Abstract
Silk-elastinlike protein polymers (SELPs) have been effectively used as controlled release matrices for the delivery of viruses for cancer gene therapy in preclinical models. However, the degradability of these polymers needs to be tuned for improved localized intratumoral gene delivery. Using recombinant techniques, systematic modifications in distinct regions of the polymer backbone, namely, within the elastin blocks, silk blocks, and adjacent to silk and elastin blocks, have been made to impart sensitivity to specific matrix metalloproteinases (MMPs) known to be overexpressed in the tumor environment. In this report we investigated the structure-function relationship of MMP-responsive SELPs for viral mediated gene therapy of head and neck cancer. These polymers showed significant degradation in vitro in the presence of MMPs. Their degradation rate was a function of the location of the MMP-responsive sequence in the polymer backbone when in hydrogel form. Treatment efficacy of the adenoviral vectors released from the MMP responsive SELP analogs in a xenograft mouse model of head and neck squamous cell carcinoma (HNSCC) was shown to be polymer structure dependent. These results demonstrate the tunable nature of MMP-responsive SELPs for localized matrix-mediated gene delivery.
Keywords: Recombinant polymers, Silk-elastinlike polymers, Drug delivery, Matrix metalloproteinase, Cancer
Graphical abstract
1. Introduction
Silk-elastinlike protein polymers (SELPs) are block copolymers composed of amino acid motifs inspired from nature in the form of silkworm silk fibroin (GAGAGS) and mammalian elastin (GVGVP). They are recombinantly produced and synthesized in E. coli using genetic engineering techniques.[1] Uniquely, SELPs are capable of a sol to gel transition in situ utilizing an increase in temperature allowing for ex vivo loading of bioactive agents while maintaining an injectable formulation. The phase transition of these materials is dictated by the ratio and sequence of silk to elastin components. A high degree of control over the polymer sequence using recombinant DNA technology enables the engineering of specific phase transition behavior and physical properties.[2–7] Previously, SELPs have been extensively characterized for their physicochemical properties and drug release characteristics.[8–14] In the context of localized, matrix mediated gene delivery to solid tumors, it has been demonstrated that an analog of SELPs, namely SELP815K (Figure 1), shows capability for localized release of adenoviruses.[15, 16]
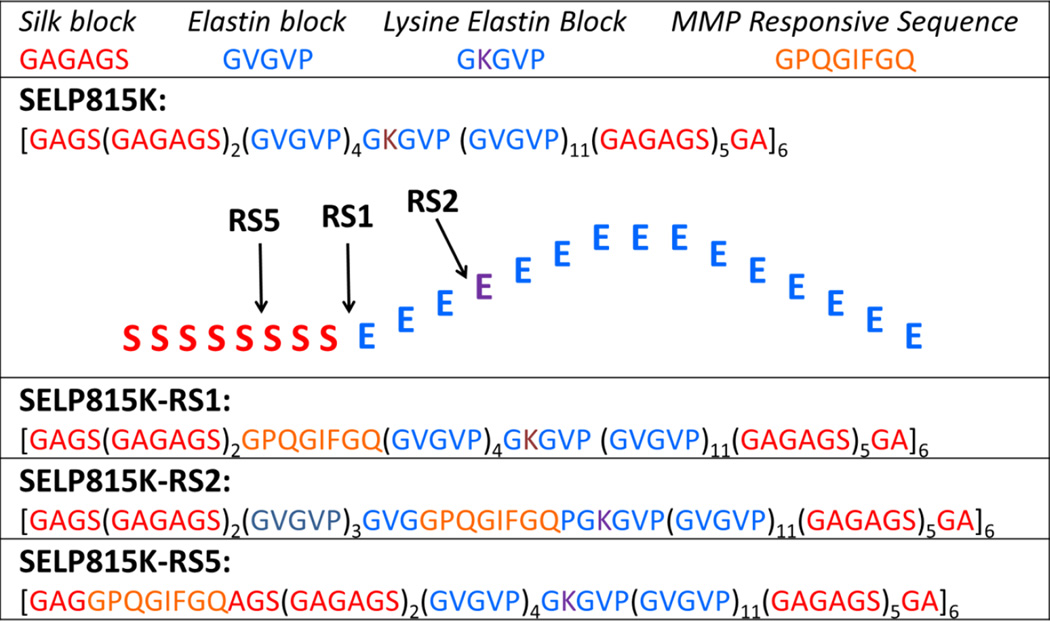
Figure 1.
Single letter amino acid sequences of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 with structural representation of matrix metalloproteinase responsive sequence insertion sites into the SELP815K monomer.
