Abstract
High population density is often associated with increased levels of stress-related hormones, such as corticosterone (CORT). Prairie voles (Microtus ochrogaster) are a socially monogamous species known for their large population density fluctuations in the wild. Although CORT influences the social behavior of prairie voles in the lab, the effect of population density on CORT has not previously been quantified in this species in the field. We validated a non-invasive hormone assay for measuring CORT metabolites in prairie vole feces. We then used semi-natural enclosures to experimentally manipulate population density, and measured density effects on male space use and fecal CORT levels. Our enclosures generated patterns of space use and social interaction that were consistent with previous prairie vole field studies. Contrary to the positive relationship between CORT and density typical of other taxa, we found that lower population densities (80 animals/ha) produced higher fecal CORT than high densities (240/ha). Combined with prior work in the lab and field, the data suggest that high prairie vole population densities indicate favorable environments, perhaps through reduced predation risk. Lastly, we found that field animals had lower fecal CORT levels than laboratory-living animals. The data emphasize the usefulness of prairie voles as models for integrating ecological, evolutionary and mechanistic questions in social behavior.
Keywords: Corticosterone, prairie vole, population density, fecal hormone assay, Microtus ochrogaster, stress
1. Introduction
Glucocorticoids function to mobilize resources and channel them to meet environmental demands, ranging from daily activity rhythms to responses to threats and stressors (Sapolsky, 2002). Many natural stressors are profoundly influenced by social factors: conspecifics may compete for territories, commit infanticide, or fuel the growth of predator populations; they may also facilitate food finding, share parental care, or satiate predators with their abundance (Adkins-Regan, 2005; Becker et al., 2002; Solomon, 1949; Wolff and Sherman, 2007). Hence, the social environment has dynamic and complex consequences for individual fitness. It seems fitting that much current attention is focused on the intersection of stress steroids, environmental variability, and social behavior (Creel et al., 2013). In the current study, we test the hypothesis that high population densities promote increases in agonistic encounters and elevate glucocorticoids in the socially monogamous prairie vole, Microtus ochrogaster.
Population density cycles are a well-investigated dimension of environmental variation. With respect to glucocorticoids, historical perspectives emphasize how density-associated predation or resource depletion can promote stress and inhibit reproduction, leading to a reduction in population growth (Christian, 1950; Wolff and Sherman, 2007). More recently, researchers have examined how predator abundance or other density-related stressors influence the developing phenotypes of young, causing cycles in stress reactivity that can alter ecological processes as offspring become adults (Breuner, 2008; Love et al., 2013). While population cycles have been examined extensively, their interactions with stress and social behavior are still not fully understood (Creel et al., 2013), and the subject remains a remarkably fruitful area of work.
Because glucocorticoids mobilize resources to deal with unfavorable conditions and limit reproduction, the effects of population density on glucocorticoids often mirror how density contributes to population growth (Christian, 1950; Creel et al., 2013). In many taxa, high population density depletes resources, drives agonistic interactions and/or promotes predation, a set of stressors that also promote glucocorticoid secretion and impair reproduction (Boonstra and Boag, 1992; Christian, 1950; Creel et al., 2013). Perhaps not surprisingly, high density is often associated with high glucocorticoid secretion (Boonstra and Boag, 1992; Christian, 1961; Creel et al., 2013; Wang et al., 2009). Alternatively, environments may actually improve with density (Allee, 1931). For example, higher densities can lower the per-capita risk of predation (Parrish and Edelstein-Keshet, 1999), and facilitate mate-finding (Crowley et al., 1991; Kokko and Rankin, 2006). When the social system includes complex social groups with dominance hierarchies, the relationship between glucocorticoids and population density becomes still more complicated (Creel et al., 2013; Sapolsky, 2005; Wingfield and Sapolsky, 2003), with glucocorticoids affecting individuals unequally depending on dominance status and the stability of social hierarchies.
To investigate the relationship between population density and glucocorticoids, we study the socially monogamous prairie vole. In the wild, most male and female prairie voles form enduring pair-bonds and participate in the rearing of young (Getz and Carter, 1996). Studies spanning decades reveal that their population density undergoes wide annual fluctuations (Getz et al., 1993; Getz et al., 2006; Getz et al., 1990), with heavy spring-summer mortality and low densities (as low as 11 animals/hectare; Getz et al., 1993), contrasted with autumn-winter population spikes (as high as 600 animals/hectare; Getz et al., 1993). The time from conception to adulthood is approximately 9 weeks (Mateo et al., 1994; Stehn and Richmond, 1975); depending on the time of year young are born, offspring may grow to live in similar densities or wildly different densities, providing an interesting challenge to the development of the young. The combination of this well-characterized natural history and the extensive use of prairie voles as laboratory subjects in social neuroscience (Carter et al., 1995; Winslow et al., 1993; Young et al., 1999; Young and Wang, 2004) make them an especially valuable model for the integrative study of stress, sociality and ecology.
The principal glucocorticoid secreted by prairie voles is corticosterone (CORT). In the lab, acute stressors and exogenous CORT facilitate social bonding in males (Blondel Thesis 2013; DeVries et al., 1996), but does not influence bonding in females (DeVries et al., 1996). Interestingly, stressed males also exhibit increased levels of paternal care (Bales et al., 2006). In several species, parental care or developmental CORT shapes the stress reactivity and social behavior of offspring as adults (Meaney, 2001). Among prairie voles, developmental CORT exposure can alter subsequent parental care and affiliation (Roberts et al., 1996).
CORT levels in the lab are traditionally quantified by invasive procedures such as retro-orbital bleeding (DeVries et al., 1995; Taymans et al., 1997), which are less appropriate for measuring CORT in the field than non-invasive fecal hormone assays (Good et al., 2003). Fecal hormone glucocorticoid assays have been validated successfully in many other mammalian taxa (Crino et al., 2010; Goymann et al., 1999; Harper and Austad, 2000; Mateo and Cavigelli, 2005; Monfort et al., 1997; Wasser et al., 1995), and are currently considered the most practical and least invasive method of measuring chronic stress (Dantzer et al., 2014; Sheriff et al., 2011). The fecal hormone assay method has considerable advantages over traditional bleeding methods (all reviewed in Dantzer et al., 2014; Goymann, 2005; Harper and Austad, 2000; Palme et al., 2005; Sheriff et al., 2011): beyond its non-invasive nature and more straightforward collection procedure, each sample also contains an averaged hormone level covering the previous several hours. Hence, measures are more representative of an individual’s general hormone exposure than the point sampling of bleeding methods.
In the current study, we begin by validating a fecal hormone assay for CORT in prairie voles, demonstrating that our assay is precise and replicable, and that it can detect acute rises in CORT induced by an arbitrary stressor. We next manipulate population density in semi-natural enclosures, to ask whether density causes changes in social interaction and CORT titers in the field. We focus on males because CORT levels are known to influence both social bonding and parental care of males in the lab, and because limited resources prevented us from looking at both sexes. By using semi-natural enclosures, we allowed social interactions while controlling for factors such as predation, food and water resources (Creel et al., 2013).
2. Material and Methods
2.1 Experimental Design Summary
We first validated a fecal CORT metabolite hormone assay using a test for assay linearity, followed by a swim challenge to confirm that the assay can detect fecal CORT responses to a standardized acute stressor. Having validated our fecal CORT assay, we conducted a field experiment using a different set of animals. We measured fecal CORT metabolites for these animals in the lab, then placed them in semi-natural enclosures in the field for 19–24 days at one of two densities: low-density (LD) = 80 animals/ha, n = 12 males and 12 females per trial, and high-density (HD) = 240 animals/ha, n = 12 males and 12 females per trial. In these enclosures we measured individual space use, male-female pairing patterns, and CORT metabolite levels over two separate replicate trials, a “summer trial” and a “fall trial”. Animals were briefly trapped and feces were collected during the trial and again at the end of the trial; thus, we collected feces at three time points: pre-field (lab), mid-way (days 8–13) and at the end of the field trial (days 19–24).
