulate crypt structure and epithelial differentiation [23]. The culture method is relatively easy, fast and robust, and suitable to study intestinal fluid secretion and CFTR channel activity. Crypts can be isolated and purified from the small intestine of adult mice or human biopsies and cultured in Matrigel [24]. Central lumen-like structures and protruding villus-like structures are formed in each enteroid, mimicking the native intestinal epithelium [25]. The interior walls of the enteroid consist of a polarized epithelial monolayer with a distinct inner luminal surface and an outer basolateral surface (Fig. 2A). An example of using a human enterosphere (the early stage in the development of an enteroid) to study CFTR-mediated fluid secretion is provided in Figure 2B-C. Forskolin (FSK), which elicits high intracellular cAMP levels, was used to activate CFTR channels and caused significant fluid secretion, represented by the volume changes of the luminal area. A CFTR inhibitor (channel blocker), CFTRinh-172, significantly abolished the FSK-induced fluid secretion (Fig. 2B). Tannic acid, which also is a CFTR inhibitor and Ca2+-activated chloride channel inhibitor, attenuated FSK-induced and CFTR-mediated fluid secretion in a dose-dependent manner (Fig. 2C). These two examples not only demonstrate that intestinal enteroids are physiologically relevant systems to study fluid secretion, but also indicate that inhibition of CFTR channel function is a possible approach to attenuate excessive intestinal fluid secretion under pathological conditions, such as in drug-induced secretory diarrhea.
Figure 2. Using human intestinal enteroids to study fluid secretion.
A. Images of an enterosphere. F-actin staining shows the apical (luminal) side of human enterosphere. DAPI is used to stain the nuclei. B. Representative phase-contrast images showing the forskolin (FSK)-induced and CFTR-mediated fluid secretion in human intestinal enterosphere, which was abolished by using a specific CFTR channel blocker CFTRinh-172. C. Tannic acid attenuated the FSK-induced and CFTR-mediated fluid secretion in human intestinal enteroids. The inhibitory effect of tannic acid was dose-dependent. The values represent the mean ± SEM (n ≥10 enteroids per group) in this bar graph.
3. Drug-induced secretory diarrhea: A role for CFTR in the pathogenic process
3.1 Drug as substrate/inhibitor of MRP4
Like CFTR, MRP4 is a member of the ABC superfamily of transporters, and it plays an important role in transporting a wide variety of endogenous and xenobiotic organic anionic compounds out of cells [26]. Some of the endogenous substrates of MRP4 play important roles in cellular communication and signaling, including cAMP, cGMP, ADP, eicosanoids, bile acids, urate, and conjugated steroid hormones [27]. MRP4 is critical for the absorption, disposition, or excretion of targeted drugs, including antivirals (e.g., adefovir, zidovudine, tenofovir), antibiotics (e.g., ceftizoxime), antihypertensives (e.g., furosmide, olsesartan), and chemotherapy agents (e.g., methotrexate, topotecan, irinotecan). All of these drugs have been reported to cause diarrheal side effects [27, 28].
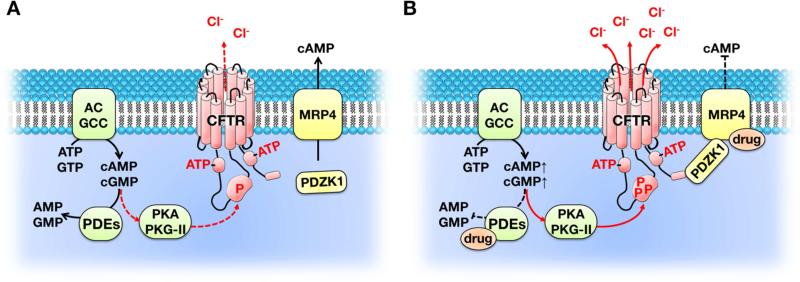
Based on previous findings that MRP4 complexes with CFTR (mediated by PDZK1) and CFTR plays an important role in intestinal fluid secretion, we recently investigated the role of CFTR in the pathogenic process of acute secretory diarrhea induced by two drugs, irinotecan (an anti-colon cancer drug) and zidovudine (an antiretroviral drug), both of which are substrates of MRP4 [25]. We found that binding of these drugs to MRP4 inhibits MRP4-mediated cAMP efflux and augments the formation of MRP4-PDZK1-CFTR complexes; the net result of which is elevated cAMP levels in close proximity to CFTR, which then hyperactivates CFTR channel function to cause excessive chloride ion efflux and fluid secretion [25]. Although we studied only these two drugs, it seems rational to speculate that MRP4-PDZK1-CFTR complexes play a critical role in other drug-induced secretory diarrhea, especially for drugs that are substrates or inhibitors of MRP4 (Fig. 3) [25].