While SELP815K has shown impressive efficacy in adenoviral gene delivery and survival elongation in xenograft models, in vivo resistance to degradation has been observed associated with fibrotic encapsulation when embedded for 12 weeks.[17] In order to promote absorption through more rapid degradation, SELP analogs with peptide sequences known to be readily cleaved by matrix metalloproteinases (MMPs) were synthesized.[18, 19] These insertions were made in both the silk and elastin blocks that represent structurally distinct regions of the polymer backbone and at the junction between these two blocks. By inserting the MMP-responsive sequence in each location, we were able to systematically evaluate the effect of the sequence on physiochemical properties as a function of insert location (Figure 1). A shear conditioning protocol was further developed to strip intramolecular secondary structures to allow for more long term inter-strand interactions and robust hydrogel formation.[19]
MMPs, a family of naturally occurring proteases that function to break down extracellular matrix proteins were selected as the target enzyme due to their frequent over expression in a variety of solid tumors.[20, 21] The influence of the location of the MMP responsive site, GPQGIFGQ in single amino acid code, in the polymer backbone on physiochemical properties was investigated previously as the sequence is known to be cleaved with high efficiency by MMP-2 and MMP-9.[18] It was shown that insertion of foreign sequences into the less structurally important elastin region and at the junction between the silk and elastin regions in SELP815K-RS2 and SELP815K-RS1 (Figure 1), respectively, resulted in little observable structural disruption with only minor increases in swelling ratio, soluble fraction, and rheological properties.[19] Insertion of foreign sequence into the main structural element of SELPs, namely the silk block, termed SELP815K-RS5, resulted in drastically increased swelling ratio, soluble fraction, minimum gel forming concentration, and poor rheological properties. Only through physical conditioning with high shear stress was SELP815K-RS5 capable of being used as a controlled release matrix at typical concentrations of 4–12% wt/wt. Here we build upon those findings to report the influence of polymer structure on in vitro degradation of the three MMP responsive SELP analogs (Figure 1) as well as on efficacy of matrix mediated viral gene delivery in a tumor xenograft model of head and neck squamous cell carcinoma (HNSCC).
Building on previous work in our lab, an adenovirus carrying the herpes simplex thymidine kinase (HSVtk) and luciferase genes was chosen for gene-directed enzyme prodrug therapy (GDEPT) in the in vivo efficacy studies.[22] Briefly, viral infection of the HNSCC cells leads to expression of HSVtk, which phosphorylates the injected prodrug ganciclovir into ganciclovir phosphate, a potent DNA synthesis inhibitor acting through chain termination. Cell proliferation is inhibited, ultimately causing cell death and tumor regression. Luciferase expression allows for bioluminescent tracking of viral expression via light generated from interaction with luciferin. Controlled release of the viral particles used from GDEPT has previously shown to increase effectiveness of the treatment. In this report controlled release from the structurally related MMP responsive SELP analogs is investigated.
2. Materials and Methods
2.1 Materials
SELP815K[9], SELP815K-RS2[18], SELP815K-RS1, and SELP815K-RS5[19] (Figure 1) were synthesized, purified and characterized as previously described. Materials for Lowry assay were purchased from Thermo Fisher Scientific (Waltham, MA). Matrix metalloproteinases were obtained from EMD Millipore (Billerica, MA). Replication deficient human adenovirus, containing E1 and E3 deletions, and the genes for thymidine kinase and firefly luciferase (Ad.Luc.HSVtk) were obtained from Vector Biolabs (Malvern, PA). Luciferin was obtained from Gold Biotechnology (St. Louis, MO). 6 week old female athymic nu/nu mice were acquired from Charles River Laboratories (Wilmington, MA). JHU-022 human oral cancer cell line was a generous gift from Prof. David Sidransky of Johns Hopkins University. Live bioluminescence imaging was performed using a Xenogen IVIS100 system from Caliper Life Sciences (Hopkinton, MA). Roswell Park Memorial Institute Advanced 1640 medium (RPMI 1640 Advanced) and supplements were purchased from Invitrogen (Carlsbad, CA). Fetal Bovine Serum was obtained from HyClone (Logan, UT). The prodrug Ganciclovir (GCV) and all other chemicals were purchased from Sigma–Aldrich (St. Louis, MO). Tissue fixation, embedding, and histological staining were performed by ARUP Laboratories (Salt Lake City, UT). Assistance in histology interpretation was provided by Animal Reference Pathology (Salt Lake City, UT).
2.2 Methods
2.2.1 Soluble Polymer MMP Digests
Lyophilized stocks of each SELP analog were solubilized to a concentration of 5mg/mL as a stock solution in deionized water. Each stock solution was formulated to a final concentration of 1mg/mL in MMP reaction buffer consisting of 50 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2, and 40nM MMP-2 at pH 7.4. Reactions were incubated at room temperature (23° C) and samples were taken at 0, 10, 20, 30, 60, and 120 minutes. Samples were immediately mixed with SDS-PAGE sample buffer (Laemmli Buffer) and heated to 95° C for 5 minutes to completely inactivate enzymes. Samples were then loaded onto a 20% SDS-PAGE protein gel and run for approximately 2 hours. Gels were washed in deionized water, stained with Coomassie Brilliant Blue gel stain, and destained in 10:40:50 acetic acid:methanol:water. Stained gels were imaged in a UVP Bioimaging system.