2.2 Subject Animals
Study animals were descendants of wild-caught voles from Illinois and Tennessee. Subjects ranged from F6 to F10 generations from the original wild-caught voles. All animals were lab born and raised, and were weaned at 21 days. Upon weaning, voles were placed in same-sex sibling groups. Housing conditions have been known to affect CORT levels, with solo-housed prairie voles exhibiting higher levels of CORT (Ruscio et al., 2007); therefore, all animals used in this study were socially housed in same-sex sib groups of 2–5. Prairie voles reach sexual maturity at 45 days (Mateo et al., 1994); only animals 45 days or older were used as subjects. All procedures were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee (IACUC) in accordance with local, state and federal regulations to minimize pain and discomfort. Our IACUC protocol number was D289.
2.3 Fecal CORT metabolite extraction and radioimmunoassays
Our sample collection, extraction and validation protocols included methods established by Harper and Austad (2000), Mateo and Cavigelli (2005), and Crino et al. (2010). For all fecal collection, we collected up to a maximum of five pellets per animal produced during a 15-minute defecation period; any feces contaminated with urine were excluded (after Cavigelli et al., 2005). Pellets were stored in a −20° C freezer.
Feces were freeze-dried and weighed. To minimize variation in weight prior to extraction and radioimmunoassay (RIA), we combined dry pellets, collected at the same time and from the same individual, to a weight of 15–20mg. If all combined pellets from a given defecation period were less than 15 mg, we still used them; if there were still remaining pellets after we reached 20 mg, we left those extra pellets out of the analysis and stored them separately. The total feces produced by an individual during a defecation period and used for analysis is hereby referred to as a “sample,” and a sample can thus consist of one or multiple pellets.
Samples were then placed in individual glass culture tubes with 1 mL 90% methanol solution, homogenized with a spatula, and then agitated at room temperature for 24 hours using a Thermo Scientific Labquake rotator. They were then centrifuged at 2000 rpm at 4°C for five minutes. We collected ~500 microliters of the supernatant from each sample, and stored the supernatant at −20°C until assayed.
Most of what is present in feces is not CORT itself, but rather CORT metabolites (Palme et al., 2005; Touma and Palme, 2005). In our study, references to fecal CORT will imply fecal CORT metabolites. For fecal glucocorticoid assays, it is standard practice to use antibodies for the original hormone (Goymann, 2005), and thus we used commercial 125I RIA kits (MP Biomedicals, Solon, OH; catalog nos. 07120102 & 07120103; minimum detectable dose of 7.7 ng CORT/mL) that have been previously validated for plasma CORT in prairie voles (DeVries et al., 1995; Grippo et al., 2007; Taymans et al., 1997). However, when doing this, it is important to first demonstrate that the antibody for the original hormone can detect the metabolites of the hormone (Goymann, 2005), which was the purpose of our fecal hormone assay validation (2.4 Fecal Hormone Assay Validation). With the exception of the test for assay linearity (Methods 2.4.1), which requires a serial dilution, we achieved our best results (i.e., consistently readable from the standard curve) using undiluted extracts for the assays. To account for differences in fecal sample mass, we divided the total CORT metabolites detected (ng/mL) by fecal mass (mg/mL).
2.4 Fecal Hormone Assay Validation
2.4.1 Test for assay linearity
Antibodies targeting CORT can be used to measure CORT metabolites if dilutions of fecal CORT produce parallel changes in binding to the RIA-antibody (Goymann, 2005). This criterion is referred to as the test for parallelism (Beehner and McCann, 2008; Crino et al., 2010; Harper and Austad, 2000; Heilmann et al., 2011; Mateo and Cavigelli, 2005; Sheriff et al., 2011; Vasconcellos et al., 2011). Using serial dilutions of a pooled fecal sample, we checked that assay values varied linearly with hormone concentration within the standard curve boundaries (Buchanan and Goldsmith, 2004; Heilmann et al., 2011) and identified appropriate sample dilutions.
We collected fecal samples from 16 animals and extracted fecal CORT as described in 2.3 Fecal CORT extraction and radioimmunoassays, and then pooled the extracted samples together. Using the pooled extracts, we performed three separate serial dilutions, each consisting of 10 dilutions ranging from 1:2 to 1:2000, using the steroid diluent provided with the RIA kit (MP Biomedicals Lot Number: RCBK1010). All dilution samples were run in duplicate in a single RIA; the intra-assay coefficient of variation (CV) was 4.35%.
We quantified CORT at each dilution level for each of the three replicates, log-logit transformed the antibody binding curves, and used an analysis of covariance to compare this with the slope of the log-logit-transformed antibody binding curves from the standards generated from stock solutions that were supplied with the RIA kits (Harper and Austad, 2000; Mateo and Cavigelli, 2005). Note that this test for assay linearity shows a dose-response relationship and is not sufficient for full demonstration of specificity, as some cross-reacting compounds could result in acceptable results (Mostl et al., 2005); therefore, we also had to demonstrate that the fecal assay could detect an acute stressor, which we did with a swim challenge.
2.4.2 Swim challenge
2.4.2.1 Swim challenge experimental design
We tested the biological validity of our assay (Touma and Palme, 2005) by demonstrating that it could detect the change in CORT associated with an acute stressor (Crino et al., 2010; Harper and Austad, 2000; Mateo and Cavigelli, 2005; Touma and Palme, 2005), a swim challenge. Prairie voles swim instinctively when placed in water; swim challenges are commonly used as standard stressors (Bosch et al., 2009; DeVries et al., 1996; Grippo et al., 2012), and are known to elevate prairie vole CORT levels (DeVries et al., 1995; Taymans et al., 1997).
Sixteen animals (eight male, eight female) were used. Four males and four females were selected at random and assigned to the control group; the remaining four males and four females were swim-treatment animals. Control and swim-treatment animals were run simultaneously to control for any effects of circadian rhythms. 48h prior to testing, all animals were removed from their sibling-housed cage and individually housed in new cages with clean bedding and ad libitum food and water. Although solo-housed voles can sometimes exhibit higher CORT levels than group-housed voles (Ruscio et al., 2007), individual housing was necessary to facilitate collection of feces. Nonetheless, because both control and treatment individuals were housed singly, any treatment-specific differences in CORT levels can be attributed to the swim challenge stressor. A full 48 hours was allowed for acclimation prior to the experiment so that any temporary CORT surge related to the cage transfer would have dissipated; we know from prior pilot studies that this time window is sufficient. We collected baseline feces from both control and swim-treatment animals 24 hours prior to the swim challenge. On the third day of individual housing, forced swim tests began between 1500 h and 1600 h; additional baseline feces were collected 1.25 hours prior to the swim challenge.
To evoke a stress response, each swim-treatment animal was placed in a ten-gallon aquarium filled with 15 cm of 22°C water for 5 minutes. This was repeated every 30 minutes for each treatment animal twice, for a total of three 5-minute swim challenges spaced over 75 minutes. Control animals were unhandled during this period; handling of the treatment animals during the swim challenge was considered part of the overall stressor. Between swimming sessions and after the swim challenge, all treatment animals were dried and returned to their cages, where they quickly resumed normal behaviors.
For 20 hours after the swim test (excluding times between 300 h and 500 h), sampling was binned into 2-hour intervals in which water bowls were removed from the cage to simplify pellet collection, interspersed with 1-hour intervals in which water was present and no feces collected. During collection periods, we monitored both swim-treatment and control animals every 15min, collecting pellets from each animal once per 2-hour interval as soon as we observed them defecating. Not all animals produced feces in every time interval, so actual sample sizes within an interval varied from 5 to10 subjects per treatment. Times are given “post-stressor” to refer to the time since an individual began its first five-minute swim challenge.
To increase our sampling of the time immediately following the stressor, we performed a second swim challenge with a subset of the animals 72 hours after the beginning of the first swim challenge (a time span which our previous pilot studies had shown was well beyond the time required for CORT levels to return to baseline). The second challenge was identical to the prior challenge, but this time we used four of the previous control animals (two male, two female) as treatment animals, and four of the previous treatment animals (two male, two female) as control animals. In this replicate, we observed the animals continuously and collected pellets from all animals as soon as they were produced and hence, had a smaller sample size; fecal pellet collection lasted 4.75 hours post-stressor. We combined the second challenge data with our first challenge data, to improve our overall statistical power. However, we also present a second statistical analysis that omits these additional samples, which avoids entirely any potential confounds of repeated testing on some, but not all, animals.