Figure 3. Molecular mechanism contributing to drug-induced fluid secretion in the intestine.
A. Under physiological conditions, intracellular cAMP and cGMP levels are spatiotemporally regulated by actions of AC, GCC, PDE, and MRP4. B. Under pathological conditions such as drug-induced secretory diarrhea, drugs inhibit the activities of PDE and/or inhibit the efflux of cAMP/cGMP through MRP4; the net result is elevated cAMP and/or cGMP levels in proximity to CFTR that hyperactivate the chloride-channel activity. The red arrows show activation of PKA/PKG-II or CFTR.
3.2 Drug as inhibitor of cyclic nucleotide phosphodiesterase 4 (PDE4)
PDE catalyzes the hydrolysis of cAMP to AMP and cGMP to GMP and thereby, inhibition of PDE increases intracellular cAMP or cGMP [29]. Thus, PDE plays important roles in regulating intracellular concentrations and downstream effects of these secondary messengers. PDE4 isoforms, such as PDE4D3 and PDE4D5, have been shown to be involved in compartmentalized cAMP signaling by forming complexes with PKA and other signaling components [30]. PDE4D has been reported to co-localize with CFTR at the apical membrane of airway epithelium, and PDE4 inhibitors (e.g., rolipram, cilomilast) stimulate CFTR channel function in excised apical patches of Calu-3 cells [31]. Targeted inhibition of PDE4 has been pursued as a way of reducing inflammation in patients with asthma and COPD. However, clinical utility of PDE4 inhibitors has been limited by adverse effects, including diarrhea. Considering that PDE4 isoforms interact with CFTR and are involved in compartmentalized cAMP signaling, the diarrheal side effect associated with PDE4 inhibitor therapies might be due to, at least in part, the hyperactivation of CFTR channel function (Fig. 3).
3.2 Miscellaneous drugs
Auranofin is an orally administrated anti-rheumatic drug that induces severe or ongoing diarrhea as a side effect [32]. The formation of Au(CN)2− is thought to be important in the metabolism of auranofin. Access of Au(CN)2− to two cysteines from the cytoplasmic side is faster in an open CFTR channel, whereas access to the same sites from the extracellular side is faster when the channel is closed [33, 34]. Therefore, auranofin activates CFTR chloride-channel function by controlling channel gating. Calcitonin is used for treating postmenopausal osteoporosis, Paget's disease of bone, and hypercalcemia [35]. Calcitonin activates the calcitonin receptor, which is a G protein-coupled receptor, and activates adenylyl cyclases (AC) to generate cAMP and subsequently induce CFTR-mediated chloride secretion in intestinal epithelial cells [36]. Clinical uses of prostaglandins based on the luteolytic effect are short cycling, estrous synchronization, treatment of persistent corporalutea, and termination of pregnancy [37]. The diarrheal side effect associated with prostaglandins can be attributed to the finding that prostaglandins bind the Gs alpha subunit and activate AC, which then activates CFTR via cAMP-mediated signaling [38]. Chenodeoxycholic acid is a bile acid used to treat gallstones that is associated with a diarrheal symptom [39]. Chenodeoxycholic acid has been shown to activate the canonical cAMP-signaling pathway to phosphorylate CFTR and increase CFTR recruitment to the apical membrane, thereby stimulating CFTR channel function [40]. Digoxin is used to treat heart failure, and its primary mechanism of action involves inhibition of Na+/K+ ATPase [3]. This inhibition causes an increase in intracellular sodium levels, resulting in a reversal of the action of sodium-calcium exchanger, which normally imports three extracellular sodium ions into the cell and transports one intracellular Ca2+ out of the cell. Increased amounts of intracellular Ca2+ lead to increased storage of calcium in the sarcoplasmic reticulum and causes activation of Ca2+-activated chloride channels, including CFTR [41].