2.2.2 Hydrogel MMP Digests
Freshly thawed SELP stocks at 12% were diluted to 4%, 5%, or 8% wt/wt and loaded into 0.5mL syringes. Syringes were incubated overnight at 37° C to allow for complete gelation. Each syringe tip was removed with an autoclaved razor blade and 25uL gel disks were cut. Each 25uL disk was placed into a separate well of a 96 well plate in 125uL of MMP reaction buffer of the above composition. Reaction buffer with MMP-2 was completely removed and replaced every 36 hours for 14 days. Total soluble polymer released was assayed via modified Lowry assay and summed over the study period.[23] Swelling ratio was determined by the ratio of wet weight to dry weight with gel disks dried by lyophilization over 3 days.
2.2.3 In Vivo Polymer Evaluation in Xenograft HNSCC Model
JHU-022 human head and neck squamous cell carcinoma was first expanded from frozen cell stocks using RPMI 1640 medium supplemented with 10% FBS and 100mM L-glutamine. Cells were harvested and suspended in cold phosphate buffered saline. Female athymic nu/nu 6 week old mice were injected subcutaneously with 2*106 cells suspended in 200uL into the right flank under anesthesia using a 1mL syringe with a 26G needle. Cells were allowed to grow for approximately 10 days until tumor size was roughly 5mm by 5mm to allow for intratumoral injection. Each SELP analog was thawed and diluted as above with adenovirus (Ad.Luc.HSVtk) and 0.9% NaCl injection saline to a final concentration of 5*108 pfu per 25uL injection and loaded into 0.5mL syringes with affixed 28G needles. On day 0, 25uL of each SELP analog with virus, virus alone, or PBS were injected into each tumor. On days 1 through 28, ganciclovir was injected intraperitoneally at a dose of 25mg/kg daily. Tumor size measurement was performed biweekly using calipers on the outside of each mouse with tumor volume computed by the formula: length*width*width/2. Bioluminescent quantification was performed via injection of luciferin followed by imaging using a Xenogen IVIS 100 under anesthesia. Animals were monitored for weight, tumor size, and health daily. Any animal exhibiting signs of pain or distress, hunched posture, vocalization, isolation from the group, greater than 10% weight loss, greater than 2000mm3 tumor burden, or ulceration of the skin at the tumor site were humanely euthanized using carbon dioxide. Tumor tissues were taken at necropsy after euthanasia or at day 50 for complete term animals and fixed in 10% neutral buffered formalin. Tissues were prepared, embedded, processed, and stained by ARUP laboratories.
2.3 Analysis
2.3.3 Statistical analysis
Student’s T-test was used to compute statistical significance between two groups. One way ANOVA with a Tukey or Bartlett post-test was used to compute statistical analysis of groups of 3 or more. A value of p ≤ 0.05 was considered statistically significant and p ≤ 0.01 was considered highly statistically significant. All error bars displayed represent standard deviation of the mean unless otherwise stated.
3. Results
3.1 Soluble Polymer MMP digests
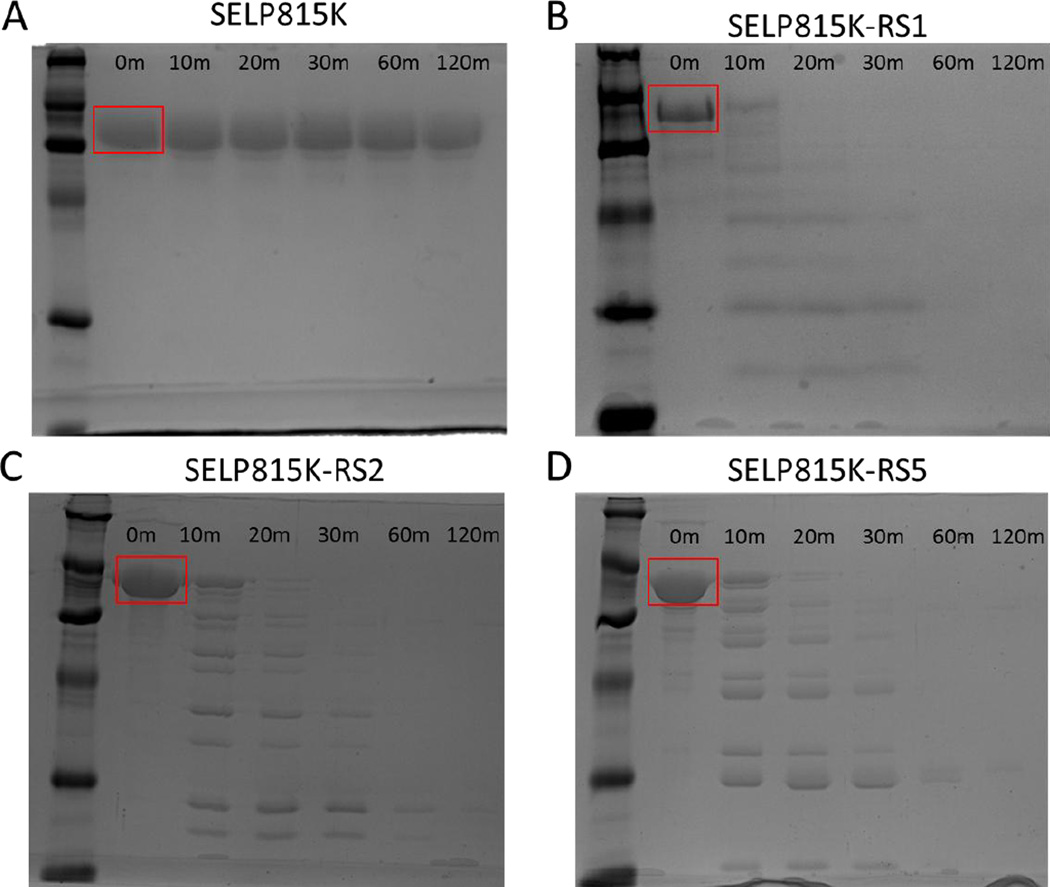
Upon digestion of soluble SELP815K and MMP-responsive SELPs with 40nM MMP-2, SDS-PAGE analysis showed rapid digestion and breakdown of MMP-responsive SELPs while SELP815K was resistant to degradation. Figure 2 shows a clear single band for all polymers at T=0 minutes, representing intact linear polymers. At T=10 minutes, SELP815K continued to show a clear single band while extensive laddering of the MMP-responsive SELPs was visible representing smaller molecular weight fragments of each polymer. At T=60 minutes, each responsive polymer was approaching complete digestion with higher molecular weight bands no longer visible. The gel showing SELP815K continued to have a single band representing intact polymer strands through the 120 minute experiment.