2.4.2.2 Swim challenge CORT quantification and data analysis
Swim-treatment and control samples were run in duplicate over two RIAs (Lot Numbers RCBK1106A, RCBK114) due to the large number of samples. To control for variation between kits and assays, we ran a subset of 9 of the 169 samples in both assays to allow for direct comparison of CORT values. Of the 169 samples, 4 were excluded because they either fell outside the range of the standard curve (n = 2), or 12 because their measured values were more than 2SD above the mean (n = 2: 2.5 and 10.3 SD)
The intra-assay average CV was 1.86%. The inter-assay average CV for nine overlapped samples that were run in both assays was 22.3%; however, the CORT measures from each assay for these nine overlapped samples were significantly positively related (simple linear regression, p < 0.0001; R2 = 0.9931). We used the resulting relationship, y = 0.663× + 15.28, to correct for inter-assay differences.
Note that the above and subsequent inter-assay average CVs are relatively high (generally ~22%). This was due to a “kit age effect” whereby older RIA kits consistently showed higher CORT values for a given sample than younger kits, despite purportedly identical standards and controls used separately in each kit (Blondel, unpublished data). There also may have been a “batch” effect in cases where kits came from different lot numbers. Since we ran a large number of samples in replicate across assays whenever using two kits (ranging from 9 to 40 overlapped samples), and had high R2 values in our regression analyses (ranging from 0.93 to 0.99), we used these regressions between assays to correct for assay-specific variation.
Next we compared the fecal CORT levels of swim-treatment animals to those of the control animals. As described above, we had two pre-challenge collection time periods (24 hours and 1.5 hours prior to the challenge); and six 2-hour collection periods spanning the 20 hours following the swim challenge. Because CORT is released in a circadian rhythm (Sapolsky, 2002), we compared swim-treatment and control treatment animals within each time interval. The CORT measurements were not normally distributed (Shapiro-Wilk, W = 0.891, p < 0.0001), thus we used non-parametric Mann-Whitney U tests to compare swim-treatment values with controls for a given time interval. To include the results of the second swim challenge in the overall analysis, we averaged CORT values for any animals in the second challenge that produced feces at more than one time point within a 2-hour sampling interval.
2.5 Semi-Natural Enclosures and Density Manipulation
In order to quantify the effect of population density on CORT levels, we created two population density treatments using semi-natural enclosures: low-density (LD; 24 animals in 0.3 hectares = 80 animals/hectare) and high-density (HD; 24 animals in 0.1 hectares = 240 animals/hectare). These density treatments fall within the range (11 to 624/hectare) reported in wild prairie vole populations (Getz et al., 1993). Our field site was located in Jackson County, Illinois, USA, well within the natural geographic range of prairie voles.
The semi-natural enclosures consist of four abutting quadrants, each measuring 0.1 hectares (Fig. 1). Quadrant walls are composed of aluminum flashing that extends 30cm below-ground and 60cm above-ground. Further, quadrants were separated by large removable gates, allowing them to be combined, thus our high-density animals were distributed in one quadrant while our low-density animals occupied three interconnected quadrants. In addition, this structure allowed us to vary the quadrant used for the high-density treatment and reduce any confound between location and density effect. Inside the enclosure were abundant tall grasses and sedges, the preferred habitat of the prairie vole (Getz, 1985). Clover was planted to supplement their mostly herbivorous diet (Batzli, 1985). Although their water requirements are typically met by the water content of vegetation and by dew, we also provided two agricultural poultry water stations in each quadrant, which were kept filled during trials and cleaned between trials. Predation-prevention measures included aviary netting covering the top and sides of the enclosure, a secondary perimeter of flashing ~3m outside the inner perimeter, snake-guards to deter entry along support posts, and a 2-cable electrified fence directly above the outer perimeter.
Figure 1.

Semi-natural enclosure facilities, demonstrating density treatments. High Density treatment location changed in different trials.
We ran two 3-week (19–24-day) field trials; Trial 1 was conducted in August 2011 (“Summer trial”) and Trial 2 was conducted in October 2011 (“Fall trial”). Each trial consisted of 12 males and 12 females assigned to high density, and another 12 males and 12 females assigned to low density (Fig. 1). All animals were ear-tagged and toe-clipped for identification and future paternity analysis, and affixed with radio-transmitters (model SOM 2028 HWSC, 2.2g or less, Wildlife Materials, Murphysboro, Illinois). Because this is a fossorial species, toe-clipping was limited to a single rear toe, in accordance with the American Society of Mammalogists guidelines (Sikes et al., 2011). In the wild, male space-use is strongly influenced by female residency (Getz and McGuire, 1993), with males dispersing until they find and join a female at her nest. Thus, we released females first, and released males two days later, to more closely replicate the natural social environment.
Prairie voles exhibit mostly crepuscular activity patterns, and beyond that also exhibit 2–4 hour ultradian activity rhythms (Calhoun, 1945; Madison, 1985). The location of animals was noted via radio-tracking from 1–3 times per day every day, with a minimum of one hour between subsequent fixes per individual. Animals were tracked at varying times of the day in order to avoid timetabling issues (Kenward, 2001), and between one and three night fixes were performed per animal over the course of each trial. Locations were estimated to within 30 cm. Radio-tracking was performed with a Telonics receiver (Telonics Inc, Mesa, Arizona) and a 3-element yagi antenna via the homing method (Kenward, 2001), by walking a grid of 3m × 3m cells. A minimum of 30 fixes per animal were collected for each trial.
Figure 2.
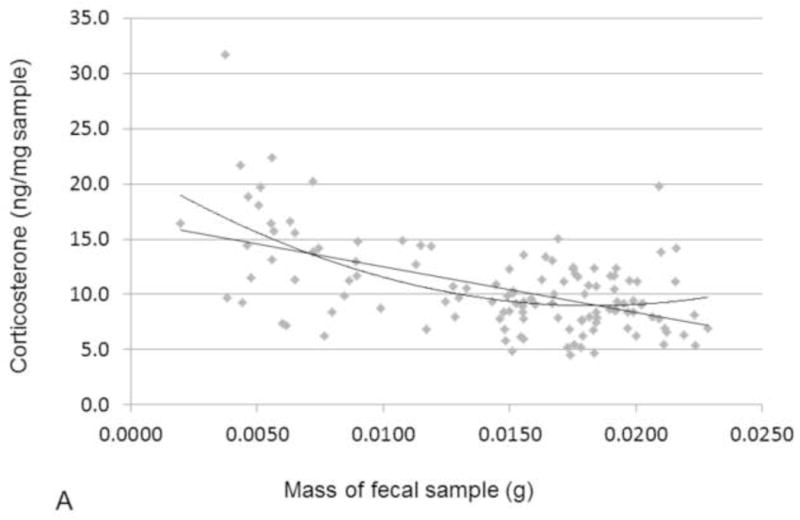
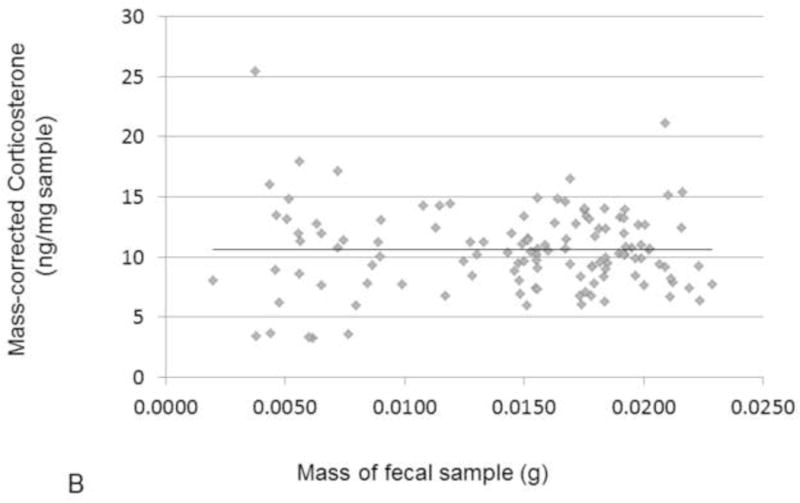
CORT sample-mass effect. A) Effect of sample mass on corticosterone concentration, showing both simple linear regression (p<0.0001, R2 = 0.28) and 2nd order polynomial (p<0.0001, R2 = 0.34. B) Effect of sample mass on mass-corrected corticosterone, simple linear regression (ns).