4. Perspective: Inhibition of CFTR for the management of drug-induced secretory diarrhea
Compelling evidence suggests a role for CFTR in the pathogenic process of drug-induced secretory diarrhea. Since CFTR is a validated drug target for cystic fibrosis therapy and combating enterotoxin-induced secretory diarrhea, inhibition of CFTR channel function is a potentially viable approach to management of drug-induced secretory diarrhea [42, 43]. Several potent small-molecule CFTR inhibitors with improved pharmacokinetics properties have been discovered, including BPO-27 and iOWH032 [44]. Also, several types of natural products have been identified as potent CFTR inhibitors, including crofelemer, LPA, and tannic acid [45-47]. Crofelemer has been tested in clinical trials for treating several types of secretory diarrhea, including cholera, AIDS-induced diarrhea, and traveler's diarrhea [48]. Tannic acid has an anti-diarrheal effect and high antioxidant capacity, and it improves intestinal epithelial barrier function [47].
5. Conclusion
Diarrhea is a common adverse effect of drug medications that can be elicited through different pathophysiological mechanisms. For drug-induced secretory diarrhea, particularly for drugs that perturb the intracellular secondary messenger signaling, compelling evidence suggests a role for CFTR in the pathogenic process. Since CFTR is a validated therapeutic target for cystic fibrosis and a target for other types of secretory diarrhea, inhibition of CFTR channel function represents a potential approach to management of drug-induced secretory diarrhea and is encouraged by the discovery and development of potent CFTR inhibitors, both synthetic small-molecules and natural products.
Supplementary Material
Acknowledgements
The authors thank J. Denise Wetzel, CCHMC Medical Writer, for review and editing of the manuscript. This work was supported by the U.S. National Institutes of Health grants R01-DK080834 and R01-DK093045 to A. P. Naren, R01HL123535 to W. Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
C. Moon and W. Zhang contributed equally to this paper. All authors participated in critical review of the manuscript, and have approved the final version.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
- 1.Chassany O, Michaux A, Bergmann JF. Drug-induced diarrhoea. Drug Saf. 2000;22:53–72. doi: 10.2165/00002018-200022010-00005. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamand BP, Sellin JH. Drug-induced, factitious, & idiopathic diarrhoea. Best Pract Res Clin Gastroenterol. 2012;26:633–648. doi: 10.1016/j.bpg.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Jones M, Hawker F, Duggin G, Falk M. Treatment of severe digoxin toxicity with digoxin-specific antibody fragments. Anaesthesia and intensive care. 1987;15:234–236. doi: 10.1177/0310057X8701500219. [DOI] [PubMed] [Google Scholar]
- 4.Donowitz M. Current concepts of laxative action: mechanisms by which laxatives increase stool water. J Clin Gastroenterol. 1979;1:77–84. doi: 10.1097/00004836-197903000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Periman P. Antibiotic-associated diarrhea. N Engl J Med. 2002;347:145. doi: 10.1056/NEJM200207113470216. author reply 145. [DOI] [PubMed] [Google Scholar]
- 6.Gibsonand RJ, Stringer AM. Chemotherapy-induced diarrhoea. Curr Opin Support Palliat Care. 2009;3:31–35. doi: 10.1097/SPC.0b013e32832531bb. [DOI] [PubMed] [Google Scholar]
- 7.DuPont HL. Guidelines on acute infectious diarrhea in adults. The Practice Parameters Committee of the American College of Gastroenterology. The American journal of gastroenterology. 1997;92:1962–1975. [PubMed] [Google Scholar]