Figure 2.
Digest of soluble (A) SELP815K, (B) SELP815K-RS1, (C) SELP815K-RS2, and (D) SELP815K-RS5 at a concentration of 1mg/mL with 40nM MMP-2 at room temperature for 0, 10, 20, 30, 60, and 120 minutes visualized by SDS-PAGE in 20% polyacrylamide gels. Ladder in lane one on all gels is PAGEmark™ protein marker with visible bands representing from bottom 16kD, 20kD, 32kD, 48kD, 67kD, and 110kD. Full length polymer band is indicated by red box.
3.2 Hydrogel MMP digests
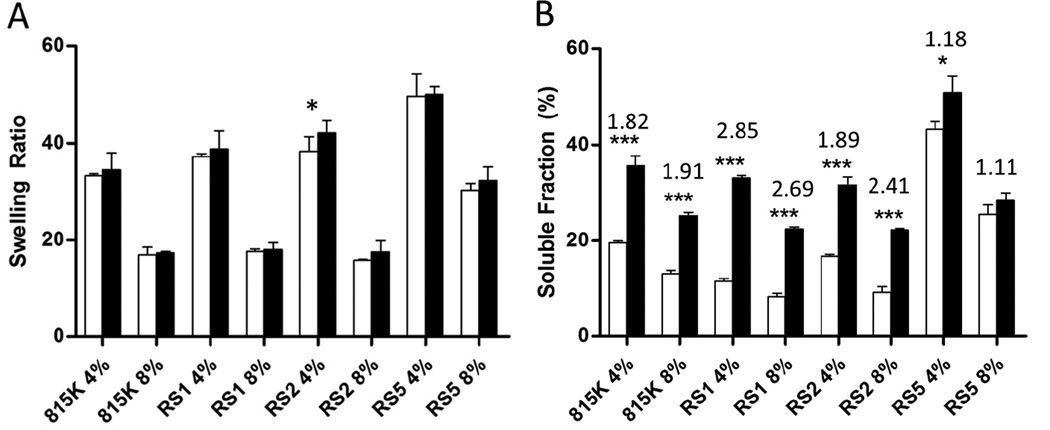
Digestion of SELP hydrogels with 40nM MMP-2 resulted in quantifiable change to both the swelling ratio and soluble fraction released at 14 days. Across all polymers and concentrations, the swelling ratio increased after digestion with MMP-2 as seen in Figure 3A (black bars indicate MMP-2 digested samples). However, these observed increases in swelling ratio were not statistically significant except in the case of SELP815K-RS2 4%. Soluble fraction released by each of the gels increased by digestion with significance or high significance achieved for all except SELP815K-RS5 8 wt%. The lowest increase was observed for SELP815K-RS5 with less than a 20% increase observed at both 4 wt% and 8 wt%. SELP815K showed an increase of up to 91% for both concentrations. The highest increases in degradation were observed for SELP815K-RS1 and SELP815K-RS2 with up to 185% increase in soluble polymer released from the hydrogels following digestion. Qualitatively, all MMP-responsive SELP hydrogels exhibited lower mechanical stability as demonstrated by the difficulty in handling the samples post-digestion.
Figure 3.
Digest of 25uL hydrogel s in 125uL of reaction buffer of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 with 40nM MMP-2 at room temperature for 14 days. White bars indicate undigested samples. Black bars indicate samples digested with MMP-2. Swelling ratio (A) defined as (wet weight/dry weight). Soluble fraction (B) assayed by modified Lowry assay, above each polymer type and weight percent is the ratio of increase of soluble fraction due to digestion. 815K:SELP815K, RS1:SELP815K-RS1, RS2:SELP815K-RS2, RS5:SELP815K-RS5. ***- p≤0.001, *-p≤0.05
3.3 In Vivo Polymer Evaluation
Each polymer was tested as a matrix for localized, viral mediated, adenoviral gene delivery in head and neck tumor models. The SELP analogs were evaluated for their ability to mediate burst release of virus and prolong gene expression as assayed by bioluminescence, tumor size reduction, and increase in median survival. These structurally related polymers were further assayed for degradation in vivo as observed in histology post necropsy.