Figure 4.
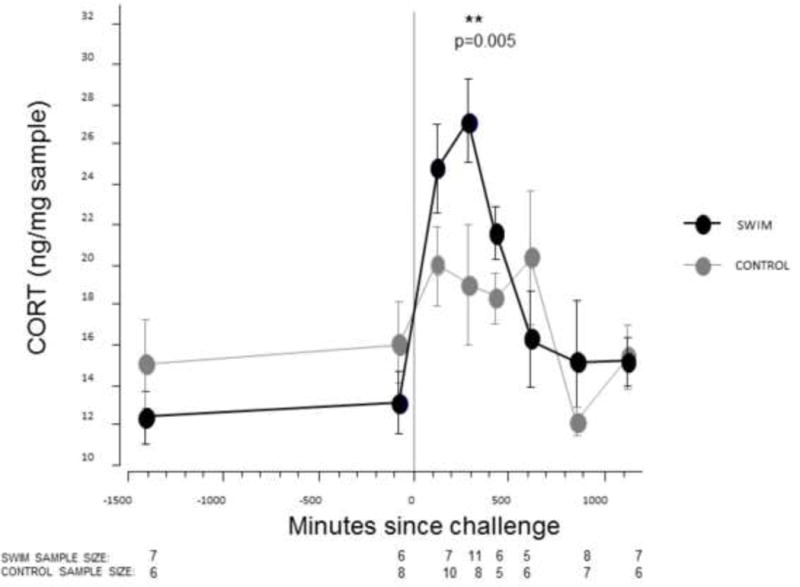
Effects of swim challenge on fecal CORT metabolites. Mean fecal CORT before and after the swim challenge, with each data point representing the midpoint of each sample collection time interval. Data presented as Mean ± SE. “Time zero” indicates the beginning of the swim challenge. Sample sizes are reported below each time window.
On days 8–13 of each trial, feces were collected from subject animals. Animals were live-trapped out between dawn and 1030 hours so as to minimize effects of circadian rhythms on CORT. 192 Sherman live-traps (model LFAHD, 3×3.5×9″) were used. These were placed in appropriate micro-habitats through-out the grid. During trap-outs, traps were checked every 15 minutes to allow calculation of time-of-capture to within 15 minutes for use in fecal CORT analysis. Animals were placed in buckets, and fecal pellets were collected as soon as they were produced, as described in 2.3 Fecal CORT metabolite extraction and radioimmunoassays. Only feces that were collected within 90 minutes of live-trap capture were used in fecal CORT analysis, as otherwise the CORT levels present in the sample may reflect the trapping event. Following fecal collection, subjects were re-released back into the enclosures at the exact location where they had been trapped. On days 19–24 of each trial, all animals were again live-trapped out, and additional feces were collected. CORT was extracted and measured from the feces as described in 2.3 Fecal CORT metabolite extraction and radioimmunoassays. Unless otherwise specified, “field” fecal CORT refers to the combined data from both mid- and end-trial feces collection; if an animal produced feces in both time periods, their CORT values were averaged.
2.5.1 Density manipulation CORT quantification
Due to their large number, samples from the enclosure experiments (n = 69) were run in duplicate over two RIAs (Lot Number RCBK1217 for both). The intra-assay average CV was 1.48%. Forty samples were run in both assays; the inter-assay average CV was 22.1%, and the relationship between the assays was y = 1.1258x + 36.173 (simple linear regression, R2 = 0.93, p < 0.0001).
Although we calculate CORT per mass of sample (ng/mg), there was still a significant effect of sample mass on CORT concentration (simple linear regression, R2 = 0.28, p < 0.0001, Fig. 2A). This is a common pattern across taxa when performing methanol-based fecal CORT extractions on samples less than 0.02g (Millspaugh and Washburn, 2004; Tempel and Gutierrez, 2004). Due to the small size of prairie voles, most of our samples were below this weight. Although the reason for such a sample mass effect is not known, it has been suggested that the effect might be caused by a higher concentration of methanol per unit mass during the extraction process (Millspaugh and Washburn, 2004). We corrected for this effect of mass by identifying the best fit second order polynomial regression (because the effect was mostly at the smaller sample weights; p < 0.0001, R2 = 0.34, Fig. 2A). For each sample, we used the sum of its residuals and the overall mean (from the original uncorrected CORT values) to create a mass-corrected CORT concentration for use in subsequent analyses. There was no effect of sample mass on these new mass-corrected CORT values (simple linear regression, Fig. 2B). All further references to our enclosure study CORT data will imply sample-mass-corrected CORT concentration.
2.5.2 Density manipulation and space use data analysis
Male prairie voles exhibit two alternative mating/space use phenotypes, resident (paired) males who live in close association with a female and (unpaired) wanderers who have larger home ranges that overlap many individuals (Getz and McGuire, 1993; Ophir et al., 2008; Solomon and Jacquot, 2002). Home ranges of individuals in our study were calculated using the Ranges6 program (http://www.anatrack.com) at the 75% kernel contour, which minimizes the distortion caused by rare foraging events that would be otherwise apparent at higher percent contours. A male and female were considered a pair if their home ranges overlapped one another more than they overlapped all other opposite sex home ranges combined (Ophir et al., 2008). For all but one pair identified in the low density, kernel centers of paired animals were also within 15 feet of one another.
Several variables were calculated for each male resident: number of home range overlaps (the number of animals whose home ranges overlapped that of the focal male, calculated by sex of other animals), percent home range overlapped by mate (the percent of the focal male’s home range that was shared with his mate), and percent home range overlapped by other animals of each sex (the cumulative percent of the focal male’s home range that was shared with animals of each sex). All of these were calculated with the Ranges6 overlap analysis function. Due to the relatively small number of male wanderers (n = 6) and extreme behavioral differences between territorial male residents and non-territorial male wanderers, the above calculations were only performed on resident males.
Enclosure space-use measures and fecal CORT values were analyzed for male residents using ANOVAs that included as factors density, trial, and factorial interactions for all predictive and response variables (i.e., CORT levels, home range size and percent overlap by animals of each sex).
3. Results
3.1 Fecal Hormone Assay Validation
3.1.1 Test for assay linearity
Log-logit transformed curves of serially diluted fecal extracts were compared with log-logit transformed standard curves in a test for parallelism (Fig. 3). The three serial dilutions and the standards had a common slope (dilutions vs standard, test for difference between slopes: DF = 3; VR = 0.6; p = 0.60; test for common slope: DF = 1; VR = 2907.6; p < 0.0001; standard curve equation y = −2.08× + 4.68, r2 = 0.996).
Figure 3.
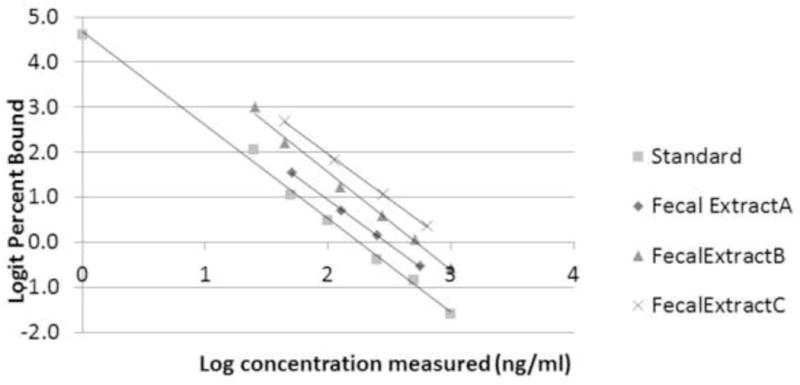
Log-logit transformed curves of serially diluted fecal extracts compared with log-logit transformed standard curves. Replicates (1:4, 1:10, 1:20, 1:40, 1:100, 1:200) are staggered for ease of viewing, but lie directly on the trendline of standard curve. Standard curve: y = −2.080x + 4.676, r2 = 0.996; Total fecal extracts: y = −2.101x + 4.710, r2 = 0.993.