- 8.Maroun JA, Anthony LB, Blais N, Burkes R, Dowden SD, Dranitsaris G, Samson B, Shah A, Thirlwell MP, Vincent MD, Wong R. Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on Chemotherapy-Induced Diarrhea. Curr Oncol. 2007;14:13–20. doi: 10.3747/co.2007.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweetser S. Evaluating the patient with diarrhea: a case-based approach. Mayo Clin Proc. 2012;87:596–602. doi: 10.1016/j.mayocp.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheppardand DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 11.Elkins JM, Gileadi C, Shrestha L, Phillips C, Wang J, Muniz JR, Doyle DA. Unusual binding interactions in PDZ domain crystal structures help explain binding mechanisms. Protein Sci. 2010;19:731–741. doi: 10.1002/pro.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liand C, Naren AP. Analysis of CFTR interactome in the macromolecular complexes. Methods Mol Biol. 2011;741:255–270. doi: 10.1007/978-1-61779-117-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frizzelland RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012;2:a009563. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo J, Pato MD, Riordan JR, Hanrahan JW. Differential regulation of single CFTR channels by PP2C, PP2A, and other phosphatases. Am J Physiol. 1998;274:C1397–1410. doi: 10.1152/ajpcell.1998.274.5.C1397. [DOI] [PubMed] [Google Scholar]
- 15.O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol. 2002;185:137–144. doi: 10.1007/s00232-001-0120-3. [DOI] [PubMed] [Google Scholar]
- 16.Kunzelmann K. CFTR: interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 17.Liand C, Naren AP. CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr Biol (Camb) 2010;2:161–177. doi: 10.1039/b924455g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naren AP, Cobb B, Li C, Roy K, Nelson D, Heda GD, Liao J, Kirk KL, Sorscher EJ, Hanrahan J, Clancy JP. A macromolecular complex of beta 2 adrenergic receptor, CFTR, and ezrin/radixin/moesin-binding phosphoprotein 50 is regulated by PKA. Proc Natl Acad Sci U S A. 2003;100:342–346. doi: 10.1073/pnas.0135434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holcomb J, Jiang Y, Lu G, Trescott L, Brunzelle J, Sirinupong N, Li C, Naren AP, Yang Z. Structural insights into PDZ-mediated interaction of NHERF2 and LPA(2), a cellular event implicated in CFTR channel regulation. Biochem Biophys Res Commun. 2014;446:399–403. doi: 10.1016/j.bbrc.2014.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Krishnamurthy PC, Penmatsa H, Marrs KL, Wang XQ, Zaccolo M, Jalink K, Li M, Nelson DJ, Schuetz JD, Naren AP. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews JB. Molecular regulation of Na+-K+-2Cl- cotransporter (NKCC1) and epithelial chloride secretion. World J Surg. 2002;26:826–830. doi: 10.1007/s00268-002-4059-z. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of Gastrointestinal Epithelial Organoids. Curr Protoc Mouse Biol. 2013;3:217–240. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moon C, Zhang W, Ren A, Arora K, Sinha C, Yarlagadda S, Woodrooffe K, Schuetz JD, Valasani KR, de Jonge HR, Shanmukhappa SK, Shata MT, Buddington RK, Parthasarathi K, Naren AP. Compartmentalized accumulation of cAMP near complexes of multidrug resistance protein 4 (MRP4) and cystic fibrosis transmembrane conductance regulator (CFTR) contributes to drug-induced diarrhea. J Biol Chem. 2015;290:11246–11257. doi: 10.1074/jbc.M114.605410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Wen J, Luo J, Huang W, Tang J, Zhou H, Zhang W. The Pharmacological and Physiological Role of Multidrug-Resistant Protein 4. J Pharmacol Exp Ther. 2015;354:358–375. doi: 10.1124/jpet.115.225656. [DOI] [PubMed] [Google Scholar]
- 28.Huynh T, Norris MD, Haber M, Henderson MJ. ABCC4/MRP4: a MYCN-regulated transporter and potential therapeutic target in neuroblastoma. Front Oncol. 2012;2:178. doi: 10.3389/fonc.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 30.Otero C, Penaloza JP, Rodas PI, Fernandez-Ramires R, Velasquez L, Jung JE. Temporal and spatial regulation of cAMP signaling in disease: role of cyclic nucleotide phosphodiesterases. Fundam Clin Pharmacol. 2014;28:593–607. doi: 10.1111/fcp.12080. [DOI] [PubMed] [Google Scholar]