3.3.1 In Vivo Efficacy in Xenograft HNSCC Model
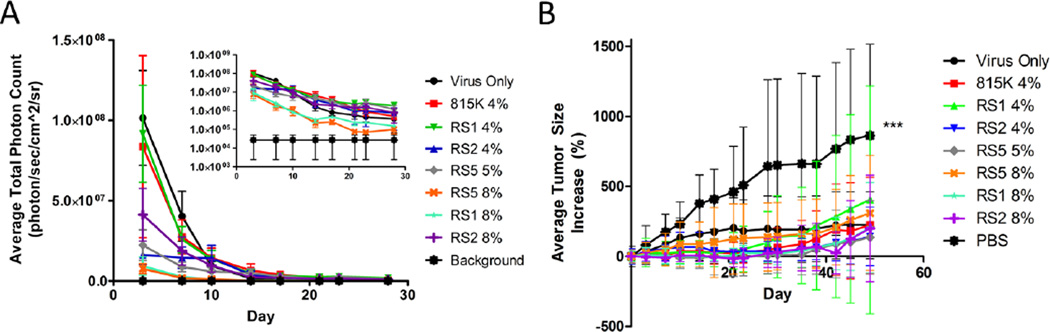
The gene expression observed as measured by bioluminescence and displayed in Figure 4A on a logarithmic scale (inset) indicated all treatment groups maintained gene expression over 28 days 10 to 1000 fold over background bioluminescence indicating adequate expression during the treatment period. As observed in the main body graph, burst expression was evident in the virus only, SELP815K, and SELP815K-RS1 4 wt% groups. Other treatment groups had lower burst release at the onset of the study with most groups normalizing to a more steady state release by day 14. No significance was obtained from bioluminescence data likely due to the high variability of the measurement technique.
Figure 4.
In vivo evaluation of (A) prolonged viral gene expression by bioluminescence generated by luciferase and (B) tumor size reduction by SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 matrix mediated local delivery. 815K:SELP815K, RS1:SELP815K-RS1, RS2:SELP815K-RS2, RS5:SELP815K-RS5. ***- p≤0.001 between control (PBS) group and treatment groups by one way ANOVA with Bartlett post-test. (A)Data displayed on normal (main graph) and log scale (inset). Error bars represent standard error of mean.
The average tumor size increase of each group (Figure 4B) indicates tumor suppression was accomplished in all treatment groups. Statistical significance was achieved between all treatment groups and the PBS control. The treatment groups maintaining the lowest average tumor size were SELP815K 4%, SELP815K-RS2 4%, SELP815K-RS1 8%, and SELP815K-RS2 8% although statistical significance was not achieved between individual treatment groups. Removal of animals due to health concerns or death prior to the end of study resulted in apparent growth stagnation in some groups such as virus only and SELP815K-RS2 4% as further evidenced in Figure 5.
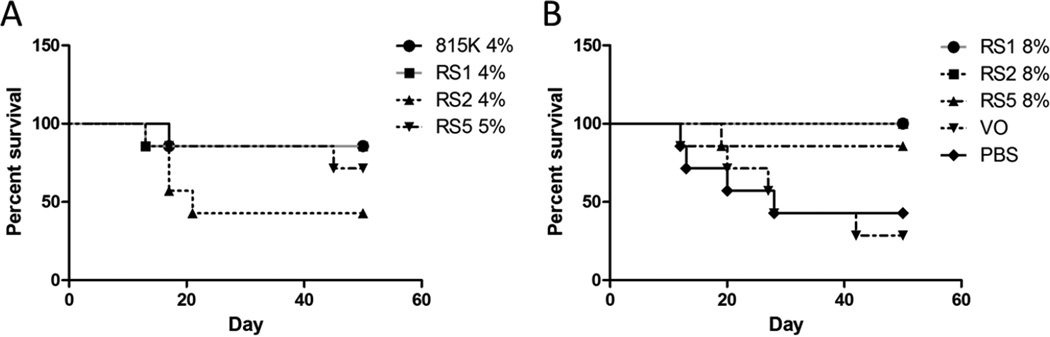
Figure 5.
Prolonged survival of animals treated with SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 matrix mediated local delivery to solid tumor xenografts at (A) 4% wt/wt, 5% RS5 and (B) 8% wt/wt. Groups split for clarity. 815K:SELP815K, RS1:SELP815K-RS1, RS2:SELP815K-RS2, RS5:SELP815K-RS5. *- p≤0.05 achieved by Mantel-Cox test. Survival was defined as absence of death, pain or distress, greater than 10% weight loss, greater than 2000mm3 tumor burden, or ulceration of the tumor site
Survival prolongation observed in the treatment groups are shown in Figure 5. Survival curves are divided between high and low weight percent test groups for clarity. SELP815K-RS1 8% and SELP815K-RS2 8% are the only groups to maintain 100% survival over the course of the 50 day study, and significance was achieved by the Mantel-Cox test. SELP815K 4%, SELP815K-RS1 4%, and SELP815K-RS5 8% maintained survival of approximately 86% throughout the study corresponding to a single death in a 7 mouse cohort. Virus only, PBS control, SELP815K-RS2 4% faired significantly worse with 29% to 43% survival.