3.1.2 Detecting Acute Stressors
The combined data from the swim challenges (Fig. 4) showed a CORT surge in the swim-treatment animals relative to time-mat Whitney U Test; U = 75, swim-treatment n = 11, control n = 8, p = 0.005). The differences in swim-treatment and control CORT levels in the first and third post-stressor collection intervals also approached significance (one-tailed Mann-Whitney U Test; U = 50.5, p = 0.070; U = 24, p = 0.058, respectively). The second swim challenge did not reveal any significant early CORT surge, and was consistent with the time-frame of the surge in the first swim challenge; as such, we combined the data to improve our statistical power. Note that when restricting the data to just the first swim challenge, we still found a significant difference in the second post-stressor collection interval (one-tailed Mann-Whitney U Test, U = 41, p = 0.019).
3.2 Density Effects on Space Use
We identified 27 males as residents (n = 10 in summer trial, n = 17 in fall trial) and 6 males as wanderers (n = 2 in summer trial, n = 4 in fall trial) in our density manipulation study (Fig. 5). In the combined high-density (HD) treatments, 83% (n = 15) of males were residents, and 17% (n = 3) of males were wanderers. In the combined low-density treatments (LD), 80% (n = 12) of males were residents, and 20% (n = 3) of males were wanderers. Hence, there were no effects of density on the expression of these alternative male tactics (χ2-test, χ2 = 0.30, DF = 1, p = 0.58). These estimates are consistent with male wanderer estimates from other studies, which range from 4% to 40% of the population (Getz and McGuire, 1993; Ophir et al., 2008; Solomon and Jacquot, 2002). We classified 37 females as residents (n = 16 in the summer trial, n = 21 in the fall trial), and 7 females as wanderers (n = 4 in the summer trial, n = 3 in the fall trial). In the combined HD treatments, 94% (n = 15) of females were residents, and 6% (n = 1) of females were wanderers. In the combined LD treatments, 72% (n = 15) of females were residents and 28% (n = 6) of females were wanderers. For females, we did see a density effect on alternative tactics, with more females pairing in the HD treatment (χ2-test, χ2 = 17.15, DF = 1, p < 0.001). As expected (Ophir et al., 2008; Solomon and Jacquot, 2002), male resident home ranges (mean 126 ± 15 SE m2) were significantly smaller than male wanderer home ranges (mean 194 ± 37 SE m2; one-tailed t-test, t31 = 1.866, p = 0.036).
Figure 5.
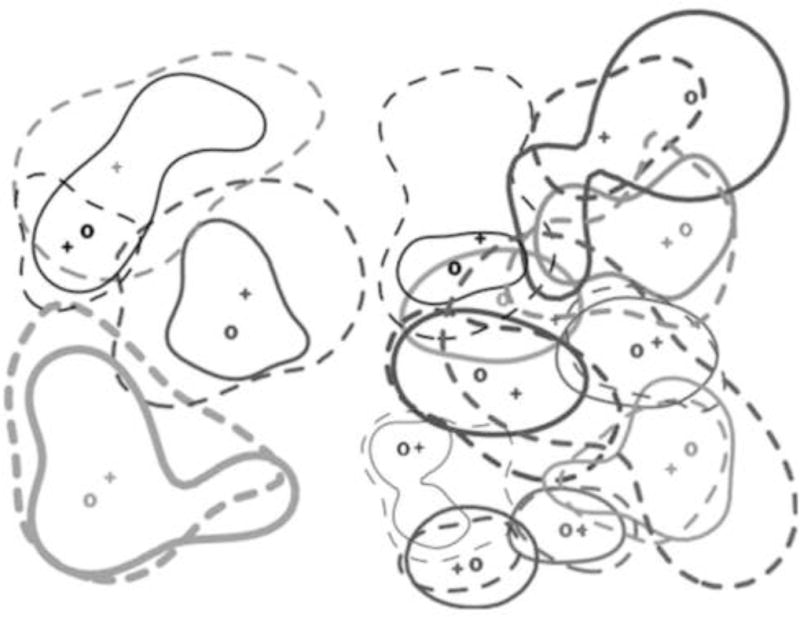
Home ranges (75% kernel contours) for the high-density quadrant and for one low-density quadrant from Trial 2 Dotted lines indicate males, solid lines indicate females. Kernel center (and likely nest site) is indicated by + for males, o for females. Where these would otherwise be overlapping, they have been staggered for visibility. Animals of the same shade have been designated pairs (“residents”) and unique shades are unpaired (“wanderers”).
Eleven males and eight females died during the summer trial, and one male and two females died during in the fall trial. The summer trial mortality was likely due to the record high temperatures in August 2011; there was no evidence of predation. All mortality occurred within the first few days of the beginning of the trial, and was thus unlikely to have had any significant or confounding effect on the space use data of the surviving animals.
The population density treatments had several significant effects on the space use of male residents. HD home ranges were significantly smaller than LD home ranges (mean HD area = 92 ± 14 SE m2, mean LD area = 169 ± 23 SE m2; N = 15 HD, N = 12 LD; ANOVA with density and trial as effect; F1,25 = 8.311; p = 0.008; Fig. 6A). Density also affected the degree of home range overlap male residents experienced. The number of males that overlapped resident male’s home ranges was significantly greater in the high-density treatment than in the low-density treatment (mean HD = 2.5 ± 0.3 SE overlaps, mean LD = 1.6 ± 0.3 SE overlaps; N = 15 HD, 12 LD; ANOVA with trial and density as effects; F2,24 = 4.796; p = 0.01; Fig. 6B). Similarly, a greater number of females overlapped male resident home ranges in the high-density treatment (mean HD = 3.2 ± 0.3 SE overlaps, mean LD = 2.2 ± 0.4 SE overlaps; N = 15 HD, 12 LD; ANOVA with trial and density as effects; F2,24 = 3.671; p = 0.03; Fig. 6C). However, population density did not have any significant effect on cumulative percent overlap by other males, cumulative percent overlap by females excluding the mate, and percent of a resident male’s home range overlapped by his mate. Thus, overall, high density animals were more tightly packed in space, but resident males in particular maintained similar degrees of home range exclusivity.
Figure 6.
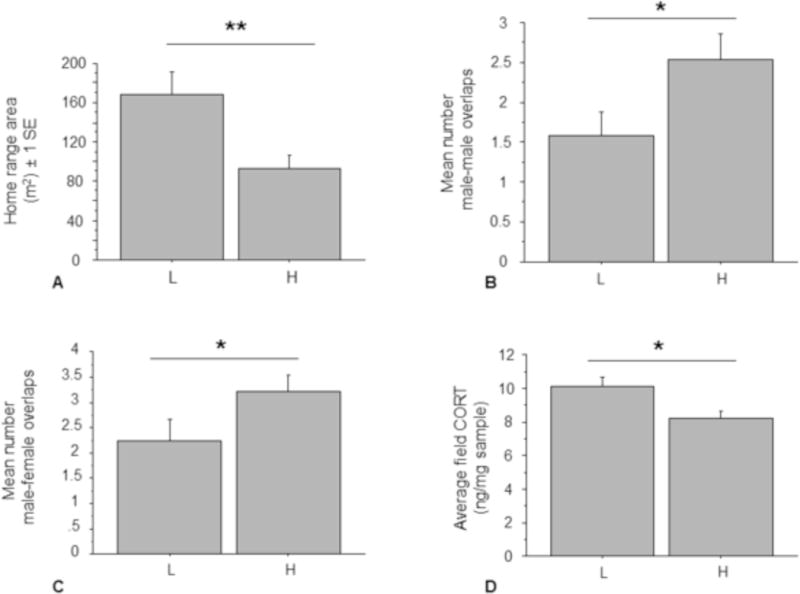
Effect of density on space use and CORT. A) Effect of density on 75% kernel home range area, male residents only. B) Effect of density on number of other males overlapping male resident home ranges. C) Effect of density on number of females overlapping male resident’s home range. D) Effect of density on average field fecal CORT metabolites. * p < 0.05, ** p < 0.01. Mean ±SE indicated, sample sizes indicated on figure.