- 31.Penmatsa H, Zhang W, Yarlagadda S, Li C, Conoley VG, Yue J, Bahouth SW, Buddington RK, Zhang G, Nelson DJ, Sonecha MD, Manganiello V, Wine JJ, Naren AP. Compartmentalized cyclic adenosine 3',5'-monophosphate at the plasma membrane clusters PDE3A and cystic fibrosis transmembrane conductance regulator into microdomains. Molecular biology of the cell. 2010;21:1097–1110. doi: 10.1091/mbc.E09-08-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magaro M, Altomonte L, Mirone L, Zoli A, Corvino G, Carelli G. Effect of oral gold salt therapy on bile acid absorption in rheumatoid arthritis patients. Clin Rheumatol. 1990;9:42–47. doi: 10.1007/BF02030239. [DOI] [PubMed] [Google Scholar]
- 33.Gaoand X, Hwang TC. Localizing a gate in CFTR. Proc Natl Acad Sci U S A. 2015;112:2461–2466. doi: 10.1073/pnas.1420676112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wangand P W. Linsdell. Conformational change opening the CFTR chloride channel pore coupled to ATP-dependent gating. Biochim Biophys Acta. 2012;1818:851–860. doi: 10.1016/j.bbamem.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Hamdyand RC, Daley DN. Oral calcitonin. Int J Womens Health. 2012;4:471–479. doi: 10.2147/IJWH.S24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Singla A, Ao M, Gill RK, Venkatasubramanian J, Rao MC, Alrefai WA, Dudeja PK. Calcitonin receptor-mediated CFTR activation in human intestinal epithelial cells. J Cell Mol Med. 2011;15:2697–2705. doi: 10.1111/j.1582-4934.2011.01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen WR, Wilsher S, Morris L, Crowhurst JS, Hillyer MH, Neal HN. Laparoscopic application of PGE2 to re-establish oviducal patency and fertility in infertile mares: a preliminary study. Equine veterinary journal. 2006;38:454–459. doi: 10.2746/042516406778400628. [DOI] [PubMed] [Google Scholar]
- 38.Fu Q, Chen X, Xiang YK. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Trends in cardiovascular medicine. 2013;23:250–256. doi: 10.1016/j.tcm.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2013;27:653–659. doi: 10.1155/2013/485631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. American journal of physiology. Cell physiology. 2013;305:C447–456. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saini-Chohan HK, Goyal RK, Dhalla NS. Involvement of sarcoplasmic reticulum in changing intracellular calcium due to Na+/K+-ATPase inhibition in cardiomyocytes. Canadian journal of physiology and pharmacology. 2010;88:702–715. doi: 10.1139/y10-055. [DOI] [PubMed] [Google Scholar]
- 42.Thiagarajah JR, Donowitz M, Verkman AS. Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol. 2015;12:446–457. doi: 10.1038/nrgastro.2015.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Awqati Q. Alternative treatment for secretory diarrhea revealed in a new class of CFTR inhibitors. J Clin Invest. 2002;110:1599–1601. doi: 10.1172/JCI17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Fujii N, Naren AP. Recent advances and new perspectives in targeting CFTR for therapy of cystic fibrosis and enterotoxin-induced secretory diarrheas. Future medicinal chemistry. 2012;4:329–345. doi: 10.4155/fmc.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tradtrantip L, Namkung W, Verkman AS. Crofelemer, an antisecretory antidiarrheal proanthocyanidin oligomer extracted from Croton lechleri, targets two distinct intestinal chloride channels. Molecular pharmacology. 2010;77:69–78. doi: 10.1124/mol.109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Penmatsa H, Ren A, Punchihewa C, Lemoff A, Yan B, Fujii N, Naren AP. Functional regulation of cystic fibrosis transmembrane conductance regulator-containing macromolecular complexes: a small-molecule inhibitor approach. The Biochemical journal. 2011;435:451–462. doi: 10.1042/BJ20101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren A, Zhang W, Thomas HG, Barish A, Berry S, Kiel JS, Naren AP. A tannic acid-based medical food, Cesinex((R)), exhibits broad-spectrum antidiarrheal properties: a mechanistic and clinical study. Digestive diseases and sciences. 2012;57:99–108. doi: 10.1007/s10620-011-1821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel TS, Crutchley RD, Tucker AM, Cottreau J, Garey KW. Crofelemer for the treatment of chronic diarrhea in patients living with HIV/AIDS. Hiv/Aids. 2013;5:153–162. doi: 10.2147/HIV.S30948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.