3.3.2 In Vivo Polymer Degradation
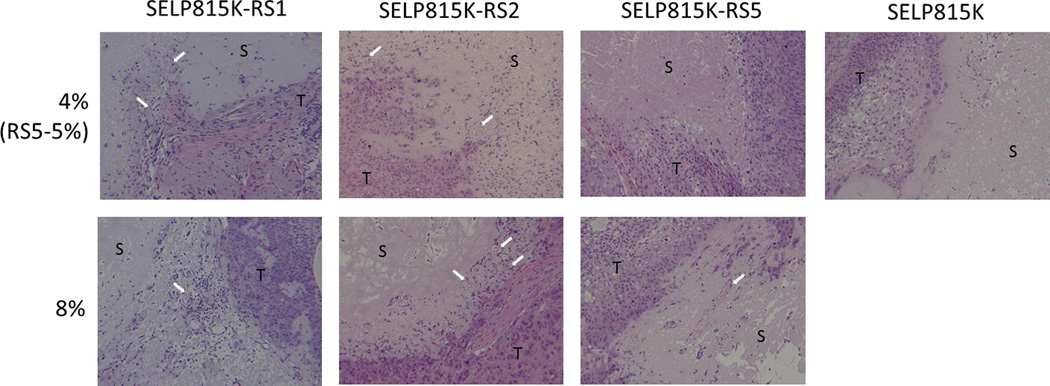
Histological examination of the polymer depots after 50 days of implantation revealed several key findings (Figure 6). In the SELP815K 4% group, limited cellular infiltration into the bulk material was observed, however a clear border region was seen between the SELP and tumor tissue regions with little apparent erosion or invagination into the bulk material by adjacent tumor tissue. In contrast, the MMP-responsive SELPs at 4% showed much higher cellular infiltration along with less clearly defined and more tortuous border region showing a mixture of hydrogel and tumor tissue. Neoplastic tissue showed penetration into the biomaterial in the MMP-responsive SELP groups. In the 8% MMP-responsive SELPs, the border regions were more clearly defined with lower cellular infiltration than 4% groups. However, there was still clearly tissue encroachment into the hydrogel as evidenced by advancing tumor tissue at the border of the hydrogel depots. Another more interesting finding was the presence of vasculature in the hydrogel in all MMP-responsive SELPs except SELP815K-RS5 at 5% not seen in any SELP815K samples as indicated by white arrows in Figure 6.
Figure 6.
Post necropsy histological evaluation of SELP815K, SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 by hematoxylin and eosin staining after 50 days of implantation. White arrows indicate vascular infiltration of the hydrogels. S: SELP hydrogel, T: tumor tissue. All images captured at 100× magnification using light microscope.
4. Discussion
Insertion of an MMP-responsive sequence in the repeating structure of SELP815K increased its degradation in the presence of MMPs. This was observed in vitro by the fragmentation of soluble polymers following incubation in MMP-2 not seen in SELP815K, and the increase in soluble protein liberated from MMP-responsive hydrogels following incubation with MMP-2. In vivo the tortuous border and penetration of vasculature into the hydrogel implants observed in MMP-responsive SELPs indicated a tumor associated degradation mechanism. Of additional note was the necessary increase in polymer concentration from SELP815K to MMP-responsive SELPs in order to impart similar efficacy and prolong survival in a model of HNSCC. This increase in concentration showed a decrease in burst release while maintaining steady state release after initial implantation. Two factors are likely the cause of this variance. First is the perturbation of structure caused by the insertion of the MMP responsive site leading to changed mechanical properties[19] and second, the increased degradation of the MMP-responsive polymer depots.
In their soluble states, the MMP-responsive SELPs, namely SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5, were degraded rapidly in the presence of MMP-2. This was in contrast to SELP815K which showed no evident fragmentation after incubation in MMP-2 for 2 hours. This indicated that the presence of the inserted sequence was sufficient to confer proteolytic cleavage by MMP-2 at any of the three positions in the SELP primary sequence. Moreover, the MMP-responsive SELPs degraded at a similar rate indicating that the site of insertion of the MMP-responsive sequence did not affect the cleavage rate.