3.3 Density and Field Effects on Fecal CORT metabolites
There was no significant difference between the average field fecal CORT of male residents and wanders (median resident = 9.4 ± 0.4 SE ng/mg, median wanderer = 10.4 ± 1.3 SE ng/mg). However, the density treatment had a significant effect on male resident fecal CORT levels. The average field-collected male resident fecal CORT levels were significantly lower in the HD treatment than in the LD treatment (mean HD = 8.2 ± 0.5 SE ng/mg, mean LD = 10.1 ± 0.6 SE ng/mg; N = 10 HD, 10 LD; ANOVA with trial and density as effects; F1,18 = 6.825; p = 0.02; Fig. 6D). Finally, the average pre-field male fecal CORT levels were significantly higher than average field-collected male fecal CORT levels, regardless of density treatment (mean pre-field = 10.8 ± 0.7 SE ng/mg, mean field = 9.2 ± 0.4 SE ng/mg; N = 18 pre-field, 20 field; unpaired t-test, t36 = 2.0188, p = 0.05).
4. Discussion
In the current study, we tested the hypothesis that high population densities promoted increases in agonistic encounters and elevated glucocorticoids. In order to test this hypothesis, it was necessary to validate our fecal measures of CORT. Our fecal CORT assay worked successfully, but our results refuted our hypothesis that high population density caused elevations in CORT. We discuss our validation and the results of our population manipulations below.
4.1 Fecal CORT Assay Validation
Our fecal CORT metabolite assay passed the test for parallelism (Fig. 3), and we demonstrated that the assay can detect changes in CORT levels in response to an acute stressor (Fig. 4). The timing of the CORT surge associated with the swim challenge, an acute stressor, suggests a gut-passage time of around five hours, possibly slightly shorter (Fig. 4). Gut-passage time has never been formally measured in prairie voles, but prairie voles exhibit ultradian activity rhythms of 2–4 hours (Calhoun, 1945; Madison, 1985) and digestion studies have suggested that this is due to a digestive bottleneck limiting food intake, which forces voles into short bouts of rest and feeding (Zynel and Wunder, 2002). Researchers intending to use this fecal CORT assay should ideally collect fecal samples as soon as possible but no later than two hours after removing a prairie vole from its home cage (in a lab context) or after live-trapping (in a field context), to avoid fecal CORT values more indicative of the handling/trapping experience than baseline values.
4.2 Density effects on social interaction as measured by space use
We found no effects of density on the probability a male would pair. Similarly, the degree of overlap between a male and his partner was similar across densities, demonstrating a stability of affiliative behavior with a mate even in the face of dramatically different population densities. However, high population density seems to provide more opportunities for extra-pair mating, as density nearly doubled the extent of overlap between home ranges of paired males and extra-pair females (Fig. 5). This is consistent with a prairie vole study that showed increased extra-pair paternity at higher densities in wild populations (Streatfeild et al., 2011).
Density had mixed effects on male-male interactions: males at high densities had smaller home ranges that overlapped more often with other males (Fig. 6A&B), but the fraction of a male’s home range overlapped by other males did not differ with density. Same-sex overlaps occur at the peripheries of home ranges, and may indicate the true boundaries of a defended territory (Maher and Lott, 1995); further, our data are consistent with previous field studies that showed non-residents interacting more at home range peripheries at higher densities (McGuire et al., 1990), yet showing no density-related increase in non-resident nest visits (McGuire and Getz, 1998). Our data suggest that male territories become constricted, but are not necessarily less exclusive at higher densities.
4.3 Density and field effects on CORT
Our density results indicate that, contrary to our predictions, high density animals have significantly lower, not higher CORT levels than low density animals (Figure 6D). Interestingly, this pattern is the opposite from that seen in most other taxa (Christian, 1961; Nelson, 2000), and would seem to conflict with data indicating increased rates of aggressive behavior among male prairie voles from higher densities (Krebs, 1970). Nevertheless, our results are consistent with some prior findings in prairie voles. Researchers found no differences in actual wounding rates of male prairie voles across densities (Rose and Gaines, 1976; Smith and Batzli, 2006), unlike comparable studies of other voles (Boonstra and Boag, 1992). Perhaps more tellingly, Getz et al. (1997) report that animals have longer life expectancy at higher densities. Moreover, in the lab males have higher CORT when housed in low density rooms than in high density rooms (Nelson et al., 1996). Taken together the CORT and longevity data suggest that high densities might actually be favorable environments, suggesting an Allee/Darling effect of population density on population growth (Allee, 1931; Darling, 1938; Lidicker, 2007).
Creel et al. (2013) reviewed the literature on population density and HPA function, and suggested that departures from the typical positive correlation between glucocorticoid secretion and density could be due to confounding effects of seasonality, recent extreme weather events, reproductive condition, or the effects of trapping and handling stress on glucocorticoids. We avoided most of these confounding effects by running our density treatments concurrently. Moreover, our density differences were concordant across seasons. Both of our trials were during the breeding season when females were in reproductive condition, and male-female pairs remained the major social group type, so we did not see, for example, the potentially confounding effects of winter formation of communal groups (Carter et al., 1995). Finally, due to our noninvasive fecal hormone assay method, trapping and handling are unlikely to have affected or confounded our results.
There are a few possible explanations for the pattern of higher CORT levels at lower densities. These are not mutually exclusive, and can be explored with future research. One explanation is that the larger home ranges observed in lower population densities (Fig. 6A) require more energy expenditure to maintain, and the higher CORT is a means of mobilizing glucose reserves to handle the additional energetic requirements. However, the previous lab density manipulations (Nelson et al., 1996), which showed elevated CORT levels at lower densities of singly housed individuals, makes this an unlikely explanation.
A second hypothesis is that at lower densities, finding a potential mate involves increased searching costs (Kokko and Rankin, 2006); rejecting a potential mate means it may cost more in energy and time to find a suitable alternative mate than it would cost at higher densities. Hence, it may be adaptive to reduce the threshold for pairing to avoid more extensive mate-search costs. We know that in male prairie voles, CORT facilitates social preference formation (Blondel et al, unpublished results; DeVries et al., 1996). We did not find density differences in the fraction of animals paired, and this is consistent with other prairie vole fieldwork (Getz et al., 1993; but see Streatfeild et al, 2011). One possible explanation is that the CORT rise facilitates partner preference and thereby compensates for lower densities. This would predict that experimentally impairing or clamping CORT function would unmask a density-dependence in pair formation.
Finally, a third explanation is that high population density signals low per capita predation risk. Annual prairie vole population density cycles seem to be driven primarily by predators, with population booms occurring during late autumn and winter months while some predators are inactive (Getz et al., 1990). The combination of low predator abundance and high population density would lead to a much lower per capita predation rate. From this perspective, the elevated CORT present at low population densities might be part of a general increase in vigilance important in high predation environments. This is consistent with the enhanced longevity exhibited at high densities (Getz et al., 1997), as well as lab data demonstrating that high densities promote lower CORT (Nelson et al., 1996). There is precedence for indirect predator cues to elevate CORT levels in prey populations (reviewed in Clinchy et al., 2013), and this is consistent with the sometimes “preparatory” nature of elevated CORT levels (Sapolsky et al., 2000), in anticipation of predictable future stressors.
One reason for studying CORT levels of resident male prairie voles is that acute stressors are known to facilitate paternal care in the lab (Bales et al., 2006). Our data suggest that males might contribute more to care at lower densities, which could in turn influence the stress reactivity of offspring born at different times of the year. While this is an exciting possibility, it is not clear whether the CORT differences we observe will persist during parental care, or whether CORT would have the same effects on paternal care in the more complex field environment.
In addition to population density differences in CORT, we were also surprised to find that lab-housed animals have significantly higher levels of CORT than they do in an outdoor enclosure. This suggests that our subjects generally experienced lab housing as a stressor, despite the fact that animals were housed with same-sex siblings and had been reared in the lab. Moreover, these animals were subject to agonistic interactions and fluctuations in environmental conditions when placed in the field. To our knowledge, this is first time that this has been demonstrated in prairie voles, and is consistent with studies in other taxa that show lower stress levels in field conditions relative to lab housing (Cooperman et al., 2004; Davis and Maerz, 2011). Our results should be kept in mind when interpreting results of laboratory studies, and should be encouraging to researchers who hope to conduct naturalistic enclosure studies.
4.4 Conclusions
We found that high population density increased measures of same-sex and opposite sex interaction in the field, but lowered the overall level of CORT. Overall, the data suggest that prairie voles may use high population density as a cue to lower predation risk, though several other hypotheses are also plausible. Future experiments are needed to explain why population density has this effect on CORT. Such studies could include sampling wild free-ranging populations at a variety of population densities, across seasons, and while measuring predation risk and fitness correlates such as survivorship and offspring. Regardless of the reason for fluctuations in CORT levels, the fact that density influences CORT levels among prairie voles raises interesting new questions regarding the possible cycling of stress-reactivity as well. By validating an assay for fecal CORT and documenting the effects of density on CORT, we hope to facilitate the future use of prairie voles as integrative models in the study of endocrinology, behavior and evolution.
Highlights.
We validate a non-invasive fecal corticosterone assay for prairie voles
We test the effect of population density on corticosterone in the field
Low population density had significantly higher CORT than high population density
Other space use measures were consistent with previous prairie vole studies
Acknowledgments
H. J. Brockmann, D. Reed, C. Wynne, C. Worman, A. Ophir, P. Campbell, A. George, B. Pasche, J. Mateo, P. Huang, C. Chaffee, M. Slot, J. Pino, & J. Luff for comments. R. Pulcher, M. Doran, S. Phelps, A Berrio, T. Lee, R. Kelly, N. Szczepanik, R. Filippini, K. Waldschmidt, S. Hilber, T. Peterson, & G. Ostrow for support in the field. G. Cua, G. Dahl, J. Hayen, T. Sha, I. Thompson, A. Monteiro, N. Jayasena, O. Crino, A. Duehl, K. Scott, L. Guillette, K. Choe, M. Wayne, C. Baer, K. Kitajima, I. Larkin, R. Kimbell, E. Wang, J. Allen, M. McCoy, J. Ferguson, & M. Salomon for support in the lab. This study was funded by NIH funds awarded to SMP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E. Hormones and Animal Social Behavior. Princeton University Press; Princeton: 2005. [Google Scholar]
- Allee WC. Animal aggregations, a study in general sociology. The University of Chicago Press; Chicago: 1931. [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiology & Behavior. 2006;87:424–429. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Batzli GO. Nutrition. In: Tamarin RH, editor. Biology of New World Microtus. American Society of Mammalogists; Stillwater, Oklahoma: 1985. pp. 779–811. [Google Scholar]
- Becker JB, Breedlove SM, Crews D, McCarthy MM. Behavioral endocrinology. Second. The MIT Press; Cambridge Massachusetts: 2002. [Google Scholar]
- Beehner JC, McCann C. Seasonal and altitudinal effects on glucocorticoid metabolites in a wild primate (Theropithecus gelada) Physiology & Behavior. 2008;95:508–514. doi: 10.1016/j.physbeh.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Boag PT. Spring declines in Microtus pennsylvanicus and the role of steroid hormones. Journal of Animal Ecology. 1992;61:339–352. [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF System Mediates Increased Passive Stress-Coping Behavior Following the Loss of a Bonded Partner in a Monogamous Rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner C. Maternal stress, glucocorticoids, and the maternal/fetal match hypothesis. Hormones and Behavior. 2008;54:485–487. doi: 10.1016/j.yhbeh.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Goldsmith AR. Noninvasive endocrine data for behavioural studies: the importance of validation. Animal Behaviour. 2004;67:183–185. [Google Scholar]
- Calhoun JB. Diel activity rhythms of the rodents, Microtus ochrogaster and Sigmodon hispidus hispidus. Ecology. 1945;26:251–273. [Google Scholar]
- Carter CS, Devries AC, Getz LL. Physiological substrates of mammalian monogamy – the prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Christian J. Phenomena associated with population density. Proceedings of the National Academy of Sciences of the United States of America. 1961;47:428–&. doi: 10.1073/pnas.47.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JJ. The adreno-pituitary system and population cycles in mammals. Journal of Mammalogy. 1950;31:247–259. [Google Scholar]
- Clinchy M, Sheriff MJ, Zanette LY. Predator-induced stress and the ecology of fear. Functional Ecology. 2013;27:56–65. [Google Scholar]
- Cooperman MD, Reed JM, Romero LM. The effects of terrestrial and breeding densities on corticosterone and testosterone levels in spotted salamanders, Ambystoma maculatum. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2004;82:1795–1803. [Google Scholar]
- Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: effects of the social environment. Functional Ecology. 2013;27:66–80. [Google Scholar]
- Crino OL, Larkin I, Phelps SM. Stress coping styles and singing behavior in the short-tailed singing mouse (Scotinomys teguina) Hormones and Behavior. 2010;58:334–340. doi: 10.1016/j.yhbeh.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Crowley PH, Travers SE, Linton MC, Cohn SL, Sih A, Sargent RC. Mate density, predation risk, and the seasonal sequence of mate choices – a dynamic game. Am Nat. 1991;137:567–596. [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conservation Physiology. 2014;2:1–18. doi: 10.1093/conphys/cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling FF. Bird flocks and the breeding cycle: a contribution to the study of avian sociality. Cambridge University Press; Cambridge: 1938. [Google Scholar]
- Davis AK, Maerz JC. Assessing Stress Levels of Captive-Reared Amphibians with Hematological Data: Implications for Conservation Initiatives. J Herpetol. 2011;45:40–44. [Google Scholar]
- DeVries AC, DeVries MB, Taymans S, Carter CS. Modulation of pair bonding in female prairie voles (Microtus ochrogaster) by corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7744–7748. doi: 10.1073/pnas.92.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz LL. Habitats. In: Tamarin RH, editor. Biology of New World Microtus. American Society of Mammalogists; Stillwater, Oklahoma: 1985. pp. 286–309. [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am Scientist. 1996;84:56–62. [Google Scholar]
- Getz LL, McGuire B. A comparison of living singly and in male-female pairs in the prairie vole, Microtus ochrogaster. Ethology. 1993;94:265–278. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) Journal of Mammalogy. 1993;74:44–58. [Google Scholar]
- Getz LL, Oli MK, Hofmann JE, McGuire B. Vole population fluctuations: factors that initiate and determine intervals between them in Microtus ochrogaster. Journal of Mammalogy. 2006;87:387–393. [Google Scholar]
- Getz LL, Simms LE, McGuire B, Snarski ME. Factors affecting life expectancy of the prairie vole, Microtus ochrogaster. Oikos. 1997;80:362–370. [Google Scholar]
- Getz LL, Solomon NG, Pizzuto TM. The effects of predation of snakes on social-organization of the prairie vole, Microtus ochrogaster. Am Midl Nat. 1990;123:365–371. [Google Scholar]
- Good T, Khan MZ, Lynch JW. Biochemical and physiological validation of a corticosteroid radioimmunoassay for plasma and fecal samples in oldfield mice (Peromyscus polionotus) Physiology & Behavior. 2003;80:405–411. doi: 10.1016/j.physbeh.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Goymann W. Noninvasive monitoring of hormones in bird droppings – Physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. In: Bauchinger U, Goymann W, JenniEiermann S, editors. Bird Hormones and Bird Migrations: Analyzing Hormones in Droppings and Egg Yolks and Assessing Adaptations in Long-Distance Migration. New York Acad Sciences; New York: 2005. pp. 35–53. [DOI] [PubMed] [Google Scholar]