As hydrogels each of the SELPs showed increased swelling ratio following digestion with MMP-2 relative to SELP815K. Soluble polymer release during degradation increased significantly compared to SELP815K for all polymers except SELP815K-RS5. There was an increase in the soluble fraction of SELP815K also, but this was likely due to the low-level non-specific gelatinase activity of MMP-2 over the long study time of 14 days. The polymer release from SELP815K-RS1 and SELP815K-RS2 gels was greater than SELP815K-RS5. A likely explanation is the location of the responsive site. The responsive site of SELP815K-RS5 resides within the silk block of the SELP monomer, the main structural unit of the hydrogel network. The associated hydrogen bonding of the silk blocks could physically impede access of MMP-2 to the responsive site, unlike SELP815K-RS1 and SELP815K-RS2 where the response sequences may be more accessible at the junction of the silk and elastin blocks and within the elastin block, respectively. The difference in degradability of SELP815K-RS5 between the soluble and hydrogel states is direct evidence of the effect higher order structure may have on the chemical attributes of SELPs. This evidence is further supported by the change in physical properties of SELP815K-RS5 even in the absence of degradation, for example the increased swelling ratio, soluble fraction release, and minimum gel forming concentration.[19]
MMP-responsive SELPs improved matrix mediated viral gene therapy. Bioluminescence data indicated a decrease of initial burst expression with 8% MMP-responsive SELPs due to the increase in concentration required to achieve similar tumor suppression and survival prolongation outcomes. The reduction in burst release also proved to be an indicator of performance in tumor size suppression. Interestingly, the luciferase expression reported throughout the study converged as the study progressed regardless of treatment modality except for the control group which received no adenoviral injection. This is likely due to the dichotomy of the adenoviral reporter and therapeutic system. The higher the achieved expression of adenoviral constructs within tumor tissue, the greater the presence of luciferase and HSVtk. This should result in higher luciferase signal, and increased efficacy. However, increased efficacy is due to greater cell death, and consequently the loss of cells which can express luciferase. This will attenuate the bioluminescence signal resulting in negative feedback between the luciferase reporter and the therapeutic gene. Due to this, the most accurate reporter of adenoviral expression is tumor size reduction, although luciferase expression can yield additional clues to the temporal release patterns of each treatment system.
Incorporation of MMP-responsive sequences within the polymer backbone did not negatively impact the ability of SELP hydrogels to deliver adenovirus to solid tumors as observed in tumor size measurements. While significance was not achieved between individual treatment groups, the most effective polymers at suppressing tumor growth were SELP815K 4%, SELP815K-RS2 4%, SELP815K-RS1 8%, and SELP815K-RS2 8%. Within the context of overall survival, SELP815K-RS1 8% and SELP815K-RS2 8% achieved 100% survival over the 50 day study, with significance achieved by the Mantel-Cox test over all other groups. The least effective controlled release group, SELP815K-RS2 4%, had the majority of animals removed from the study due to ulceration of the tumor site rather than excessive tumor burden. This could be interpreted as excessive therapeutic gene expression in a short time frame which could lead to destruction of surrounding tissue including the skin. This interpretation is fortified by the gene expression measurements in Figure 4A showing the highest gene expression in SELP815K-RS2 4% except for virus only injection. Further, the variance in survival between the controlled delivery groups can likely be traced to the range of expression levels seen between the groups. This was expected as past studies have shown that an increase in polymer concentration slows the expression profile exhibited by the depot system.[24]
Enhanced in vivo degradation of the MMP-responsive SELPs was apparent from the observed increase in tumor cell infiltration into the hydrogel. Typical hallmarks of in vivo gel degradation in a non-tumor environment include the presence of active inflammatory cells at the implant border including macrophages, monocytes, and lymphocytes, whereas a non-degradable implant would more likely be surrounded by less active cells such as histiocytes and fibroblasts. However, in the tumor microenvironment, which is known to be immune suppressed,[25] the primary signs of gel degradation are from the tumor tissue itself. As observed previously,[17] SELP815K 4% showed little evidence of degradation in vivo as demonstrated by a clear border region between tumor tissue and gel implant with fibrosis and limited cellular infiltration. To the contrary, all MMP-responsive SELPs at 4% or 5% showed increased cellular infiltration as well as apparent encroachment of neoplastic tissue into the biomaterial as visualized by a tortuous appearance of border regions containing both tissue and biomaterial. These observations represent the successful application of a hydrogel responsive to tumor invasion as increased degradation of the hydrogel will increase the release rate of constrained adenoviruses.
Increased vascularization was also identified in many of the MMP-responsive hydrogel samples as evidenced by the appearance of vessels carrying red blood cells within the gel mass. The vasculature may be tumor associated, which is driven in part by the production of MMPs.[26] This was consistent with the observed increase of degradation of the MMP-responsive hydrogels by an MMP-mediated mechanism. These findings further the conclusion that the in vivo degradation is occurring in MMP-responsive SELPs due to interaction with host tissues. Additionally, these results represent a potential for the use of MMP responsive SELPs as a biomaterial in applications which require remodeling of the material such as wound healing as evidenced by vascular penetration into the gel mass.