- Goymann W, Mostl E, Van’t Hof T, East ML, Hofer H. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. General and Comparative Endocrinology. 1999;114:340–348. doi: 10.1006/gcen.1999.7268. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Sgoifo A, Jepson AJ, Bates SL, Chandler DL, McNeal N, Preihs K. The Integration of Depressive Behaviors and Cardiac Dysfunction During an Operational Measure of Depression: Investigating the Role of Negative Social Experiences in an Animal Model. Psychosomatic Medicine. 2012;74:612–619. doi: 10.1097/PSY.0b013e31825ca8e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Austad SN. Fecal glucocorticoids: A noninvasive method of measuring adrenal activity in wild and captive rodents. Physiological and Biochemical Zoology. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- Heilmann RM, Lanerie DJ, Ruaux CG, Grutzner N, Suchodolski JS, Steiner JM. Development and analytic validation of an immunoassay for the quantification of canine S100A12 in serum and fecal samples and its biological variability in serum from healthy dogs. Veterinary Immunology and Immunopathology. 2011;144:200–209. doi: 10.1016/j.vetimm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Kenward RE. A Manual for Wildlife Radio Tagging. Academic Press; London: 2001. [Google Scholar]
- Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philosophical Transactions of the Royal Society B-Biological Sciences. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ. Microtus population biology – behavioral changes associated with population cycle in M. ochrogaster and M. pennsylvanicus. Ecology. 1970;51:34–&. [Google Scholar]
- Lidicker WZJ. Issues in Rodent Conservation. In: Wolff JO, Sherman PW, editors. Rodent Societies: An Ecological and Evolutionary Perspective. University of Chicago Press; Chicago: 2007. pp. 453–462. [Google Scholar]
- Love OP, McGowan PO, Sheriff MJ. Maternal adversity and ecological stressors in natural populations: the role of stress axis programming in individuals, with implications for populations and communities. Functional Ecology. 2013;27:81–92. [Google Scholar]
- Madison DM. Activity Rhythms and Spacing. In: Tamarin RH, editor. Biology of New World Microtus. American Society of Mammalogists; Stillwater, Oklahoma: 1985. pp. 373–419. [Google Scholar]
- Maher CR, Lott DF. Definitions of territoriality used in the study of variation in vertebrate spacing systems. Animal Behaviour. 1995;49:1581–1597. [Google Scholar]
- Mateo JM, Cavigelli SA. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiological and Biochemical Zoology. 2005;78:1069–1084. doi: 10.1086/432855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo JM, Holmes WG, Bell AM, Turner M. Sexual maturation in male prairie voles – effects of the social environment. Physiology & Behavior. 1994;56:299–304. doi: 10.1016/0031-9384(94)90198-8. [DOI] [PubMed] [Google Scholar]
- McGuire B, Getz LL. The nature and frequency of social interactions among free-living prairie voles (Microtus ochrogaster) Behav Ecol Sociobiol. 1998;43:271–279. [Google Scholar]
- McGuire B, Pizzuto T, Getz LL. Potential for social-interaction in a natural-population of prairie voles (Microtus ochrogaster) Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1990;68:391–398. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE. Use of fecal glucocorticold metabolite measures in conservation biology research: considerations for application and interpretation. General and Comparative Endocrinology. 2004;138:189–199. doi: 10.1016/j.ygcen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Monfort SL, Wasser SK, Mashburn KL, Burke M, Brewer BA, Creel SR. Steroid metabolism and validation of noninvasive endocrine monitoring in the African wild dog (Lycaon pictus) Zoo Biology. 1997;16:533–548. [Google Scholar]
- Mostl E, Rettenbacher S, Palme R. Measurement of corticosterone metabolites in birds’ droppings: An analytical approach. In: Bauchinger U, Goymann W, JenniEiermann S, editors. Bird Hormones and Bird Migrations: Analyzing Hormones in Droppings and Egg Yolks and Assessing Adaptations in Long-Distance Migration. New York Acad Sciences; New York: 2005. pp. 17–34. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An Introduction to Behavioral Endocrinology. Second. Sinauer Associates, Inc; Sunderland, Massachusetts: 2000. [Google Scholar]
- Nelson RJ, Fine JB, Demas GE, Moffatt CA. Photoperiod and population density interact to affect reproductive and immune function in male prairie voles. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1996;270:R571–R577. doi: 10.1152/ajpregu.1996.270.3.R571. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Animal Behaviour. 2008;75:1143–1154. [Google Scholar]
- Palme R, Rettenbacher S, Touma C, El-Bahr SM, Mostl E. Stress hormones in mammals and birds – Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. In: Vaudry H, Roubos E, Schoofs L, Fiik G, Larhammar D, editors. Trends in Comparative Endocrinology and Neurobiology. 2005. pp. 162–171. [DOI] [PubMed] [Google Scholar]
- Parrish JK, Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–101. doi: 10.1126/science.284.5411.99. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Zullo A, Gustafson EA, Carter CS. Perinatal steroid treatments alter alloparental and affiliative behavior in prairie voles. Hormones and Behavior. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. [DOI] [PubMed] [Google Scholar]
- Rose RK, Gaines MS. Levels of aggression in fluctuating populations of prairie vole, Microtus ochrogaster, in eastern Kansas. Journal of Mammalogy. 1976;57:43–57. [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Hormones and Behavior. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology of the Stress-Response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd. The MIT Press; Cambridge, Massachusetts: 2002. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia. 2011;166:869–887. doi: 10.1007/s00442-011-1943-y. [DOI] [PubMed] [Google Scholar]
- Sikes RS, Gannon WL, Amer Soc M. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy. 2011;92:235–253. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JE, Batzli GO. Dispersal and mortality of prairie voles (Microtus ochrogaster) in fragmented landscapes: a field experiment. Oikos. 2006;112:209–217. [Google Scholar]
- Solomon ME. The natural control of animal populations. Journal of Animal Ecology. 1949;18:1–35. [Google Scholar]
- Solomon NG, Jacquot JJ. Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 2002;80:951–955. [Google Scholar]
- Stehn RA, Richmond ME. Male-induced pregnancy termination in prairie vole, Microtus ochrogaster. Science. 1975;187:1211–1213. doi: 10.1126/science.1114340. [DOI] [PubMed] [Google Scholar]
- Streatfeild CA, Mabry KE, Keane B, Crist TO, Solomon NG. Intraspecific variability in the social and genetic mating systems of prairie voles, Microtus ochrogaster. Animal Behaviour. 2011;82:1387–1398. [Google Scholar]
- Taymans SE, DeVries AC, DeVries MB, Nelson RJ, Friedman TC, Castro M, DeteraWadleigh S, Carter CS, Chrousos GP. The hypothalamic-pituitary-adrenal axis of prairie voles (Microtus ochrogaster): Evidence for target tissue glucocorticoid resistance. General and Comparative Endocrinology. 1997;106:48–61. doi: 10.1006/gcen.1996.6849. [DOI] [PubMed] [Google Scholar]
- Tempel DJ, Gutierrez RJ. Factors related to fecal corticosterone levels in California Spotted Owls: Implications for assessing chronic stress. Conservation Biology. 2004;18:538–547. [Google Scholar]
- Touma C, Palme R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. In: Bauchinger U, Goymann W, JenniEiermann S, editors. Bird Hormones and Bird Migrations: Analyzing Hormones in Droppings and Egg Yolks and Assessing Adaptations in Long-Distance Migration. New York Acad Sciences; New York: 2005. pp. 54–74. [DOI] [PubMed] [Google Scholar]
- Vasconcellos AS, Chelini MOM, Palme R, Guimaraes M, Oliveira CA, Ades C. Comparison of two methods for glucocorticoid evaluation in maned wolves. Pesquisa Veterinaria Brasileira. 2011;31:79–83. [Google Scholar]
- Wang DW, Wang ZL, Zhang JX, Zhang JJ, Zhang ZB. Fecal hormone variation during prolonged social interaction in male Tscheskia triton. Physiology & Behavior. 2009;97:347–352. doi: 10.1016/j.physbeh.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Velloso AD, Rodden MD. Using fecal steroids to evaluate reproductive function in female maned wolves. Journal of Wildlife Management. 1995;59:889–894. [Google Scholar]
- Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: When and how. Journal of Neuroendocrinology. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wolff JO, Sherman PW. Rodent Societies: An Ecological and Evolutionary Perspective. The University of Chicago Press; Chicago, Illinois: 2007. [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V-1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zynel CA, Wunder BA. Limits to food intake by the Prairie Vole: effects of time for digestion. Functional Ecology. 2002;16:58–66. [Google Scholar]