5. Conclusion
SELP815K along with three MMP-responsive analogs, namely SELP815K-RS1, SELP815K-RS2, and SELP815K-RS5 were evaluated for degradation in vitro in the presence of MMP-2 and in vivo in the presence of head and neck cancer solid tumors. All MMP-responsive SELPs showed increased degradation in the presence of MMP-2 in both soluble and hydrogel forms. SELP815K-RS1 and SELP815K-RS2 showed significantly higher degradation compared to SELP815K while the likely steric hindrance of the silk block insertion site of SELP815-RS5 in the hydrogel form limited the impact of MMP-2 digestion. SELP815K-RS1 and SELP815K-RS2 at 8% wt/wt showed the most effective cancer treatment as a matrix to mediate viral gene therapy by increasing survival from 29% to 100% over 50 days compared to the control group and maintaining lower tumor sizes. Further, histological examination of polymer depots following implantation revealed evidence of degradation in MMP-responsive SELPs not seen in SELP815K. The next logical steps in this research are to evaluate the in vivo fate of degradation products of the polymers and the safety and efficacy of lead compositions in translational preclinical models of head and neck cancer.
Acknowledgements
Financial Support was provided by the NIH (2R01-CA107621, F30 CA176922), Utah Science Technology and Research (USTAR) Initiative, University of Utah Intramural Research Instrumentation Grant, and Pharmaceutical Research Manufacturers of America Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnology Progress. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 3.Frandsen JL, Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chemical Society Reviews. 2012;41(7):2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- 4.MacEwan SR, Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94(1):60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- 5.Qi Y, Chilkoti A. Growing polymers from peptides and proteins: a biomedical perspective. Polymer Chemistry. 2014;5(2):266–276. [Google Scholar]
- 6.Ngo JT, Tirrell DA. Noncanonical amino acids in the interrogation of cellular protein synthesis. Accounts of Chemical Research. 2011;44(9):677–685. doi: 10.1021/ar200144y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson JA, Lu YY, Van Deventer JA, Tirrell DA. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Current Opinion in Chemical Biology. 2010;14(6):774–780. doi: 10.1016/j.cbpa.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cresce AW, Dandu R, Burger A, Cappello J, Ghandehari H. Characterization and real-time imaging of gene expression of adenovirus embedded silk-elastinlike protein polymer hydrogels. Molecular Pharmaceutics. 2008;5(5):891–897. doi: 10.1021/mp800054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandu R, Cresce AV, Briber R, Dowell P, Cappello J, Ghandehari H. Silk-elastinlike protein polymer hydrogels: Influence of monomer sequence on physicochemical properties. Polymer. 2009;50(2):366–374. [Google Scholar]
- 10.Dandu R, Ghandehari H, Cappello J. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. Journal of Bioactive and Compatible Polymers. 2008;25:441–454. [Google Scholar]
- 11.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Progress in Polymer Science. 2007;32(8–9):1008–1030. [Google Scholar]
- 12.Dinerman AA, Cappello J, El-Sayed M, Hoag SW, Ghandehari H. Influence of solute charge and hydrophobicity on partitioning and diffusion in a genetically engineered silk-elastinlike protein polymer hydrogel. Macromolecular Bioscience. 2010;10(10):1235–1247. doi: 10.1002/mabi.201000061. [DOI] [PubMed] [Google Scholar]
- 13.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002;23(21):4203–4210. doi: 10.1016/s0142-9612(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 14.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Solute diffusion in genetically engineered silk–elastinlike protein polymer hydrogels. Journal of Controlled Release. 2002;82(2–3):277–287. doi: 10.1016/s0168-3659(02)00134-7. [DOI] [PubMed] [Google Scholar]
- 15.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: from molecular definition to controlled gene expression. Journal of Controlled Release. 2009;140(3):256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafson JA, Ghandehari H. Silk-elastinlike protein polymers for matrix-mediated cancer gene therapy. Advanced Drug Delivery Reviews. 2010;62(15):1509–1523. doi: 10.1016/j.addr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Price R, Gustafson J, Greish K, Cappello J, McGill L, Ghandehari H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. International Journal of Pharmaceutics. 2012;427(1):97–104. doi: 10.1016/j.ijpharm.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafson JA, Price RA, Frandsen J, Henak CR, Cappello J, Ghandehari H. Synthesis and characterization of a matrix-metalloproteinase responsive silk–elastinlike protein polymer. Biomacromolecules. 2013;14(3):618–625. doi: 10.1021/bm3013692. [DOI] [PubMed] [Google Scholar]
- 19.Price R, Poursaid A, Cappello J, Ghandehari H. Effect of shear on physicochemical properties of matrix metalloproteinase responsive silk-elastinlike hydrogels. Journal of Controlled Release. 2014;195:92–98. doi: 10.1016/j.jconrel.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews: Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 21.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson JA, Price RA, Greish K, Cappello J, Ghandehari H. Silk-elastin-like hydrogel improves the safety of adenovirus-mediated gene-directed enzyme-prodrug therapy. Molecular Pharmaceutics. 2010;7(4):1050–1056. doi: 10.1021/mp100161u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 24.Gustafson J, Greish K, Frandsen J, Cappello J, Ghandehari H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: From molecular definition to controlled gene expression. Journal of Controlled Release. 2009;140(3):256–261. doi: 10.1016/j.jconrel.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V. Seminars in Cancer Biology. Elsevier; 2012. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. [DOI] [PubMed] [Google Scholar]
- 26.Rundhaug JE. Matrix metalloproteinases and angiogenesis. Journal of cellular and molecular medicine. 2005;9(2):267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]